Introduction
Prostate cancer is one of the most common types of
cancer in men worldwide (1,2). Although stage migration of prostate
cancer has been reported, when metastatic disease occurs it usually
involves the bones (3,4). In addition to bone metastases, bone
demineralization from previous castration (medical or surgical)
contributes to an increased risk of skeletal complications (e.g.,
fracture and pain) (5–8). Complications from bone metastases are
a major cause of morbidity in patients with prostate cancer,
causing intractable debilitating pain, spinal cord compression,
pathologic fractures and abnormalities in serum calcium levels
(9). Thus, patients with metastatic
hormone-refractory prostate cancer (HRPC) are particularly prone to
incapacitating progressive bone disease (10).
Zoledronic acid (ZOL) is a new generation
nitrogen-containing bisphosphonate (BP) with improved efficacy
benefits over pamidronate in preclinical testing. In addition, ZOL
is superior to pamidronate in the treatment of hypercalcemia of
malignancy (11). It is also the
first BP to demonstrate efficacy in patients with bone metastases
from solid tumors other than breast cancer, including prostate
cancer, non-small cell lung cancer and a variety of other tumor
types (12,13). Although not an oral agent, ZOL can
be safely administered intravenously (14) (Maxwell et al, Convenience of
a 15-minute infusion of zoledronic acid. Presented at the 3rd
European Oncology Nursing Symposium Spring Convention, Venice,
Italy, April 12–14, 2002).
ZOL has been evaluated in phase I trials in patients
with a variety of cancer types and bone metastases (15,16).
In a previous comparative phase III trial (17), a 4-mg infusion of ZOL was as
effective as a 90-mg infusion of pamidronate in reducing skeletal
complications in patients with multiple myeloma or breast cancer.
Previously, Saad et al reported that ZOL treatment for
metastatic hormone-refractory prostate cancer significantly reduced
the incidence of skeletal-related events (SRE) by 36%, and delayed
the first SRE by >5 months as compared to the placebo used
(18). Moreover, BPs are well
tolerated with only minimal side effects (e.g. influenza-like
syndrome, arthralgia and gastrointestinal symptoms). We
investigated ZOL treatment in 17 Japanese men with advanced
prostate cancer, treated at the Aichi Medical University Hospital
between August 2006 and November 2007. Specifically, we evaluated
the effects of ZOL on SRE, bone pain, adverse events, serum
calcium, creatinine and prostate-specific antigen (PSA) in subjects
with advanced prostate cancer.
Patients and methods
Study population
Our cohort comprised of 17 non-consecutive patients
with biopsy-confirmed stage D2 prostate cancer and bone metastasis;
verified by bone scintigraphy. Table
I lists the demographic and clinicopathological characteristics
of the cohort. Of the 17 patients, 12 had HRPC, while 5 were
sensitive to androgen manipulation. HRPC patients had received
docetaxel-based chemotherapy or steroidal therapy. Non-HRPC
patients had received combined androgen blockade therapy. Patients
had been prescribed morphine and non-steroidal anti-inflammatory
drugs (NSAIDS) for moderate to severe bone pain. Six patients were
previously treated with incadronate disodium. Three patients had
SRE (1 patient with a compression fracture and paralysis and 2
patients with pending fractures, which were treated with radiation
therapy). No patients were found to have hypercalcemia of
malignancy.
 | Table IClinicopathological characteristics of
the cohort. |
Table I
Clinicopathological characteristics of
the cohort.
| Patient no. | 17 |
| Age (years) | 58–86 (mean age
74.4) |
| TNM classification
(UICCa) |
| T2aN0M1b | 1 |
| T2bN0M1b | 5 |
| T3aN1M1b | 3 |
| T3bN1M1b | 1 |
| T4N0M1b | 2 |
| T4N1M1b | 5 |
| Peformance status
(ECOGb) |
| PS 0 | 7 |
| PS 1 | 7 |
| PS 2 | 1 |
| PS 3 | 2 |
| HRPCc | 12 |
| Non-HRPC | 5 |
| Previous SREd |
| Yes | 3 |
| No | 14 |
| Prior to BPe |
| Yes | 6 (incadronate
disodium) |
| No | 11 |
Methods
ZOL (Zometa®; Novartis Pharma AG, Basel,
Switzerland/Novartis Pharmaceuticals Corp., East Hanover, NJ) was
administered intravenously at a dose of 4 mg over 15 min every 4
weeks (one cycle). Treatments ranged from 1 to 13 cycles (median of
6 cycles). Hydration was not performed. Clinical and hospital
records were reviewed for several key factors including laboratory
results and SRE. Statistical analysis of the laboratory results was
evaluated using the Wilcoxon and Friedman’s tests. The patients
were assessed for any adverse effects using the National Cancer
Institute’s Common Toxicity Criteria, version 2.0. Pain complaints
were evaluated. The median follow-up was 11 months.
Results
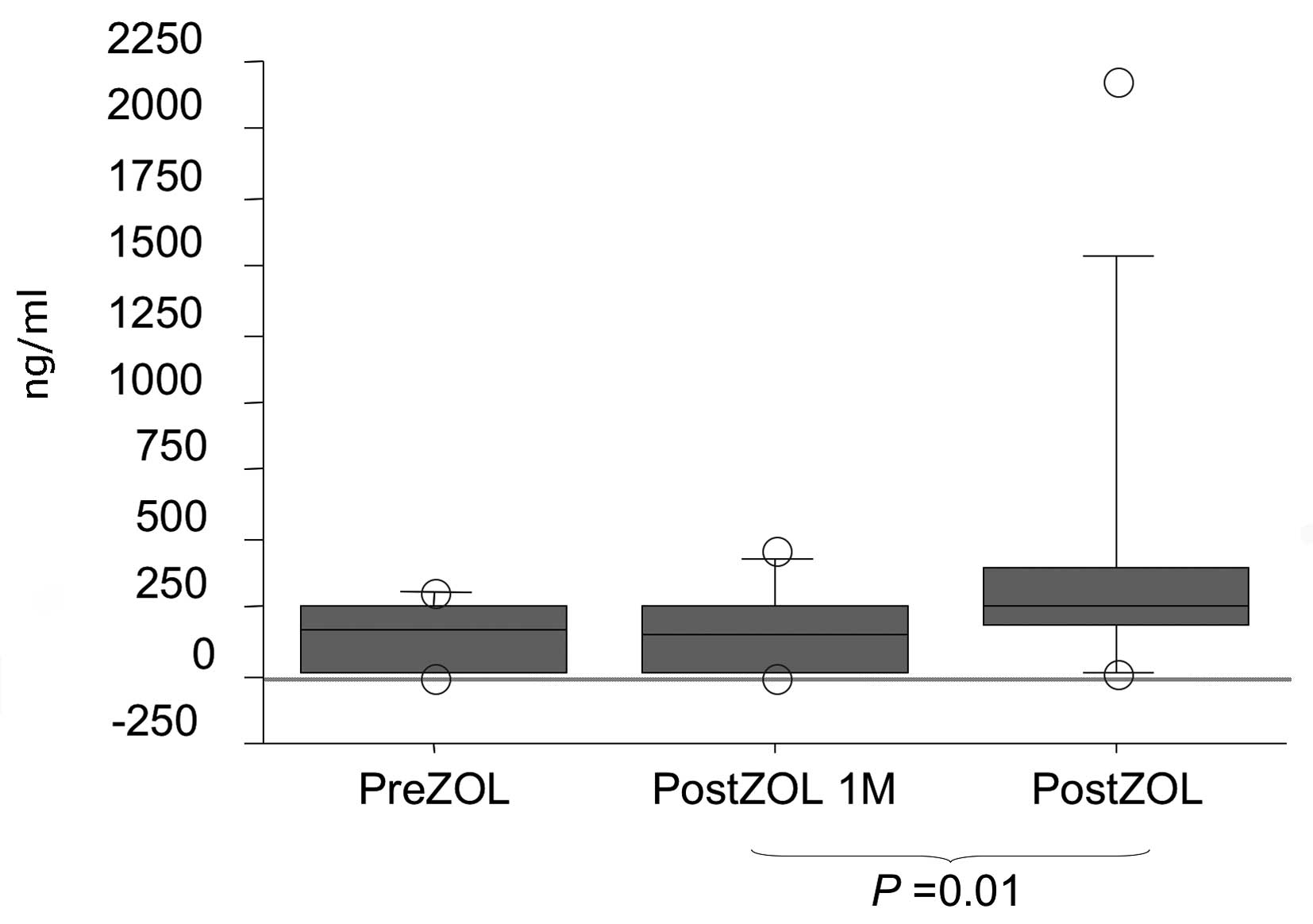
Although serum PSA levels were not altered (Fig. 1), a significant reduction in
subjective bone pain was evident. Subjects previously utilizing
morphine and NSAIDS for moderate to severe bone pain, reported that
bone pain decreased in (14 subjects, 82.4%) with ZOL treatment. The
other three subjects, however, reported a dose escalation of
morphine to control bone pain. No new SRE was evident in the
cohort.
ZOL therapy was well tolerated in our cohort. Mild
renal dysfunction (i.e., serum creatinine 1.4–2.0 mg/dl) was
detected in two patients (11.8%). However, no statistically
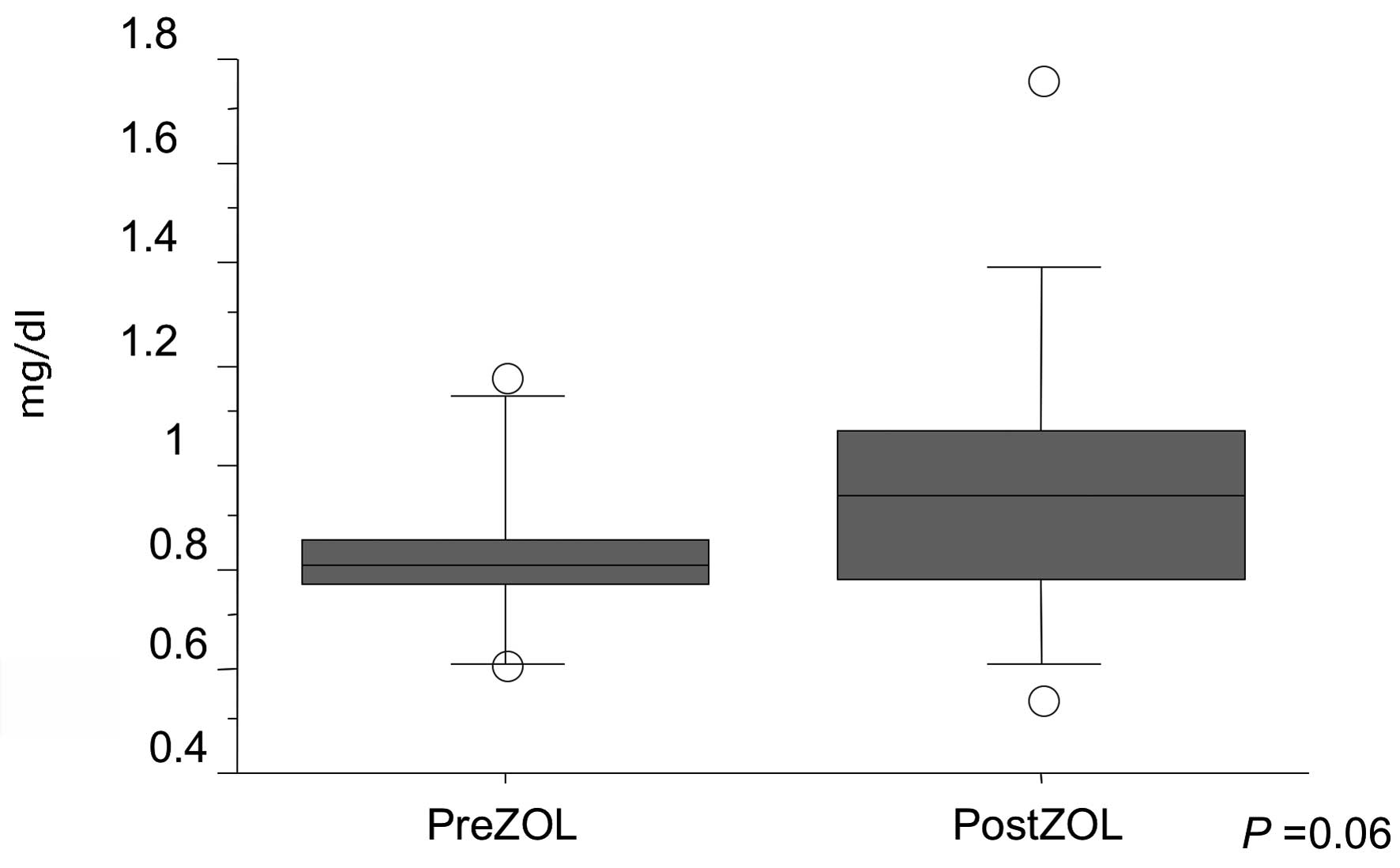
significant differences were noted in serum creatinine levels
between pre- and post-ZOL injection (P=0.06) (Fig. 2). Skin rash was developed in one
patient (5.8%). The two patients with renal dysfunction were on
medication for hypertension. No significant side effects were
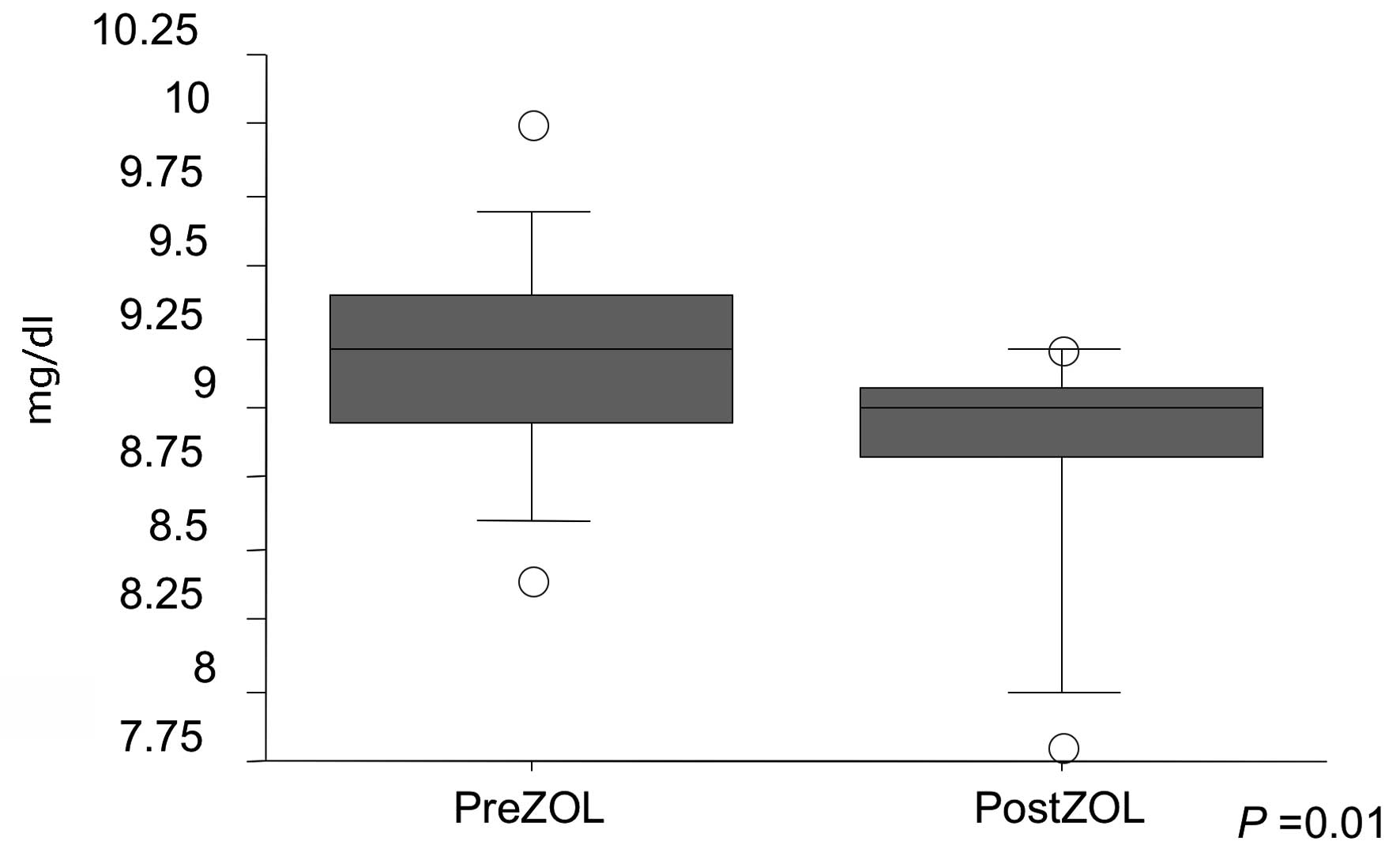
noted. As for abnormalities in serum chemistry, serum calcium
decreased post-ZOL injection (P=0.01), but there was no evidence of
severe hypocalcemia (Fig. 3).
Discussion
Bone metastasis in prostate cancer can lead to
significant skeletal morbidity. In randomized controlled trials, BP
therapy has been associated with a reduction in SRE. BPs are known
to reduce excessive bone turnover while preserving bone structure
and mineralization in patients with metastatic disease to the
skeleton (15). We investigated the
efficacy of ZOL in a cohort of Japanese men with stage D2 prostate
cancer.
We found no SRE in the small cohort of Japanese men
with stage D2 prostate cancer treated with ZOL. Previously, Saad
et al reported that ZOL significantly reduced the incidence
of SRE by 36% and delayed the first SRE by >5 months as compared
to the placebo used (18).
Furthermore, ZOL provided significant long-term reductions in bone
pain as compared to the placebo used (19). Unlike ZOL, first generation BPs
(etidronate, clodronate and pamidronate) were unable to demonstrate
a statistically significant clinical benefit in patients with bone
metastatic prostate cancer (15).
Previous reports and the present study show that ZOL significantly
lowers the incidence of skeletal complications and helps in
alleviating bone pain. Thus, ZOL is currently the most commonly
chosen BP treatment for patients with hormone-refractory bone
metastatic prostate cancer. ZOL subjectively improved bone pain in
14 patients (82.4%) who were previously treated with morphine and
NSAIDS. In 5 cases, morphine and NSAID treatment decreased.
However, in our cohort, 3 patients reported an increase in morphine
use to alleviate bone pain. Notably, SRE reduction was greatest in
patients without pain. Thus, it is advised that ZOL therapy be
started before patients present symptoms (20).
Several issues related to BP therapy are worth
mentioning. First, it is not uncommon for patients to experience an
increase in creatinine levels while on therapy. Vogel and others
noted a mild increase in serum creatinine in 7.7% of patients,
previously treated with BP therapy, as well as 4.5% of BP-naïve
patients (16). In our cohort
(11.8%), ZOL therapy resulted in mild and transient renal
dysfunction (increase in serum creatinine from 1.4 to 2.0 mg/dl).
Second, BP therapy has been associated with severe hypocalcemia
(21). We found a non-significant
decrease in serum calcium levels noted within a few days of BP
therapy. Severe hypocalcemia, however, was not noted. On the other
hand, Gulley et al reported that following a single dose of
zoledronic acid, one patient developed hypocalcemia which persisted
for approximately 60 days (21).
Third, osteonecrosis of the jaw has been linked to BP therapy
(22). In our study, BP treatment
was delayed in one subject to accommodate for dental evaluation and
treatment. It is therefore critical that patients receiving BP be
taught meticulous dental care to prevent osteonecrosis of the jaw.
Fourth, ZOL can halt bone turnover associated with metastatic
disease to the bones. Previously, Vordos and colleagues reported
that ZOL therapy in combination with docetaxel was associated with
a decrease in serum PSA by more than 50% at 2 months in more than
50% of the patients (23). Thus, a
significant reduction in PSA may be associated with burden of
disease within the skeleton. We noted no decrease in patient serum
PSA levels during ZOL therapy. Significant clinical benefits of ZOL
therapy were demonstrated in this retrospective analysis of 17
Japanese men with bone metastasis from prostate cancer. It is
therefore critical that urologists understand the benefits and
potential implications of ZOL in the treatment of stage D2 prostate
cancer.
References
|
1
|
Parkin DM, Laara E and Muir CS: Estimates
of the worldwide frequency of sixteen major cancers in 1980. Int J
Cancer. 41:184–197. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parker SL, Tong T, Bolden S and Wingo PA:
Cancer statistics. CA Cancer J Clin. 46:5–27. 1996.
|
|
3
|
Carlin BI and Andriole GL: The natural
history, skeletal complications, and management of bone metastases
in patients with prostate carcinoma. Cancer. 88:2989–2994. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pentyala SN, Lee J, Hsieh K, Waltzer WC,
Trocchia A, Musacchia L, et al: Prostate cancer: a comprehensive
review. Med Oncol. 17:85–105. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Townsend MF, Sanders WH, Northway RO and
Graham SD Jr: Bone fractures associated with luteinizing
hormone-releasing hormone agonists used in the treatment of
prostate carcinoma. Cancer. 79:545–550. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Collinson MP, Tyrell CJ and Hutton C:
Osteoporosis occurring in two patients receiving LHRH analogs for
carcinoma of the prostate (letter). Calcif Tissue Int. 54:327–328.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daniell HW: Osteoporosis after orchiectomy
for prostate cancer. J Urol. 157:439–444. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clarke NW, McClure J and George NJ: The
effects of orchiectomy on skeletal metabolism in metastatic
prostate cancer. Scand J Urol Nephrol. 27:475–483. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scher HI and Chung LW: Bone metastases:
improving the therapeutic index. Semin Oncol. 21:630–656.
1994.PubMed/NCBI
|
|
10
|
Berruti A, Dogliotti L, Bitossi R, Fasolis
G, Gorzegno G, Bellina M, et al: Incidence of skeletal
complications in patients with bone metastatic prostate cancer and
hormone refractory disease: predictive role of bone resorption and
formation markers evaluated at baseline. J Urol. 164:1248–1253.
2000. View Article : Google Scholar
|
|
11
|
Major P, Lortholary A, Hon J, et al:
Zoledronic acid is superior to pamidronate in the treatment of
hypercalcemia of malignancy: a pooled analysis of two randomized,
controlled clinical trials. J Clin Oncol. 19:558–567.
2001.PubMed/NCBI
|
|
12
|
Rosen LS, Gordon D, Tchekmedyian S, et al:
Zoledronic acid versus placebo in the treatment of skeletal
metastases in patients with lung cancer and other solid tumors: A
phase III, double-blind, randomized trial - The Zoledronic Acid
Lung Cancer and Other Solid Tumors Study Group. J Clin Oncol.
21:3150–3157. 2003. View Article : Google Scholar
|
|
13
|
Saad F, Gleason DM, Murray R, et al: A
randomized, placebo-controlled trial of zoledronic acid in patients
with hormone-refractory metastatic prostate carcinoma: for the
Zoledronic Acid Prostate Cancer Study Group. J Natl Cancer Inst.
94:1458–1468. 2002. View Article : Google Scholar
|
|
14
|
Green JR, Müller K and Jaeggi KA:
Preclinical pharmacology of CGP 42′446, a new, potent, heterocyclic
bisphosphonate compound. J Bone Miner Res. 9:745–751.
1994.PubMed/NCBI
|
|
15
|
Aapro M, Abrahamsson PA, Body JJ, et al:
Guidance on the use of bisphosphonates in solid tumours:
recommendations of an international expert panel. Ann Oncol.
19:420–432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vogel CL, Yanagihara RH, Wood AJ, et al:
Safety and pain palliation of zoledronic acid in patients with
breast cancer, prostate cancer, or multiple myeloma who previously
received bisphosphonate therapy. Oncologist. 9:687–695. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rosen LS, Gordon D, Antonio BS, et al:
Zoledronic acid versus pamidronate in the treatment of skeletal
metastases in patients with breast cancer or osteolytic lesions of
multiple myeloma: a phase III, double-blind, comparative trial.
Cancer J. 7:377–387. 2001.
|
|
18
|
Saad F, Gleason DM, Murray R, et al:
Long-term efficacy of zoledronic acid for the prevention of
skeletal complications in patients with metastatic
hormone-refractory prostate cancer. J Natl Cancer Inst. 96:879–882.
2004. View Article : Google Scholar
|
|
19
|
Saad F: Clinical benefit of zoledronic
acid for the prevention of skeletal complications in advanced
prostate cancer. Clin Prostate Cancer. 4:31–37. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saad F: Bisphosphonates can prevent
skeletal complications of malignant bone disease from prostate
cancer and renal cell carcinoma. Eur Urol Suppl. 6:683–688. 2007.
View Article : Google Scholar
|
|
21
|
Gulley JL, Wu S, Arlen PM and Dahut WL:
Persistent hypocalcemia induced by zoledronic acid in a patient
with androgen-independent prostate cancer and extensive bone
metastases. Clin Genitourin Cancer. 5:403–405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bagan JV, Murillo J, Jimenez Y, et al:
Vascular jaw osteonecrosis in association with cancer chemotherapy:
series of 10 cases. J Oral Pathol Med. 34:120–123. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vordos D, Paule B, Vacherot F, et al:
Docetaxel and zoledronic acid in patients with metastatic
hormone-refractory prostate cancer. BJU Int. 94:524–527. 2004.
View Article : Google Scholar : PubMed/NCBI
|