Introduction
Cervical cancer remains one of the most prevalent
gynecological malignancies among women, ranking fourth globally in
terms of both incidence and mortality (1,2). It is
the second leading cause of cancer-associated mortalities in women
aged 20–39 years (3). Women of
lower socioeconomic status experience higher incidence and
mortality rates of cervical cancer (4). Moreover, in China, cervical cancer is
the second most common malignancy among women after breast cancer
(5), with an increasingly younger
patient demographic (6), posing
notable challenges to their quality of life (QoL) and
prognosis.
For patients with early-stage cervical cancer,
surgery is the recommended first-line treatment (7). However, in cases of locally advanced
cervical cancer, where surgery is not viable, the standard
treatment protocol is definitive concurrent chemoradiotherapy
(8,9). Whole pelvic radiotherapy serves a
pivotal role in treating locally advanced cervical cancer. Commonly
utilized techniques include conformal radiation therapy,
intensity-modulated radiation therapy (IMRT) and helical
tomotherapy (HT) (10,11). Due to the unique anatomical
structures in the pelvis, conventional conformal radiotherapy often
exposes significant portions of pelvic structures, such as the
rectum, intestines, bladder, and femoral heads-to radiation doses
approaching or exceeding the prescribed therapeutic levels for the
target tumor This frequently results in severe acute and chronic
toxicities (12). By contrast, IMRT
has demonstrated its capability to reduce doses to organs-at-risk
(OARs) and minimize adverse effects to a certain extent (13). However, limitations remain, as
certain patients still experience substantial radiation-related
toxicities (14). HT, a novel
radiotherapy technology, employs unique binary pneumatic multileaf
collimators and a 360° rotational beam delivery. It features wide
treatment fields, robust modulation capabilities, high conformity
in target dose distribution and enhanced protection of normal
tissues (15,16). HT achieves precise treatment of
tumor targets whilst sparing normal tissues, showcasing notable
advantages in several malignancies, particularly head and neck
tumors (17,18). Despite these benefits, limited
studies have explored the application of HT in whole pelvic
radiotherapy for cervical cancer, and evidence supporting its
clinical superiority over IMRT remains insufficient.
In locally advanced cervical cancer, the extensive
target area and complex anatomical structures present a major
challenge in balancing high-dose tumor control with the protection
of OARs. Given its technical attributes, HT holds the potential to
address this challenge. Therefore, the present study aimed to
compare HT and IMRT in terms of dosimetric characteristics, adverse
effect profiles and long-term prognostic outcomes in patients with
locally advanced cervical cancer. By providing a multidimensional
comparison, the present research seeks to offer scientific guidance
for optimizing radiotherapy strategies, ultimately improving
treatment outcomes and patient QoL.
Materials and methods
General information
The present retrospective study analyzed the
clinical data of patients with locally advanced cervical cancer
treated at the Cangzhou Integrated Traditional Chinese and Western
Medicine Hospital (Cangzhou, China) from January 2015 to December
2023. All patients had histologically- or cytologically-confirmed
cervical cancer and received definitive concurrent
chemoradiotherapy. The inclusion criteria were as follows: i)
Cervical cancer diagnosed through histological or cytological
examination; ii) clinical staging of IIB-IVA according to the 2018
revised International Federation of Gynecology and Obstetrics
(FIGO) staging system (19,20), as the present study focused on
patients with locally advanced cervical cancer for whom concurrent
chemoradiotherapy is the standard treatment; iii) definitive
concurrent chemoradiotherapy administered as first-line therapy as
the uniform treatment protocol; iv) availability of complete and
comprehensive imaging data [including computed tomography (CT),
magnetic resonance imaging (MRI) or positron emission tomography
(PET)/CT] and standardized treatment planning data; and v) signed
informed consent permitting the use of patient data for the present
study. The exclusion criteria were as follows: i) Concurrent second
primary malignancies; ii) organ dysfunction: Severe pulmonary,
hepatic, renal or cardiovascular dysfunctions (such as respiratory
failure, decompensated cirrhosis, renal failure or recent
myocardial infarction); iii) severe gynecological diseases:
Conditions such as significant uterine fibroids, uterine polyps or
ovarian cysts that interfere with defining the radiation target
area; iv) prior therapy received, such as prior radiation therapy
or chemotherapy, potentially influencing the study outcomes; v)
radiation contraindications, such as inability to tolerate
radiotherapy or active infections; vi) incomplete records, such as
lacking of essential imaging or treatment planning data; and vii)
loss to follow-up, including failure to complete the prescribed
treatment or provide sufficient follow-up data.
A total of 112 patients were initially reviewed for
eligibility and 12 patients were excluded due to incomplete imaging
or treatment planning data, resulting in a final cohort of 100
patients included for analysis. These patients were evenly divided
into HT (n=50) and IMRT (n=50) groups. All exclusions were made
during the initial data collection phase and no additional patients
were removed after that point. Based on available demographic and
clinical information, excluded patients did not exhibit systematic
differences from those included in the final cohort, and their
exclusion is unlikely to have introduced selection bias. Clinical
characteristics, including age, body mass index (BMI), Eastern
Cooperative Oncology Group (ECOG) score (21), clinical stage, histological type,
parametrial invasion, lymph node metastasis, differentiation grade,
SCC antigen level, HPV infection status and tumor diameter , were
collected for analysis. Treatment allocation was not randomized: In
general, patients with more complex disease presentations (such as
those with a more advanced clinical stage or extensive lymph node
involvement) were more likely to receive HT, owing to its superior
dose conformity and ability to spare OARs in complex pelvic
irradiation (11). However,
treatment selection was not determined by a standardized protocol
and may have been influenced by physician preference, equipment
availability or other logistical considerations. Notably, baseline
imbalances in tumor-related characteristics between the groups were
addressed through propensity score matching (PSM), resulting in
well-balanced cohorts suitable for comparative outcome
analysis.
Treatment methods
Radiotherapy preparation
All patients underwent standardized pretreatment
preparation, including bowel preparation and bladder filling. Bowel
preparation: Patients adopted a low-residue diet for ≥3 days prior
to treatment, followed by enema administration on the morning of
radiotherapy to minimize bowel content interference. Bladder
filling: Patients were instructed to drink 800–1,500 ml water 1 h
before treatment to ensure optimal bladder filling, facilitating
improved dose distribution between targets and OARs.
Simulation and positioning
Patients were positioned supine with arms raised and
immobilized using body frames and thermoplastic molds (Ready
Medical Srl) to ensure positional reproducibility. Simulation CT
scans (Siemens AG) covered the area from the inferior border of the
T10 vertebra to the mid-femoral region, with a slice thickness of 3
mm. CT images were transferred via the Digital Imaging and
Communication in Medicine protocol to the MONACO system (version
5.1; Elekta Instrument AB) for IMRT design and to the Precision
planning system (version 2.0; Accuray Incorporated) for HT
planning.
Target and OAR delineation
Target delineation adhered to the Radiation Therapy
Oncology Group (RTOG) guidelines for cervical cancer (22). A total of two senior radiation
oncologists jointly completed and reviewed the delineations. The
following target definitions were used: Gross tumor volume, defined
as the macroscopic tumor region, including primary lesions and
positive lymph nodes, identified via clinical examination and
imaging modalities such as CT, MRI or PET; and clinical target
volume (CTV), defined as the areas at risk of microscopic disease
spread, including the cervix, uterine body, parametrium, upper
vaginal segment, pelvic lymphatic drainage regions and para-aortic
lymph nodes (PALNs). For positive lymph nodes, the CTV extended 2
cm beyond their boundaries. For patients with
radiologically-confirmed metastases in the common iliac and/or
PALNs, pelvic field extension and PALN irradiation were implemented
in both groups based on identical institutional criteria. In total,
11 patients in the HT group and 9 patients in the IMRT group
received PALN irradiation. Target volume delineation and dose
prescription for the para-aortic region followed the same protocol
in both groups, in accordance with the Chinese Society of Clinical
Oncology (CSCO) and National Comprehensive Cancer Network (NCCN)
guidelines for cervical cancer radiotherapy (23,24).
Planning target volume (PTV) was defined as a 0.5 cm expansion
around the CTV to account for positional and motion errors during
treatment. OAR definitions included the rectum, small intestine,
bladder, pelvic bones, femoral heads and other normal tissues.
Radiotherapy plan design
Both HT and IMRT plans were created and
independently validated for each patient, applying identical
dose-volume constraints for targets and OARs (Table I). The IMRT plan was designed using
the MONACO system and delivered using the Elekta Synergy
accelerator (Elekta Instrument AB). Plans utilized nine evenly
distributed coplanar beams optimized iteratively. Prescription
doses were PTV 45–50.4 Gy in 25–28 fractions (5 fractions/week),
with simultaneous boosts of 10–20 Gy for positive lymph node
regions and 5–10 Gy for parametrial or pelvic wall involvement. The
HT plan was generated using the Precision planning system and
delivered using Radixact® X5 equipment (Accuray
Incorporated). Parameters such as field width, pitch and modulation
factor were iteratively adjusted to optimize dose distribution.
Prescription doses mirrored those of IMRT.
 | Table I.Dose-volume constraints for targets
and organs-at-risk. |
Table I.
Dose-volume constraints for targets
and organs-at-risk.
| Structure | Dose volume
constraints |
|---|
| PTV | Minimal dose, 47.5
Gy; Maximal dose, 55 Gy; ≥95% receiving 50 Gy |
| Bowel | ≤35% receiving ≥35
Gy |
| Bladder | ≤50% receiving ≥50
Gy |
| Rectum | ≤50% receiving ≥50
Gy |
| Pelvic bones | ≤50% receiving ≥20
Gy |
| Femoral head | ≤5% receiving ≥50
Gy |
| Normal tissue | EUD, 18 Gy |
| Ringa | EUD, 38 Gy |
Treatment quality assurance (QA)
All radiotherapy plans underwent rigorous QA prior
to treatment delivery. For both HT and IMRT groups,
patient-specific QA was performed by experienced medical physicists
using a 3D diode array phantom system (ArcCHECK®; Sun
Nuclear Corporation). Verification was performed before the first
treatment session for each patient, and dose accuracy was evaluated
using gamma index analysis (criteria: 3% dose difference/3 mm
distance-to-agreement). A gamma passing rate of ≥95% was required
for clinical implementation. In addition, daily image-guided
radiotherapy was employed to ensure accurate patient positioning.
For IMRT, orthogonal kilovoltage (kV) imaging was used, whilst
megavoltage computed tomography was used for HT. These image-guided
procedures were performed immediately prior to each treatment
session and reviewed by attending radiation oncologists. All QA
procedures followed institutional protocols based on
recommendations from the American Association of Physicists in
Medicine Task Groups 119 and 142 (25,26),
ensuring high precision and reproducibility in dose delivery.
Concurrent chemotherapy
Patients received weekly cisplatin (40
mg/m2 per dose) administered via intravenous infusion,
for 4–6 consecutive weeks (one cycle per week), concurrent with
external beam radiotherapy. For cisplatin-intolerant patients,
carboplatin [area under the curve (AUC)=2, weekly] or
platinum-based combination regimens were employed. Preventive
antiemetics were routinely administered. Combination regimens,
whilst recognized in both NCCN and CSCO guidelines as an
alternative option for concurrent chemoradiotherapy, are associated
with higher toxicity and are less commonly adopted in routine
clinical practice for locally advanced cervical cancer. Therefore,
in the present study, weekly cisplatin monotherapy was prioritized
to reduce treatment-related toxicity and maintain uniformity in
systemic therapy across the cohort, thereby minimizing potential
confounding in survival outcome analysis.
Brachytherapy
After 20 external beam therapy sessions,
high-dose-rate brachytherapy was integrated. A high dose rate
brachytherapy system was used. The target design was as follows:
High-risk clinical target volume (HRCTV) was adjusted dynamically
based on pelvic MRI before and during treatment. Moreover, the
prescription dose was as follows: HRCTV D90 was 30 Gy in 5
fractions (6 Gy per fraction). Combined with external beam therapy,
the equivalent total dose (EQD2) for HRCTV D90 was 87–92 Gy.
Observational metrics
Dosimetric parameters included PTV coverage,
homogeneity index (HI), conformity index (CI) and OAR dose-volume
metrics (V5, V10, V20, V30, V40 and V50). The efficacy metrics were
based on Response Evaluation Criteria in Solid Tumors (RECIST) 1.1
criteria (27), and short-term
outcomes were categorized as complete response (CR), partial
response (PR), stable disease (SD) or progressive disease (PD), and
the objective response rate (ORR) was calculated. Long-term
outcomes included 5-year overall survival (OS) and progression-free
survival (PFS). The toxicity metrics were acute and chronic
radiation-related toxicities, graded per the RTOG criteria
(28).
Follow-up
Patients were systematically followed to assess
efficacy, prognosis and radiation-related adverse effects. The
schedule was as follows: Follow-ups were performed at 1, 3 and 6
months post-radiotherapy, then semi-annually for the first 5 years
and annually thereafter. The content of the follow-ups included
routine pelvic MRI, chest CT and abdominal ultrasonography,
supplemented with PET/CT when necessary. Blood parameters,
including squamous cell carcinoma (SCC) antigen, liver and kidney
function tests, were monitored. All patients underwent HPV testing
as part of the standardized diagnostic protocol. Radiation-related
toxicities were assessed using the RTOG criteria. During treatment,
toxicities were evaluated weekly by at least one senior attending
radiation oncologist (associate chief physician or above), based on
clinical examination and patient-reported symptoms, and documented
in structured toxicity assessment forms. After treatment
completion, toxicities were recorded at each scheduled follow-up
visit. All toxicity data used for analysis in the present study
were retrospectively extracted from electronic medical records by
two independent researchers and cross-verified to ensure
consistency. OS was defined as the time from radiotherapy
initiation to death from any cause. PFS was defined as the time to
disease progression or death. Median follow-up: was 74 months
(range, 21–108 months). Among the 100 patients included in the
present study, 96 completed the full follow-up protocol. A total of
four patients were lost to follow-up after completing initial
treatment, and their outcomes were unknown beyond their last
documented clinical visit. These patients were included in the
Kaplan-Meier survival analyses using right-censoring at the time of
their last follow-up.
Statistical analysis
Data analysis was performed using SPSS 27.0 software
(IBM Corp.). Continuous variables were expressed as mean ± standard
deviation, and comparisons between groups employed independent
t-tests or χ2 tests. Non-parametric data were analyzed
using the Mann-Whitney U test. Efficacy and adverse event
differences were assessed using χ2 or Fisher's exact
tests. Survival analyses were performed using Kaplan-Meier curves,
with log-rank tests evaluating group differences. P<0.05 was
considered to indicate a statistically significant difference.
A sample size calculation was performed using PASS
software (version 15.0; NCSS, LLC) based on a two-sided log-rank
test. Assuming a 3-year PFS rate of 70% for the HT group and 50%
for the IMRT group (a 20% absolute difference), with α=0.05 and
power=0.80, the estimated required sample size was 90 patients (45
in each group). To allow for potential loss to follow-up, 100
patients (50 per group) were recruited, which is sufficient to
ensure statistical power for primary outcome comparisons. Although
differences in dosimetric parameters were statistically significant
and favored HT, PFS was considered as the primary clinical endpoint
for power analysis, as it best reflects meaningful therapeutic
benefit to patients.
To minimize selection bias, 1:1 PSM was performed
using nearest-neighbor matching with a caliper of 0.2. A total of
five baseline variables were included in the matching model: Age
(≤60 vs. >60 years), ECOG performance status (0 vs. 1), FIGO
stage (II vs. III/IV), tumor diameter (≤4 vs. >4 cm) and lymph
node metastasis (yes vs. no). After matching, 39 patients were
retained in each group (HT and IMRT), with well-balanced baseline
characteristics. Following PSM, survival analysis using
Kaplan-Meier curves and multivariate Cox proportional hazards
regression was performed to further adjust for residual confounding
and identify independent prognostic factors for PFS and OS. The
multivariate Cox regression model included the following
covariates: Age (≤60 vs. >60 years), ECOG performance status (0
vs. 1), tumor diameter (≤4 vs. >4 cm), parametrial invasion (yes
vs. no), lymph node metastasis (yes vs. no), differentiation grade
(high, moderate and low), FIGO stage (II, III and IV), treatment
modality (HT vs. IMRT), HPV status (positive vs. negative) and SCC
antigen level (normal vs. elevated, using 1.5 ng/ml as the
institutional cutoff). The proportional hazards assumption was
verified prior to model fitting.
Results
Clinical characteristics analysis
A total of 100 patients with locally advanced
cervical cancer were enrolled in the present study, with 50
patients in each of the HT and IMRT groups. There were no
statistically significant differences in baseline characteristics
such as age, BMI, ECOG score, tumor stage, histological type, SCC
antigen levels or HPV infection status between the two groups
(P>0.05), indicating good comparability (Table II).
 | Table II.Baseline clinical characteristics of
the helical tomotherapy and intensity-modulated radiation therapy
groups. |
Table II.
Baseline clinical characteristics of
the helical tomotherapy and intensity-modulated radiation therapy
groups.
| Characteristic | HT Group
(n=50) | IMRT Group
(n=50) | χ2 | P-value |
|---|
| Age |
|
| 1.980 | 0.159 |
| ≤60
years | 19 (38.0) | 26 (52.0) |
|
|
| >60
years | 31 (62.0) | 24 (48.0) |
|
|
| BMI |
|
| 2.683 | 0.261 |
|
Normal | 24 (48.0) | 28 (56.0) |
|
|
|
Overweight | 15 (30.0) | 17 (34.0) |
|
|
|
Obese | 11 (22.0) | 5 (10.0) |
|
|
| ECOG score |
|
| 1.714 | 0.190 |
| 0 | 38 (76.0) | 32 (64.0) |
|
|
| 1 | 12 (24.0) | 18 (36.0) |
|
|
| Tumor diameter
(MRI) |
|
| 0.932 | 0.334 |
| ≤4
cm | 13 (26.0) | 9 (18.0) |
|
|
| >4
cm | 37 (74.0) | 41 (82.0) |
|
|
| Parametrial
invasion |
|
| 0.250 | 0.617 |
| No | 9 (18.0) | 11 (22.0) |
|
|
|
Yes | 41 (82.0) | 39 (78.0) |
|
|
| Lymph node
metastasis |
|
| 0.386 | 0.534 |
| No | 33 (66.0) | 30 (60.0) |
|
|
|
Yes | 17 (34.0) | 20 (40.0) |
|
|
| Histological
type |
|
|
| 0.912 |
|
Squamous cell carcinoma | 48 (96.0) | 47 (94.0) |
|
|
|
Others | 2 (4.0) | 3 (6.0) |
|
|
| Differentiation
grade |
|
| 1.462 | 0.481 |
|
High | 9 (18.0) | 14 (28.0) |
|
|
|
Moderate | 31 (62.0) | 28 (56.0) |
|
|
|
Low | 10 (20.0) | 8 (16.0) |
|
|
| Clinical stage
(FIGO) |
|
| 0.915 | 0.633 |
| Stage
II | 17 (34.0) | 18 (36.0) |
|
|
| Stage
III | 19 (38.0) | 22 (44.0) |
|
|
| Stage
IV | 14 (28.0) | 10 (20.0) |
|
|
| SCC antigen
level |
|
|
| 0.487 |
|
Normal | 6 (12.0) | 3 (6.0) |
|
|
|
Elevated | 44 (88.0) | 47 (94.0) |
|
|
| HPV infection |
|
| 2.060 | 0.151 |
|
Negative | 16 (32.0) | 23 (46.0) |
|
|
|
Positive | 34 (68.0) | 27 (54.0) |
|
|
Target dose distribution
The dosimetric distribution of the target areas in
the HT and IMRT groups is presented in Table III. Both groups achieved
satisfactory prescription dose coverage of the PTV, but the HT
group demonstrated notable advantages in dose homogeneity and
conformity. The mean CI in the HT group was 0.94, significantly
higher than 0.86 in the IMRT group (t=−3.29; P<0.05). Moreover,
the mean HI in the HT group was 1.05, significantly lower than 1.12
in the IMRT group (t=4.98; P<0.05), indicating a more uniform
dose distribution in the HT plans. Additionally, the
PTV95 values, defined as the percentage of the planning
target volume (PTV) receiving at least 95% of the prescribed
dose-for the HT and IMRT groups were 99.3 and 99.8%, respectively,
with no statistically significant difference between the groups
(t=0.25; P=0.62). However, the PTV105 and
PTV110 values were significantly lower in the HT group
(t=8.36 and t=5.02, respectively; both P<0.05), highlighting the
superior control of HT of high-dose regions within the target area.
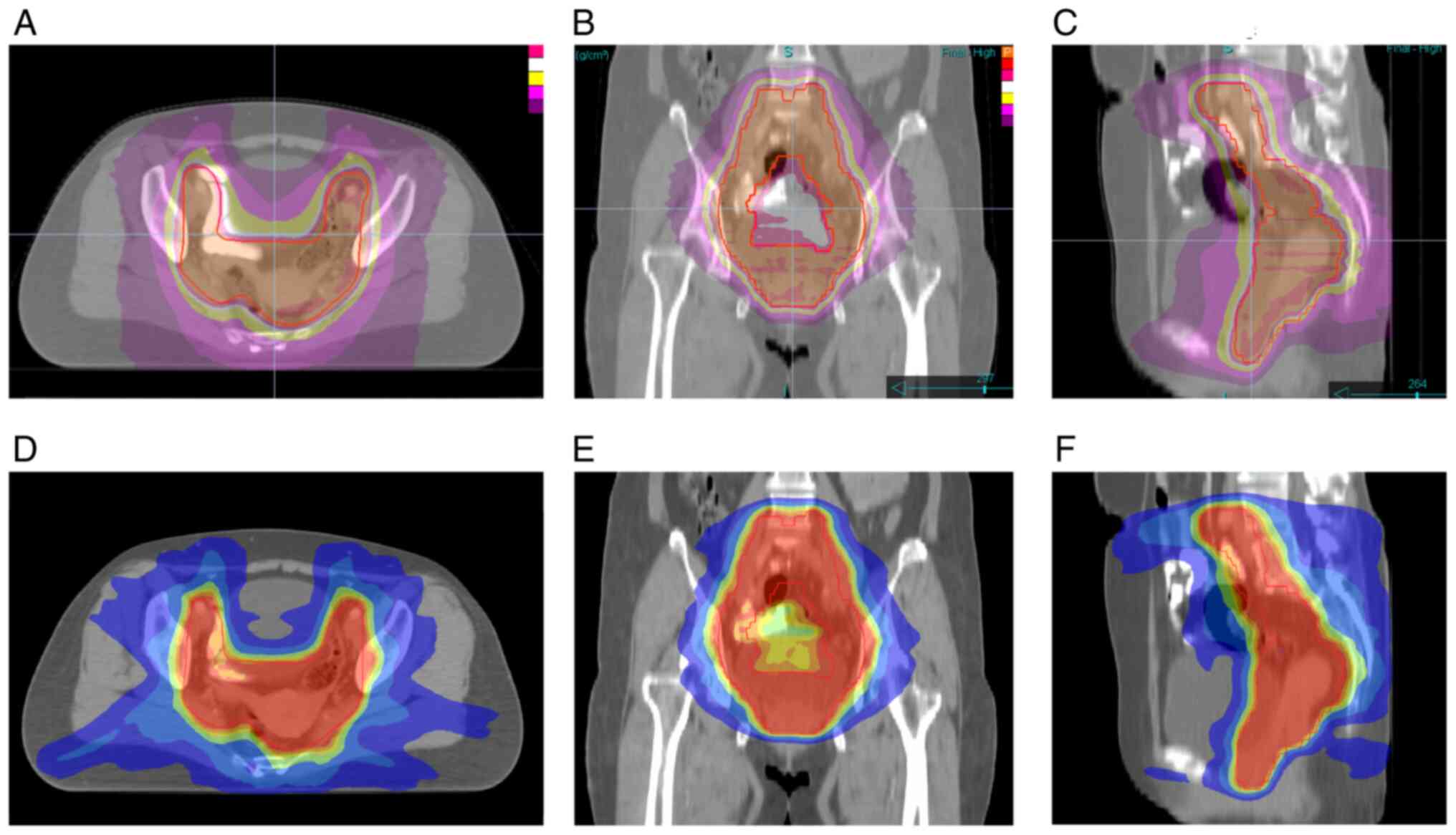
Representative dose distribution images for typical patients are
shown in Fig. 1.
 | Table III.Planning target volume dose
distribution in helical tomotherapy and intensity-modulated
radiation therapy plans. |
Table III.
Planning target volume dose
distribution in helical tomotherapy and intensity-modulated
radiation therapy plans.
| Plan | IMRT | HT | t-value | P-value |
|---|
| PTV95,
% | 99.84±0.03 | 99.33±0.02 | 0.25 | 0.628 |
| PTV100,
% | 95.75±0.16 | 95.31±0.24 | 0.39 | 0.543 |
| PTV105,
% | 47.92±9.62 | 13.57±2.92 | 8.36 | <0.001 |
| PTV110,
% | 8.28±4.77 | 0.59±0.26 | 5.02 | <0.001 |
| Dmean,
Gy | 52.82±0.43 | 51.43±0.27 | 6.47 | <0.001 |
| CI | 0.86±0.04 | 0.94±0.02 | −3.29 | <0.001 |
| HI | 1.12±0.02 | 1.05±0.01 | 4.98 | <0.001 |
Dose distribution to OARs and normal
tissues
Significant differences were demonstrated for in the
dose distribution to OARs and normal tissues between the HT and
IMRT groups (Table IV and Table V). For the rectum, the
V30, V40 and V50 values,
representing the percentage of rectal volume receiving at least 30,
40, and 50 Gy, respectively, in the HT group were 84.7, 56.2 and
24.1, respectively, with a mean dose of 41.2 Gy compared with the
IMRT group (93.2, 64.1 and 28.2%, and 43.4 Gy, respectively;
t=3.05; P=0.01), indicating improved rectal protection by HT. For
the bladder, the V30, V40 and V50
values in the HT group were 50.6, 28.9 and 13.4%, respectively,
with a mean dose of 32.7 Gy compared with the IMRT group (68.8,
38.3 and 17.8%, and 36.4 Gy, respectively; t=6.95; P<0.05),
demonstrating the advantage that HT has in bladder protection. The
V40 and V50 values were significantly reduced
for both femoral heads in the HT group compared with the IMRT
group, with mean doses of 14.6 and 16.6% (t=4.92; P<0.001; left)
and 13.7 and 15.3% (t=3.83; P<0.05; right), respectively.
Moreover, in the HT group, low-to-mid-dose exposure was slightly
higher and high-dose exposure (V30, V40 and
V50) was slightly lower in the bowel compared with the
IMRT group, but the differences in the were not statistically
significant (t=0.80; P>0.05). In the pelvic bones, the HT group
demonstrated higher exposure in the low-dose regions
(V5, V10 and V20; t=−6.00, −6.70
and −2.12, respectively; P<0.05), whilst high-dose region
exposure (V30, V40 and V50) was
reduced, in comparison with the IMRT group. Finally, in normal
tissue, V5, V10 and V20 values
were 12.5, 17.5 and 5.2% higher in the HT group, compared with in
the IMRT group, with a significantly higher mean dose (20.1 vs.
18.6 Gy; t=−7.31; P<0.001). This reflects the wider low-dose
exposure characteristic of the rotational beam delivery of
HT.8.
 | Table IV.Dosimetric distribution of
organs-at-risk in helical tomotherapy and intensity-modulated
radiation therapy plans. |
Table IV.
Dosimetric distribution of
organs-at-risk in helical tomotherapy and intensity-modulated
radiation therapy plans.
| A, Bowel |
|---|
|
|---|
| Plan | IMRT | HT | t-value | P-value |
|---|
| V5,
% | 93.2±5.1 | 96.3±1.2 | −1.09 | 0.264 |
| V10,
% | 85.2±6.4 | 88.3±7.2 | −1.32 | 0.198 |
| V20,
% | 63.2±6.1 | 64.3±8.2 | −0.33 | 0.742 |
| V30,
% | 38.7±6.3 | 32.6±9.1 | 2.69 | 0.039 |
| V40,
% | 21.2±2.3 | 17.4±6.2 | 1.67 | 0.133 |
| V50,
% | 8.8±2.1 | 8.0±3.8 | 0.92 | 0.384 |
| Dmean,
Gy | 26.7±6.0 | 26.2±7.1 | 0.80 | 0.455 |
|
| B,
Rectum |
|
| Plan | IMRT | HT | t-value | P-value |
|
| V5,
% | 99.2±0.5 | 100.0±0.0 | −1.17 | 0.271 |
| V10,
% | 98.2±1.1 | 100.0±0.0 | −1.62 | 0.145 |
| V20,
% | 96.8±2.6 | 97.4±1.2 | −1.30 | 0.246 |
| V30,
% | 93.2±5.3 | 84.7±8.1 | 3.32 | <0.001 |
| V40,
% | 64.1±12.2 | 56.2±7.4 | 3.07 | 0.017 |
| V50,
% | 28.2±10.2 | 24.1±5.6 | 2.39 | 0.045 |
| Dmean,
Gy | 43.4±6.5 | 41.2±5.3 | 3.05 | 0.014 |
|
| C,
Bladder |
|
| Plan | IMRT | HT | t-value | P-value |
|
| V5,
% | 100.0±0.0 | 100.0±0.0 | – | – |
| V10,
% | 99.3±0.6 | 100.0±0.0 | −1.73 | 0.112 |
| V20,
% | 89.2±2.5 | 86.1±4.2 | 0.86 | 0.421 |
| V30,
% | 68.8±5.4 | 50.6±6.7 | 4.92 | <0.001 |
| V40,
% | 38.3±15.1 | 28.9±7.4 | 5.77 | <0.001 |
| V50,
% | 17.8±8.5 | 13.4±3.4 | 4.65 | <0.001 |
| Dmean,
Gy | 36.4±5.2 | 32.7±6.6 | 6.95 | <0.001 |
|
| D, Pelvic
bones |
|
| Plan | IMRT | HT | t-value | P-value |
|
| V5,
% | 92.2±4.7 | 97.4±2.7 | −6.00 | <0.001 |
| V10,
% | 85.6±7.4 | 92.3±3.3 | −6.70 | <0.001 |
| V20,
% | 65.4±9.1 | 68.3±4.5 | −2.12 | 0.079 |
| V30,
% | 45.6±5.8 | 43.4±2.1 | 1.41 | 0.197 |
| V40,
% | 24.7±4.1 | 23.6±2.8 | 0.43 | 0.682 |
| V50,
% | 9.4±5.5 | 9.1±2.2 | 1.63 | 0.146 |
| Dmean,
Gy | 27.5±1.3 | 28.2±0.7 | −1.44 | 0.264 |
|
| E, Left femoral
head |
|
| Plan | IMRT | HT | t-value | P-value |
|
| V5,
% | 100.0±0.0 | 100.0±0.0 | – | – |
| V10,
% | 99.7±0.3 | 100.0±0.0 | −0.39 | 0.763 |
| V20,
% | 88.6±7.5 | 86.7±5.4 | 0.92 | 0.453 |
| V30,
% | 67.9±2.7 | 66.4±1.8 | 0.85 | 0.467 |
| V40,
% | 9.2±2.0 | 7.8±1.9 | 2.55 | 0.048 |
| V50,
% | 0.37±1.4 | 0.16±2.1 | 3.61 | <0.001 |
| Dmean,
Gy | 16.6±4.0 | 14.6±3.4 | 4.92 | <0.001 |
|
| F, Right femoral
head |
|
| Plan | IMRT | HT | t-value | P-value |
|
| V5,
% | 99.6±0.4 | 100.0±0.0 | −0.16 | 0.899 |
| V10,
% | 98.3±1.1 | 99.7±0.6 | −0.32 | 0.741 |
| V20,
% | 90.2±3.2 | 87.7±6.4 | 1.06 | 0.268 |
| V30,
% | 67.0±2.1 | 66.8±1.0 | 0.45 | 0.685 |
| V40,
% | 13.4±0.1 | 12.2±1.5 | 1.84 | 0.057 |
| V50,
% | 0.92±3.4 | 0.48±1.0 | 2.91 | 0.023 |
| Dmean,
Gy | 15.3±4.5 | 13.7±2.8 | 3.83 | <0.001 |
 | Table V.Dosimetric distribution of normal
tissues in helical tomotherapy and intensity-modulated radiation
therapy plans. |
Table V.
Dosimetric distribution of normal
tissues in helical tomotherapy and intensity-modulated radiation
therapy plans.
| Plan | IMRT | HT | t-value | P-value |
|---|
| V5,
% | 79.7±4.3 | 92.2±2.4 | −8.25 | <0.001 |
| V10,
% | 63.4±5.7 | 80.9±3.8 | −9.13 | <0.001 |
| V20,
% | 37.3±4.6 | 42.5±4.2 | −4.92 | <0.001 |
| V30,
% | 18.5±4.4 | 17.6±2.7 | 0.99 | 0.335 |
| V40,
% | 7.2±1.4 | 6.3±2.2 | 0.56 | 0.667 |
| V50,
% | 1.3±0.1 | 1.0±0.2 | 0.69 | 0.516 |
| Dmean,
Gy | 18.6±3.9 | 20.1±5.1 | −7.31 | <0.001 |
Comparison of OS and PFS
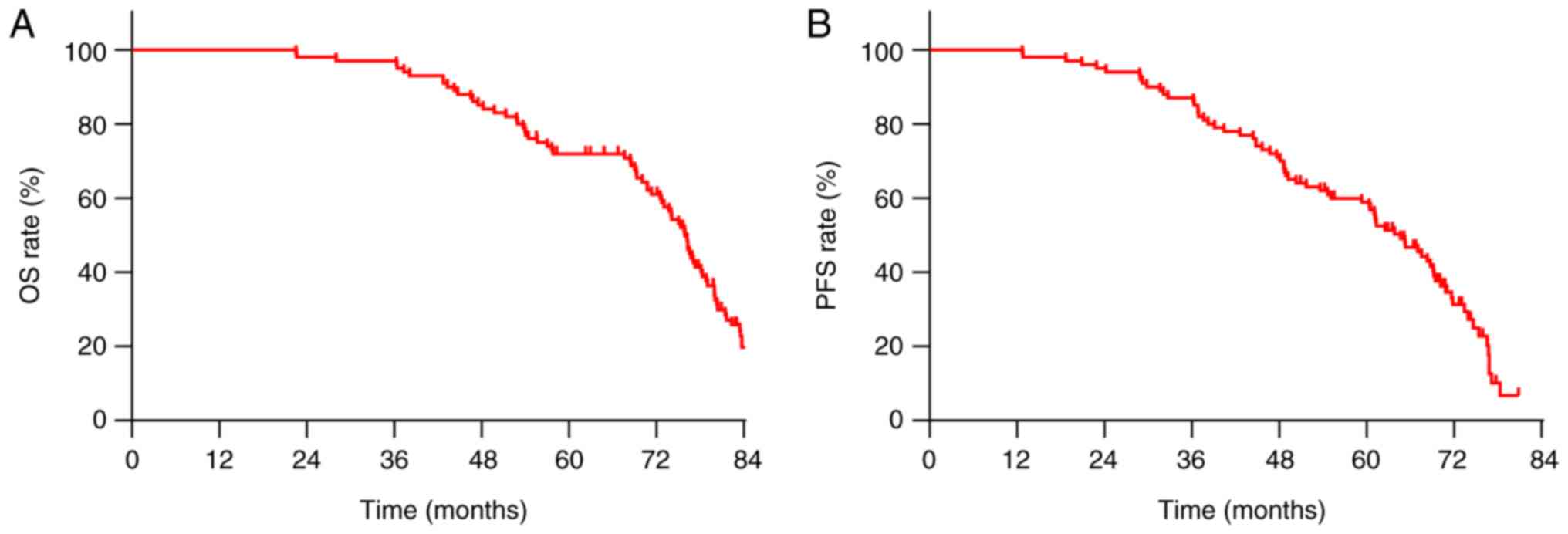
The median OS and PFS for all patients were 73.9 and
61.2 months, respectively (Fig. 2).
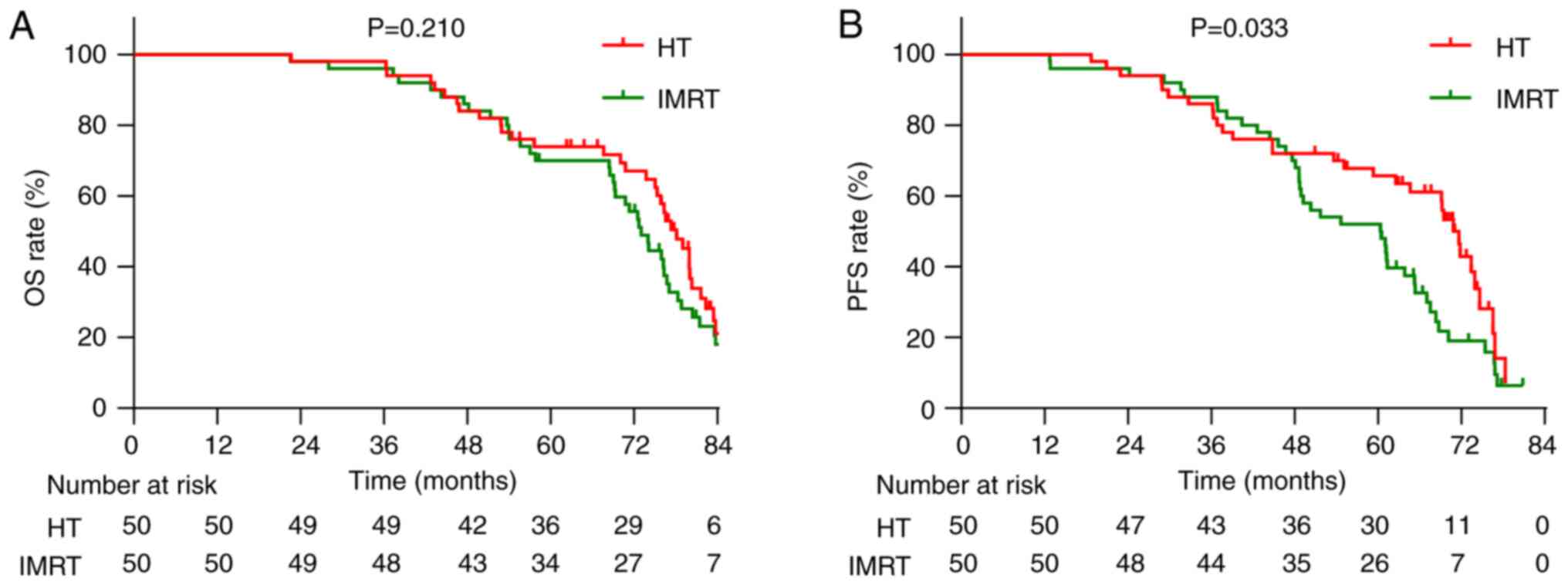
Furthermore, the median OS was 75.8 months in the HT group and 72.4
months in the IMRT group, with 5-year OS rates of 72.0 and 68.0%,
respectively. There was no statistically significant difference
between the groups [hazard ratio (HR), 0.756; 95% confidence
interval (CI), 0.486–1.175; χ2=1.570; P=0.210; Fig. 3A]. The median PFS was 67.2 months in
the HT group, significantly longer than 60.3 months in the IMRT
group. The 5-year PFS rates were 60.0 and 52.0%, respectively (HR,
0.612; 95% CI, 0.386–0.971; χ2=4.539; P=0.033; Fig. 3B). Recurrence pattern analysis
revealed that the overall recurrence rate was lower in the HT group
(25/50; 50.0%) compared with in the IMRT group (34/50; 68.0%);
however, the difference was not statistically significant
(χ2=3.348; P=0.067). Specifically, local recurrence
occurred in 3 patients (6.0%) in the HT group and 6 patients
(12.0%) in the IMRT group; and regional recurrence was observed in
8 patients (16.0%) in the HT group and 13 patients (26.0%) in the
IMRT group. Moreover, distant metastases occurred in 14 patients
(28.0%) in the HT group and 15 patients (30.0%) in the IMRT group.
Although none of the individual recurrence patterns reached
statistical significance, the overall trend suggests a potential
benefit of HT in reducing local and regional recurrence,
potentially contributing to the observed improvement in PFS.
Local tumor control
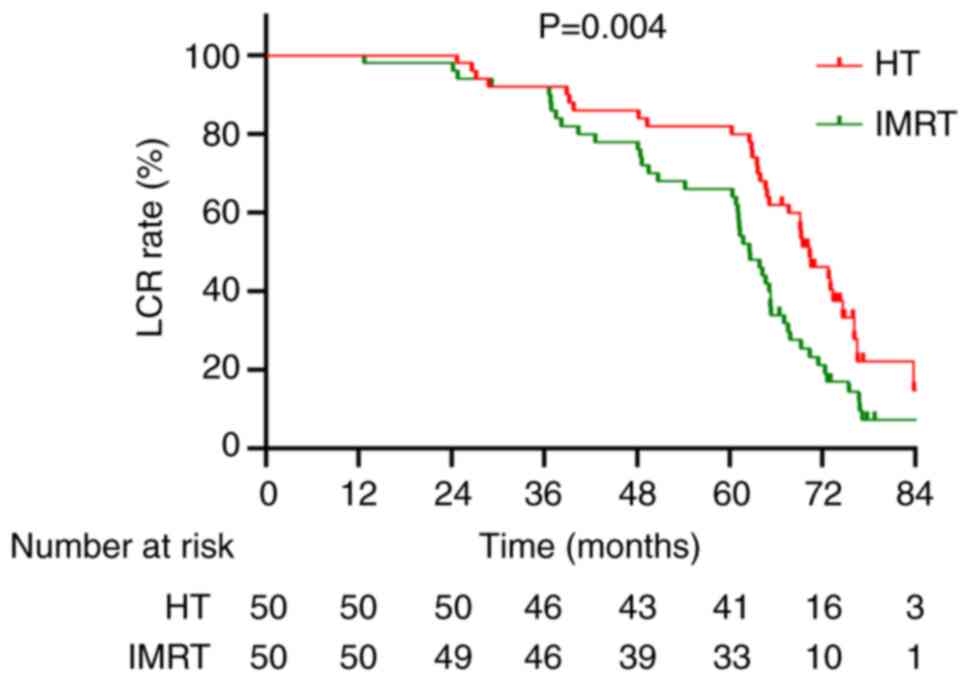
The 5-year local control rate (LCR) was 82.0% in the
HT group and 66.0% in the IMRT group. Moreover, the median local
control duration was 69.2 months (95% CI, 24.7–86.7) in the HT
group and 62.6 months (95% CI, 15.7–84.9) in the IMRT group.
Kaplan-Meier analysis demonstrated a statistically significant
improvement in LCR in the HT group compared with in the IMRT group
(HR, 0.529; 95% CI, 0.339–0.826; χ2=8.246; P=0.004;
Fig. 4).
Treatment efficacy
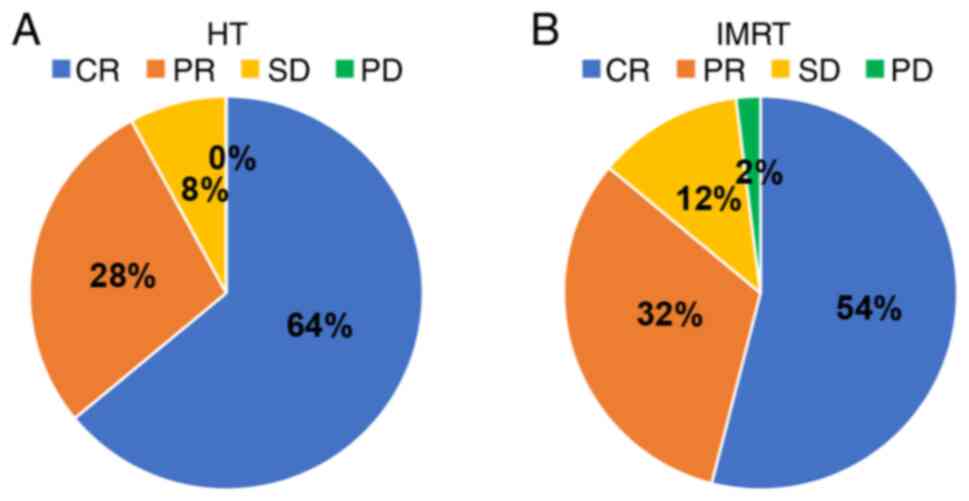
According to RECIST 1.1 criteria, the HT group
achieved a CR rate of 64.0% (32/50), PR rate of 28.0% (14/50) and
SD rate of 8.0% (4/50), with no PD cases. The ORR was 92.0%. In the
IMRT group, CR, PR, SD and PD rates were 54.0% (27/50), 32.0%
(16/50), 12.0% (6/50) and 2.0% (1/50), respectively, with an ORR of
86.0%. There was no significant difference in ORR between the two
groups (χ2=0.919; P=0.338; Fig. 5).
Radiation-related toxicity
Radiation-related adverse events were reported in
both groups, with differences summarized in Table VI. Grade 1–2 toxicity occurred in
68.0% (34/50) of HT patients compared with 84.0% (42/50) of IMRT
patients. Grade 3 toxicity occurred in 8.0% (4/50) of HT patients
compared with 12.0% (6/50) of IMRT patients, with no significant
difference (χ2=0.444; P=0.505). However, HT exhibited
superior dosimetric protection for the rectum and bladder (Table IV), though the incidence of severe
adverse events (Grade ≥3) was comparable between the two groups
(Table VI).
 | Table VI.Comparison of radiotherapy-related
toxicities in helical tomotherapy and intensity-modulated radiation
therapy groups. |
Table VI.
Comparison of radiotherapy-related
toxicities in helical tomotherapy and intensity-modulated radiation
therapy groups.
| Toxicity type | HT group
(n=50) | IMRT group
(n=50) | P-value |
|---|
| Radiation
enteritis |
|
| 0.319 |
| Grade
1–2 | 17 (34.0) | 24 (48.0) |
|
| Grade
≥3 | 1 (2.0) | 2 (4.0) |
|
| Radiation
cystitis |
|
| 0.109 |
| Grade
1–2 | 19 (38.0) | 27 (54.0) |
|
| Grade
≥3 | 0 (0.0) | 1 (2.0) |
|
| Radiation
vaginitis |
|
| 0.087 |
| Grade
1–2 | 15 (30.0) | 32 (64.0) |
|
| Grade
≥3 | 0 (0.0) | 0 (0.0) |
|
| Radiation
dermatitis |
|
| 0.682 |
| Grade
1–2 | 21 (42.0) | 18 (36.0) |
|
| Grade
≥3 | 0 (0.0) | 0 (0.0) |
|
| Bone marrow
suppression |
|
| 0.057 |
| Grade
1–2 | 26 (52.0) | 37 (74.0) |
|
| Grade
≥3 | 3 (6.0) | 3 (6.0) |
|
Treatment compliance and chemotherapy
tolerance
Of the 100 patients, 87 (87.0%) received the
institutional standard regimen of weekly cisplatin (40
mg/m2). Among them, 76 patients (87.4%) completed the
full course of 5–6 cycles without modification. The remaining 11
patients (12.6%) experienced notable toxicity, including
gastrointestinal side effects (n=3) and hematologic toxicity (n=8),
necessitating dose reductions or treatment delays. A total of 13
patients (13.0%) were deemed unsuitable for cisplatin due to
baseline renal impairment or severe gastrointestinal intolerance
and were switched to weekly carboplatin (AUC=2) as an alternative
regimen. No patients received combined chemotherapy regimens such
as cisplatin plus paclitaxel. All patients successfully completed
the planned radiotherapy regimen, including both external beam
radiation and brachytherapy, without any dose reductions. However,
4 patients (4.0%) required minor adaptive planning during treatment
due to anatomical changes such as variations in bladder filling or
weight loss. These adaptations did not affect the prescribed dose
and were implemented under image-guided verification with a weekly
plan review.
Post-PSM analysis of baseline
characteristics, survival outcomes and toxicities
After performing 1:1 PSM with a caliper of 0.2, 39
matched pairs from the HT and IMRT groups were included in the
final analysis. The baseline characteristics, including age, ECOG
performance status, FIGO stage, tumor diameter and lymph node
metastasis, were well-balanced between the two groups following
matching. Detailed comparisons of these characteristics are
presented in Table VII, with no
significant differences observed (all P>0.05). Moreover,
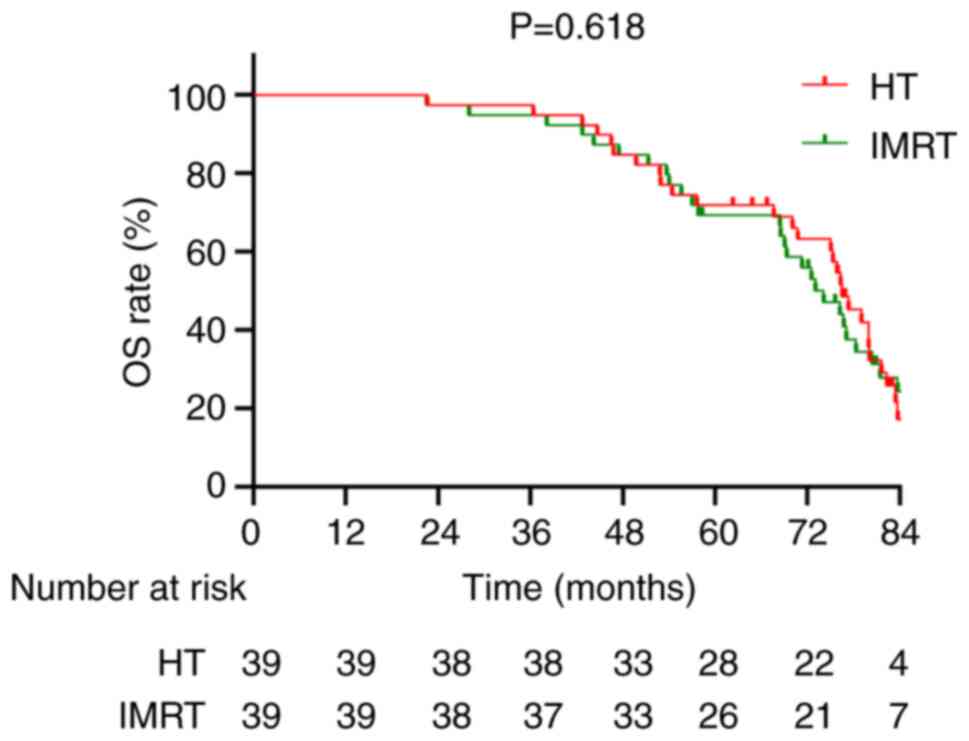
Kaplan-Meier survival curves demonstrated no significant difference
in OS between the two groups (χ2=0.249; P=0.618;
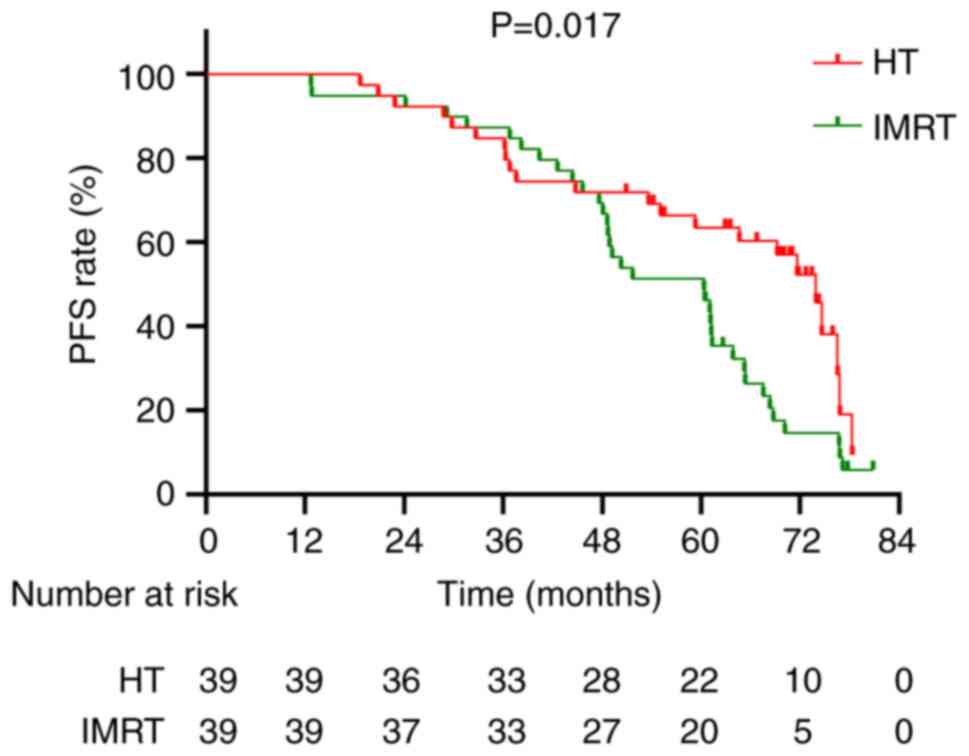
Fig. 6); however, there was a
significantly improved PFS rate in the HT group compared with in
the IMRT group (χ2=5.737; P=0.017; Fig. 7).
 | Table VII.Comparison of baseline
characteristics between helical tomotherapy and intensity-modulated
radiation therapy groups after propensity score matching. |
Table VII.
Comparison of baseline
characteristics between helical tomotherapy and intensity-modulated
radiation therapy groups after propensity score matching.
| Characteristic | HT group
(n=39) | IMRT group
(n=39) | χ2 | P-value |
|---|
| Age |
|
| 0.473 | 0.492 |
| ≤60
years | 15 (38.5) | 18 (46.2) |
|
|
| >60
years | 24 (61.5) | 21 (53.8) |
|
|
| ECOG score |
|
| 1.258 | 0.262 |
| 0 | 33 (84.6) | 29 (74.4) |
|
|
| 1 | 6 (15.4) | 10 (25.6) |
|
|
| Tumor diameter
(MRI) |
|
| 0.831 | 0.362 |
| ≤4
cm | 8 (20.5) | 5 (12.8) |
|
|
| >4
cm | 31 (79.5) | 34 (87.2) |
|
|
| Lymph node
metastasis |
|
| 0.867 | 0.352 |
| No | 26 (66.7) | 22 (56.4) |
|
|
|
Yes | 13 (33.3) | 17 (43.6) |
|
|
| Clinical stage
(FIGO) |
|
| 0.748 | 0.688 |
| Stage
II | 13 (33.3) | 14 (35.9) |
|
|
| Stage
III | 17 (43.6) | 19 (48.7) |
|
|
| Stage
IV | 9 (23.1) | 6 (15.4) |
|
|
Multivariate Cox regression analysis revealed that,
for OS, FIGO stage (HR, 6.024; 95% CI, 1.961–10.258; P<0.001)
and lymph node metastasis (HR, 1.703; 95% CI, 1.080–2.673; P=0.039)
were significant independent prognostic factors, whilst treatment
modality revealed a nonsignificant trend (HR, 1.517; 95% CI,
0.784–2.361; P=0.089) (Table
VIII). Moreover, SCC antigen level (HR, 1.327; 95% CI,
0.758–3.015; P=0.194) and HPV status (HR, 1.269; 95% CI,
0.692–2.438; P=0.461) were not significantly associated with OS
(Table VIII). Multivariate Cox
regression analysis also revealed that treatment modality (HT vs.
IMRT) was an independent predictor for PFS (HR, 2.193; 95% CI,
1.188–4.049; P=0.012) (Table IX).
In addition, FIGO stage (P<0.001) and lymph node metastasis
P=0.027) were significant predictors of PFS. However, SCC antigen
level (HR, 1.203; 95% CI, 0.759–2.681; P=0.614) and HPV status (HR,
1.182; 95% CI, 0.527–2.284) were not significantly associated with
PFS, although both were retained in the model for completeness
(Table IX).
 | Table VIII.Univariate and multivariate Cox
regression analysis for overall survival after propensity score
matching. |
Table VIII.
Univariate and multivariate Cox
regression analysis for overall survival after propensity score
matching.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age |
|
|
|
|
|
|
| ≤60
years | 1.000 |
|
|
|
|
|
| >60
years | 1.059 | 0.578–1.601 | 0.802 |
|
|
|
| ECOG score |
|
|
|
|
|
|
| 0 | 1.000 |
|
|
|
|
|
| 1 | 1.271 | 0.847–1.986 | 0.457 |
|
|
|
| Tumor diameter
(MRI) |
|
|
|
|
|
|
| ≤4
cm | 1.000 |
|
|
|
|
|
| >4
cm | 1.216 | 0.686–1.914 | 0.502 |
|
|
|
| Parametrial
invasion |
|
|
|
|
|
|
| No | 1.000 |
|
|
|
|
|
|
Yes | 1.393 | 0.864–2.209 | 0.162 |
|
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
| No | 1.000 |
|
| 1.000 |
|
|
|
Yes | 1.826 | 1.138–2.812 | 0.021 | 1.703 | 1.080–2.673 | 0.039 |
| Differentiation
grade |
|
|
|
|
|
|
|
High | 1.000 |
|
|
|
|
|
|
Moderate | 1.086 | 0.653–1.852 | 0.758 |
|
|
|
|
Low | 1.404 | 0.817–2.328 | 0.152 |
|
|
|
| Clinical stage
(FIGO) |
|
|
|
|
|
|
| Stage
II | 1.000 |
| <0.001 | 1.000 |
| <0.001 |
| Stage
III | 4.139 | 1.364–8.927 | <0.001 | 4.627 | 1.627–9.364 | <0.001 |
| Stage
IV | 5.657 | 1.620–9.534 | <0.001 | 6.024 | 1.961–10.258 | <0.001 |
| Treatment
modality |
|
|
|
|
|
|
| HT | 1.000 |
|
| 1.000 |
|
|
|
IMRT | 1.662 | 0.988–2.567 | 0.048 | 1.517 | 0.784–2.361 | 0.089 |
| HPV status |
|
|
|
|
|
|
|
Negative | 1.000 |
|
|
|
|
|
|
Positive | 1.269 | 0.692–2.438 | 0.461 |
|
|
|
| SCC antigen
level |
|
|
|
|
|
|
|
Normal | 1.000 |
|
|
|
|
|
|
Elevated | 1.327 | 0.758–3.015 | 0.194 |
|
|
|
 | Table IX.Univariate and multivariate Cox
regression analysis for progression-free survival after propensity
score matching. |
Table IX.
Univariate and multivariate Cox
regression analysis for progression-free survival after propensity
score matching.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age |
|
|
|
|
|
|
| ≤60
years | 1.000 |
|
|
|
|
|
| >60
years | 1.245 | 0.645–2.523 | 0.510 |
|
|
|
| ECOG score |
|
|
|
|
|
|
| 0 | 1.000 |
|
|
|
|
|
| 1 | 1.397 | 0.785–2.387 | 0.259 |
|
|
|
| Tumor diameter
(MRI) |
|
|
|
|
|
|
| ≤4
cm | 1.000 |
|
| 1.000 |
|
|
| >4
cm | 1.935 | 0.937–3.262 | 0.044 | 1.623 | 0.752–2.967 | 0.115 |
| Parametrial
invasion |
|
|
|
|
|
|
| No | 1.000 |
|
|
|
|
|
|
Yes | 1.426 | 0.628–3.024 | 0.212 |
|
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
| No | 1.000 |
|
| 1.000 |
|
|
|
Yes | 2.373 | 0.927–4.285 | 0.008 | 1.984 | 0.751–4.128 | 0.027 |
| Differentiation
grade |
|
|
|
|
|
|
|
High | 1.000 |
|
|
|
|
|
|
Moderate | 1.009 | 0.536–2.614 | 0.967 |
|
|
|
|
Low | 1.158 | 0.546–2.935 | 0.852 |
|
|
|
| Clinical stage
(FIGO) |
|
|
|
|
|
|
| Stage
II | 1.000 |
| <0.001 | 1.000 |
| <0.001 |
| Stage
III | 2.849 | 0.945–5.694 | <0.001 | 4.213 | 1.568–8.657 | <0.001 |
| Stage
IV | 3.591 | 1.317–7.658 | <0.001 | 5.683 | 2.301–9.657 | <0.001 |
| Treatment
modality |
|
|
|
|
|
|
| HT | 1.000 |
|
| 1.000 |
|
|
|
IMRT | 2.692 | 0.859–4.627 | <0.001 | 2.193 | 0.932–5.128 | 0.012 |
| HPV status |
|
|
|
|
|
|
|
Negative | 1.000 |
|
|
|
|
|
|
Positive | 1.182 | 0.527–2.284 | 0.793 |
|
|
|
| SCC antigen
level |
|
|
|
|
|
|
|
Normal | 1.000 |
|
|
|
|
|
|
Elevated | 1.203 | 0.759–2.681 | 0.614 |
|
|
|
Additionally, treatment-related toxicities were
compared between the HT and IMRT groups after PSM (Table X). Although no statistically
significant differences were demonstrated, the HT group exhibited a
lower incidence of grade 1–2 gastrointestinal, hematological and
vaginal toxicities compared with that of the IMRT group. Moreover,
no significant differences in grade ≥3 toxicities were observed
between the two groups.
 | Table X.Comparison of radiotherapy-related
toxicities between helical tomotherapy and intensity-modulated
radiation therapy groups after propensity score matching. |
Table X.
Comparison of radiotherapy-related
toxicities between helical tomotherapy and intensity-modulated
radiation therapy groups after propensity score matching.
| Toxicity type | HT group
(n=39) | IMRT group
(n=39) | P-value |
|---|
| Radiation
enteritis |
|
| 0.208 |
| Grade
1–2 | 14 (35.9) | 21 (53.8) |
|
| Grade
≥3 | 1 (2.6) | 1 (2.6) |
|
| Radiation
cystitis |
|
| 0.109 |
| Grade
1–2 | 18 (46.2) | 25 (64.1) |
|
| Grade
≥3 | 0 (0.0) | 1 (2.6) |
|
| Radiation
vaginitis |
|
| 0.057 |
| Grade
1–2 | 13 (33.3) | 29 (74.4) |
|
| Grade
≥3 | 0 (0.0) | 0 (0.0) |
|
| Radiation
dermatitis |
|
| 0.647 |
| Grade
1–2 | 18 (46.2) | 15 (38.5) |
|
| Grade
≥3 | 0 (0.0) | 0 (0.0) |
|
| Bone marrow
suppression |
|
| 0.068 |
| Grade
1–2 | 24 (61.5) | 35 (89.7) |
|
| Grade
≥3 | 2 (2.6) | 3 (7.7) |
|
Discussion
With the widespread implementation and continuous
refinement of HT, its advantages in target dose homogeneity,
conformity and protection of OARs have been assessed in the
treatment of several malignancies, including head and neck cancers,
intracranial tumors and prostate cancer (29,30).
Sheng et al (31) reported
that, compared with fixed gantry angle IMRT, HT improved dose
conformity and homogeneity whilst providing superior protection for
critical structures such as the parotid glands in nasopharyngeal
carcinoma. Lee et al (32)
arrived at similar conclusions. However, the unique characteristics
of whole pelvic radiotherapy for cervical cancer (including target
and OAR geometry, dose-volume prescriptions and OAR tolerances)
make it distinct from other malignancies (33). Limited studies have addressed the
dosimetric characteristics of HT in this context (34–36).
The present study systematically compared HT and IMRT in the
treatment of locally advanced cervical cancer. It analyzed
dosimetric distributions in targets and OARs, as well as clinical
outcomes and adverse event profiles, to provide quantitative data
for improving treatment planning and assessing the potential
clinical advantages of HT.
The results of the present study demonstrated that
HT outperformed IMRT in terms of target dose conformity and
homogeneity, as shown by a higher CI and a lower HI. These findings
indicate that HT delivers more precise target coverage whilst
reducing excessive high-dose regions within the target. These
improvements stem from the 360° rotational delivery of HT, which
enables highly uniform dose distribution (37). Optimizing target dose distribution
not only enhances tumor control but also mitigates potential risks
of normal tissue damage from dose hotspots (38). HT demonstrated significant
advantages in reducing high-dose exposure (V40 and
V50) to critical structures such as the rectum, bladder
and femoral heads, with corresponding reductions in mean dose.
High-dose protection of OARs is crucial for minimizing both acute
and late radiation injuries (39).
These results suggest that HT may have a potential clinical
advantage in reducing radiation-induced toxicities, particularly in
the rectum and bladder. However, due to its rotational beam
delivery, HT exhibited greater exposure of normal tissues to
low-dose radiation (V5, V10 and
V20), which may increase long-term risks such as bone
marrow suppression, fractures and secondary malignancies (40,41).
This warrants particular attention in younger patients.
In the present study, HT demonstrated a significant
improvement in PFS, with a median PFS of 67.2 compared with 60.3
months for IMRT (χ2=4.539; P=0.033). However, there was
no statistically significant difference in OS between the two
groups. In addition to prolonged PFS, the HT group also
demonstrated a significantly higher 5-year LCR (82.0 vs. 66.0%) and
longer median local control duration (69.2 vs. 62.6 months). These
results indicate that the dosimetric advantages of HT
(specifically, improved conformity and homogeneity of dose
distribution) may translate into more effective local tumor
control. This is supported by the recurrence pattern analysis,
which revealed a lower overall recurrence rate in the HT group
(50.0 vs. 68.0%) and fewer local and regional relapses. Although
individual recurrence types did not reach statistical significance,
the overall trend suggests that HT may provide greater locoregional
control, which likely contributed to the observed improvement in
PFS.
Given the critical role of adequate dose coverage
and hotspot avoidance in pelvic radiotherapy (42), this finding further supports the
clinical relevance of optimizing radiotherapy techniques beyond
traditional endpoints such as toxicity or PFS. Moreover, whilst
this analysis was based on the full cohort before PSM, the
magnitude of difference in local control suggests a true
therapeutic gain attributable to the physical precision of HT. The
5-year OS rates for HT and IMRT were 72.0 and 68.0%, respectively.
The higher ORR observed in the HT group (92.0 vs. 86.0%) further
highlights its potential clinical benefits. Furthermore, the
improvement in PFS observed with HT likely reflects its superior
local control and reduced treatment-related toxicity. By contrast,
OS can be influenced by a wider range of factors beyond initial
local treatment efficacy, including patterns of distant metastasis,
salvage therapies, comorbidities and post-progression care
(43). The relatively small sample
size and limited number of survival events may also reduce the
statistical power to detect OS differences.
PFS may serve as a more sensitive and immediate
endpoint for evaluating the clinical benefits of advanced
radiotherapy techniques such as HT, particularly in retrospective
cohort studies with long follow-up durations. Given the clinical
relevance of PFS in reflecting disease control, and its
statistically significant difference observed in the present study,
PFS was selected as the primary endpoint for power and sample size
estimation. Whilst dosimetric parameters, such as CI and
V40, provide technical insight into treatment planning
quality, they do not directly translate to patient outcomes. The
observed improvement in PFS with HT likely reflects enhanced
short-term local tumor control due to superior dose conformity and
reduced toxicity.
However, OS is multifactorial and heavily influenced
by systemic therapies, including concurrent chemotherapy, salvage
treatments and control of distant micrometastases (44). Although all patients received
concurrent platinum-based chemoradiotherapy in the present study,
variations in disease biology, treatment response and
post-progression management may have contributed to the lack of a
significant OS difference. This highlights the complementary role
of systemic therapy in determining long-term survival outcomes.
Therefore, sample size justification was based on expected
differences in PFS, which improves the representation of the
clinically meaningful therapeutic benefit (45). The findings of the present study
align with those of Li et al (45), who reported comparable ORR rates
between HT and IMRT but no significant differences in 1- or 3-year
OS and PFS rates. The extended follow-up in the present study
(median, 74 months) may explain the observed improvement in
long-term PFS with HT, suggesting that HT could provide incremental
benefits in the long-term management of locally advanced cervical
cancer. To reduce the potential impact of selection bias inherent
in retrospective studies, PSM was performed based on clinically
relevant baseline characteristics. After matching, the two
treatment groups were well balanced in terms of age, ECOG score,
FIGO stage, tumor diameter and lymph node status. Notably, survival
analyses in the matched cohort yielded consistent results, with HT
still demonstrating a significant improvement in PFS. Furthermore,
multivariate Cox regression confirmed that treatment modality
remained an independent predictor of PFS, along with FIGO stage and
lymph node metastasis. These findings enhance the robustness of the
results of the present study and support the potential clinical
value of HT in improving disease control. HPV status and SCC
antigen levels were also included in the multivariate analysis,
given their established relevance in cervical cancer prognosis.
However, neither variable was revealed to be significantly
associated with OS or PFS in the cohort. This may reflect limited
statistical power or lack of subtype stratification, but their
inclusion strengthens the transparency and completeness of the
prognostic model. However, larger, multi-center studies with longer
follow-up durations are necessary to validate these findings.
Reducing the irradiated volume of critical OARs, such as the rectum
and bladder, is essential for mitigating both acute and chronic
radiation injuries.
In the present study, the HT group exhibited lower
incidences of Grade 1–2 radiation-related toxicities, particularly
for rectal and bladder toxicity. However, there were no significant
differences in the incidence of severe (Grade ≥3) toxicities
between the two groups, possibly due to the limited sample size.
The wider low-dose radiation exposure associated with HT, resulting
from its rotational delivery pattern, warrants further optimization
in treatment planning. Whilst no significant increase in acute
gastrointestinal toxicity was observed in the present study, the
extended low-dose exposure to intestinal tissues in the HT group
raises potential concerns regarding late radiation enteritis.
Chronic gastrointestinal complications, such as radiation-induced
enteritis, can develop months or even years after treatment,
causing long-term bowel dysfunction, diarrhea and malabsorption,
which notably impair patient QoL. The broader low-dose exposure
pattern in HT, which affects the small and large intestines,
increases the risk of such complications over time (46). Although the present study did not
assess these late effects comprehensively, the risk of delayed
gastrointestinal toxicities, including bowel stenosis, chronic
diarrhea and enteritis, should be considered when evaluating the
long-term safety of HT. These complications, whilst not immediately
life-threatening, can have a profound impact on patient well-being
and functional status. Given the potential for delayed
gastrointestinal toxicities due to low-dose radiation exposure, it
is crucial that future studies incorporate structured assessments
of long-term gastrointestinal sequelae, particularly when
evaluating radiotherapy modalities with broader low-dose
distribution patterns such as HT. Implementing advanced dose-volume
constraints, such as those that specifically limit low-dose
exposure to critical gastrointestinal tissues, will be essential to
minimize these risks. Moreover, long-term monitoring of
gastrointestinal function, including the use of validated
instruments for QoL assessment, will help to evaluate the true
patient-centered benefits of HT. Further investigation into the
incidence of radiation enteritis, chronic bowel dysfunction and
other late effects in a larger cohort with longer follow-up is
urgently needed to fully understand the biological consequences of
low-dose radiation exposure in HT.
Moreover, although the differences in grade ≥3
toxicities between HT and IMRT groups were not statistically
significant, the HT group exhibited lower rates of mild-to-moderate
gastrointestinal, hematologic and vaginal toxicities. These
findings, whilst not definitive, suggest a trend toward improved
tolerability with HT, which may be clinically meaningful,
particularly in younger patients or those with borderline treatment
tolerance. Furthermore, although Grade 1–2 toxicities are generally
considered clinically manageable, they may still have meaningful
effects on patient comfort, psychological well-being and overall
QoL, especially when symptoms are persistent (47). Previous studies have indicated that
mild genitourinary and gastrointestinal toxicities, even when not
classified as severe, can markedly impair daily functioning and
patient-reported outcomes. Lapierre et al (48) emphasized the importance of reducing
late toxicity to improve QoL following radiotherapy. Similarly, the
RAPIDO trial reported that lower-grade symptoms, such as mild
diarrhea or urinary irritation, were associated with reductions in
QoL scores in pelvic radiotherapy patients (49). Although the present study did not
incorporate a formal QoL instrument, the reduced incidence of
low-grade toxicities in the HT group may suggest a potential
patient-centered benefit that warrants further investigation in
future prospective studies using validated QoL scales.
Although HT significantly reduces high-dose exposure
to critical structures, its rotational delivery pattern leads to
broader low-dose radiation exposure (V5-V20)
in normal tissues, including the pelvic bone marrow and soft
tissues. This raises concerns about potential long-term effects
such as chronic bone marrow suppression, secondary malignancies or
radiation-induced fibrosis. In the present study, no secondary
cancers or significant differences in late toxicities were observed
during the median 74-month follow-up. However, the relatively small
cohort and limited event rate preclude definitive conclusions.
Future prospective studies with longer follow-up are needed to
evaluate the biological consequences of low-dose radiation exposure
in HT. Additionally, PALN irradiation was selectively applied to
patients with confirmed metastases in the common iliac or
para-aortic regions, in accordance with institutional criteria and
CSCO/NCCN guidelines. The number of patients receiving PALN
irradiation was similar between groups (HT, n=11; IMRT, n=9), and
the delineation protocols and dose prescriptions were standardized.
Therefore, differences in PALN coverage are unlikely to have
influenced the comparative efficacy outcomes between radiotherapy
techniques.
The present study is among the first to
systematically compare HT and IMRT in the treatment of locally
advanced cervical cancer, to the best of our knowledge. By
assessing target dose uniformity, conformity and OAR protection, it
provides comprehensive insights into the dosimetric advantages of
HT. By incorporating PSM and multivariate regression analysis, the
present study also strengthens the validity of the clinical outcome
comparisons between the two modalities. Additionally, with a median
follow-up of 74 months, the present study evaluated the potential
value of HT in improving both short-term and long-term outcomes.
However, several limitations must be acknowledged: i) The
single-center study may have limitations in terms of
generalizability. However, treatment protocols, radiotherapy
equipment and diagnostic workflows in the Cangzhou Hospital of
Integrated Traditional Chinese and Western Medicine are largely
consistent with national and international standards, which
enhances the comparability and potential generalizability of the
findings; ii) The relatively small sample size and limited
follow-up may affect the assessment of rare events, such as severe
adverse reactions or long-term outcomes; iii) Variations in
parameter optimization between HT and IMRT plans may have
influenced the results; iv) The study exclusively included patients
with FIGO stages IIB-IVA, consistent with the standard definition
of locally advanced cervical cancer. As early-stage patients (FIGO
I) are typically treated surgically rather than with
chemoradiotherapy, the findings may not be generalizable to this
population; v) the present study did not perform subgroup analyses
by FIGO stage (such as Stage II vs. III/IV) due to the limited
number of patients within each subgroup. As a result, potential
stage-specific differences in the efficacy of HT could not be
evaluated. Future prospective studies with larger cohorts should
perform FIGO stage-specific subgroup analyses to determine whether
the clinical benefits of HT are consistent across different tumor
stages; vi) Although the median follow-up duration in the present
study was relatively long (74 months), data on specific late
complications such as rectal stenosis, bladder fibrosis or sexual
dysfunction were not systematically collected and thus could not be
analyzed. The present study did not specifically include follow-up
assessments of pelvic bone mineral density, femoral head necrosis
or fractures. However, during the median follow-up period of 74
months, no cases of clinically reported pelvic fractures or femoral
head necrosis were documented in either group. However, the
increased low- to mid-dose exposure to pelvic bones observed with
HT indicates that long-term bone toxicity is an important
consideration. Future studies incorporating imaging-based bone
density evaluations and musculoskeletal toxicity monitoring will be
valuable in improving the characterization of these risks. These
complications are clinically important in pelvic radiotherapy,
particularly for patients with long-term survival. Future
prospective studies should incorporate structured long-term
toxicity assessments, including functional outcomes and QoL
domains, to comprehensively evaluate the impact of advanced
radiotherapy techniques. Furthermore, the present study did not
include formal patient-reported QoL assessments, such as the EORTC
QLQ-CX24 or FACT-Cx (50,51). Whilst prior studies have reported
that reduced toxicity may translate into improved QoL (52–54),
the absence of direct patient-reported outcomes limited the ability
of the present study to confirm this association in the study
cohort. Future prospective studies should integrate validated QoL
instruments to comprehensively evaluate the symptomatic and
functional impact of advanced radiotherapy techniques such as HT.
Future research should also focus on multi-center, large-scale and
prospective randomized controlled trials to further validate the
clinical advantages of HT. Prolonged follow-up is necessary to
assess long-term toxicity and efficacy comprehensively.
Furthermore, advancements in artificial intelligence (AI)-assisted
treatment planning hold promise for improving dose distribution
whilst minimizing normal tissue exposure, enabling more
personalized and effective radiotherapy.
In conclusion, the present study compared the
dosimetric distribution, clinical efficacy and radiation-related
toxicity of HT and IMRT in the treatment of locally advanced
cervical cancer. HT demonstrated significant advantages in target
dose homogeneity, conformity and protection of high-dose regions in
OARs. It also revealed potential benefits in improving PFS and ORR.
However, the increased exposure of normal tissues to low-dose
radiation with HT remains a limitation, necessitating further
optimization and investigation.
As an advanced radiotherapy technology, HT provides
a promising option for personalized treatment of locally advanced
cervical cancer. Future studies incorporating optimized HT planning
and long-term, multi-center clinical research will help realize its
full potential in enhancing patient survival outcomes and
minimizing radiation toxicity. In addition, AI-assisted treatment
planning, particularly using deep learning and knowledge-based
optimization algorithms, has shown potential to enhance the
efficiency and consistency of HT planning. By integrating
large-scale clinical data and patient-specific anatomy, AI-based
systems may further reduce OAR dose exposure, improve plan quality
and support individualized radiotherapy strategies. Although not
applied in the present study, such technologies represent a
promising direction for future clinical implementation and
research. Despite the potential clinical advantages of HT, it
requires specialized equipment, infrastructure and expertise, which
may limit its availability in low- and middle-income settings. The
present study did not include a formal cost-effectiveness analysis.
However, the long-term reduction in toxicity and improvement in
disease control associated with HT may contribute to downstream
cost savings. Comprehensive health economic evaluations, including
direct and indirect costs, are warranted in future research to
assess the feasibility and sustainability of HT in different
clinical and resource contexts. Finally, a multicenter prospective
trial is warranted to generate high-level evidence confirming the
therapeutic benefits of HT and to evaluate its generalizability
across different clinical environments. Such efforts may also
facilitate the standardization of HT protocols, promote
cost-benefit analysis and support more widespread clinical adoption
in both high- and low-resource settings.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Self-funded Project under
the Key Research and Development Program of Cangzhou City (Project
Number: 222106021).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TX performed the data analysis and paper writing. YS
was responsible for the research design and guided the revision of
the paper. JZ designed the study and analyzed data. BW and LX
interpreted data. TX and YS confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The current study was performed in accordance with
the Declaration of Helsinki and approved by the local ethics
committee of the Cangzhou Hospital of Integrated Traditional
Chinese and Western Medicine-Hebei Province (Cangzhou, China;
approval no. CZX2023062). Each patient provided written informed
consent for participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CI
|
conformity index
|
|
HI
|
homogeneity index
|
|
PTV
|
planning target volume
|
|
RTOG
|
Radiation Therapy Oncology Group
|
|
RECIST
|
Response Evaluation Criteria in Solid
Tumors
|
|
ORR
|
objective response rate
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
BMI
|
body mass index
|
|
MRI
|
magnetic resonance imaging
|
|
SCC
|
squamous cell carcinoma
|
|
HPV
|
human papillomavirus
|
|
FIGO
|
International Federation of Gynecology
and Obstetrics
|
|
HRCTV
|
high-risk clinical target volume
|
|
CT
|
computed tomography
|
|
PET
|
positron emission tomography
|
|
IMRT
|
intensity-modulated radiation
therapy
|
|
HT
|
helical tomotherapy
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bhatla N, Aoki D, Sharma DN and
Sankaranarayanan R: Cancer of the cervix uteri: 2021 update. Int J
Gynaecol Obstet. 155:28–44. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wright JD, Matsuo K, Huang Y, Tergas AI,
Hou JY, Khoury-Collado F, St Clair CM, Ananth CV, Neugut AI and
Hershman DL: Prognostic performance of the 2018 international
federation of gynecology and obstetrics cervical cancer staging
guidelines. Obstet Gynecol. 134:49–57. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Diao X, Guo C, Jin Y, Li B, Gao X, Du X,
Chen Z, Jo M, Zeng Y, Ding C, et al: Cancer situation in China: An
analysis based on the global epidemiological data released in 2024.
Cancer Commun (Lond). 45:178–197. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bizzarri N, Querleu D, Dostálek L, van
Lonkhuijzen LRCW, Giannarelli D, Lopez A, Salehi S, Ayhan A, Kim
SH, Ortiz DI, et al: Survival associated with extent of radical
hysterectomy in early-stage cervical cancer: A subanalysis of the
Surveillance in Cervical CANcer (SCCAN) collaborative study. Am J
Obstet Gynecol. 229:428.e1–428.e12. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cibula D, Abu-Rustum NR, Benedetti-Panici
P, Köhler C, Raspagliesi F, Querleu D and Morrow CP: New
classification system of radical hysterectomy: Emphasis on a
three-dimensional anatomic template for parametrial resection.
Gynecol Oncol. 122:264–268. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de la Rochefordiere A, Kamal M, Floquet A,
Thomas L, Petrow P, Petit T, Pop M, Fabbro M, Kerr C, Joly F, et
al: PIK3CA pathway mutations predictive of poor response following
standard Radiochemotherapy ± Cetuximab in cervical cancer patients.
Clin Cancer Res. 21:2530–2537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pujade-Lauraine E, Tan DSP, Leary A, Mirza
MR, Enomoto T, Takyar J, Nunes AT, Chagüi JDH, Paskow MJ and Monk
BJ: Comparison of global treatment guidelines for locally advanced
cervical cancer to optimize best care practices: A systematic and
scoping review. Gynecol Oncol. 167:360–372. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Loiselle C and Koh WJ: The emerging use of
IMRT for treatment of cervical cancer. J Natl Compr Canc Netw.
8:1425–1434. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang AJ, Richardson S, Grigsby PW and
Schwarz JK: Split-field helical tomotherapy with or without
chemotherapy for definitive treatment of cervical cancer. Int J
Radiat Oncol Biol Phys. 82:263–269. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ahamad A, D'Souza W, Salehpour M, Iyer R,
Tucker SL, Jhingran A and Eifel PJ: Intensity-modulated radiation
therapy after hysterectomy: Comparison with conventional treatment
and sensitivity of the normal-tissue-sparing effect to margin size.
Int J Radiat Oncol Biol Phys. 62:1117–1124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang J, Gao J, Zhang F, Gu F, Ding S,
Yang Q, Bai Y and Li G: Pelvic bone marrow sparing intensity
modulated radiation therapy reduces the bone mineral density loss
of patients with cervical cancer. Int J Radiat Oncol Biol Phys.
121:107–117. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu CN, Wang JD, Chen WC, Lin CY, Chiu TJ,
Yang YH, Chang JT, Luo SD and Wang YM: Intensity-modulated proton
therapy versus volumetric-modulated ARC therapy in patients with
nasopharyngeal carcinoma: A long-term, multicenter cohort study.
Radiother Oncol. 202:1106482025. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roy S, MacRae R, Grimes S, Malone J, Lock
M, Mehra P, Morgan SC and Malone S: Helical tomotherapy Versus
3-dimensional conformal radiation therapy in High-risk prostate
cancer: A phase 3 randomized controlled trial. Int J Radiat Oncol
Biol Phys. 120:1386–1393. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Q, Fan S, Xu X, Du S, Zhu G, Jiang
C, Xia SA, Li Q, Wang Q, Qian D, et al: Efficacy and toxicity of
moderately hypofractionated radiation therapy with helical
tomotherapy versus conventional radiation therapy in patients with
unresectable stage III Non-small cell lung cancer receiving
concurrent chemotherapy: A multicenter, randomized phase 3 trial.
Int J Radiat Oncol Biol Phys. 120:422–431. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feliciani G, Licciardello T, Guidi C, Del
Duca M, Mazzotti G, Bellia SR, Ghigi G, Romeo A and Sarnelli A:
Comparison of HDR-brachytherapy and tomotherapy for the treatment
of non-melanoma skin cancers of the head and neck. Radiother Oncol.
204:1107032024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Vulpen M, Field C, Raaijmakers CP,
Parliament MB, Terhaard CH, MacKenzie MA, Scrimger R, Lagendijk JJ
and Fallone BG: Comparing step-and-shoot IMRT with dynamic helical
tomotherapy IMRT plans for head-and-neck cancer. Int J Radiat Oncol
Biol Phys. 62:1535–1539. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bhatla N, Berek JS, Cuello Fredes M, Denny
LA, Grenman S, Karunaratne K, Kehoe ST, Konishi I, Olawaiye AB,
Prat J, et al: Revised FIGO staging for carcinoma of the cervix
uteri. Int J Gynaecol Obstet. 145:129–135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sehnal B, Sláma J, Kmoníčková E, Dubová O
and Zikán M: The changes in FIGO staging for carcinoma of the
cervix uteri. Ceska Gynekol. 84:216–221. 2019.PubMed/NCBI
|
|
21
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 6:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Small W Jr, Mell LK, Anderson P,
Creutzberg C, De Los Santos J, Gaffney D, Jhingran A, Portelance L,
Schefter T, Iyer R, et al: Consensus guidelines for delineation of
clinical target volume for intensity-modulated pelvic radiotherapy
in postoperative treatment of endometrial and cervical cancer. Int
J Radiat Oncol Biol Phys. 71:428–434. 2028. View Article : Google Scholar
|
|
23
|
National Comprehensive Cancer Network
(NCCN), . NCCN clinical practice guidelines in oncology. Cervical
cancer. Version 3. 2024.http://www.nccn.org
|
|
24
|
Cervical Cancer Committee of China
Anti-Cancer Association, . Guidelines for the Treatment of Locally
Advanced Cervical Cancer (2025 edition). Chin J Practical Gynecol
Obstet. 2:186–193. 2025.
|
|
25
|
Ezzell GA, Burmeister JW, Dogan N, LoSasso
TJ, Mechalakos JG, Mihailidis D, Molineu A, Palta JR, Ramsey CR,
Salter BJ, et al: IMRT commissioning: Multiple institution planning
and dosimetry comparisons, a report from AAPM Task Group 119. Med
Phys. 36:5359–5373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klein EE, Hanley J, Bayouth J, Yin FF,
Simon W, Dresser S, Serago C, Aguirre F, Ma L, Arjomandy B, et al:
Task Group 142 report: Quality assurance of medical accelerators.
Med Phys. 36:4197–212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ko CC, Yeh LR, Kuo YT and Chen JH: Imaging
biomarkers for evaluating tumor response: RECIST and beyond.
Biomark Res. 9:522021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cox JD, Stetz J and Pajak TF: Toxicity
criteria of the radiation therapy oncology Group (RTOG) and the
european organization for research and treatment of cancer (EORTC).
Int J Radiat Oncol Biol Phys. 31:1341–1346. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang X, Wang T, Xiao X, Li X, Wang CY,
Huang B, He L and Song Y: Radiotherapy for head and neck tumours
using an oral fixation and parameter acquisition device and TOMO
technology: A randomised controlled study. BMJ Open.
11:e0525422021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Keiler L, Dobbins D, Kulasekere R and
Einstein D: Tomotherapy for prostate adenocarcinoma: A report on
acute toxicity. Radiother Oncol. 84:171–176. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sheng K, Molloy JA and Read PW:
Intensity-modulated radiation therapy (IMRT) dosimetry of the head
and neck: A comparison of treatment plans using linear
accelerator-based IMRT and helical tomotherapy. Int J Radiat Oncol
Biol Phys. 65:917–923. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee TF, Fang FM, Chao PJ, Su TJ, Wang LK
and Leung SW: Dosimetric comparisons of helical tomotherapy and
step-and-shoot intensity-modulated radiotherapy in nasopharyngeal
carcinoma. Radiother Oncol. 89:89–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hsieh CH, Wei MC, Lee HY, Hsiao SM, Chen
CA, Wang LY, Hsieh YP, Tsai TH, Chen YJ and Shueng PW: Whole pelvic
helical tomotherapy for locally advanced cervical cancer: Technical
implementation of IMRT with helical tomotherapy. Radiat Oncol.
4:622009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang R, Xu S, Jiang W, Xie C and Wang J:
Integral dose in three-dimensional conformal radiotherapy,
intensity-modulated radiotherapy and helical tomotherapy. Clin
Oncol (R Coll Radiol). 21:706–712. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shang H, Pu Y, Wang W, Dai Z and Jin F:
Evaluation of plan quality and robustness of IMPT and helical IMRT
for cervical cancer. Radiat Oncol. 15:342020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cheng YK, Kuo SH, Yen HH, Wu JH, Chen YC
and Huang MY: The prognostic significance of pretreatment squamous
cell carcinoma antigen levels in cervical cancer patients treated
by concurrent chemoradiation therapy and a comparison of dosimetric
outcomes and clinical toxicities between tomotherapy and volumetric
modulated arc therapy. Radiat Oncol. 17:912022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Y, Chen Z, Zhou Q, Shang X, Zhao W,
Zhang G and Xu S: A feasibility study of dose-band prediction in
radiation therapy: Predicting a spectrum of plan dose. Radiother
Oncol. 202:1105932025. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fu Q, Chen X, Liu Y, Zhang J, Xu Y, Yang
X, Huang M, Men K and Dai J: Improvement of accumulated dose
distribution in combined cervical cancer radiotherapy with deep
learning-based dose prediction. Front Oncol. 14:14070162024.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yu M, Jang HS, Jeon DM, Cheon GS, Lee HC,
Chung MJ, Kim SH and Lee JH: Dosimetric evaluation of Tomotherapy
and four-box field conformal radiotherapy in locally advanced
rectal cancer. Radiat Oncol J. 31:252–259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cihoric N, Tsikkinis A, Tapia C, Aebersold
DM, Zlobec I and Lössl K: Dose escalated intensity modulated
radiotherapy in the treatment of cervical cancer. Radiat Oncol.
10:2402015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Engels B, De Ridder M, Tournel K, Sermeus
A, De Coninck P, Verellen D and Storme GA: Preoperative helical
tomotherapy and megavoltage computed tomography for rectal cancer:
Impact on the irradiated volume of small bowel. Int J Radiat Oncol
Biol Phys. 74:1476–1480. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Marnitz S, Lukarski D, Köhler C,
Wlodarczyk W, Ebert A, Budach V, Schneider A and Stromberger C:
Helical tomotherapy versus conventional intensity-modulated
radiation therapy for primary chemoradiation in cervical cancer
patients: An intraindividual comparison. Int J Radiat Oncol Biol
Phys. 81:424–430. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu J, Tang G, Zhou Q and Kuang W:
Outcomes and prognostic factors in patients with locally advanced
cervical cancer treated with concurrent chemoradiotherapy. Radiat
Oncol. 17:1422022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gao X, Kong Y, Ning Y, Tian T, Gai X, Lei
K and Cui Z: The prognosis of patients with locally advanced
cervical cancer undergoing surgical versus non-surgical treatment:
A retrospective cohort study based on SEER database and a
single-center data. Int J Surg. 1:1619–1623. 2025. View Article : Google Scholar
|
|
45
|
Li D, Wang D, Feng S, Chen Q, Sheng X, Jia
J, Yan X, Zhu J and Yin Y: Comparing dosimetric and cancer control
outcomes after intensity-modulated radiation therapy and
tomotherapy for advanced cervical cancer. Oncol Lett. 24:2392022.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Renard-Oldrini S, Guinement L, Salleron J,
Brunaud C, Huger S, Grandgirard N, Villani N, Marchesi V, Oldrini G
and Peiffert D: Dosimetric comparaison between VMAT and tomotherapy
with Para-aortic irradiation for cervix carcinoma. Cancer
Radiother. 19:733–738. 2015.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yeung R, McConnell Y, Warkentin H, Graham
D, Warkentin B, Joseph K and Doll CM: Intensity-modulated
radiotherapy (IMRT) vs helical tomotherapy (HT) in concurrent
chemoradiotherapy (CRT) for patients with anal canal carcinoma
(ACC): An analysis of dose distribution and toxicities. Radiat
Oncol. 10:922015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lapierre A, Bourillon L, Larroque M,
Gouveia T, Bourgier C, Ozsahin M, Pèlegrin A, Azria D and Brengues
M: Improving patients' life quality after radiotherapy treatment by
predicting late toxicities. Cancers (Basel). 14:20972022.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dijkstra EA, Hospers GAP, Kranenbarg EM,
Fleer J, Roodvoets AGH, Bahadoer RR, Guren MG, Tjalma JJJ, Putter
H, Crolla RMPH, et al: Quality of life and late toxicity after
short-course radiotherapy followed by chemotherapy or
chemoradiotherapy for locally advanced rectal cancer-The RAPIDO
trial. Radiother Oncol. 171:69–76. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Greimel ER, Kuljanic Vlasic K, Waldenstrom
AC, Duric VM, Jensen PT, Singer S, Chie W, Nordin A, Bjelic Radisic
V and Wydra D: European Organization for Research and Treatment of
Cancer Quality-of-Life Group: European organization for research
and treatment of cancer Quality-of-Life Group: The european
organization for research and treatment of cancer (EORTC)
Quality-of-Life questionnaire cervical cancer module: EORTC
QLQ-CX24. Cancer. 107:1812–1822. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ding Y, Hu Y and Hallberg IR: Psychometric
properties of the Chinese version of the Functional Assessment of
Cancer Therapy-Cervix (FACT-Cx) measuring health-related quality of
life. Health Qual Life Outcomes. 10:1242012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Schörghofer A, Groher M, Karner J, Kopp A,
Kametriser G, Kunit T, Holzinger J, Sedlmayer F and Wolf F:
Risk-adapted moderate hypofractionation of prostate cancer: A
prospective analysis of acute toxicity, QOL and outcome in 221
patients. Strahlenther Onkol. 10:894–901. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fransson P, Nilsson P, Gunnlaugsson A,
Beckman L, Tavelin B, Norman D, Thellenberg-Karlsson C, Hoyer M,
Lagerlund M, Kindblom J, et al: Ultra-hypofractionated versus
conventionally fractionated radiotherapy for prostate cancer
(HYPO-RT-PC): Patient-reported quality-of-life outcomes of a
randomised, controlled, Non-inferiority, phase 3 trial. Lancet
Oncol. 22:235–245. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Schuurhuizen CSEW, Braamse AMJ, Konings
IRHM, Sprangers MAG, Ket JCF, Dekker J and Verheul HMW: Does severe
toxicity affect global quality of life in patients with metastatic
colorectal cancer during palliative systemic treatment? A
systematic review. Ann Oncol. 28:478–486. 2017. View Article : Google Scholar : PubMed/NCBI
|