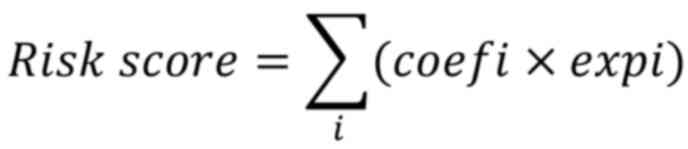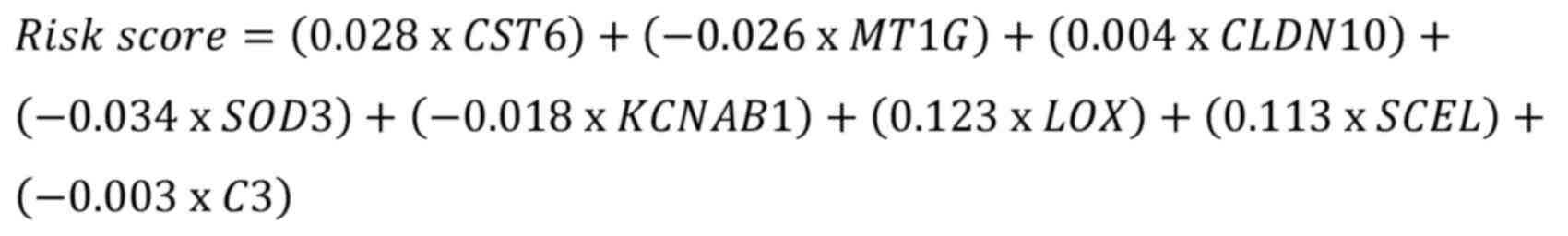Introduction
Thyroid carcinoma (THCA) is the fastest-growing
malignancy globally, with papillary thyroid carcinoma (PTC) being
the most prevalent subtype (1,2).
Although PTC generally has a favorable prognosis due to its
indolent nature, lymph node metastasis (LNM) in the neck can occur
early, most often first affecting the central compartment. Central
LNM (CLNM) is frequently identified only postoperatively, termed
occult CLNM (3). In patients with
PTC, cervical LNMs markedly increase the risk of recurrence and
distant metastasis, making accurate preoperative assessment
critical for determining the need for prophylactic central neck
dissection (pCLND).
International guidelines recommend therapeutic lymph
node dissection for patients with clinically evident central
compartment metastasis (cN1 stage). However, the role of pCLND in
patients with clinical lymph node negative (cN0) remains
controversial. Japanese guidelines support routine pCLND, citing
benefits in precise postoperative staging, treatment guidance and
potential recurrence reduction (4).
Conversely, the 2015 American Thyroid Association (5), 2019 European Society for Medical
Oncology (6) and 2022 Chinese
Anti-Cancer Association (7)
guidelines advise pCLND only for cN0 patients with high-risk
features, such as T3-T4 tumors, multicentricity, family history,
childhood radiation exposure, or lateral cervical LNM. The 2022
National Comprehensive Cancer Network guidelines do not recommend
routine pCLND (8). Although pCLND
may decrease cervical lymph node recurrence by 34%, postoperative
complication rates can reach 17.7% (9), highlighting the need for
individualized preoperative assessment and predictive biomarkers
for CLNM.
Currently, pCLND decisions rely heavily on surgical
experience. Neck ultrasound, the primary imaging modality, has a
sensitivity of only 15–40% for detecting CLNM, per the 8th edition
of the AJCC manual (10–12). Computed tomography can be used as a
supplement, however the indolent progression of PTC often limits
detectable imaging changes associated with CLNM (13,14).
Fine-needle aspiration cytology (FNAC) remains the most direct,
accurate, and cost-effective preoperative diagnostic tool, enabling
detection of molecular biomarkers such as BRAF, RAS and TERT
mutations. The BRAF V600E mutation, present in 40–80% of
cases, is associated with aggressive PTC features. While some
studies suggest it may predict CLNM in patients with cN0 (15), current evidence does not support
using BRAF V600E alone to guide pCLND. Integrating
additional indicators may enhance predictive accuracy and inform
surgical planning (16,17).
To elucidate the relationship between BRAF
V600E, clinicopathological features and CLNM in PTC, the present
study analyzed gene expression profiles and clinical data from The
Cancer Genome Atlas (TCGA) and the Gene Expression Omnibus (GEO)
databases. It aimed to develop a risk scoring model for CLNM based
on differentially expressed genes (DEGs) stratified by BRAF
V600E status. Furthermore, patients with cN0 PTC treated at the
General Surgery Department of Shunde Hospital of Guangzhou
University of Chinese Medicine (GUCM) were included, with
preoperative CLNM tissues collected via ultrasound-guided FNAC and
postoperative thyroid tissue analyzed for BRAF V600E. The
present study sought to support personalized preoperative
assessment, optimize surgical decisions, reduce unnecessary lymph
node dissection and improve postoperative quality of life.
Materials and methods
Public data collection and
processing
mRNA expression profiles and clinical data for THCA
patients were obtained from TCGA database. Level 3 HTSeq-FPKM data
were normalized to transcripts per million reads. For external
validation, GSE60542 and GSE29265 datasets were retrieved from the
Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/).
Tumor mutation analysis
The prevalence of BRAF mutations across
cancer types was initially assessed using cBioPortal (http://www.cbioportal.org). Somatic mutation data from
TCGA were analyzed with the R package ‘maftools’ to visualize
mutation frequencies and types, focusing on THCA. After confirming
BRAF V600E as the predominant mutation in THCA, samples
harboring this mutation and their associated clinical data were
downloaded from cBioPortal for further analysis.
DEGs' analysis
Data of patients with THCA obtained from TCGA were
stratified into BRAF V600E mutant and wild-type groups.
Differential expression analysis was performed using the R package
limma 3.52.2 (18). DEGs were
defined by an adjusted P-value <0.05 and |log2-fold-change
(FC)|>1.5.
Functional enrichment analysis
To explore the biological significance of DEGs, Gene
Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway analyses were conducted using the ClusterProfiler R package
4.4.4 (18).
Construction and validation of the
diagnostic risk model
A diagnostic risk model for predicting CLNM in THCA
was established using DEGs from both BRAF V600E mutant and
wild-type cases via Lasso-Cox regression with the glmnet package
3.0 (glmnet.stanford.edu/). The model was formulated as:
where ‘expi’ denotes gene expression and ‘coefi’
represents the corresponding gene risk coefficient. Model
performance was evaluated using Receiver Operating Characteristic
(ROC) and Precision-Recall (PR) curves. External validation was
performed using the GSE60542 and GSE29265 datasets.
It should be noted that genes such as CST6 and LOX
were included solely as part of the predictive model, and their
downstream mechanistic roles in CLNM were not experimentally
investigated in the present study, representing a limitation.
Immune infiltration analysis
The relative enrichment of 24 immune cell types in
THCA was assessed using single-sample Gene Set Enrichment Analysis
(ssGSEA) via the R package GSVA 3.19 (19). Spearman's correlation analysis was
performed to evaluate associations between the risk score and
immune cell infiltration. P-values from the 24 parallel tests were
adjusted using the Benjamini-Hochberg false discovery rate (FDR),
with significance defined as FDR<0.05. Differences in immune
infiltration between high- and low-risk groups were analyzed using
the Wilcoxon rank-sum test. Additionally, immune, stromal and
ESTIMATE scores were calculated using the ESTIMATE algorithm
(20).
The correlation between the risk score and
expression of interleukins, chemokines and immune checkpoints was
further examined. Immunophenoscore (IPS) was used to predict
potential responses to immunotherapy (anti-PD-1 and anti-CTLA-4)
based on gene expression profiles, leveraging data from The Cancer
Immunome Atlas (https://tcia.at/).
Construction and validation of the
nomogram
A binary logistic regression model was constructed
using the glm function in R to predict overall survival
probability. The RMS package was used to develop and visualize the
nomogram. Calibration curves and restricted cubic spline plots were
employed to evaluate the nomogram's performance. The concordance
index (C-index) quantified its discriminative ability and decision
curve analysis was performed to assess net clinical benefit.
Retrospective analyses of pathological
samples
Preoperative assessment of BRAF V600E mutation in
central lymph nodes via PCR
Clinical data and ultrasound-guided FNAC results
were collected from 32 patients with cN0 PTC who underwent
preoperative assessment at Shunde Hospital of GUCM between August
2022 and November 2023 (Foshan, China). Inclusion criteria were: i)
FNAC-confirmed or highly suspected PTC with TI-RADS 5 nodules; ii)
No LNM on preoperative ultrasound/CT, classified as cN0 per
Kowalski criteria; iii) Central lymph node diameter >0.5 cm.
Exclusion criteria included prior thyroid surgery, history of
thyroid disease treatments (including I-131 therapy), and
incomplete clinical data. FNAC was performed by an experienced
physician, and the specimens were sent to Guangzhou Da'an Clinical
Laboratory for BRAF V600E mutation detection using ARMS-PCR (cat.
no. 56404 LOT:026ABB01; Wuxi Shenrui Bio-Pharmaceuticals Co.
Ltd.).
Postoperative immunohistochemical
(IHC) analysis of BRAF V600E mutation
Clinical and pathological data from 222 patients
with PTC treated between January 2022 and November 2023 were
collected. Inclusion criteria were: i) age ≥18 years; ii)
histologically confirmed PTC; and iii) first diagnosis of THCA.
Exclusion criteria included incomplete data, non-primary THCA, or
presence of other malignancies. Collected variables included age,
sex, histopathological subtype, tumor location and size, central
lymph node status, IHC results, and surgical approach. CLNM was
independently assessed by two blinded pathologists to minimize
observer bias. IHC was used to detect BRAF V600E protein
expression, with positivity determined by staining intensity and
cytoplasmic localization.
Ethics statement
The present study was approved by the Ethics
Committee of Shunde Hospital of Guangzhou University of Chinese
Medicine (approval nos. KY2022066 and KY2023127; Foshan, China).
All procedures were performed in accordance with relevant
guidelines and regulations.
Statistical analysis
Group differences were evaluated using the Wilcoxon
test. Associations between categorical variables were assessed
using the Chi-squared test or Fisher's exact test, as appropriate;
Fisher's exact test was applied when more than 20% of the cells in
a contingency table had an expected count of less than 5. Spearman
correlation analysis was used to examine associations between risk
score and immune infiltration. P<0.05 was considered to indicate
a statistically significant difference, with multiple testing
corrections applied using the Benjamini-Hochberg FDR method.
Results
Characteristics of the TCGA
cohort
A total of 508 TCGA patients pathologically
diagnosed with PTC with available clinical and RNA-sequencing data
were analyzed. Among these, 490 samples had documented BRAF
mutation status and were included in further analyses.
Clinicopathological characteristics of the cohort are summarized in
Table I.
 | Table I.Clinical pathological characteristics
of patients with papillary thyroid carcinoma from TCGA. |
Table I.
Clinical pathological characteristics
of patients with papillary thyroid carcinoma from TCGA.
|
|
| BRAF
V600E |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Overall, no.
(%) | Mut, no. (%) | Non-mut, no.
(%) | P-value |
|---|
| n | 508 | 285 | 205 |
|
| Sex (%) |
|
|
| 0.822 |
|
Female | 371 (73.2) | 208 (73.2) | 152 (74.1) |
|
|
Male | 136 (26.8) | 76 (26.8) | 53 (25.9) |
|
| Age, n (%) |
|
|
| 0.772 |
|
≤45 | 239 (47.1) | 152 (53.5) | 107 (52.2) |
|
|
>45 | 268 (52.9) | 132 (46.5) | 98 (47.8) |
|
| Pathologic T stage,
n (%) |
|
|
| 0.001 |
| T1 | 144 (28.5) | 17 (6) | 5 (2.5) |
|
| T2 | 167 (33.1) | 79 (27.9) | 82 (40.2) |
|
| T3 | 171 (33.9) | 76 (26.9) | 64 (31.4) |
|
| T4 | 23 (4.6) | 111 (39.2) | 53 (26) |
|
| Pathologic N stage,
n (%) |
|
|
| <0.001 |
| N0 | 231 (50.5) | 155 (59.2) | 63 (35.2) |
|
| N1 | 226 (49.5) | 107 (40.8) | 116 (64.8) |
|
| Pathologic M stage,
n (%) |
|
|
| 0.735a |
| M0 | 283 (96.9) | 170 (97.1) | 104 (96.3) |
|
| M1 | 9 (3.1%) | 5 (2.9) | 4 (3.7) |
|
| Pathologic stage, n
(%) |
|
|
| <0.001 |
| Stage
I | 285 (56.4) | 38 (13.4) | 15 (7.4) |
|
| Stage
II | 52 (10.3) | 18 (6.4) | 32 (15.7) |
|
| Stage
III | 113 (22.4) | 152 (53.7) | 122 (59.8) |
|
| Stage
IV | 55 (10.9) | 75 (26.5) | 35 (17.2) |
|
| Ethnicity (%) |
|
|
| 0.478 |
| Asian
and Black or African American | 80 (19.3) | 45 (18.3) | 32 (21.2) |
|
|
White | 334 (80.7) | 201 (81.7) | 119 (78.8) |
|
| Histological type,
n (%) |
|
|
| <0.001 |
|
Classical | 359 (77.9) | 232 (93.9) | 111 (56.3) |
|
|
Follicular | 102 (22.1) | 15 (6.1) | 86 (43.7) |
|
| Residual tumor
(%) |
|
|
| 0.113* |
| R0 | 389 (87.4) | 215 (85) | 163 (91.6) |
|
| R1 | 52 (11.7) | 35 (13.8) | 14 (7.9) |
|
| R2 | 4 (0.9) | 3 (1.2) | 1 (0.6) |
|
| Primary neoplasm
focus type, n (%) |
|
|
| 0.551 |
|
Multifocal | 228 (45.9) | 149 (53) | 111 (55.8) |
|
|
Unifocal | 269 (54.1) | 132 (47) | 88 (44.2) |
|
| Neoplasm location,
n (%) |
|
|
| 0.158 |
|
Bilateral | 86 (17.2) | 111 (39.5) | 97 (48%) |
|
|
Isthmus | 22 (4.4) | 102 (36.3) | 70 (34.7) |
|
| Left
lobe | 178 (35.5) | 52 (18.5) | 29 (14.4) |
|
| Right
lobe | 215 (42.9) | 16 (5.7) | 6 (3) |
|
| Thyroid gland
disorder history (%) |
|
| 0.047 |
|
|
Lymphocytic Thyroiditis | 72 (16.1) | 12 (4.8) | 12 (6.6) |
|
| Nodular
Hyperplasia | 69 (15.4) | 31 (12.4) | 35 (19.2) |
|
|
Normal | 281 (62.7) | 36 (14.3) | 34 (18.7) |
|
| Other,
specify | 26 (5.8) | 172 (68.5) | 101 (55.5) |
|
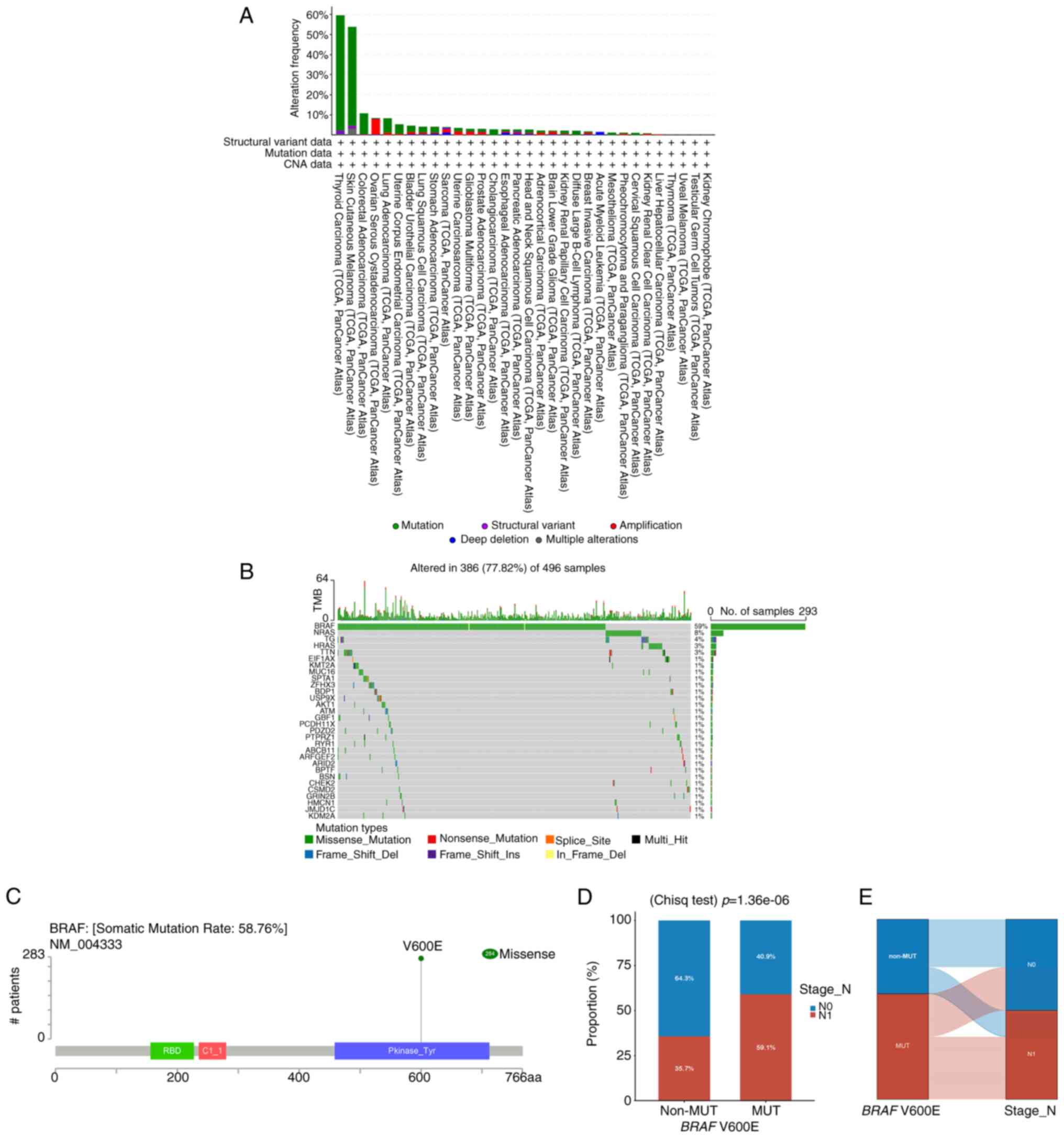
BRAF genetic variations in the TCGA
cohort
The mutational landscape of BRAF was analyzed
across 10,967 samples from 32 cancer types using cBioPortal. THCA
exhibited the highest BRAF mutation frequency, accounting
for 59.6% of cases (Fig. 1A).
Examination of THCA mutations, illustrated by a waterfall plot
(Fig. 1B), showed that missense
mutations were predominant (77.73%). Analysis of 284 mutation sites
across amino acids 0–766, including one duplicate, confirmed that
BRAF V600E mutations were exclusively missense (Fig. 1C). Chi-squared analysis revealed a
significant association between BRAF V600E and CLNM. The
mutation group exhibited a CLNM rate of 59.1%, significantly higher
than 35.7% in the non-mutation group (Fig. 1D). A Sankey diagram (Fig. 1E) further illustrated the strong
link between BRAF V600E mutations and higher CLNM incidence,
whereas the non-mutation group displayed minimal CLNM.
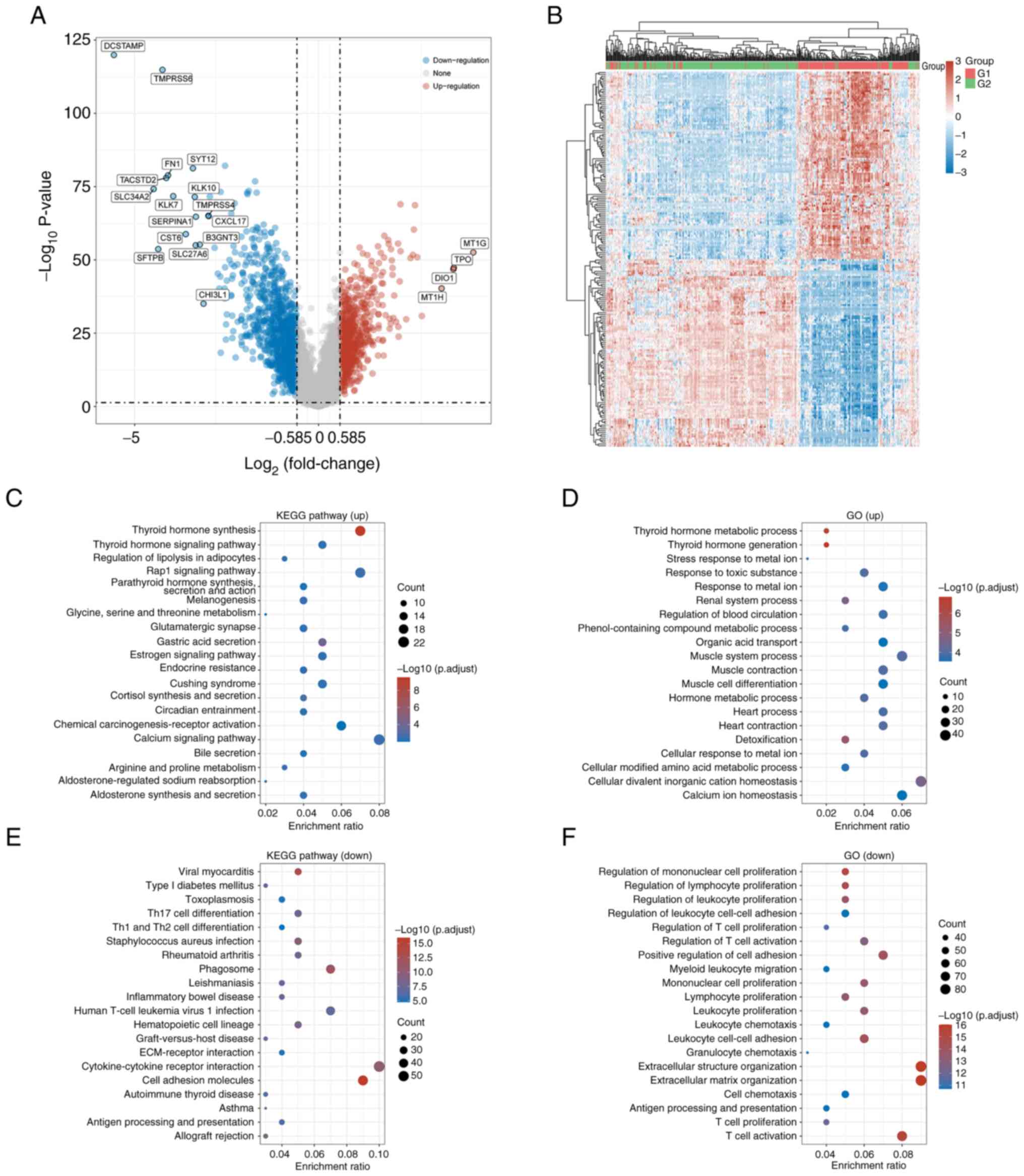
Identification of DEGs in THCA
A total of 229 DEGs were identified between
BRAF V600E mutant and wild-type groups, including 57
upregulated (24.9%) and 172 downregulated genes (75.1%), using
thresholds of adjusted P<0.05 and |log2-FC|>1.5 (Fig. 2A and B; Table SI).
KEGG pathway analysis indicated that upregulated
genes were significantly enriched in ‘thyroid hormone synthesis,
thyroid hormone signaling and Rap1 signaling pathways (Fig. 2C). GO analysis showed enrichment in
thyroid hormone metabolism, hormone generation, and metal ion
stress response processes (Fig.
2D). By contrast, downregulated genes in the non-BRAF
V600E group were associated with KEGG pathways including viral
myocarditis, type I diabetes mellitus, and toxoplasmosis (Fig. 2E). GO analysis highlighted their
involvement in regulating monocyte, lymphocyte and leukocyte
proliferation (Fig. 2F).
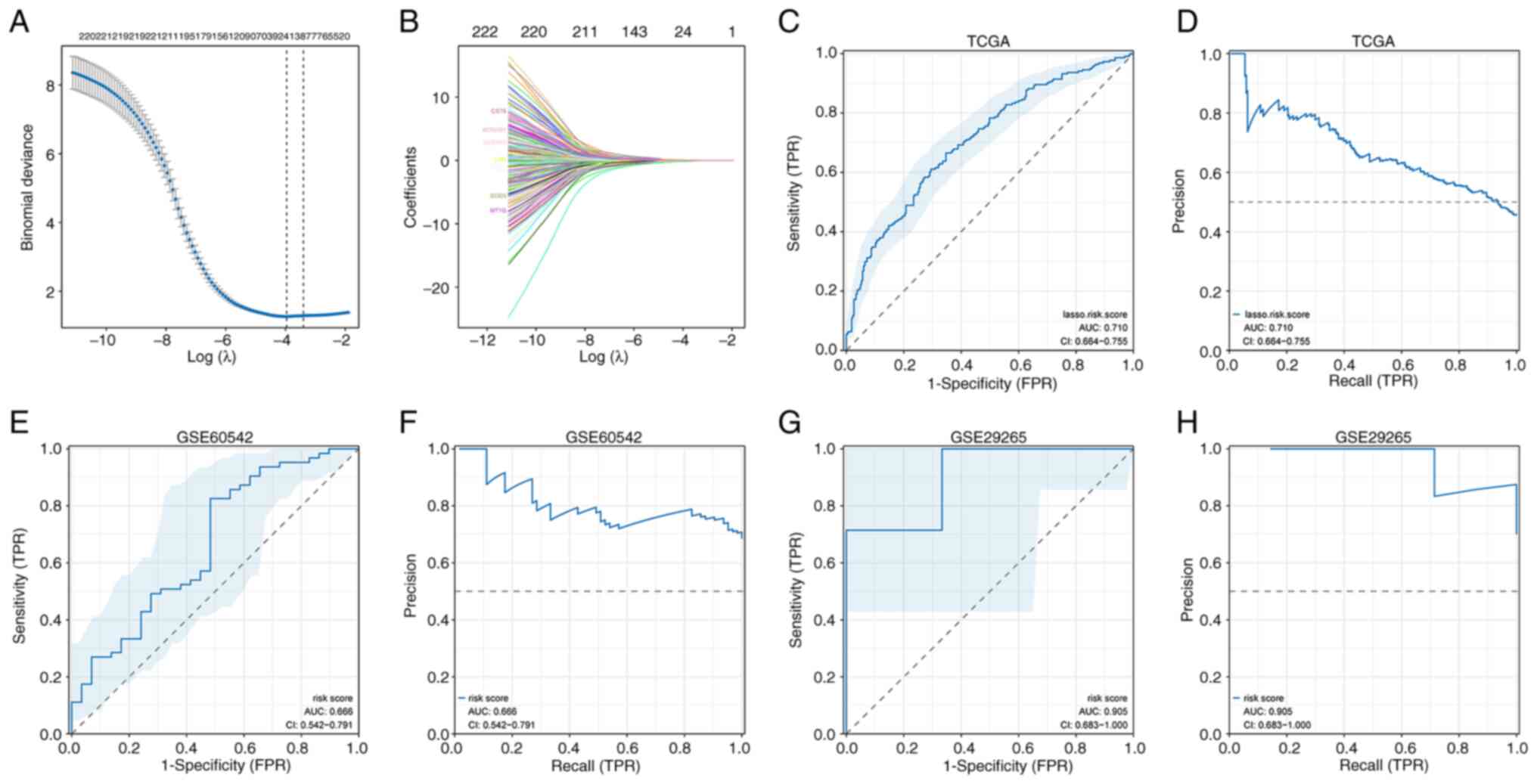
Development and validation of a
diagnostic model using DEGs
Based on the identified association between
BRAF V600E mutation and CLNM (Fig. 3A and B), the authors evaluated
whether the 229 DEGs could serve as a diagnostic signature. Lasso
regression was used to construct a risk score model:
This model stratified THCA samples into high- and
low-risk groups and achieved a ROC AUC of 0.710 (95% CI:
0.664–0.755), indicating acceptable accuracy (Fig. 3C and D). External validation in GEO
datasets yielded ROC AUCs of 0.666 (95% CI: 0.542–0.791) in
GSE60542 and 0.905 (95% CI: 0.683–1.0) in GSE29265, demonstrating
moderate to high predictive performance (Fig. 3E-H).
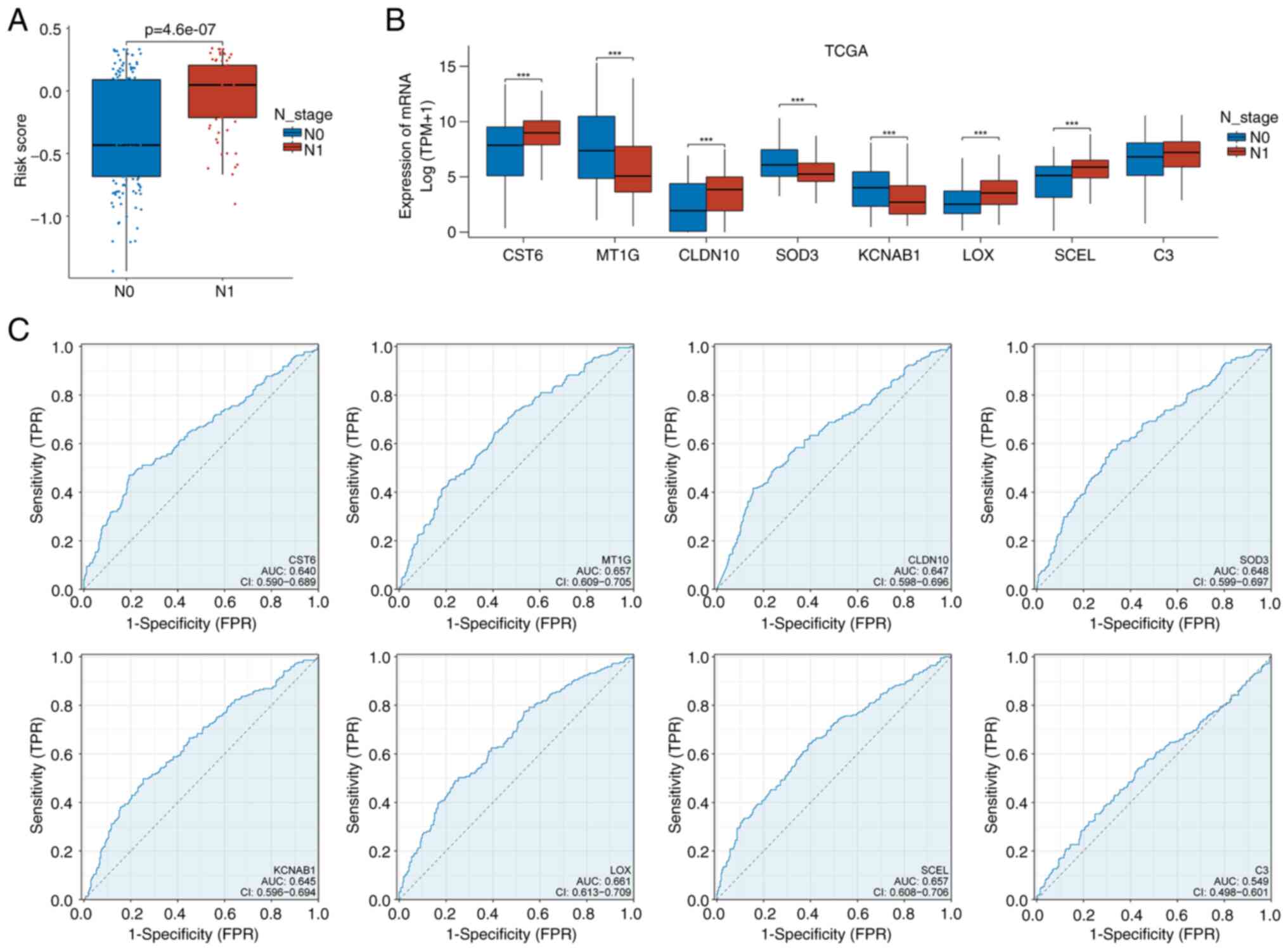
Incorporation of CLNM status showed significantly
higher risk scores in the N1 group, reflecting elevated CLNM rates
in high-risk samples (Fig. 4A).
Analysis of BRAF V600E-related signature genes revealed that
CST6, CLDN10, LOX, and SCEL were upregulated in the LNM group,
whereas MT1G, SOD3, and KCNAB1 were higher in the N0 group
(Fig. 4B). Individual gene ROC AUCs
for LNM prediction were: CST6 (0.64), MT1G (0.657), CLDN10 (0.647),
SOD3 (0.648), KCNAB1 (0.645), LOX (0.661), SCEL (0.657) and C3
(0.549), indicating moderate discriminative ability (Fig. 4C).
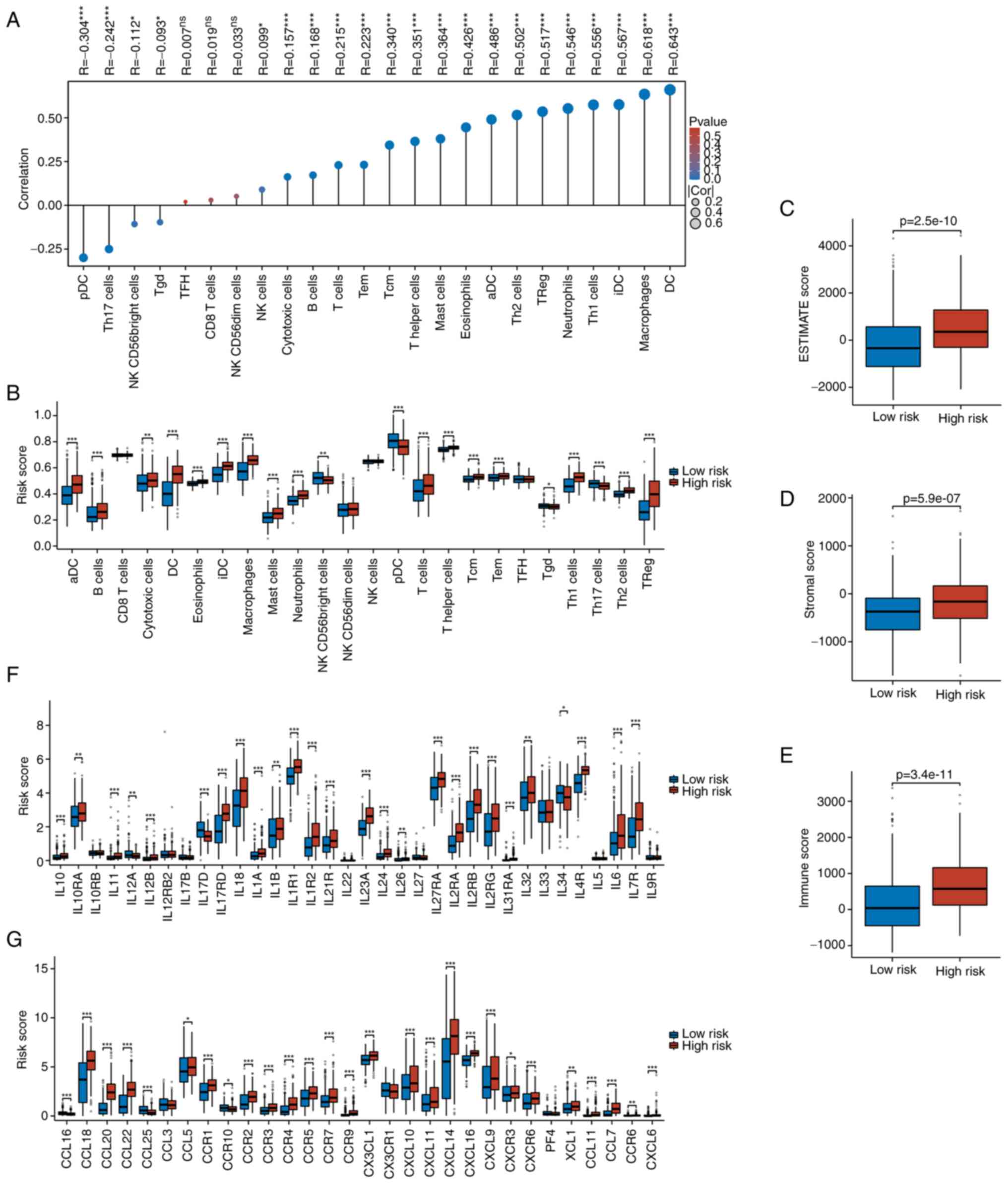
Association between risk score and
immune landscape
The relationship between risk scores and the immune
landscape in THCA were evaluated by comparing immune cell
composition between low- and high-risk groups. ssGSEA demonstrated
a strong positive correlation (R>0.5) between risk score and
multiple immune cell types, including dendritic cells (DCs),
macrophages, immature DCs (iDCs), Th1 cells, neutrophils, Treg
cells, and Th2 cells, with significance maintained after
Benjamini–Hochberg FDR correction (Fig.
5A). High-risk samples exhibited higher proportions of these
immune cells (Fig. 5B), as well as
elevated ESTIMATE, stromal and immune scores (Fig. 5C-E), indicating increased immune
infiltration.
Analysis of the tumor immune microenvironment
revealed that most interleukins, except IL12A, IL17D and IL34, were
upregulated in the high-risk group (Fig. 5F). Similarly, chemokines and their
receptors were generally elevated, except for CCL16, CCL25 and
CCR10 (Fig. 5G). Immune checkpoint
analysis showed higher expression of inhibitory molecules,
including BTLA, CD244, CD274, CSF1R, CTLA4, HAVCR2, IL10, KDR,
LGALS9, PDCD1LG2, TGFβ1, TGFβR1, TIGIT and VTCN1, in the high-risk
group (Fig. 6A), alongside
increased levels of most stimulatory checkpoint molecules (Fig. 6B). These findings suggest that
high-risk THCA patients exhibit enhanced immune infiltration with
both inhibitory and stimulatory components, highlighting potential
responsiveness to immunotherapy.
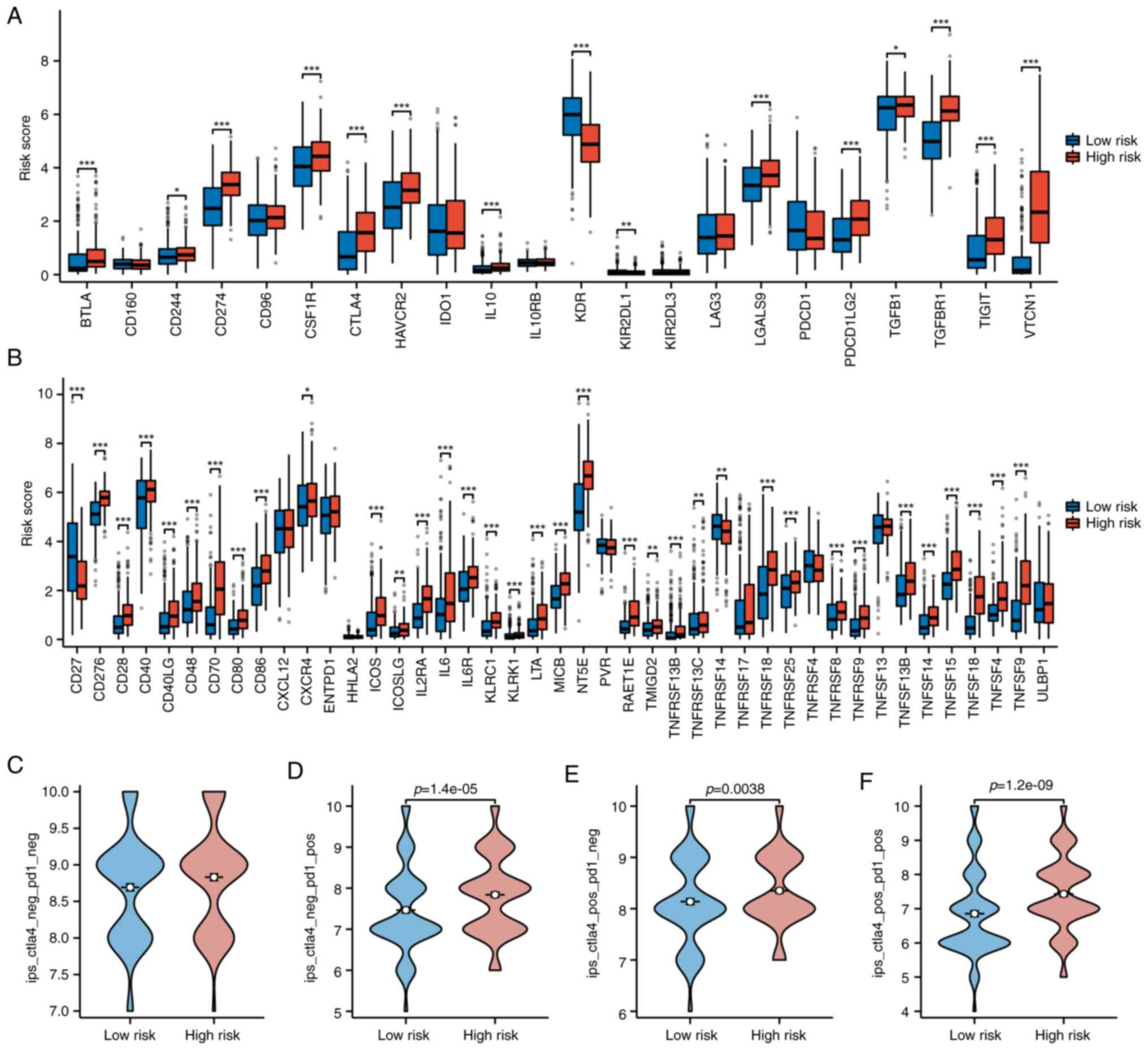 | Figure 6.Immune checkpoint expression and IPS
comparison between risk groups. (A and B) Differential expression
of immune checkpoint inhibitors and stimulators in high- vs.
low-risk patients, with upregulation in the high-risk group. (C-F)
IPS analyses under four immunotherapy conditions
(PD-1+/CTLA4+, PD-1+/CTLA4−,
PD-1−/CTLA4+,
PD-1−/CTLA4−), indicating enhanced
immunogenicity in high-risk patients. IPS, immunophenoscore; PD-1,
programmed cell death protein 1; CTLA-4, cytotoxic T
lymphocyte-associated protein 4. *P<0.05, **P<0.01,
***P<0.001. |
Finally, IPS were higher in the high-risk group
across CTLA4-negative PD-1-positive, CTLA4-positive PD-1-negative,
and CTLA4-positive PD-1-positive conditions (Fig. 6C-F), indicating a more active and
potentially immunotherapy-responsive tumor microenvironment
(TME).
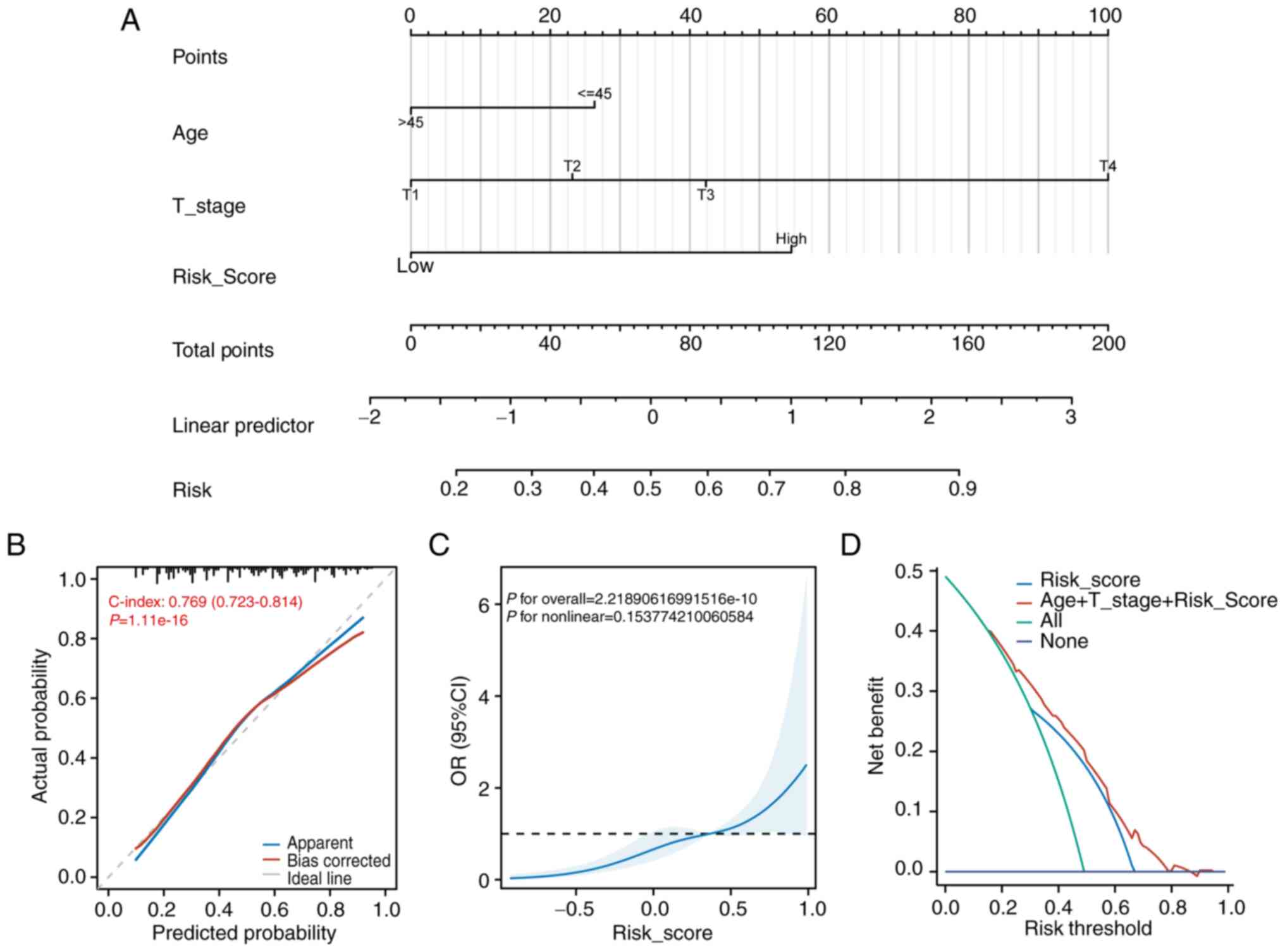
Nomogram development for risk score in
independent diagnostic analysis
Logistic regression analysis of TCGA clinical data
identified several factors significantly associated with increased
CLNM risk: age (HR=2.032, 95% CI: 1.298–3.179), T2 stage (HR=1.902,
95% CI: 1.086–3.331), T3 stage (HR=2.709, 95% CI: 1.555–4.719), T4
stage (HR=12.940, 95% CI: 3.309–50.602), and risk score (HR=3.910,
95% CI: 2.518–6.071; Table
II).
 | Table II.Univariate and multivariate analyses
of central lymph node metastasis status in patients with papillary
thyroid carcinoma. |
Table II.
Univariate and multivariate analyses
of central lymph node metastasis status in patients with papillary
thyroid carcinoma.
| Characteristic | Total (n=424) | OR (95% CI)
univariate analysis | P-value | OR (95% CI)
multivariate analysis | P-value |
|---|
| Age, years |
| |
|
|
|
|
>45 | 225 | Reference |
| Reference |
|
|
≤45 | 199 | 1.664
(1.133–2.445) | 0.009 | 2.032
(1.298–3.179) | 0.002 |
| Sex |
|
|
|
|
|
|
Male | 112 | Reference |
| Reference |
|
|
Female | 312 | 0.612
(0.395–0.947) | 0.027 | 0.716
(0.435–1.178) | 0.188 |
| Focus type |
| |
|
|
|
|
Unifocal | 227 | Reference |
| Reference |
|
|
Multifocal | 197 | 1.601
(1.090–2.352) | 0.016 | 1.469
(0.898–2.404) | 0.126 |
| Location |
|
|
|
|
|
| Right
lobe | 176 | Reference |
| Reference |
|
| Left
lobe | 152 | 1.343
(0.868–2.078) | 0.186 | 1.250
(0.763–2.046) | 0.376 |
|
Bilateral | 77 | 2.281
(1.317–3.953) | 0.003 | 1.723
(0.868–3.423) | 0.120 |
|
Isthmus | 19 | 1.895
(0.727–4.943) | 0.191 | 1.504
(0.539–4.197) | 0.436 |
| T |
|
|
|
|
|
| T1 | 124 | Reference |
| Reference |
|
| T2 | 131 | 1.659
(0.998–2.758) | 0.051 | 1.902
(1.086–3.331) | 0.024 |
| T3 | 149 | 3.176
(1.930–5.228) | <0.001 | 2.709
(1.555–4.719) | <0.001 |
| T4 | 20 | 11.472
(3.180–41.388) | <0.001 | 12.940
(3.309–50.602) | <0.001 |
| Group |
|
|
| |
|
|
Low | 205 | Reference |
| Reference |
|
|
High | 219 | 4.613
(3.063–6.948) | <0.001 | 3.910
(2.518–6.071) | <0.001 |
Using these variables, a nomogram was constructed to
estimate the probability of CLNM in patients with THCA (Fig. 7A). Calibration analysis showed a
C-index of 0.769 (95% CI: 0.723–0.814), indicating favorable model
fit (Fig. 7B). Further validation
using a restricted cubic spline plot demonstrated overall
significance and indicated a non-significant predominantly linear
relationship between predictors and outcome (Fig. 7C). Decision curve analysis confirmed
that the combined model of age, T stage and risk score provided the
highest net clinical benefit (Fig.
7D).
Predictive value of preoperative BRAF
V600E mutation for CLNM
To evaluate the predictive value of BRAF
V600E for CLNM in PTC, clinical data from 36 patients with cN0 PTC
were analyzed who underwent preoperative ultrasound-guided central
lymph node FNAC at Shunde Hospital, GUCM, between August 2022 and
November 2023 were analyzed. After excluding four patients with
insufficient samples, 32 patients were included, all of whom
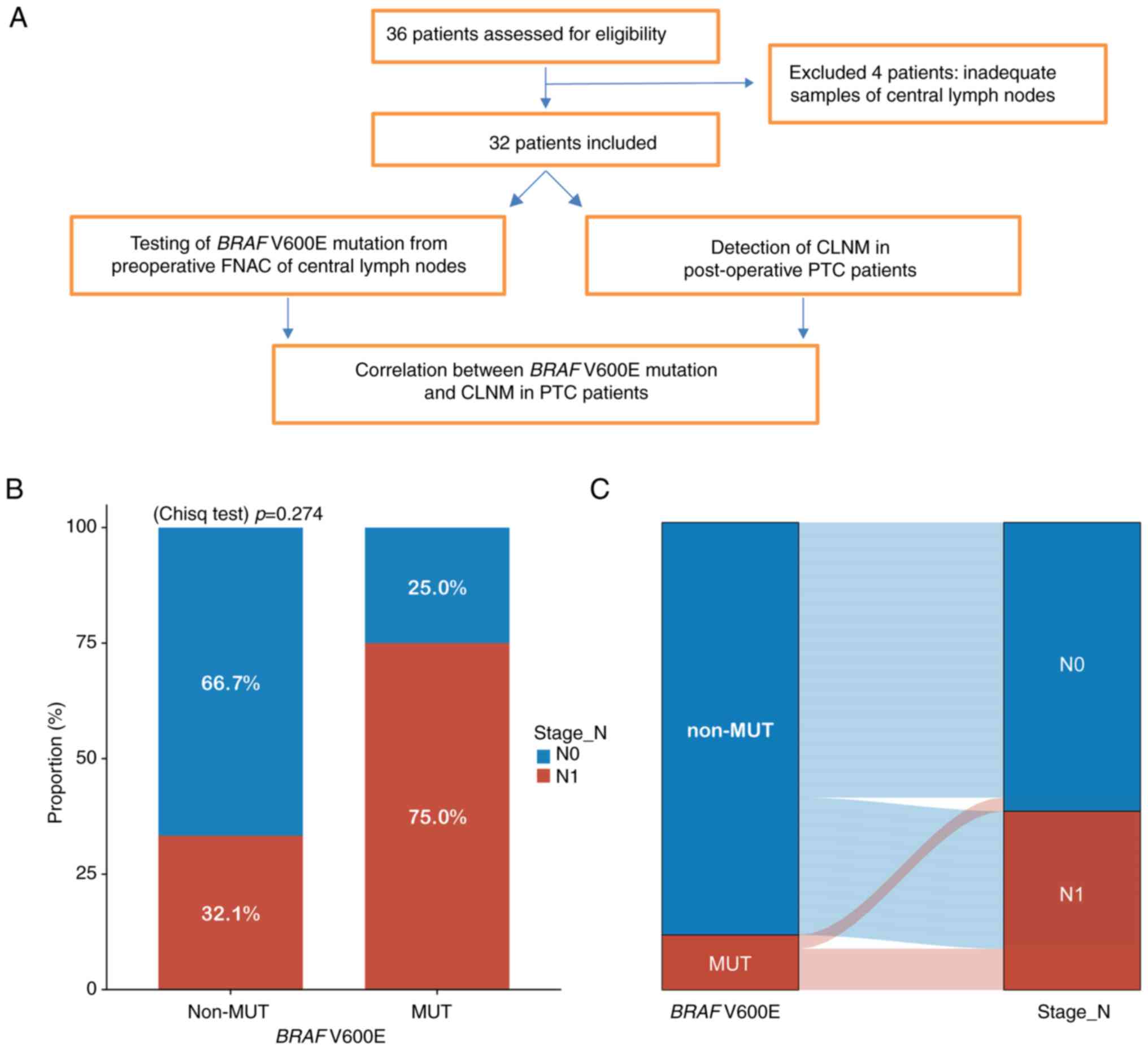
underwent total thyroidectomy (TT) and pCLND (Fig. 8A).
BRAF V600E mutation was detected in four
patients using ARMS-PCR, three of whom had CLNM (Table III). Patients were categorized
into non-mutation (n=28) and mutation (n=4) groups. CLNM rates were
32.1% in the non-mutation group and 75.0% in the mutation group
(Fig. 8B). Although the mutation
group showed higher CLNM incidence, the difference was not
statistically significant. A Sankey diagram (Fig. 8C) illustrated that most BRAF
V600E-positive patients experienced CLNM, whereas most non-mutation
patients did not.
 | Table III.Correlation between BRAF V600E
mutation and CLNM in post-operative in patients with papillary
thyroid carcinoma. |
Table III.
Correlation between BRAF V600E
mutation and CLNM in post-operative in patients with papillary
thyroid carcinoma.
|
| Lymph node
metastasis, n (%) |
|---|
|
|
|
|---|
| Group | Yes | No |
|---|
| non-Mut (n=28) | 9 (32.1) | 19 (67.9) |
| Mut (n=4) | 3 (75.0) | 1 (25.0) |
Postoperative BRAF V600E mutation and
CLNM risk
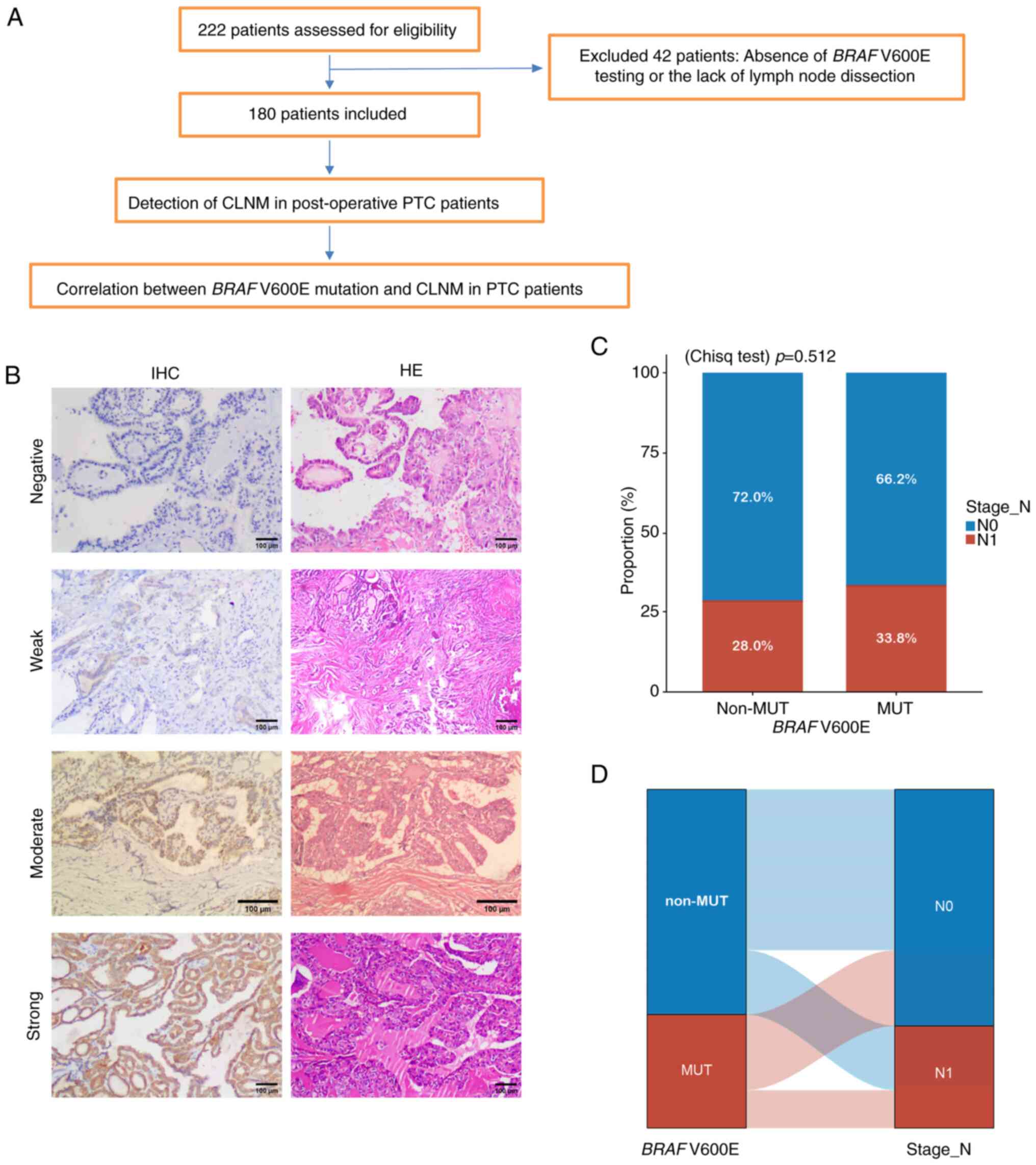
A retrospective analysis of 222 PTC cases was
performed to assess the association between BRAF V600E and
CLNM risk. After excluding 42 cases lacking BRAF testing or
lymph node dissection, 180 patients were analyzed, all of whom
underwent TT with ipsilateral pCLND (Fig. 9A).
Patients were grouped by BRAF V600E status
into non-mutation (n=100) and mutation (n=80) groups (Table IV). Representative IHC images of
BRAF V600E expression in four patients are demonstrated in
Fig. 9B, with additional details in
Table V. CLNM occurred in 28.0% of
non-mutation and 33.8% of mutation patients, with no statistically
significant difference (Fig. 9C). A
Sankey diagram (Fig. 9D) shows a
higher proportion of CLNM in the BRAF V600E mutation group
compared with the non-mutation group.
 | Table IV.Clinical pathological characteristics
of patients with papillary thyroid carcinoma (n=180) from Shunde
Hospital of GUCM. |
Table IV.
Clinical pathological characteristics
of patients with papillary thyroid carcinoma (n=180) from Shunde
Hospital of GUCM.
|
|
| BRAF
V600E |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Overall no.
(%) | Mut, no. (%) | Non-mut, no.
(%) | P-value |
|---|
| n |
| 80 | 100 |
|
| Sex, n (%) |
|
|
| 0.480 |
|
Female | 149 (82.8) | 68 (37.8) | 81 (45) |
|
|
Male | 31 (17.2) | 12 (6.7) | 19 (10.6%) |
|
| Age, n (%) |
|
|
| 0.070 |
|
>45 | 81 (45) | 42 (23.3) | 39 (21.7) |
|
|
≤45 | 99 (55) | 38 (21.1) | 61 (33.9) |
|
| T stage, n (%) |
|
|
| 0.408a |
| T1 | 173 (96.1) | 76 (42.2) | 97 (53.9) |
|
| T2 | 6 (3.3) | 4 (2.2) | 2 (1.1) |
|
| T3 | 1 (0.6) | 0 (0) | 1 (0.6) |
|
| N stage, n (%) |
|
|
| 0.405 |
| N1 | 55 (30.6) | 27 (15) | 28 (15.6) |
|
| N0 | 125 (69.4) | 53 (29.4) | 72 (40) |
|
| Primary neoplasm
focus type, n (%) |
|
|
| 0.506 |
|
Unifocal | 137 (76.1) | 59 (32.8) | 78 (43.3) |
|
|
Multifocal | 43 (23.9) | 21 (11.7) | 22 (12.2) |
|
| Neoplasm location,
n (%) |
|
|
| 0.307a |
|
Right | 78 (43.3) | 29 (16.1) | 49 (27.2) |
|
|
Left | 70 (38.9) | 36 (20) | 34 (18.9) |
|
|
Bilateral | 27 (15) | 12 (6.7) | 15 (8.3) |
|
|
Isthmus | 5 (2.8) | 3 (1.7) | 2 (1.1) |
|
 | Table V.Clinical data and description of
immunohistochemistry results. |
Table V.
Clinical data and description of
immunohistochemistry results.
| Protein | Tissue | Age, years | Sex | Location | Quantity | Intensity |
|---|
| BRAF
V600E | PTC | 54 | Female | - | - | Negative |
| BRAF
V600E | PTC | 35 | Male | Cytoplasmic | 40% | Weak |
| BRAF
V600E | PTC | 27 | Male | Cytoplasmic | 10% | Moderate |
| BRAF
V600E | PTC | 55 | Female | Cytoplasmic | 95% |
|
Discussion
The rising incidence of THCA is largely attributable
to improved detection methods, yet overall mortality remains low,
and numerous thyroid nodules exhibit indolent behavior (21). Nonetheless, LNM markedly increases
recurrence and mortality risk, making its prediction and management
a central clinical concern. In classical PTC, CLNM is particularly
relevant to prognosis. Reported prevalence of CLNM ranges from
20–90%, while preoperative ultrasound demonstrates limited
sensitivity, occasionally as low as 12.1% (22). In total, ~60% of patients with PTC
are confirmed to have CLNM during initial surgery despite negative
preoperative imaging (23),
highlighting the need for reliable preoperative predictive models
to better guide surgical decision-making.
Routine pCLND remains controversial. Critics argue
that it offers limited survival or recurrence benefit while
increasing postoperative complications. A total of ~14% of patients
undergoing TT with pCLND develop temporary hypoparathyroidism, and
4% experience permanent hypoparathyroidism (24). The modest reduction in recurrence
(0.66%) is counterbalanced by a 1.83% increase in temporary
hypoparathyroidism (25), and risks
of parathyroid and recurrent laryngeal nerve injury (26). Meta-analyses report permanent
hypoparathyroidism at 1.1%, permanent recurrent laryngeal nerve
injury at 0.5%, and recurrence at 2.8% in the pCLND group (27), with vocal cord paralysis ranging
3.28–27.8% (28). Taken together,
these data highlight that indiscriminate pCLND may cause more harm
than benefit, thereby emphasizing the potential utility of
preoperative diagnostic models to refine indications for CLND and
minimize morbidity.
The present study identified age, T stage and
BRAF V600E mutation status as significant predictors of
CLNM, consistent with established risk stratification frameworks.
The ATA risk system guides postoperative management, particularly
for radioactive iodine therapy and follow-up intensity (5). CLNM is a key determinant in assigning
intermediate or high ATA risk categories. Ghaznavi et al
(29) demonstrated that integrating
AJCC staging, ATA risk, and age refines disease-specific survival
estimates, especially in younger patients with high-risk features.
Building on this, our nomogram, incorporating preoperative
variables (BRAF V600E, age and tumor size), provides an
additional tool to stratify risk before surgery. Importantly, the
model showed favorable calibration and discrimination in the TCGA
cohort, with decision curve analysis confirming superior clinical
benefit compared with individual predictors. Thus, preoperative
BRAF-based models and postoperative ATA risk stratification
may be complementary, together enabling more individualized
treatment strategies. Importantly, our prognostic model did not
include classical genes such as TERT or KRAS, consistent with
recent literature (30),
emphasizing the unique predictive value of BRAF V600E.
Moreover, the LASSO-derived gene signature demonstrated strong
performance, with external validation in GSE29265 achieving an AUC
of 0.905.
The relationship between age, tumor size and CLNM
remains debated. Some studies report no significant association
(31), whereas others, including
Ahn et al (32), identify age ≤45 years as an independent
risk factor, consistent with the present findings. Tumor size is
also recognized as a risk factor, though thresholds vary: ≥1 cm per
Ahn et al (32) versus ≥0.25
cm per Yan et al (33). Our
dataset included few nodules ≥4 cm, which may limit statistical
accuracy. These discrepancies highlight the need for larger
datasets to clarify cutoff values for more robust clinical
applications.
Molecular insights have highlighted the significance
of BRAF mutations in PTC. BRAF is the most frequently
mutated gene in THCA, with V600E promoting cell proliferation and
tumor aggressiveness (34,35). Preoperative detection of BRAF
V600E informs surgical planning, recurrence risk stratification and
potential I-131 therapy (36–38).
The results of the present study confirmed that BRAF
V600E-positive patients show higher recurrence and metastasis
rates, supporting more intensive follow-up and, where appropriate,
more aggressive management.
The link between BRAF V600E and CLNM,
however, remains controversial. Xing et al (39,40) suggested
that this mutation significantly increases the likelihood of CLNM
in patients with PTC. In the present study, preoperative FNAC with
ARMS-PCR enabled detection of BRAF V600E in patients with
cN0, suggesting potential utility in guiding prophylactic CLND.
Further analysis of BRAF V600E-associated DEGs identified
LOX and CST6 as potential mediators: LOX mediates extracellular
matrix remodeling and metastasis (41–43),
whereas CST6 may modulate the TME and metastatic potential
(44). These genes could act as
downstream or parallel mediators of BRAF-driven MAPK
signaling, rationalizing their association with elevated CLNM
risk.
Immune microenvironment analysis reinforced the
clinical implications of our risk model. High-risk patients with
THCA displayed a distinctive ‘inflamed’ immune phenotype, with
substantial infiltration of DCs, macrophages, neutrophils, Th1/Th2
cells and Tregs, along with higher stromal and immune scores. This
pattern, consistent with Xie et al (45), indicates an activated yet
functionally constrained immune context. The broad upregulation of
cytokines and chemokines, including immunosuppressive mediators
such as IL-10 and TGF-β (46),
together with increased expression of multiple immune checkpoint
molecules (for example, PD-L1, CTLA-4, TIGIT and HAVCR2) (47), reflects T-cell exhaustion. Notably,
IPS analysis suggested that high-risk patients may respond more
favorably to immune checkpoint inhibitors, particularly combined
PD-1/CTLA-4 blockade, suggesting that despite worse prognosis, they
could derive substantial benefit from immunotherapy (48). Thus, the current findings not only
delineate metastatic risk but also identify an immunological subset
of patients potentially suited for targeted immunotherapies.
Several methodological and cohort-related
limitations warrant consideration. Because of the limited volume of
FNAC specimens, ARMS-PCR was used for BRAF V600E detection, whereas
IHC was applied to postoperative samples owing to its practicality
in routine pathology. As IHC is generally less sensitive than
molecular methods such as PCR or NGS, this methodological
difference may introduce false negatives, and the relatively small
FNAC cohort, particularly the limited number of BRAF-positive
cases, further reduced statistical power. In addition, the
detection rates of CLNM varied across cohorts (TCGA: BRAF+ 59.1%
vs. BRAF- 35.7%, P<0.001; FNAC: 75% vs. 32.1%, P>0.05; IHC:
33.8% vs. 28.0%, P>0.05), which may reflect differences in
detection techniques, population heterogeneity (Western vs. Chinese
patients), and sample sizes; although the nomogram demonstrated
acceptable discrimination in TCGA (AUC=0.710), its performance in
external validation in GSE60542 was modest (AUC=0.666),
underscoring the need for larger and more homogeneous cohorts as
well as more sensitive molecular approaches (49). Finally, the nomogram of the present
study was designed as a preoperative supplementary tool but was not
directly compared with the ATA Risk Stratification System, an
important limitation that should be addressed in future prospective
studies.
Ultimately, these limitations highlight the need for
future studies that incorporate larger and more homogeneous
cohorts, employ sensitive molecular detection methods, and evaluate
head-to-head comparisons with established clinical tools. Such
efforts will facilitate the integration of molecularly informed
predictive models into routine practice, thereby improving
preoperative risk stratification, surgical decision-making, and
individualized management of THCA patients.
In summary, the present study identified age, T
stage and BRAF V600E-associated high metastatic risk as
independent predictors of CLNM in patients with PTC. The nomogram
developed herein provides a practical visual tool for preoperative
estimation of CLNM risk, supporting more precise surgical planning.
For classical patients with PTC with TI-RADS ≥4a nodules and no
radiologic evidence of cervical LNM, integrated assessment
combining ultrasound-guided fine-needle aspiration with BRAF
V600E mutation testing is recommended. This approach enables
individualized risk stratification, informing surgical
decision-making, reducing unnecessary procedures, and potentially
improving patient outcomes and quality of life.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Self-funded Science and
Technology Projects of Foshan (grant no. 2220001005113).
Availability of data and materials
The data generated and/or analyzed in the present
study may be found in the TCGA database or at the following URL:
(https://portal.gdc.cancer.gov).
Authors' contributions
JP and ZW contributed to conceptualization and study
design and were involved in writing the original draft. YH
performed the bioinformatic analysis and curated the data. RP was
responsible for specimen collection and statistical analysis. ZF
conducted lymph node punctures. JW performed the postoperative
immunohistochemical analysis of thyroid and lymph nodes. ZW and YH
confirm the authenticity of all the raw data. All authors reviewed
the data, provided critical revisions, read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Shunde Hospital of Guangzhou University of Chinese
Medicine (approval nos. KY2022066 and KY2023127; Foshan, China).
Written informed consent was obtained from all participants for the
use of their clinical and pathological data in the present
research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wiltshire JJ, Drake TM, Uttley L and
Balasubramanian SP: Systematic review of trends in the incidence
rates of thyroid cancer. Thyroid. 26:1541–1552. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stonell R, Bannister P and Memon A:
Changing epidemiology and trends in incidence of thyroid cancer in
England, 1985–2019. Eur J Public Health. 32 (Suppl 3):ckac130.053.
2022. View Article : Google Scholar
|
|
3
|
Yao F, Yang Z, Li Y, Chen W, Wu T, Peng J,
Jiao Z and Yang A: Real-world evidence on the sensitivity of
preoperative ultrasound in evaluating central lymph node metastasis
of papillary thyroid carcinoma. Front Endocrinol (Lausanne).
13:8659112022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang H: Interpretation of surgical update
in 2018 Japanese clinical practice guidelines for thyroid tumors.
Chin J Pract Surg. 39:52019.
|
|
5
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Filetti S, Durante C, Hartl D, Leboulleux
S, Locati LD, Newbold K, Papotti MG and Berruti A; ESMO Guidelines
Committee. Electronic address, : simpleclinicalguidelines@esmo.org:
Thyroid cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up†. Ann Oncol. 30:1856–1883. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han L and Xu H: China anti-cancer
association guidelines for holistic integrative management of
thyroid cancer (2022 abridged version). Chin J Clin Oncol.
50:325–330. 2023.
|
|
8
|
Haddad RI, Bischoff L, Ball D, Bernet V,
Blomain E, Busaidy NL, Campbell M, Dickson P, Duh QY, Ehya H, et
al: Thyroid carcinoma, version 2.2022, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 20:925–951. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao W, You L, Hou X, Chen S, Ren X, Chen
G and Zhao Y: The effect of prophylactic central neck dissection on
locoregional recurrence in papillary thyroid cancer after total
thyroidectomy: A systematic review and meta-analysis: pCND for the
locoregional recurrence of papillary thyroid cancer. Ann Surg
Oncol. 24:2189–2198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yeh MW, Bauer AJ, Bernet VA, Ferris RL,
Loevner LA, Mandel SJ, Orloff LA, Randolph GW and Steward DL;
American Thyroid Association Surgical Affairs Committee Writing
Task Force, : American thyroid association statement on
preoperative imaging for thyroid cancer surgery. Thyroid. 25:3–14.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao H and Li H: Meta-analysis of
ultrasound for cervical lymph nodes in papillary thyroid cancer:
Diagnosis of central and lateral compartment nodal metastases. Eur
J Radiol. 112:14–21. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai Q, Tao Y, Liu D, Zhao C, Sui D, Xu J,
Shi T, Leng X and Lu M: Ultrasound radiomics models based on
multimodal imaging feature fusion of papillary thyroid carcinoma
for predicting central lymph node metastasis. Front Oncol.
13:12610802023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seo YL, Yoon DY, Lim KJ, Cha JH, Yun EJ,
Choi CS and Bae SH: Locally advanced thyroid cancer: Can CT help in
prediction of extrathyroidal invasion to adjacent structures? AJR
Am J Roentgenol. 195:W240–W244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zou Y, Zhang H, Li W, Guo Y, Sun F, Shi Y,
Gong Y, Lu X, Wang W and Xia S: Prediction of ipsilateral lateral
cervical lymph node metastasis in papillary thyroid carcinoma: A
combined dual-energy CT and thyroid function indicators study. BMC
Cancer. 21:2212021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Attia AS, Hussein M, Issa PP, Elnahla A,
Farhoud A, Magazine BM, Youssef MR, Aboueisha M, Shama M, Toraih E
and Kandil E: Association of BRAFV600E mutation with the
aggressive behavior of papillary thyroid microcarcinoma: A
meta-analysis of 33 studies. Int J Mol Sci. 23:156262022.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Parvathareddy SK, Siraj AK, Ahmed SO,
DeVera F, Al-Sobhi SS, Al-Dayel F and Al-Kuraya KS: Risk factors
for central lymph node metastases and benefit of prophylactic
central lymph node dissection in middle eastern patients with cN0
papillary thyroid carcinoma. Front Oncol. 11:8198242022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hlozek J, Pekova B, Rotnágl J, Holý R and
Astl J: Genetic changes in thyroid cancers and the importance of
their preoperative detection in relation to the general treatment
and determination of the extent of surgical intervention-A review.
Biomedicines. 10:15152022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bindea G, Mlecnik B, Tosolini M,
Kirilovsky A, Waldner M, Obenauf AC, Angell H, Fredriksen T,
Lafontaine L, Berger A, et al: Spatiotemporal dynamics of
intratumoral immune cells reveal the immune landscape in human
cancer. Immunity. 39:782–795. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshihara K, Shahmoradgoli M, Martínez E,
Vegesna R, Kim H, Torres-Garcia W, Treviño V, Shen H, Laird PW,
Levine DA, et al: Inferring tumour purity and stromal and immune
cell admixture from expression data. Nat Commun. 4:26122013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li N, Du XL, Reitzel LR, Xu L and Sturgis
EM: Impact of enhanced detection on the increase in thyroid cancer
incidence in the United States: Review of incidence trends by
socioeconomic status within the surveillance, epidemiology, and end
results registry, 1980–2008. Thyroid. 23:103–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park CH, Song CM, Ji YB, Pyo JY, Yi KJ,
Song YS, Park YW and Tae K: Significance of the extracapsular
spread of metastatic lymph nodes in papillary thyroid carcinoma.
Clin Exp Otorhinolaryngol. 8:289–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao L, Wang J, Jiang Y, Gao Q, Wang Y, Xi
X and Zhang B: The number of central lymph nodes on preoperative
ultrasound predicts central neck lymph node metastasis in papillary
thyroid carcinoma: A prospective cohort study. Int J Endocrinol.
2020:26986592020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ywata de Carvalho A, Chulam TC and
Kowalski LP: Long-term results of observation vs prophylactic
selective level VI neck dissection for papillary thyroid carcinoma
at a cancer center. JAMA Otolaryngol Head Neck Surg. 141:599–606.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang J, Han Y, Min Y, Chen C, Chen J,
Xiang K, Liao J, Feng Y, Hu D and Yin G: Prophylactic central neck
dissection for cN0 papillary thyroid carcinoma: Is there any
difference between western countries and China? A systematic review
and meta-analysis. Front Endocrinol (Lausanne). 14:11765122023.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pereira JA, Jimeno J, Miquel J, Iglesias
M, Munné A, Sancho JJ and Sitges-Serra A: Nodal yield, morbidity,
and recurrence after central neck dissection for papillary thyroid
carcinoma. Surgery. 138:1095–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu LS, Liang J, Li JH, Liu X, Jiang L,
Long JX, Jiang YM and Wei ZX: The incidence and risk factors for
central lymph node metastasis in cN0 papillary thyroid
microcarcinoma: A meta-analysis. Eur Arch Otorhinolaryngol.
274:1327–1338. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Enomoto K, Uchino S, Watanabe S, Enomoto Y
and Noguchi S: Recurrent laryngeal nerve palsy during surgery for
benign thyroid diseases: Risk factors and outcome analysis.
Surgery. 155:522–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ghaznavi SA, Ganly I, Shaha AR, English C,
Wills J and Tuttle RM: Using the American thyroid association
risk-stratification system to refine and individualize the American
joint committee on cancer eighth edition disease-specific survival
estimates in differentiated thyroid cancer. Thyroid. 28:1293–1300.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ji J and Shi X: Gene mutations as
predictors of central lymph mode metastasis in cN0 PTC: A
meta-analysis. Clin Genet. 105:130–139. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Khan H, Ullah H and Nabavi SM: Mechanistic
insights of hepatoprotective effects of curcumin: Therapeutic
updates and future prospects. Food Chem Toxicol. 124:182–191. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahn BH, Kim JR, Jeong HC, Lee JS, Chang ES
and Kim YH: Predictive factors of central lymph node metastasis in
papillary thyroid carcinoma. Ann Surg Treat Res. 88:63–68. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan H, Zhou X, Jin H, Zheng M, Ming X,
Wang R and Liu J: A study on central lymph node metastasis in 543
cN0 papillary thyroid carcinoma patients. Int J Endocrinol.
2016:18781942016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi EK, Chong A, Ha JM, Jung CK O, JH and
Kim SH: Clinicopathological characteristics including BRAF V600E
mutation status and PET/CT findings in papillary thyroid carcinoma.
Clin Endocrinol (Oxf). 87:73–79. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lasolle H, Schiavo A, Tourneur A, Gillotay
P, de Faria da Fonseca B, Ceolin L, Monestier O, Aganahi B,
Chomette L, Kizys MML, et al: Dual targeting of MAPK and PI3K
pathways unlocks redifferentiation of Braf-mutated thyroid cancer
organoids. Oncogene. 43:155–170. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song Y, Xu G, Ma T, Zhu Y, Yu H, Yu W, Wei
W, Wang T and Zhang B: Utility of a multigene testing for
preoperative evaluation of indeterminate thyroid nodules: A
prospective blinded single center study in China. Cancer Med.
9:8397–8405. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang K, Wang H, Liang Z, Liang J, Li F and
Lin Y: BRAFV600E mutation associated with non-radioiodine-avid
status in distant metastatic papillary thyroid carcinoma. Clin Nucl
Med. 39:675–679. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng W, Liu R, Zhu G, Wang H and Xing M:
Robust thyroid gene expression and radioiodine uptake induced by
simultaneous suppression of BRAF V600E and histone deacetylase in
thyroid cancer cells. J Clin Endocrinol Metab. 101:962–971. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xing M, Alzahrani AS, Carson KA, Shong YK,
Kim TY, Viola D, Elisei R, Bendlová B, Yip L, Mian C, et al:
Association between BRAF V600E mutation and recurrence of papillary
thyroid cancer. J Clin Oncol. 33:42–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xing M, Alzahrani AS, Carson KA, Viola D,
Elisei R, Bendlova B, Yip L, Mian C, Vianello F, Tuttle RM, et al:
Association between BRAF V600E mutation and mortality in patients
with papillary thyroid cancer. JAMA. 309:1493–1501. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boufraqech M, Patel D, Nilubol N, Powers
A, King T, Shell J, Lack J, Zhang L, Gara SK, Gunda V, et al: Lysyl
oxidase is a key player in BRAF/MAPK pathway-driven thyroid cancer
aggressiveness. Thyroid. 29:79–92. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jolly LA, Novitskiy S, Owens P, Massoll N,
Cheng N, Fang W, Moses HL and Franco AT: Fibroblast-mediated
collagen remodeling within the tumor microenvironment facilitates
progression of thyroid cancers driven by BrafV600E and Pten loss.
Cancer Res. 76:1804–1813. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu H, Sun X, Dong B, Zhang J, Zhang J, Gu
Y, Chen L, Pang X, Ye J, Wang X and Rong Z: Systematic
characterisation and analysis of lysyl oxidase family members as
drivers of tumour progression and multiple drug resistance. J Cell
Mol Med. 29:e705362025. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Oler G, Camacho CP, Hojaij FC, Michaluart
P Jr, Riggins GJ and Cerutti JM: Gene expression profiling of
papillary thyroid carcinoma identifies transcripts correlated with
BRAF mutational status and lymph node metastasis. Clin Cancer Res.
14:4735–4742. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xie Z, Li X, He Y, Wu S, Wang S, Sun J, He
Y, Lun Y and Zhang J: Immune cell confrontation in the papillary
thyroid carcinoma microenvironment. Front Endocrinol (Lausanne).
11:5706042020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ferrari SM, Fallahi P, Galdiero MR,
Ruffilli I, Elia G, Ragusa F, Paparo SR, Patrizio A, Mazzi V,
Varricchi G, et al: Immune and inflammatory cells in thyroid cancer
microenvironment. Int J Mol Sci. 20:44132019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kurachi M: CD8+ T cell
exhaustion. Semin Immunopathol. 41:327–337. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zander AD, Erbe R, Liu Y, Jin A, Hyun SW,
Mukhopadhyay S, Terdich B, Rosasco MG, Patel N, Mahon BM, et al:
Development and validation of the immune profile score (IPS), a
novel multiomic algorithmic assay for stratifying outcomes in a
real-world cohort of patients with advanced solid cancer treated
with immune checkpoint inhibitors. J Immunother Cancer.
13:e0113632025. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fu G, Chazen RS, MacMillan C and Witterick
IJ: Development of a molecular assay for detection and
quantification of the BRAF variation in residual tissue from
thyroid nodule fine-needle aspiration biopsy specimens. JAMA Netw
Open. 4:e21272432021. View Article : Google Scholar : PubMed/NCBI
|