Introduction
Small cell neuroendocrine carcinoma (SCNEC) is a
highly aggressive malignancy. Although it most commonly arises in
the lungs, its occurrence in the gynaecological tract is rare
(1). When SCNEC does affect
gynaecological organs, the uterine cervix is the most frequently
involved site (1). Cervical SCNEC
accounts for <1% of all gynaecological malignancies and <5%
of all cervical cancers (2,3). Compared to cervical squamous cell
carcinoma (SCC) and adenocarcinoma, cervical SCNEC has a
significantly worse prognosis (1–4).
Therefore, early detection and multimodal therapy are crucial for
improving patient outcomes.
Cytological examination is one of the gold standards
for detecting cervical lesions because it is convenient, minimally
invasive, and cost-effective (5).
Thus, cytological diagnosis plays a crucial role in the early
detection of cervical SCNEC. However, due to its rarity, diagnosing
SCNEC cytologically can be challenging. In one study, only one out
of 13 patients was suspected of having cervical SCNEC based on
cytological findings (6). In
another cytological series, none of the patients were accurately
diagnosed with cervical SCNEC (7).
Instead, patients were often misdiagnosed with squamous lesions,
which are far more common (6,7). The
cytological features of cervical SCNEC are similar to those of
other organs, such as the lungs. These features include loose
cohesive clusters and/or single small, round neoplastic cells with
a high nuclear-to-cytoplasmic ratio, oval-to-round nuclei with
finely granular chromatin and inconspicuous nucleoli, nuclear
moulding in a necrotic background (6–10).
Moreover, cervical SCNEC may coexist with other histological types
of non-neuroendocrine neoplasms, including squamous intraepithelial
lesions (SIL), SCC, and adenocarcinoma (11–15).
The presence of these additional components may lead to a
misdiagnosis of SCNEC components in cytological specimens, as SIL,
SCC, or adenocarcinoma components can overshadow the SCNEC features
(16–19).
This study retrospectively analysed the cytological
features of cervical SCNEC, including combined SCNEC, and discussed
the clinicocytological features of this rare tumour, with a focus
on identifying coexisting carcinoma components.
Materials and methods
Patient selection
Patients diagnosed with cervical SCNEC based on
pathological examination of biopsy and/or surgically resected
specimens at Osaka Medical and Pharmaceutical University Hospital
(Osaka, Japan) between January 2016 and December 2024 were included
in this study.
This retrospective, single-institution study was
conducted in accordance with The Declaration of Helsinki
guidelines. The study protocol was approved by the Institutional
Review Board of Osaka Medical and Pharmaceutical University
Hospital (approval no. 2023-073). All data were anonymised.
Informed consent was obtained from the patients using an opt-out
methodology, as this retrospective study involved the use of
medical records and archived samples with no risk to participants.
This study did not include children. Information regarding study
inclusion criteria and opt-out options was provided on the
institutional website (https://www.ompu.ac.jp/u-deps/path/img/file19.pdf).
Cytological analysis
Cervical smear specimens were conventionally stained
with Papanicolaou stain. No liquid-based cytology was applied in
the present study. Cytological characteristics, including
background features (presence of necrotic material or inflammation)
and nuclear and cytoplasmic features of neoplastic cells, were
evaluated. The cytological features were re-evaluated by at least
two researchers in a blind manner. The cytological diagnosis was
performed according to the criteria of the Bethesda System for
Reporting Cervical Cytology (5). No
statistical analysis was performed in the present study.
Histopathological analysis
Surgically resected or biopsied uterine specimens
were fixed in 10% buffered neutral formalin, dehydrated, embedded
in paraffin, sectioned, and stained with haematoxylin and eosin.
The histopathological features of all specimens were independently
assessed by at least two researchers. Histopathological features,
such as nuclear and cytoplasmic features, were evaluated and
compared with the cytological features observed in cervical smear
specimens.
Immunohistochemical analysis
Immunohistochemical analysis was performed using an
autostainer (Discovery Ultra System; Roche Diagnostics,
Switzerland) according to the manufacturer's instructions;
4-micrometre sections were incubated with the following antibodies:
rabbit monoclonal antibody against CD56 (MRQ-42, pre-diluted
Roche), mouse monoclonal antibody against chromogranin A (LK2H10,
pre-diluted, Roche), mouse monoclonal antibody against p16 (E6H4,
pre-diluted, Roche), and rabbit monoclonal antibody against
synaptophysin (MRQ-40, pre-diluted, Roche). Secondary antibodies
were prediluted and incubated at room temperature using the
Optiview DAB Universal Kit (cat. no. 518-11427; Roche).
Results
Patient characteristics
Table I summarises
the clinicocytological features of the study cohort, which included
six female patients (median age, 55 years; range, 39–70 years).
Three patients had pure SCNEC, while two had combined SCNEC (in
both cases, the other carcinoma component was adenocarcinoma). The
remaining patient had carcinosarcoma, in which the carcinoma
component was SCNEC, and the sarcomatous component was homologous
sarcoma. Patient 6 (carcinosarcoma) received neoadjuvant
chemotherapy, followed by radical hysterectomy. Additionally, for
Patient 3 (pure SCNEC), only biopsy specimens were available. Human
papillomavirus (HPV) testing was not performed on any of the
patients.
 | Table I.Clinicocytological features of small
neuroendocrine carcinoma of the cervix. |
Table I.
Clinicocytological features of small
neuroendocrine carcinoma of the cervix.
|
|
|
|
|
| Cytological
features |
|---|
|
|
| Histological
features |
|
|---|
| Patient no. | Age, years |
| Initial
diagnosis | Background | Clusters | Nuclear moulding | Other components |
|---|
| Histology | Other component
(%) | Stage |
|---|
| 1 | 59 | Combined SCNEC | Adenocarcinoma in
situ (10%) | pT1b1 | SCNEC | Inflammatory | Small and
large | + | Adenocarcinoma |
| 2 | 41 | Pure SCNEC | - | pT1b1 | SCNEC | Inflammatory | Small | + | - |
| 3 | 70 | Pure SCNEC | - | NA | SCNEC | Inflammatory | Small and
large | + | - |
| 4 | 39 | Pure SCNEC | - | pT1b1 | SCNEC | Necrotic | Small and
large | + | - |
| 5 | 52 | Combined SCNEC | Adenocarcinoma
(30%) | pT1b2 | SCC | Necrotic | Small and
large | + | - |
| 6 | 58 | Carcinosarcoma | SCNEC and
homologous sarcoma components (10%) | ypT1b1 | SCNEC | Necrotic | Small and
large | + | Sarcoma |
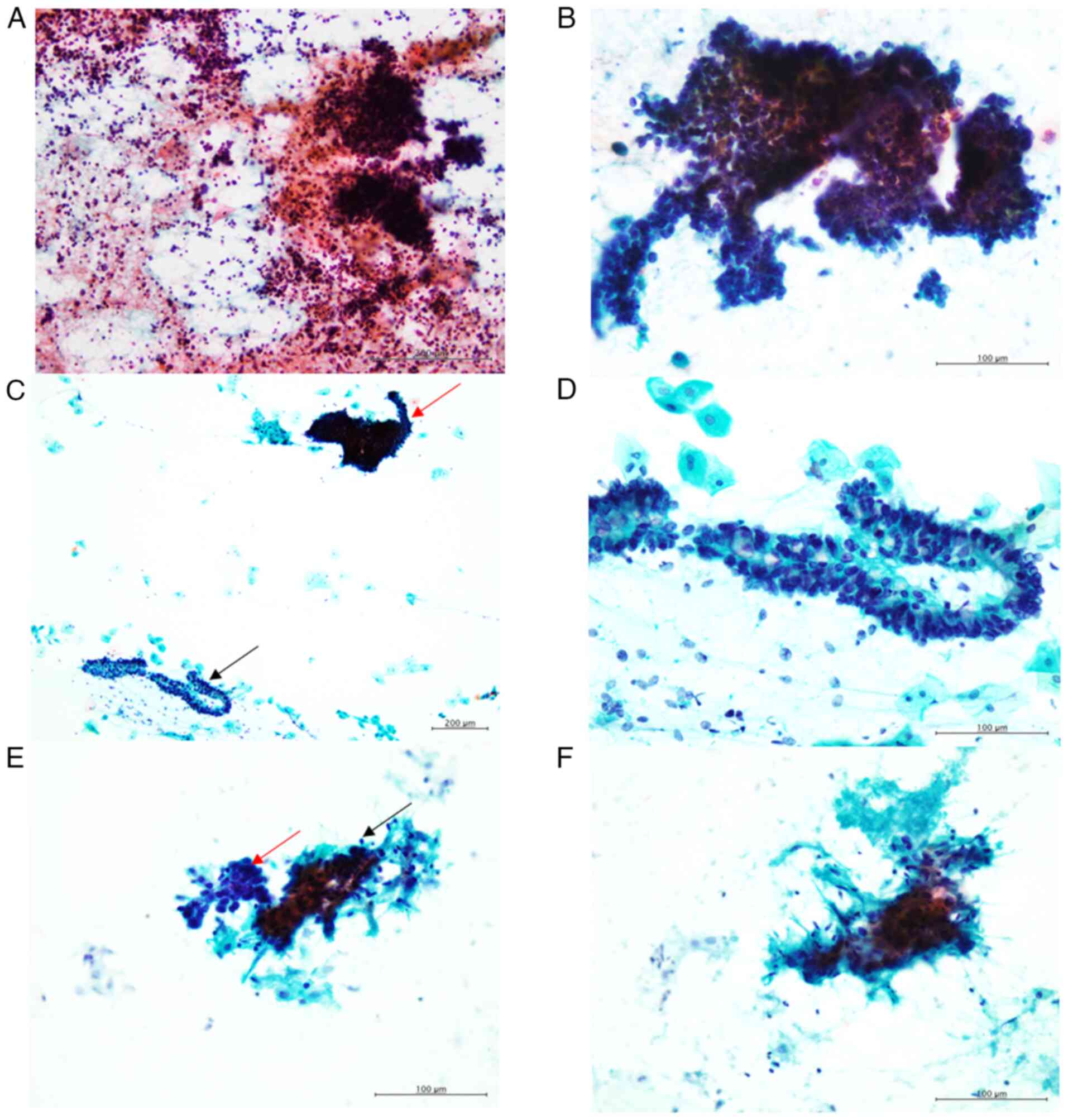
Cytological features
The characteristic cytological features of cervical
SCNEC include the presence of small and/or large clusters of small,
round neoplastic cells with round-to-oval nuclei, exhibiting a
granular chromatin pattern without conspicuous nucleoli in an
inflammatory or necrotic background (Fig. 1A and B). These neoplastic cells also
displayed nuclear moulding (Fig.
1B).
In this study, all cytological specimens contained
SCNEC components. In patients with pure SCNEC, only SCNEC
components were present in the cytological specimens. Among the two
patients with combined SCNEC, adenocarcinoma was identified in the
cytological specimen of one patient, while the other patient's
specimen showed no adenocarcinoma component upon retrospective
evaluation. In Patient 1, an adenocarcinoma component was observed,
characterised by clusters of columnar cells with peripherally
located round-to-oval nuclei and intracytoplasmic mucin (Fig. 1C and D).
For Patient 6 (carcinosarcoma), besides the SCNEC
component, small clusters of spindle-shaped cells were observed.
These cells had large, oval-to-short spindle nuclei with
hyperchromasia and small nucleoli (Fig.
1E and F), suggesting a sarcomatous component.
The initial cytological diagnosis is presented in
Table I. According to the Bethesda
System for Reporting Cervical Cytology (5), five of the six patients were
categorised as having ‘other malignant neoplasms (SCNEC)’. The
remaining patient (Patient 5) was cytodiagnosed with SCC.
Retrospective review revealed that nuclear moulding was present but
not apparent in the cytological specimens of Patient 5 compared to
the other five patients. The adenocarcinoma component (Patient 1)
and the sarcoma component (Patient 6) were not initially detected
cytologically. However, in Patient 6, the presence of atypical
spindle cells was noted in the cytological report.
Histopathological and
immunohistochemical results
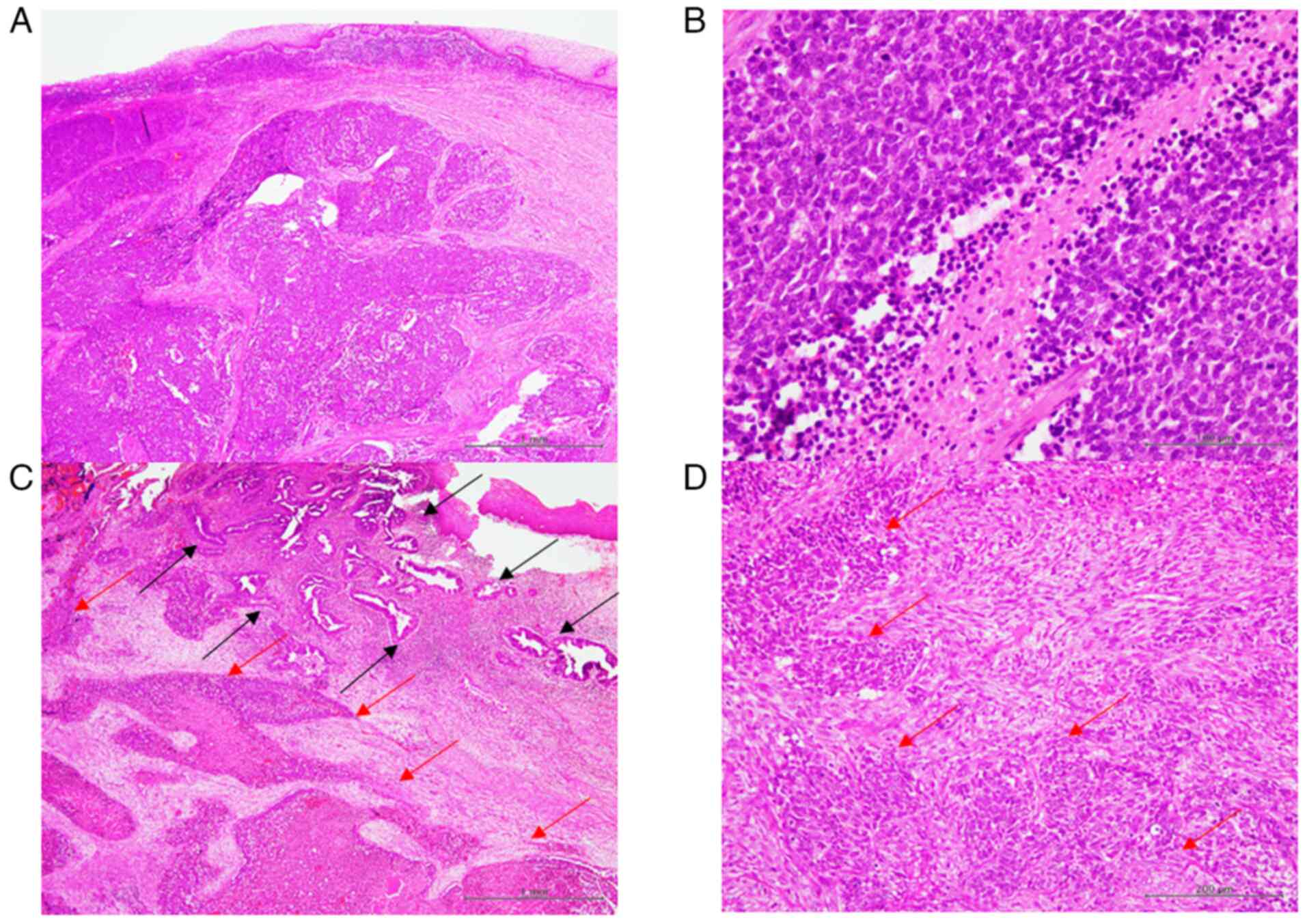
Fig. 2 illustrates
the typical histopathological features of cervical SCNEC. The
proliferation of small, round neoplastic cells with scant cytoplasm
and a high nuclear-to-cytoplasmic ratio without conspicuous
nucleoli were characteristic of SCNEC (Fig. 2A). These neoplastic cells exhibited
nuclear moulding, while mitotic figures and necrosis were also
readily observed (Fig. 2B). In
patients with combined SCNEC (Patients 1 and 5), an adenocarcinoma
component was identified. The adenocarcinoma exhibited glandular
formation by neoplastic columnar cells with large, round-to-oval
nuclei and prominent nucleoli (Fig.
2C). In Patient 1, no invasive growth was noted (adenocarcinoma
in situ), whereas infiltrative growth was observed in
Patient 5 (Fig. 2C). Proliferation
of neoplastic spindle-shaped cells around the SCNEC component was
noted in Patient 6 (carcinosarcoma). These spindle-shaped cells had
large, oval-to-short spindle nuclei with nucleoli, which were
considered to the sarcomatous component (Fig. 2D).
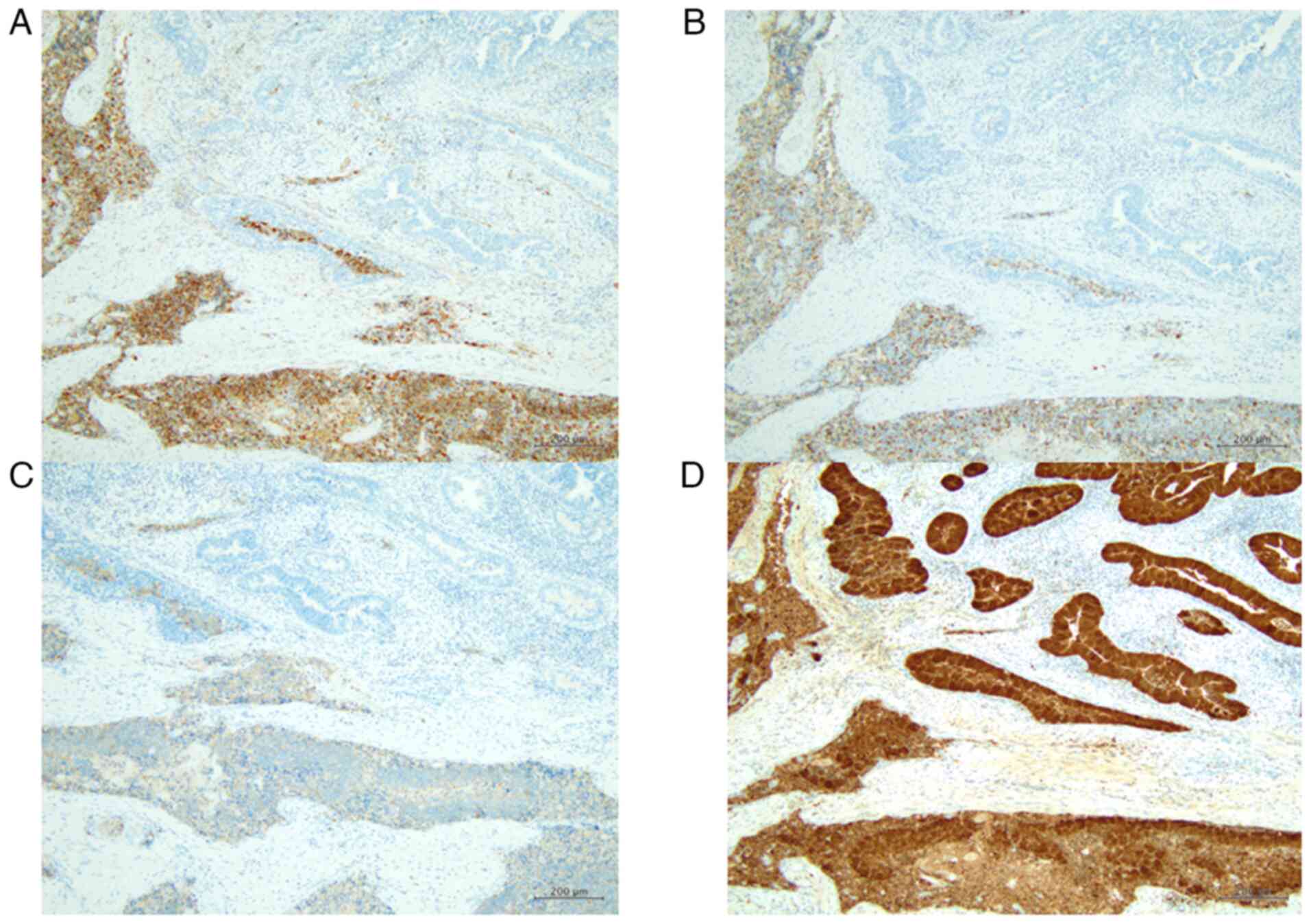
Immunohistochemically, CD56 was diffusely expressed
in the SCNEC of all six patients (Fig.
3A). Chromogranin A and synaptophysin were also expressed in
the SCNEC component of five patients, except Patient 4 (Fig. 3B and C). Additionally, p16 was
diffusely expressed in the SCNEC component of all six patients
(100% of SCNEC cells) (Fig. 3D).
The adenocarcinoma component did not express CD56, chromogranin A,
or synaptophysin; however, p16 was diffusely expressed in the
adenocarcinoma components of Patients 1 and 5 (100% of
adenocarcinoma cells).
Discussion
This study described the cytological features of six
patients with cervical SCNEC. To our knowledge, this is the second
cytological series on cervical SCNEC to include combined SCNEC. The
cytological features of SCNEC are consistent across various organs,
including the lungs, bile duct, uterine cervix, endometrium, and
urinary bladder (6–10). Thus, the cytological diagnosis of
SCNEC using cervical smears should not be particularly challenging.
However, due to the rarity of cervical SCNEC, the rate of accurate
cytodiagnosis remains low (6,7). For
instance, Liu et al (7)
retrospectively analysed cytological cases of cervical SCNEC,
including combined SCNEC. Their study comprised seven pure SCNEC
cases and four combined SCNEC, and none of the patients were
correctly diagnosed with SCNEC based on the initial cytological
examination. Instead, three cases were suspected to have high-grade
SIL, while two were misdiagnosed as atypical glandular cells.
Additionally, only one cytological specimen contained high-grade
SIL, but no adenocarcinoma and SCC components were present by
retrospective review of combined SCNEC (7). Cervical SCNEC is mainly missed or
misdiagnosed as SIL or SCC (6,7,20–22).
In the present series, five of six patients were initially
diagnosed with SCNEC, and the remaining patients were diagnosed
with SCC. Liu et al summarised the causes of misdiagnosis of
SCNEC by the cervical cytological examination as follows: i) the
rarity of this type of carcinoma, ii) SCNEC can be overlooked in
the presence of squamous and/or glandular lesions, iii) SCNEC can
be mimicked by the other neoplasms, such as SIL and SCC
(cytological differential diagnostic considerations are discussed
later), iv) SCNEC is not present in the cytological specimens
because this carcinoma component can be present in the submucosal
region or necrosis and/or heavy inflammatory may result in
insufficient sampling (7).
Interestingly, the present series included two patients with
combined SCNEC and one patient with carcinosarcoma. Retrospective
cytological analysis revealed that the adenocarcinoma component was
present in the cytological specimen of one of the two patients with
combined SCNEC, and both the sarcomatous component and SCNEC were
present in the cytological specimen of the carcinosarcoma. Previous
reports have suggested that SCNEC, as well as combined
adenocarcinoma and/or SIL components, can be detected in cervical
cytological specimens (16,17). According to these results, careful
observation of cervical cytological specimens may lead to the
correct diagnosis of SCNEC as well as combined tumour lesions.
Carcinosarcomas are rare biphasic malignant tumours
composed of carcinomatous and sarcomatous components. Although
rare, SCNEC can serve as the carcinomatous component in cervical
carcinoma (23), as seen in our
study (Patient 6). The sarcomatous component is often homologous
(e.g., fibrosarcoma, endometrioid stromal sarcoma) but can also
exhibit heterologous differentiation (e.g., osteosarcoma and
rhabdomyosarcoma) (23). The
accuracy of initial cytological diagnosis for cervical
carcinosarcoma is generally low. A previous study reported that
only two of 23 cases (21 endometrial and two cervical
carcinosarcomas) were correctly identified as carcinosarcoma
through cytological examination, while 14 cases were misdiagnosed
as carcinoma (24). The detection
of sarcomatous components in endometrial and cervical cytological
specimens is notably more challenging (24,25).
In Patient 6 (carcinosarcoma), the SCNEC component was identified
by initial cytological examination. However, retrospective
cytological analysis was able to detect both SCNEC and sarcomatous
components. These findings emphasise the importance of meticulous
cytological evaluation for achieving an accurate diagnosis.
It is well-recognised that HPV is associated with
the development of a large proportion of cervical neoplasms,
including SIL, SCC, and adenocarcinoma (5). Infection with HPV, particularly HPV18,
a common high-risk strain, is correlated with cervical SCNEC
(1,7). Consequently, cervical SCNEC may
contain SIL, SCC, and/or adenocarcinoma components, although their
precise incidence remains unknown. Immunohistochemical staining for
p16 is widely used as a surrogate marker for detecting high-risk
HPV, and most cervical SCNECs exhibit positive immunoreactivity for
this marker (1,7). Additionally, it has been reported that
combined SIL, SCC, and adenocarcinoma components exhibit positive
immunoreactivity for p16 (11,16,17).
In the present study, all SCNEC showed positive immunoreactivity
for p16. Although HPV testing results were not available for all
patients in the present cohort, all likely had a high-risk HPV
infection, particularly HPV18 (1,7).
The primary differential cytological diagnoses for
cervical SCNEC are SIL and SCC. A small proportion of patients with
cervical SCNEC are misdiagnosed with SIL and SCC (6,7,20–22),
and one of the six patients in the present cohort was initially
diagnosed with SCC. Key distinguishing features of SCNEC include
finely granular (salt-and-pepper) chromatin, inconspicuous
nucleoli, and nuclear moulding (6,7,20–22).
In contrast, SIL and SCC typically exhibit richer and denser
cytoplasm, clearer cell boundaries, and a lack of definitive
nuclear moulding (6,7,20–22).
These cytological features may help in differential diagnosis. In
one patient initially diagnosed with SCC in the present study,
nuclear moulding was not apparent; therefore, SCC was initially
considered. Positive immunocytochemical staining for CD56 may aid
in the cytodiagnosis of SCNEC (20); however, immunocytochemical staining
for neuroendocrine markers was not performed in the present
study.
The present study had some limitations. First, this
study included relatively few patients with SCNEC, although this
type of carcinoma is relatively rare. Second, HPV testing results
were not available for all patients in the present cohort. Further
studies are needed to clarify the clinicocytological and molecular
features of cervical SCNEC.
In conclusion, the results of the present study
demonstrated that most SCNEC could be detected in cervical
cytological specimens. Moreover, other combined carcinoma
components, as well as sarcomatous components, could be identified
in cervical cytological specimens. Thus, cervical cytological
examination may be helpful in SCNEC diagnosis. The detection of
SCNEC in cervical cytological specimens is crucial for accurate and
early diagnosis and timely treatment of this aggressive
carcinoma.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MO and MI conceived the study. MO, MI, HO, MT, KA,
IK, MU, CD, NK, SO, RT, YT, TT, and YH analysed the cytological
and/or clinicopathological data, and MO and MI prepared the
figures. MO and MI wrote the original draft and edited the
manuscript. MO and MI confirm the authenticity of all the raw data.
All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
tenets of The Declaration of Helsinki, and the study protocol was
approved by the Institutional Review Board of Osaka Medical and
Pharmaceutical University (approval no. 2023-073; Takatsuki, Osaka,
Japan). All data were anonymised. Informed consent was obtained
from the patients using an opt-out methodology because of the
retrospective study design, with no risk of patient identity
exposure. In addition, this study did not include children.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HPV
|
human papillomavirus
|
|
SCC
|
squamous cell carcinoma
|
|
SCNEC
|
small cell neuroendocrine
carcinoma
|
|
SIL
|
squamous intraepithelial lesion
|
References
|
1
|
Alvarado-Cabrero I, Euscher ED, Ganesan R
and Howitt BE: Small cell neuroendocrine carcinoma. WHO
Classification of Tumours. Female Genital Tumours. 5th Edition.
Volume 4. IARC Publications; Lyon, France: pp. 455–456. 2020
|
|
2
|
Rouzbahman M and Clarke B: Neuroendocrine
tumors of the gynecologic tract: Select topics. Semin Diagn Pathol.
30:224–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tempfer CB, Tischoff I, Dogan A, Hilal Z,
Schultheis B, Kern P and Rezniczek GA: Neuroendocrine carcinoma of
the cervix: A systematic review of the literature. BMC Cancer.
18:5302018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chu T, Meng Y, Wu P, Li Z, Wen H, Ren F,
Zou D, Lu H, Wu L, Zhou S, et al: The prognosis of patients with
small cell carcinoma of the cervix: A retrospective study of the
SEER database and a Chinese multicentre registry. Lancet Oncol.
24:701–708. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nayar R and Wilbur DC: The Bethesda system
for reporting cervical cytology. 3rd Edition. Springer;
Switzerland: 2015, View Article : Google Scholar
|
|
6
|
Zhou C, Hayes MM, Clement PB and Thomson
TA: Small cell carcinoma of the uterine cervix: Cytologic findings
in 13 cases. Cancer. 84:281–288. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Li M, Liu Y, Wan Y, Yang B, Li D
and Wang S: Liquid-based cytology of small cell carcinoma of the
cervix: A multicenter retrospective study. Onco Targets Ther.
17:557–565. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ebisu Y, Ishida M, Okano K, Sandoh K,
Mizokami T, Kita M, Okada H and Tsuta K: Small-cell neuroendocrine
carcinoma in directly sampled endometrial cytology: A monocentric
retrospective study of six cases. Diagn Cytopathol. 47:1297–1301.
2019. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishida M, Okano K, Sandoh K, Ito H, Ikeura
T, Mitsuyama T, Miyoshi H, Shimatani M, Takaoka M, Okazaki K and
Tsuta K: Neuroendocrine carcinoma diagnosis from bile duct
cytological specimens: A retrospective single-center study. Diagn
Cytopathol. 48:154–158. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshida K, Ishida M, Kagotani A, Iwamoto
N, Iwai M and Okabe H: Small cell carcinoma of the urinary bladder
and prostate: Cytological analyses of four cases with emphasis on
the usefulness of cytological examination. Oncol Lett. 7:369–372.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alvarado-Cabrero I, Euscher ED, Ganesan R
and Howitt BE: Carcinoma admixed with neuroendocrine carcinoma. WHO
Classification of Tumours, Female Genital Tumours. 5th Edition.
Volume 4. IARC Press; Lyon, France: pp. 4592020
|
|
12
|
Masuda M, Iida K, Iwabuchi S, Tanaka M,
Kubota S, Uematsu H, Onuma K, Kukita Y, Kato K, Kamiura S, et al:
Clonal origin and lineage ambiguity in mixed neuroendocrine
carcinoma of the uterine cervix. Am J Pathol. 194:415–429. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pei X, Xiang L, Chen W, Jiang W, Yin L,
Shen X, Zhou X and Yang H: The next generation sequencing of
cancer-related genes in small cell neuroendocrine carcinoma of the
cervix. Gynecol Oncol. 161:779–786. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li S and Zhu H: Twelve cases of
neuroendocrine carcinomas of the uterine cervix: Cytology,
histopathology and discussion of their histogenesis. Acta Cytol.
57:54–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Giordano G, D'Adda T, Pizzi S, Campanini
N, Gambino G and Berretta R: Neuroendocrine small cell carcinoma of
the cervix: A case report. Mol Clin Oncol. 14:922021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okabe A, Ishida M, Noda Y, Okano K, Sandoh
K, Fukuda H, Kita M, Okada H and Tsuta K: Small-cell neuroendocrine
carcinoma of the cervix accompanied by adenocarcinoma and
high-grade squamous intraepithelial lesion. Diagn Cytopathol.
50:E285–E288. 2022. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishiumi Y, Nishimura T, Kashu I, Aoki T,
Itoh R, Tsuta K and Ishida M: Adenocarcinoma in situ admixed with
small cell neuroendocrine carcinoma of the cervix: A case report
with cytological features. Diagn Cytopathol. 46:752–755. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimojo N, Hirokawa YS, Kanayama K, Yoneda
M, Hashizume R, Hayashi A, Uchida K, Imai H, Kozuka Y and Shiraishi
T: Cytological features of adenocarcinoma admixed with small cell
neuroendocrine carcinoma of the uterine cervix. Cytojournal.
14:122017.PubMed/NCBI
|
|
19
|
Alphandery C, Dagrada G, Frattini M,
Perrone F and Pilotti S: Neuroendocrine small cell carcinoma of the
cervix associated with endocervical adenocarcinoma: A case report.
Acta Cytol. 51:589–593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta P, Gupta N, Suri V, Rai B and
Rajwanshi A: Cytomorphological features of cervical small cell
neuroendocrine carcinoma in SurePath™ liquid-based cervical
samples. Cytopathology. 32:813–818. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim MJ, Kim NR, Cho HY, Lee SP and Ha SY:
Differential diagnostic features of small cell carcinoma in the
uterine cervix. Diagn Cytopathol. 36:618–623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim Y, Ha HJ, Kim JS, Chung JH, Koh JS,
Park S and Lee SS: Significance of cytologic smears in the
diagnosis of small cell carcinoma of the uterine cervix. Acta
Cytol. 46:637–644. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yemelyanova A, Kong CS and Srinivasan R:
Carcinosarcoma. WHO Classification of Tumours. Female Genital
Tumours. 5th Edition. Volume 4; IARC Press; Lyon, France: pp.
3822020
|
|
24
|
Costa MJ, Tidd C and Willis D:
Cervicovaginal cytology in carcinosarcoma [malignant mixed
Mullerian (mesodermal) tumor] of the uterus. Diagn Cytopathol.
8:33–40. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Okano K, Ishida M, Sandoh K, Mizokami T,
Kita M, Okada H and Tsuta K: Cytological features of uterine
carcinosarcoma: A retrospective study of 20 cases with an emphasis
on the usefulness of endometrial cytology. Diagn Cytopathol.
47:547–552. 2019. View
Article : Google Scholar : PubMed/NCBI
|