Introduction
Melanoma is a highly aggressive malignancy arising
from the malignant transformation of melanocytes, neural
crest-derived cells located in the basal epidermis (1). Cutaneous melanoma (CM) is the
predominant subtype, accounting for >90% of new melanoma
diagnoses and ~75% of skin cancer-associated mortalities due to its
rapid systemic dissemination and high metastatic potential
(2,3). In autopsy studies, brain metastases
(MTS) have been observed in up to 74% of patients with advanced
melanoma (4).
Pregnancy-associated melanoma (PAM) is rare, with an
estimated incidence rate ranging from 1.4 to 8.5 cases per 100,000
pregnancies, based on data from European and U.S. studies (5–8).
Melanoma is also one of the few cancer types capable of
metastasizing to the placenta and fetus, accounting for
approximately one-third of all reported placental MTS (9). With placental involvement, the
transmission risk to the fetus is ~22%, placing such neonates at
high risk (10).
The therapeutic landscape of advanced melanoma has
been transformed by targeted inhibitors of the mitogen-activated
protein kinase (MAPK) pathway and immune checkpoint blockade
(11–13). However, systemic treatment during
pregnancy remains challenging due to concerns regarding
teratogenicity and limited available data. Only a few case reports
have described the use of the B-Raf proto-oncogene serine/threonine
kinase (BRAF) inhibitor vemurafenib during pregnancy (14–17);
however, to the best of our knowledge, the present study is the
first report documenting combined BRAF and mitogen-activated
protein kinase kinase (MEK) inhibition with dabrafenib and
trametinib (D + T) in a pregnant patient with melanoma brain
MTS.
The current report presents the clinical course of a
35-year-old woman diagnosed at 24 weeks of gestation with multiple
brain MTS from melanoma, who was managed with a multidisciplinary
approach, including neurosurgery, targeted therapy and subsequent
immunotherapy.
Case report
Patient history
The current study presents the case of a 35-year-old
pregnant woman with a history of superficial spreading melanoma on
the scalp. The primary tumor showed ulceration, vertical growth
phase, Clark's level IV (18) and a
Breslow thickness of 1.1 mm (19).
Initial treatment consisted of an excisional biopsy with narrow
resection margins (May 2021). Despite medical recommendations, a
radical re-excision of the tumor bed and sentinel lymph node biopsy
were not performed, mainly due to circumstances related to the
SARS-CoV-2 pandemic.
At the follow-up in January 2023, a whole-body
18F-FDG PET/CT revealed a non-specific pulmonary nodule
in the left anterior segment (S3) of the lung, without evidence of
MTS. The melanoma was staged as IIA (T2bN0M0) according to the
American Joint Committee on Cancer system (20). Systemic treatment was not indicated,
and routine surveillance was continued.
Presentation during pregnancy
In January 2024, ~3 years later, the patient
presented to the Academician Ladislav Dérer Hospital (University
Hospital Bratislava; Bratislava, Slovakia) at 24+2 weeks of
gestation with a brief episode of expressive aphasia that resolved
within 15 min. Upon admission, the neurological examination was
normal.
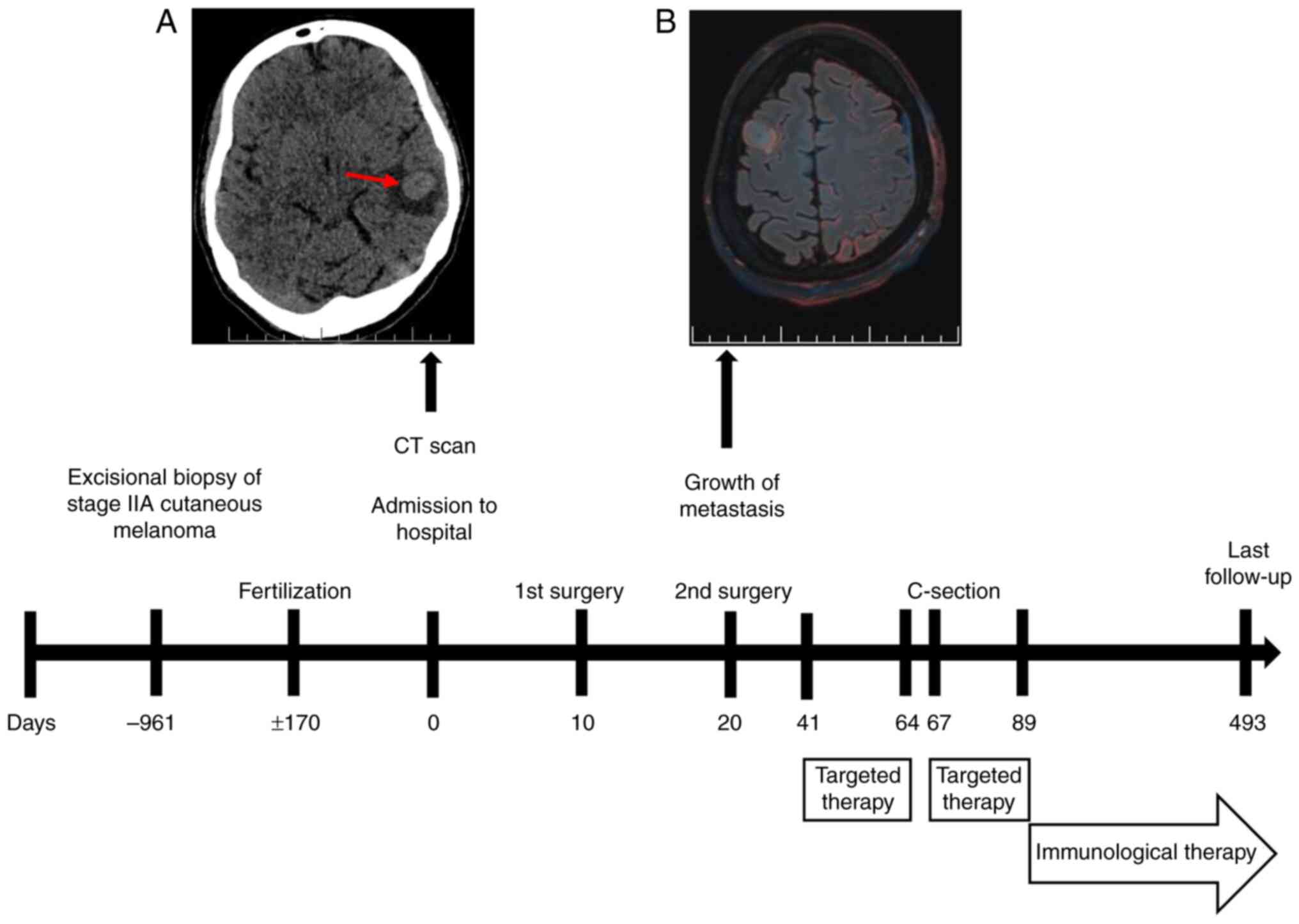
A non-contrast low-dose CT scan of the brain
revealed an intra-axial lesion in the left temporal lobe, with
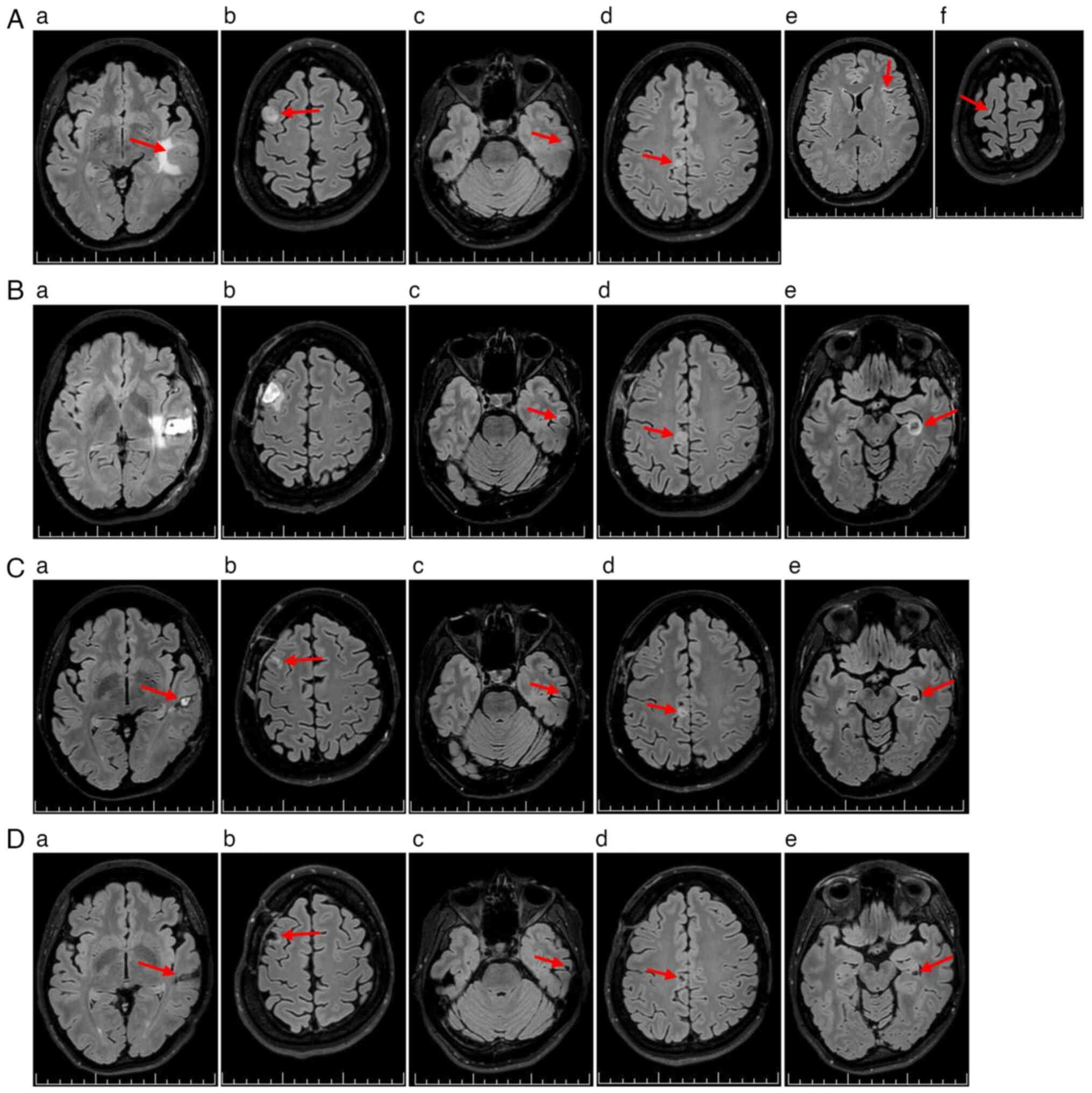
perifocal edema (Fig. 1A). The
following day, non-contrast 3T brain MRI identified six
supratentorial lesions. A total of four lesions showed radiological
features consistent with MTS (Fig.
2Aa-Ad), while two small cortical hyperintensities on
fluid-attenuated inversion recovery sequences were atypical but
suspicious for metastatic disease (Fig.
2Ae and Af).
For systemic staging, whole-body (WB)-MRI with
diffusion-weighted imaging demonstrated a small solid lesion in the
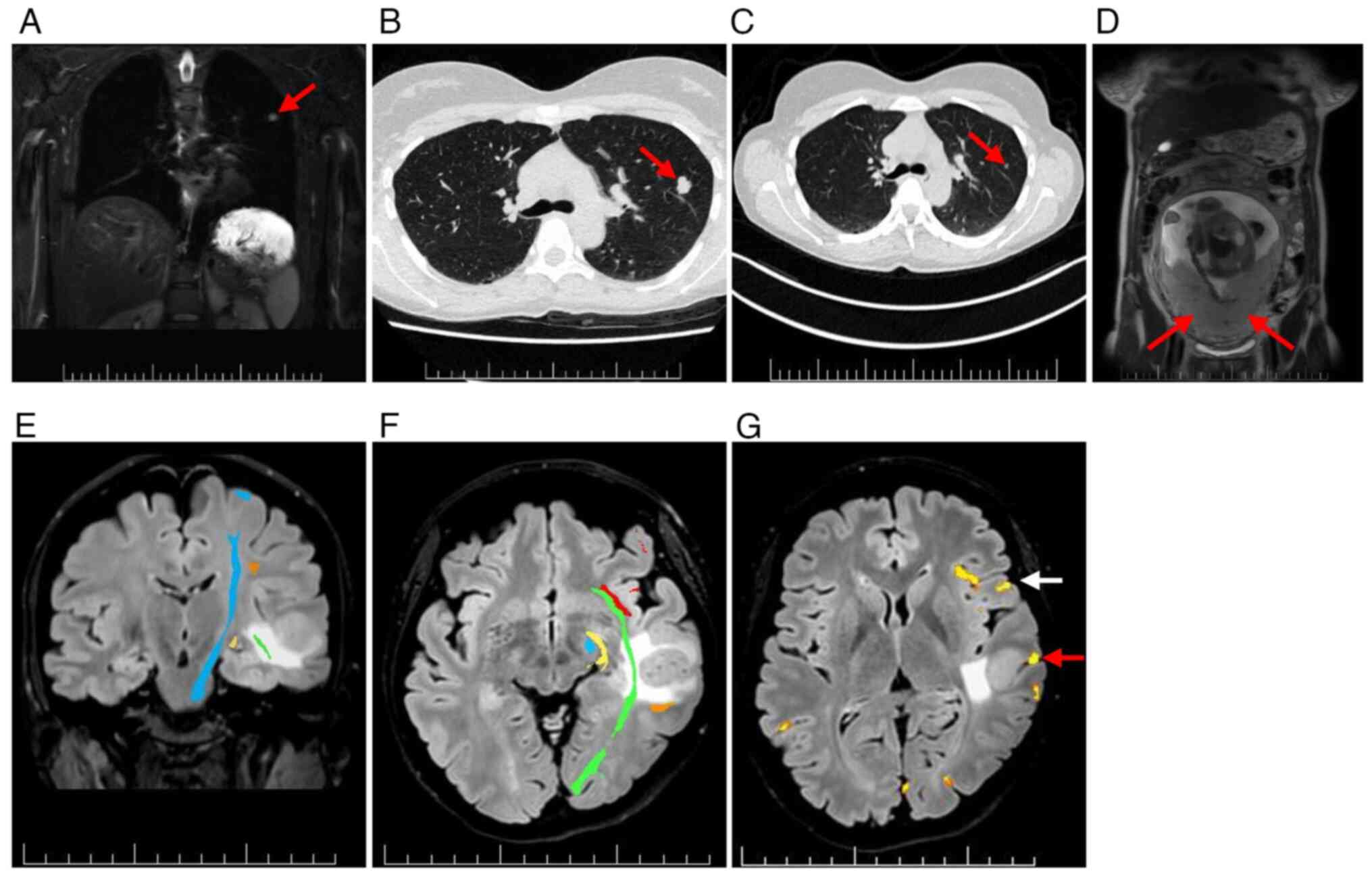
left upper lung lobe (Fig. 3A).
High-resolution CT confirmed a lobulated nodule in the left S3
region (Fig. 3B). Retrospective
comparison with a CT scan from January 2023, showed interval growth
of 12 mm (Fig. 3C). No uterine or
placental involvement was detected using WB-MRI (Fig. 3D).
Neurosurgical management
After multidisciplinary counseling and obtaining
informed consent, a pro-fetus strategy was adopted. Due to
symptomatic peritumoral edema from brain MTS, the patient received
intravenous dexamethasone (4 mg every 8 h for 12 doses; total, 48
mg), followed by oral methylprednisolone (16 mg every 8 h), which
was gradually reduced and discontinued after 6 weeks.
Preoperative MRI mapping with diffusion tensor
imaging and functional MRI showed the largest left temporal MTS
abutting eloquent language and motor areas (Fig. 3E-G). In January 2024, an awake
craniotomy with resection of the left middle temporal gyrus MTS was
performed (Fig. 2Ba).
Histopathological analysis of formalin-fixed and paraffin-embedded
samples confirmed metastatic melanoma (Fig. S1) (21). Immunohistochemistry demonstrated the
following results: Cytokeratin AE1/3(−), S100(+), SOX10(+),
melan-A(+), HMB-45(+), preferentially expressed antigen in
melanoma(+), with a Ki-67 proliferation index up to 30% (Fig. S1) (22,23).
Molecular testing detected a BRAF V600E mutation using the CE-IVD
certified PNAClamp™ BRAF Mutation Detection Kit (HLP
Panagene Co., Ltd.), performed according to the manufacturer's
instructions. No epileptiform activity was observed on preoperative
electroencephalography or intraoperative electrocorticography.
Due to interval progression, a second craniotomy was
undertaken in January 2024, to resect the right superior frontal
gyrus MTS (Figs. 1B and 2Bb). Both procedures were uneventful. The
patient and fetus remained clinically stable without neurological
deficit, and the patient was discharged to close outpatient
surveillance in February 2024.
Initiation of targeted therapy
A follow-up MRI in February 2024 demonstrated
progression of brain MTS (Fig. 2Bc and
Bd) and the emergence of a new unresectable left hippocampal
MTS, with perifocal edema (Fig.
2Be). At 30+1 weeks' gestation, the patient was counseled
regarding three potential management strategies: i) Induction of
preterm delivery to permit systemic therapy; ii) initiation of
systemic therapy during pregnancy; or iii) a conservative
watch-and-wait approach.
A multidisciplinary board, chaired by the hospital
director and including an oncologist, neonatologist, gynecologist,
neurosurgeon, neurologist and clinical psychologist, reviewed the
case. Counseling addressed maternal neurological risks, potential
teratogenicity of targeted therapy, expected neonatal outcomes at
different gestational ages, the rationale for delaying vs.
expediting delivery and the scope of fetal surveillance. The
patient was given >24 h to reflect on the information and had
the opportunity to consult with their husband and family.
After providing informed consent, the patient
elected to continue the pregnancy and initiate systemic targeted
therapy. In February 2024, oral dabrafenib (150 mg twice daily) and
trametinib (2 mg once daily) were commenced (Fig. 1).
A subsequent MRI in March 2024 demonstrated
favorable postoperative changes (Fig.
2Ca and Cb), along with a marked reduction in brain MTS volume
compared with that in February 2024 (Fig. 2Cc-Ce).
Peripartum events
To allow for fetal maturation, delivery was planned
for ~34 weeks' gestation. Oncological therapy was interrupted 3
days beforehand, and intramuscular dexamethasone (6 mg every 12 h;
four doses) was administered for fetal lung maturation.
In March 2024 (33+6 weeks), the patient experienced
a focal impaired consciousness seizure, necessitating an urgent
cesarean section. A eutrophic premature male neonate was delivered,
weighing 2,030 g and measuring 46 cm, with Apgar scores of 9/9/9
(24). The patient resumed targeted
oncological therapy postoperatively and was started on
levetiracetam, titrated to 500 mg twice daily, for seizure
control.
Neonatal findings and follow-up
At birth, the neonate exhibited delayed cranial
ossification with widely open anterior and posterior fontanelles
and a broad sagittal suture. Cutaneous findings included fragile
vasculature with dark discoloration, most pronounced in the cubital
veins, and several isolated dark gray macules (4–5 mm in
diameter).
The neonate developed respiratory insufficiency
requiring admission to the neonatal intensive care unit (NICU) and
continuous positive airway pressure support. Transient hypotension
was managed with intravenous crystalloids. Progression to
respiratory distress syndrome necessitated surfactant replacement
and 6 days of mechanical ventilation. Pulmonary hypertension was
treated with inhaled nitric oxide and vasopressors. Cranial
ultrasound revealed a grade I intraventricular hemorrhage and stage
I hypoxic-ischemic encephalopathy. No congenital malformations were
identified. Histological examination of the placenta and umbilical
cord showed no evidence of metastatic disease (Fig. S1). The neonate was discharged 27
days after birth.
The child has been followed up at the National
Institute of Children's Diseases (Bratislava, Slovakia).
Neurodevelopmental assessment at 11 months of chronological age
(corrected age, 9 months) included standardized testing with the
Bayley Scales of Infant and Toddler Development, Fourth Edition
(Bayley-4) (25) for cognitive,
communication and motor domains, and the Bayley-III (26) for social-emotional behavior.
Cognitive function (37th percentile), communication (50th
percentile) and motor function (73rd percentile) were all within
the average range (16th-84th percentile) for corrected age, while
social-emotional behavior was in the higher-average range (75th
percentile). Repeated screening with the S-PMV test (Slovakian,
Skríning psychomotorického vývoja) was also normal. At the
latest follow-up (May 2025), growth and psychomotor development
were within normal limits.
Maternal oncological course and
follow-up
The patient's targeted therapy with D + T was
terminated after 7 weeks (April 2024) due to pyrexia and fatigue.
The patient was subsequently transitioned to combined immune
checkpoint inhibitor therapy as second-line treatment (Fig. 1). The concurrent regimen of
ipilimumab (3 mg per kg) and nivolumab (1 mg per kg) was
administered intravenously every 3 weeks for four doses, followed
by maintenance nivolumab (240 mg) every 2 weeks.
In May 2024, the patient experienced a second
epileptic seizure of the focal to bilateral tonic-clonic type, and
the levetiracetam dosage was increased to 750 mg twice daily. As of
May 2025, the patient remains on maintenance nivolumab therapy with
partial disease remission and no evidence of treatment-related
toxicity (Fig. 2Da-De).
Cognitive function remained intact, with a Montreal
Cognitive Assessment (27) score of
30 out of 30. Quality of life, assessed with the Patient-Weighted
Quality of Life in Epilepsy Inventory (version 2) (28), was 70.6 out of 100. Psychological
evaluation revealed moderate anxiety, with a Generalized Anxiety
Disorder-7 (29) score of 8 out of
21 and mild depressive symptoms, with a Patient Health
Questionnaire-9 (30) score of 9
out of 27, without suicidal ideation.
The patient remains under regular oncological
follow-up, receiving maintenance nivolumab every 2 weeks and under
neurological surveillance every 3 months. The partial remission
response could be durable as demonstrated in the CheckMate 204
study (12); however, long-term
vigilance is warranted.
Discussion
CM is a highly aggressive malignancy responsible for
>60,000 mortalities annually (31). Over the past decade, innovative
systemic therapies, including MAPK pathway inhibitors (BRAF and
MEK) and immune checkpoint blockers (cytotoxic T-lymphocyte
associated protein 4 and programmed cell death protein 1), have
notably improved melanoma prognosis, with 3-year overall survival
rates reaching 41.3 and 58.4%, respectively. During the first year
of treatment, the combination of BRAF and MEK inhibitors has shown
superior efficacy compared with immune checkpoint blockade, with a
rapid onset of response even in brain MTS, although typically
limited to 6 months (11,32). In patients with symptomatic brain
MTS, intracranial responses to vemurafenib (a BRAF inhibitor) were
seen in only 16% of cases (33). By
contrast, D + T therapy achieved an intracranial response rate of
58% in patients with BRAF V600-mutant melanoma brain MTS, compared
with 31% in patients treated with dabrafenib monotherapy (11).
Human placental cotyledon models indicate notable
passage of D + T molecules through the placental barrier, with
higher fetal transfer for dabrafenib (14.9%) compared with
trametinib (8.6%) (34).
Preclinical animal studies have shown that BRAF inhibitors
(vemurafenib, dabrafenib and encorafenib) possess teratogenic
potential, while trametinib (a MEK inhibitor) has been associated
with possible teratogenic and embryotoxic effects (35). Dabrafenib demonstrated teratogenic
and embryotoxic properties at doses three times higher compared
with standard human exposure (36,37). D
+ T may disrupt fetal growth and development by inhibiting the
RAS/MAPK signaling pathway, potentially causing congenital defects
(including cardiomyopathies, and facial and skeletal anomalies),
developmental delay, intellectual disability and tumor
predisposition (35,38). Furthermore, the MAPK/ERK pathway
serves a crucial role in trophoblast proliferation, making BRAF/MEK
inhibitor use particularly concerning during early pregnancy
(5,39).
As of May 2025, to the best of our knowledge, no
reports have documented the combined use of BRAF and MEK inhibitors
during pregnancy. Available data and well-controlled studies on the
safety of D + T in pregnant women remain limited, precluding
definitive conclusions (35).
Current European Society for Medical Oncology (ESMO) and American
Society of Clinical Oncology guidelines advise against combined
BRAF/MEK inhibition during pregnancy due to teratogenic and
embryotoxic risks. As alternatives, interferon-α or BRAF inhibitor
monotherapy may be considered as temporizing measures if urgent
systemic treatment cannot be delayed (40–43).
In the present case of advanced metastatic melanoma
during pregnancy, two surgical resections of the largest
symptomatic brain MTS were prioritized. However, due to further
inoperable progression and the patient's refusal of preterm
delivery, systemic therapy with D + T was initiated, based on its
superior efficacy over vemurafenib or dabrafenib monotherapy
(11,33,44).
The immediacy of life-threatening brain MTS justified short-term
dual BRAF/MEK inhibition despite guideline cautions, supported by
multidisciplinary consensus, comprehensive maternal counseling and
close materno-fetal monitoring. Therapy was initiated only after
the period of fetal organogenesis had been completed. Histological
examination of the placenta and umbilical cord, in line with ESMO
guidance, revealed no metastatic involvement.
There are isolated reports of BRAF inhibitor use in
pregnancy-associated stage IV melanoma (Table I). In all 4 cases (including one set
of twins), vemurafenib was administered (14–17),
with low-level transplacental transfer (37). Administration occurred between 17
and 25 weeks' gestation, and all 5 infants were born prematurely
(26–36 weeks). A total of 3 had a low birth weight (1,028, 950 and
900 g), 1 weighed 2,510 g and 1 had an unspecified weight, with 3
of the newborns requiring NICU admission. With regard to the
mothers, 1 patient with a solitary brain metastasis in the temporal
lobe died from an intracranial hemorrhage 78 days after treatment
initiation (16). Another was
diagnosed with cerebral and dural MTS postpartum and died 3.5
months after initiating vemurafenib therapy (15). The present case therefore contrasts
with previous vemurafenib-only reports, being the first to describe
combined BRAF/MEK inhibition in pregnancy. Notably, the patient
remains alive in partial remission at >14 months (452 days)
after the initiation of D + T.
 | Table I.Case reports of targeted therapy in
pregnancy-associated stage IV melanoma. |
Table I.
Case reports of targeted therapy in
pregnancy-associated stage IV melanoma.
| First author,
year | Drug | Patient age,
years | Weeks gestation at
initiation of treatment | Weeks gestation at
delivery | Neonate birth
weight, g | NICU admission of
neonate | Metastatic
involvement in patient | (Refs.) |
|---|
| Pagan et al,
2019 | Vemurafenib | 25 | 25 | 34 | 2,510 | Yes | Lungs, subcutaneous
and lymph node | (14) |
| Maleka et
al, 2013 | Vemurafenib | 37 | 25 | 30 | 1,028 | No | Subcutaneous,
liver, lungs, brain and meninges | (15) |
| de Haan et
al, 2018 | Vemurafenib | 30 | 22 | 26 | 950 and 900 | Yes (both) | Cutaneous, bowel,
mesentery, breast and brain | (16) |
| Marcé et al,
2019 | Vemurafenib | 29 | 17 | 36 | Not mentioned | No | Liver, lymph node,
subcutaneous and bone | (17) |
| Present case | Dabrafenib +
trametinib | 35 | 30 | 33 | 2,030 | Yes | Brain |
|
Checkpoint inhibitors introduced postpartum in the
present case provided additional long-term disease control.
Previous research highlights the heterogeneity of the melanoma
immune microenvironment and identifies a prognostic NOD-like
receptor gene signature strongly associated with survival in skin
CM (45). Such insights into
tumor-immune interactions may help explain the durable partial
remission observed in the present patient under nivolumab
maintenance.
The neonate in the present case exhibited delayed
cranial ossification, fragile vasculature and respiratory
complications requiring admission to the NICU. These findings are
most likely multifactorial, reflecting prematurity and possible
in utero exposure to targeted therapy. While inhibition of
the MAPK pathway provides a plausible mechanistic link to abnormal
ossification, causality cannot be established. A limitation is that
therapeutic drug monitoring of D + T plasma levels was not
available at the Academician Ladislav Dérer Hospital (University
Hospital Bratislava, Bratislava, Slovakia) and therefore was not
performed.
The present case provides rare insight into the
management of PAM with brain MTS, illustrating that, in selected
situations with immediate maternal risk, combined BRAF/MEK
inhibition may be justified following multidisciplinary evaluation,
despite current guideline cautions. The present report emphasizes
the importance of individualized decision-making, careful
materno-fetal monitoring and long-term follow-up of both mother and
child.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Associate Professor
Andrej Steno, Head of the Department of Neurosurgery, Faculty of
Medicine, Comenius University (Bratislava, Slovakia), for
performing both neurosurgical procedures and for expert guidance.
The authors also thank Dr Robert Godal, oncologist at the 2nd
Department of Oncology, Faculty of Medicine, Comenius University
(Bratislava, Slovakia), for providing valuable input regarding
oncological management and follow-up care.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JM, MJ, SM, KKG, MKa and GT collected and analyzed
clinical data. JM drafted the manuscript. PV and GT contributed to
the study conception and supervised the project. IS and MKr
participated in data interpretation and provided critical revisions
of the manuscript. MJ, SM, KKG, MKa, IS, MKr, PV and GT reviewed
and edited the manuscript. JM and GT confirm the authenticity of
all the raw data. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present case study was performed in accordance
with the Declaration of Helsinki. Ethical review and approval were
not required for this type of study on human participants,
according to local legislation and institutional requirements. The
patient provided written informed consent to participate in all the
diagnostic, neurosurgical and oncological treatment procedures
described in the present report.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of clinical data, accompanying images
and any potentially identifiable information included in the
present article. The patient was informed that the published
material would be available in print and online, and consented to
publication under these terms.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BRAF
|
B-Raf proto-oncogene serine/threonine
kinase
|
|
CM
|
cutaneous melanoma
|
|
D + T
|
dabrafenib and trametinib
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MEK
|
mitogen-activated protein kinase
kinase
|
|
MTS
|
metastases
|
|
NICU
|
neonatal intensive care unit
|
|
PAM
|
pregnancy-associated melanoma
|
|
WB
|
whole-body
|
References
|
1
|
Leonardi GC, Falzone L, Salemi R, Zanghì
A, Spandidos DA, Mccubrey JA, Candido S and Libra M: Cutaneous
melanoma: From pathogenesis to therapy (Review). Int J Oncol.
52:1071–1080. 2018.PubMed/NCBI
|
|
2
|
Chang AE, Karnell LH and Menck HR: The
national cancer data base report on cutaneous and noncutaneous
melanoma: A summary of 84,836 cases from the past decade. The
American college of surgeons commission on cancer and the American
cancer society. Cancer. 83:1664–1678. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shenenberger DW: Cutaneous malignant
melanoma: A primary care perspective. Am Fam Physician. 85:161–168.
2012.PubMed/NCBI
|
|
4
|
Sampson JH, Carter JH Jr, Friedman AH and
Seigler HF: Demographics, prognosis, and therapy in 702 patients
with brain metastases from malignant melanoma. J Neurosurg.
88:11–20. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ziogas DC, Diamantopoulos P, Benopoulou O,
Anastasopoulou A, Bafaloukos D, Stratigos AJ, Kirkwood JM and Gogas
H: Prognosis and management of BRAF V600E-mutated
pregnancy-associated melanoma. Oncologist. 25:e1209–e1220. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Serrano-Ortega S and Buendía-Eisman A:
Melanoma and pregnancy. Actas Dermosifiliogr. 102:647–649. 2011.(In
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O'Meara AT, Cress R, Xing G, Danielsen B
and Smith LH: Malignant melanoma in pregnancy: A population-based
evaluation. Cancer. 103:1217–1226. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dillman RO, Vandermolen LA, Barth NM and
Bransford KJ: Malignant melanoma and pregnancy ten questions. West
J Med. 164:156–161. 1996.PubMed/NCBI
|
|
9
|
Hepner A, Negrini D, Hase EA, Exman P,
Testa L, Trinconi AF, Filassi JR, Francisco RPV, Zugaib M, O'Connor
TL and Martin MG: Cancer during pregnancy: The oncologist overview.
World J Oncol. 10:28–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alexander A, Samlowski WE, Grossman D,
Bruggers CS, Harris RM, Zone JJ, Noyes RD, Bowen GM and Leachman
SA: Metastatic melanoma in pregnancy: Risk of transplacental
metastases in the infant. J Clin Oncol. 21:2179–2186. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Davies MA, Saiag P, Robert C, Grob JJ,
Flaherty KT, Arance A, Chiarion-Sileni V, Thomas L, Lesimple T,
Mortier L, et al: Dabrafenib plus trametinib in patients with
BRAFV600-mutant melanoma brain metastases (COMBI-MB): A
multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol.
18:863–873. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tawbi HA, Forsyth PA, Hodi FS, Algazi AP,
Hamid O, Lao CD, Moschos SJ, Atkins MB, Lewis K, Postow MA, et al:
Long-term outcomes of patients with active melanoma brain
metastases treated with combination nivolumab plus ipilimumab
(CheckMate 204): Final results of an open-label, multicentre, phase
2 study. Lancet Oncol. 22:1692–1704. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ascierto PA, Mandalà M, Ferrucci PF,
Guidoboni M, Rutkowski P, Ferraresi V, Arance A, Guida M, Maiello
E, Gogas H, et al: Sequencing of ipilimumab plus nivolumab and
encorafenib plus binimetinib for untreated BRAF-mutated metastatic
melanoma (SECOMBIT): A randomized, three-arm, open-label phase II
trial. J Clin Oncol. 41:212–221. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pagan M, Jinks H and Sewell M: Treatment
of metastatic malignant melanoma during pregnancy with a BRAF
kinase inhibitor. Case Rep Womens Health. 24:e001422019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maleka A, Enblad G, Sjörs G, Lindqvist A
and Ullenhag GJ: Treatment of metastatic malignant melanoma with
vemurafenib during pregnancy. J Clin Oncol. 31:e192–e193. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Haan J, van Thienen JV, Casaer M,
Hannivoort RA, Van Calsteren K, van Tuyl M, van Gerwen MM, Debeer
A, Amant F and Painter RC: Severe adverse reaction to vemurafenib
in a pregnant woman with metastatic melanoma. Case Rep Oncol.
11:119–124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marcé D, Cornillier H, Denis C,
Jonville-Bera AP and Machet L: Partial response of metastatic
melanoma to BRAF-inhibitor-monotherapy in a pregnant patient with
no fetal toxicity. Melanoma Res. 29:446–447. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Clark WH Jr, From L, Bernardino EA and
Mihm MC: The histogenesis and biologic behavior of primary human
malignant melanomas of the skin. Cancer Res. 29:705–727.
1969.PubMed/NCBI
|
|
19
|
Breslow A: Thickness, cross-sectional
areas and depth of invasion in the prognosis of cutaneous melanoma.
Ann Surg. 172:902–908. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Keung EZ and Gershenwald JE: The eighth
edition American joint committee on cancer (AJCC) melanoma staging
system: Implications for melanoma treatment and care. Expert Rev
Anticancer Ther. 18:775–784. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suvarna KS, Layton C and Bancroft JD:
Bancroft's Theory and Practice of Histological Techniques. 8th
edition. Elsevier; Philadelphia, PA: 2018
|
|
22
|
Ramos-Vara JA: Technical aspects of
immunohistochemistry. Vet Pathol. 42:405–426. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ohsie SJ, Sarantopoulos GP, Cochran AJ and
Binder SW: Immunohistochemical characteristics of melanoma. J Cutan
Pathol. 35:433–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Apgar V: A proposal for a new method of
evaluation of the newborn infant. Curr Res Anesth Analg.
32:260–267. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bayley N and Aylward GP: Bayley Scales of
Infant and Toddler Development. 4th edition. Pearson Education,
Inc.; San Antonio, TX: 2019
|
|
26
|
Bayley N: Bayley scales of Infant and
Toddler Development. 3rd edition. Harcourt Assessment; San Antonio,
TX: 2006
|
|
27
|
Nasreddine ZS, Phillips NA, Bédirian V,
Charbonneau S, Whitehead V, Collin I, Cummings JL and Chertkow H:
The montreal cognitive assessment, MoCA: A brief screening tool for
mild cognitive impairment. J Am Geriatr Soc. 53:695–699. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cramer JA and Van Hammée G; N132 Study
Group, : Maintenance of improvement in health-related quality of
life during long-term treatment with levetiracetam. Epilepsy Behav.
4:118–123. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Spitzer RL, Kroenke K, Williams JBW and
Löwe B: A brief measure for assessing generalized anxiety disorder:
The GAD-7. Arch Intern Med. 166:1092–1097. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kroenke K, Spitzer RL and Williams JBW:
The PHQ-9: Validity of a brief depression severity measure. J Gen
Intern Med. 16:606–613. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Global Burden of Disease Cancer
Collaboration, . Fitzmaurice C, Abate D, Abbasi N, Abbastabar H,
Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I,
et al: Global, regional, and national cancer incidence, mortality,
years of life lost, years lived with disability, and
disability-adjusted life-years for 29 cancer groups, 1990 to 2017:
A systematic analysis for the global burden of disease study. JAMA
Oncol. 5:1749–1768. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ugurel S, Röhmel J, Ascierto PA, Becker
JC, Flaherty KT, Grob JJ, Hauschild A, Larkin J, Livingstone E,
Long GV, et al: Survival of patients with advanced metastatic
melanoma: The impact of MAP kinase pathway inhibition and immune
checkpoint inhibition-update 2019. Eur J Cancer. 130:126–138. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dummer R, Goldinger SM, Turtschi CP,
Eggmann NB, Michielin O, Mitchell L, Veronese L, Hilfiker PR,
Felderer L and Rinderknecht JD: Vemurafenib in patients with
BRAF(V600) mutation-positive melanoma with symptomatic brain
metastases: Final results of an open-label pilot study. Eur J
Cancer. 50:611–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Heggarty E, Combarel D, Vialard F,
Rodriguez Y, Grassin-Delyle S, Lamy E, Mir O, Paci A and Berveiller
P: 36 Evaluation of transplacental transfer of cancer therapies in
melanoma via the perfused human placental model. Am J Obstet
Gynecol. 230 (Suppl 1):S28–S29. 2024. View Article : Google Scholar
|
|
35
|
Hassel JC, Livingstone E, Allam JP, Behre
HM, Bojunga J, Klein HH, Landsberg J, Nawroth F, Schüring A, Susok
L, et al: Fertility preservation and management of pregnancy in
melanoma patients requiring systemic therapy. ESMO Open.
6:1002482021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pelczar P, Kosteczko P, Wieczorek E,
Kwieciński M, Kozłowska A and Gil-Kulik P: Melanoma in
pregnancy-diagnosis, treatment, and consequences for fetal
development and the maintenance of pregnancy. Cancers (Basel).
16:21732024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lorenzi E, Simonelli M, Persico P,
Dipasquale A and Santoro A: Risks of molecular targeted therapies
to fertility and safety during pregnancy: A review of current
knowledge and future needs. Expert Opin Drug Saf. 20:503–521. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tajan M, Paccoud R, Branka S, Edouard T
and Yart A: The RASopathy family: Consequences of germline
activation of the RAS/MAPK pathway. Endocr Rev. 39:676–700. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Grunewald S and Jank A: New systemic
agents in dermatology with respect to fertility, pregnancy, and
lactation. J Dtsch Dermatol Ges. 13:277–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Peccatori FA, Azim HA Jr, Orecchia R,
Hoekstra HJ, Pavlidis N, Kesic V and Pentheroudakis G; ESMO
Guidelines Working Group, : Cancer, pregnancy and fertility: ESMO
clinical practice guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 24 (Suppl 6):vi160–vi170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Amaral T, Ottaviano M, Arance A, Blank C,
Chiarion-Sileni V, Donia M, Dummer R, Garbe C, Gershenwald JE,
Gogas H, et al: Cutaneous melanoma: ESMO clinical practice
guideline for diagnosis, treatment and follow-up. Ann Oncol.
36:10–30. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Silverstein J, Post AL, Chien AJ, Olin R,
Tsai KK, Ngo Z and Loon KV: Multidisciplinary management of cancer
during pregnancy. JCO Oncol Pract. 16:545–557. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sorouri K, Loren AW, Amant F and Partridge
AH: Patient-centered care in the management of cancer during
pregnancy. Am Soc Clin Oncol Educ Book. 43:e1000372023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Garzón-Orjuela N, Prieto-Pinto L, Lasalvia
P, Herrera D, Castrillón J, González-Bravo D, Castañeda-Cardona C
and Rosselli D: Efficacy and safety of dabrafenib-trametinib in the
treatment of unresectable advanced/metastatic melanoma with
BRAF-V600 mutation: A systematic review and network meta-analysis.
Dermatol Ther. 33:e131452020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Geng Y, Sun YJ, Song H, Miao QJ, Wang YF,
Qi JL, Xu XL and Sun JF: Construction and identification of an
NLR-associated prognostic signature revealing the heterogeneous
immune response in skin cutaneous melanoma. Clin Cosmet Investig
Dermatol. 16:1623–1639. 2023. View Article : Google Scholar : PubMed/NCBI
|