Introduction
Radiofrequency ablation (RFA) is performed worldwide
for patients with hepatocellular carcinoma (HCC) as a curative
local therapy because of the low rates of morbidity and mortality,
and the high level of efficacy (1–5).
Developments in ultrasonography (US) and contrast-enhanced US
(CEUS) agents for hepatic tumors have enabled the diagnosis of HCC
in the early stage, as CEUS makes it easier to detect small HCC
tumors that are invisible to B-mode US.
In January 2007, Sonazoid® (Perflubutane,
Daiichi Sankyo Co., Ltd., Japan) was approved as a new CEUS agent
in Japan (6). Hatanaka et al
found that this contrast agent has higher sensitivity and accuracy
for the diagnosis of hepatic malignancy (7). We reported on the diagnostic efficacy
of CEUS with Sonazoid in cases of HCC which were <2 cm (8). The primary characteristic of this
agent is the ability to repeatedly obtain a continuous enhanced
view in the Kupffer phase. Numata et al also noted that
repeated and continuous scanning in the Kupffer phase using CEUS
with Sonazoid allowed for the easy detection of target lesions
during RFA procedures (9). Thus,
this contrast enhancement agent is considered to have a positive
effect on therapeutic strategy. However, there are no known reports
of changes in therapies prior to and following the introduction of
CEUS with Sonazoid.
In order to evaluate the usefulness of Sonazoid as a
treatment of patients with HCC, we investigated the changes in
therapies for the period before and after its introduction at our
institution.
Materials and methods
Patients
This study included 1142 patients who were treated
with surgical resection, RFA, percutaneous ethanol injection (PEIT)
(10) or transcatheter arterial
chemoembolization (TACE) (11,12)
from January 2005 to December 2008 at our institution. Those
treated with chemotherapy or supportive care were excluded.
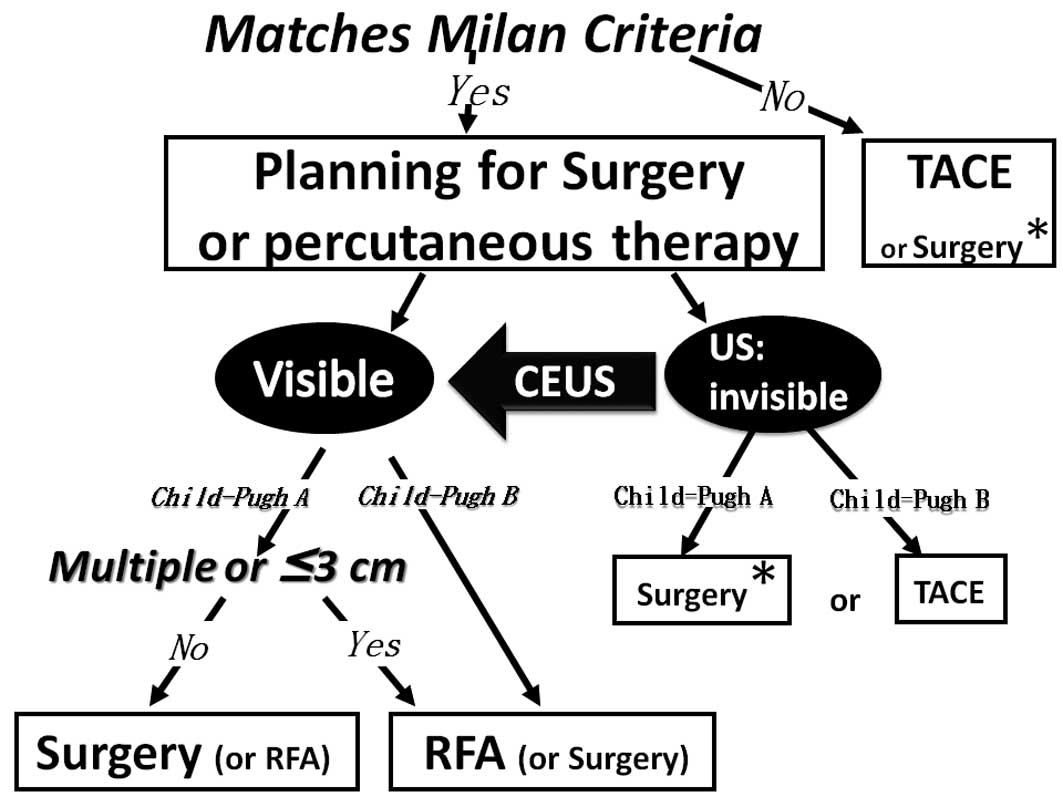
Patients were admitted to Ehime Prefectural Central Hospital and
were treated based on our strategy (Fig. 1), which is based on established
guidelines for the treatment of HCC in Japan (13). HCC diagnosis was based on histology,
past history of HCC and/or cytological findings, or imaging
evidence of tumor formation in the liver (with arterial
hyper-vascularization) using at least 2 imaging modalities [US,
dynamic computed tomography (CT), angiography and CT
angio-portography (CTAP)] (14).
Assistance with CEUS was considered in cases when the HCC tumors
were visible by other modalities (e.g., dynamic CT and CTAP) and
invisible by conventional B-mode US. RFA was performed when the
target lesions were clearly visible by conventional B-mode US or
CEUS with Sonazoid.
The patients were divided into 2 groups based on
when CEUS with Sonazoid was introduced (pre-CEUS group, January
2005 to December 2006, n=451; post-CEUS group, January 2007 to
December 2008, n=691). The results and patient backgrounds were
compared. In addition, the backgrounds of 130 naïve cases in the
pre-CEUS group were investigated and compared to those of 171 naïve
cases in the post-CEUS group. Clinical features, etiology,
Child-Pugh classification (15),
tumor node metastasis (TNM) stage (16), Japan Integrated Staging (JIS) score
(17), percentage of patients
matched with Milan criteria (single lesion ≤5 cm or 2–3 lesions
each ≤3 cm) (18), and frequencies
of each therapeutic modality selected (surgical resection, RFA,
PEIT or TACE) were analyzed in the pre- and post-CEUS groups.
CEUS
Prior to CEUS, the SSD5500 machine(Aloka Co., Ltd.,
Tokyo, Japan), which had no program for CEUS was used to perform
RFA. Aloka prosound α-10 and EUB7500 (Hitachi Medical Corp., Tokyo,
Japan) were then introduced in 2007. These machines have a program
that allows them to perform CEUS with Sonazoid. Sonazoid was used
as the CEUS agent (0.5 ml/body) in all of the examinations. The
target lesions were scanned following injection in the arterial and
Kupffer phases. The arterial phase of CEUS imaging was defined as
that which occurred from 10 to 60 sec after injection of Sonazoid,
and the Kupffer phase as that occurring 10 min after injection
(19). Fifteen frames/sec were
usually used to scan for a low mechanical index (0.2–0.3). A nodule
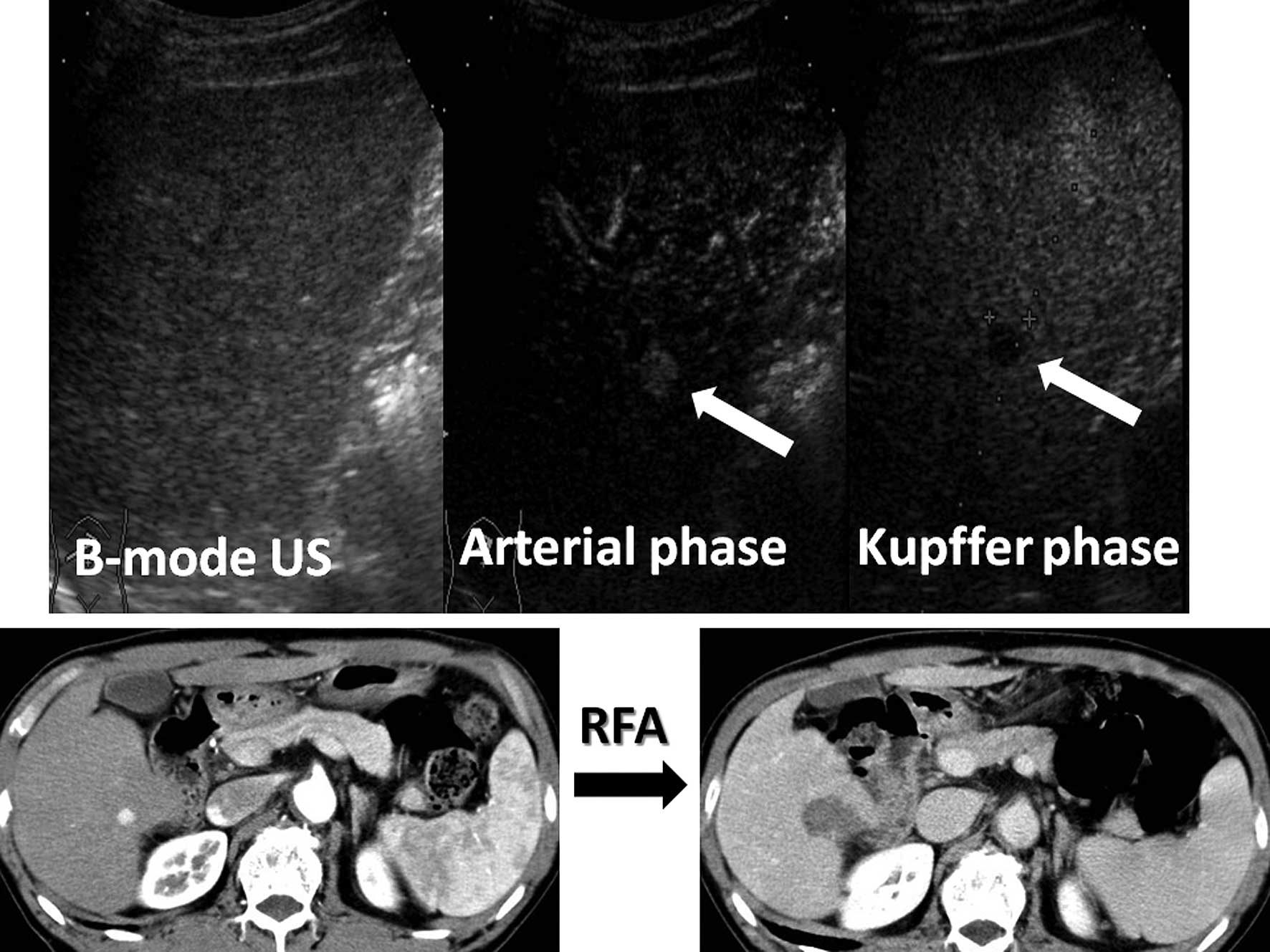
was diagnosed as typical HCC when it was shown to be hypervascular
in the arterial phase, and was revealed to be a defect lesion in
the Kupffer phase (Fig. 2) by CEUS
(20,21). In invisible cases, RFA was performed
using continuous imaging in the Kupffer phase of CEUS.
RFA
Prior to RFA treatment, 15 mg of pentazocine
hydrochloride and 25 mg of hydroxyzine hydrochloride were
administered intramuscularly. Local anesthesia was induced by 5 ml
of 1% lidocaine injected through the skin into the peritoneum along
a predetermined puncture line. In cases with HCCs located near the
lung, gallbladder or gastrointestinal tract, artificial pleural
effusion (22) and artificial
ascites were used as assistant methods for RFA. Midazolam
(Dormicum®, Astellas Pharma Inc., Japan) was injected
intravenously at the start of ablation (0.1 mg/kg). Most
hypervascular nodules were subjected to TACE (11,12)
with epirubicin-lipiodol emulsion and multiporous gelatine
particles (Gelpart®, Astellas Pharma Inc.) prior to RFA,
for which we inserted a 20-cm long 17-gauge radiofrequency
electrode equipped with a 2- or 3-cm long exposed metallic tip
(Radionics Cool-tip, Burlington, MA, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Statistical analyses were performed using Student’s t-test for
unpaired data and a Mann-Whitney U test as appropriate. Statistical
analyses were performed using SPSS 16.0J (SPSS Japan Inc., Japan).
P<0.05 was considered to be statistically significant.
Results
For the patients studied, there were no significant
differences in the clinical backgrounds between the groups
(Table I). HCC patient percentages,
with the Milan criteria in the two groups, did not show a
significant difference. However, the ratio of patients treated with
RFA increased after CEUS was introduced [pre-CEUS vs. post-CEUS, 21
(95/451) vs. 32% (219/691); P<0.01]. Furthermore, of the
patients treated with RFA in the post-CEUS group, 18.4% (41/219)
received RFA with Sonazoid.
 | Table IBackground and frequency for 1142
patients with HCC. |
Table I
Background and frequency for 1142
patients with HCC.
| Pre-CEUS group
(n=451) | Post-CEUS group
(n=691) | P-value |
|---|
| Etiology (HCV:HBV:
HBV+HCV:nonBnonC) | 355:20:14:62 | 534:45:12:100 | 0.619 |
| Child-Pugh class
(A:B:C) | 266:169:16 | 428:238:25 | 0.452 |
| TNM stage
(I:II:III:IV) | 75:177:160:39 | 123:250:275:43 | 0.547 |
| JIS score
(0:1:2:3:4:5) |
46:144:142:94:21:4 |
84:192:243:146:23:3 | 0.414 |
| Milan criteria
(within:without) | 244:207 | 349:342 | 0.259 |
| Frequency of RFA | 21% (n=95) | 32% (n=219) | <0.010 |
| RFA with CEUS | none | 18.7% (41/219) | |
There were 130 naïve HCC patients in the pre-CEUS
group and 171 in the post-CEUS group, with no significant
differences in clinical background between the two groups (Table II). Naïve HCC patient percentages
with the Milan criteria in the two groups were similar. However,
the ratio of patients treated with RFA increased after CEUS was
introduced [pre-CEUS vs. post-CEUS, 32 (41/130) vs. 52% (89/171);
P<0.01]. During the RFA procedure, assistance with CEUS was used
in 15.7% (14/89) of naïve patients treated with RFA in the
post-CEUS group. The clinical backgrounds of naïve HCC patients
treated with RFA in the two groups were not significantly
different, except for the number of tumors (pre-CEUS vs. post-CEUS,
1.15±0.48 vs. 1.40±0.67; P<0.01; Table III). In addition, the percentage
of tumors <1 cm in diameter in naïve patients increased after
the introduction of CEUS with Sonazoid, though the difference was
not significant (0 vs. 8%; P=0.097).
 | Table IIBackground and frequency for 301
patients with naïve HCC. |
Table II
Background and frequency for 301
patients with naïve HCC.
| Pre-CEUS group
(n=130) | Post-CEUS group
(n=171) | P-value |
|---|
| Etiology (HCV:HBV:
HBV+HCV:nonBnonC) | 88:9:3:30 | 125:10:0:36 | 0.371 |
| Child-Pugh class
(A:B:C) | 86:41:3 | 119:47:5 | 0.506 |
| TNM stage
(I:II:III:IV) | 27:72:20:11 | 40:73:51:7 | 0.545 |
| JIS score
(0:1:2:3:4:5) | 18:58:37:9:7:1 | 30:60:50:28:3:0 | 0.584 |
| Milan criteria
(within:without) | 94:36 | 119:52 | 0.700 |
| Frequency of RFA | 32% (n=41) | 52% (n=89) | <0.010 |
| RFA with CEUS | none | 15.7% (14/89) | |
 | Table IIIClinical features of naïve HCC
patients treated with RFA. |
Table III
Clinical features of naïve HCC
patients treated with RFA.
| Pre-CEUS group
(n=41) | Post-CEUS group
(n=89) | P-value |
|---|
| Age (year) | 71.4±8.3 | 69.0±9.2 | 0.184 |
| Gender
(male:female) | 28:13 | 68:21 | 0.445 |
| Tumor numbers | 1.15±0.48 | 1.40±0.67 | <0.010 |
| Tumor diameter
(cm) | 2.15±0.7 | 1.96±0.67 | 0.848 |
| Number of tumors
<1 cm | none | 7 (8%) | 0.097 |
| AFP (ng/ml) | 84.8±243.8 | 124.8±524.8 | 0.423 |
| AFP-L3 (%) | 9.3±18.4 | 6.5±13.3 | 0.168 |
| PIVKA-II
(mAU/ml) | 71.1±99.0 | 515.5±2224.3 | 0.030 |
| Etiology (HCV:HBV:
HBV+HCV:nonBnonC) | 30:2:1:8 | 75:5:0:9 | 0.210 |
| Child-Pugh class
(A:B:C) | 27:14:0 | 67:22:0 | 0.192 |
| TNM stage
(I:II:III:IV) | 17:21:3:0 | 37:35:17:0 | 0.429 |
| JIS score
(0:1:2:3:4:5) | 11:19:11:0:0:0 | 30:30:24:5:0:0 | 0.850 |
Discussion
CEUS with Sonazoid, which is able to provide
continuous imaging of target lesions in the Kupffer phase, has been
reported to have a 94.7% rate of sensitivity and 81.8% rate of
specificity for diagnosing small HCCs (<2 cm), while the
positive and negative predictive values for those were 94.7 and
81.8%, respectively (8). Minami
et al reported that CEUS with Levovist®
(Schering, Berlin, Germany) was useful as an indicator and guide
for RFA (23). However, the
combination of CEUS with another agent besides Sonazoid does not
reveal the Kupffer phase continuously, because imaging during that
phase is performed by bursting microbubbles that accumulate in
Kupffer cells by sound waves (24).
Continuous imaging in the Kupffer phase with Sonazoid occurs
because the images are obtained with vibration rather than bursting
microbubbles by sound waves, making it easy to observe abnormal
areas continuously (9). Although
RFA guided by CEUS with Sonazoid has been reported (9), there are no known reports of changes
in therapies at any institution prior to and following the
introduciton of this procedure.
No differences in clinical backgrounds, including
the ratio of patients with Milan criteria, hepatic reserve function
and TNM stage were noted between the groups in the present study.
In contrast, the RFA ratio for 1142 subjects, as well as the naïve
cases significantly increased after CEUS was introduced. We
consider that these differences were attributable to continuous
imaging in the Kupffer phase with Sonazoid, which enabled us to
detect invisible HCCs by conventional B-mode US. Sonazoid allowed
us to confirm that a greater number of target lesions were more
visible by additional CEUS examination. As a result, we were able
to perform the RFA procedure with confidence in the tumor location
in those cases.
Livraghi et al reported that the same
therapeutic effect was obtained after comparing patients, who had
undergone surgical resection or RFA, with single HCC tumors ≤2.0 cm
in diameter (25). We previously
reported that RFA was safer and had a similar effect to surgical
resection, though the number of patients with liver cirrhosis
Child-Pugh B class treated with RFA was greater than that of
patients treated with surgical resection (5). The results encouraged us to select RFA
for liver cirrhosis patients with HCC. Sonazoid is a powerful agent
for screening and performing RFA procedures for HCC nodules, which
can be detected by other modalities such as dynamic CT but are
difficult to detect by conventional B-mode US.
With the increasing number of small HCCs detectable
by CEUS with Sonazoid, the number of patients that can potentially
be treated with RFA will also increase, indicating that curative
and low invasive RFA procedures will be performed in a larger
number of HCC patients in the near future. We found that CEUS with
Sonazoid allowed for the use of RFA for a considerable number of
patients with HCCs, which would have otherwise been invisible by
conventional B-mode US.
References
|
1
|
Rossi S, Di Stasi M, Buscarini E, et al:
Percutaneous RF interstitial thermal ablation in the treatment of
hepatic cancer. Am J Roentgenol. 167:759–768. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tateishi R, Shiina S, Teratani T, et al:
Percutaneous radio-frequency ablation for hepatocellular carcinoma.
An analysis of 1000 cases. Cancer. 103:1201–1209. 2005.PubMed/NCBI
|
|
3
|
Shiina S, Teratani T, Obi S, Hamamura K,
Koike Y and Omata M: Nonsurgical treatment of hepatocellular
carcinoma: from percutaneous ethanol injection therapy and
percutaneous microwave coagulation therapy to radiofrequency
ablation. Oncology. 62:64–68. 2002. View Article : Google Scholar
|
|
4
|
Ng KK and Poon RT: Role of radiofrequency
ablation for liver malignancies. Surg Practice. 9:94–103. 2005.
View Article : Google Scholar
|
|
5
|
Hiraoka A, Horiike N, Yamashita Y, et al:
Efficacy of radio-frequency ablation therapy compared to surgical
resection in 164 patients in Japan with single hepatocellular
carcinoma smaller than 3 cm, along with report of complications.
Hepatogastroenterology. 55:2171–2174. 2008.
|
|
6
|
Kindberg GM, Tolleshaug H, Roos N, et al:
Hepatic clearance of Sonazoid perfluorobutane microbubbles by
Kupffer cells does not reduce the ability of liver to phagocytose
or degrade albumin microspheres. Cell Tissue Res. 312:49–54.
2003.
|
|
7
|
Hatanaka K, Kudo M, Minami Y and Maekawa
K: Sonazoid-enhanced ultrasonography for diagnosis of hepatic
malignancies: comparison with contrast-enhanced CT. Oncology.
75:42–47. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kan M, Horiike N, Hiraoka A, et al:
Comparison diagnostic efficacy of contrast enhances ultrasonography
with Perflubutane and dynamic computed tomography in patients with
liver tumors smaller than 2 cm. Hepatol Int. 2:A2552008.
|
|
9
|
Numata K, Morimoto M, Ogura M, et al:
Ablation therapy guided by contrast-enhanced sonography with
Sonazoid for hepatocellular carcinoma lesions not detected by
conventional sonography. J Ultrasound Med. 27:395–406. 2008.
|
|
10
|
Shiina S, Teratani T, Obi S, et al:
Percutaneous ethanol injection therapy for liver tumors. Eur J
Ultrasound. 13:95–106. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takayasu K, Arii S, Ikai I, et al:
Prospective cohort study of transarterial chemoembolization for
unresectable hepatocellular carcinoma in 8510 patients.
Gastroenterology. 131:461–469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hiraoka A, Kumagi T, Hirooka M, et al:
Prognosis following transcatheter arterial embolization for 121
patients with unresectable hepatocellular carcinoma with or without
a history of treatment. World J Gastroenterol. 12:2075–2079.
2006.
|
|
13
|
Makuuchi M and Kokudo N: Clinical practice
guidelines for hepatocelluar carcinoma: the first evidence based
guidelines from Japan. World J Gastroenterol. 12:828–829.
2006.PubMed/NCBI
|
|
14
|
Bruix J and Sherman M: Management of
hepatocellular carcinoma. Hepatology. 42:1208–1236. 2005.
View Article : Google Scholar
|
|
15
|
Pugh RN, Murray-Lyon IM, Dawson JL, et al:
Transection of the oesophagus for bleeding oesophageal varices. Br
J Surg. 60:646–649. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liver Cancer Study of Japan. The General
Rules for the Clinical and Pathological Study of Primary Liver
Cancer. 4th edition. Kanehara; Tokyo: pp. 192000
|
|
17
|
Kudo M, Chung H, Haji S, et al: Validation
of a new prognostic staging system for hepatocellular carcinoma:
the JIS score compared with CLIP score. Hepatology. 40:1396–1405.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mazzafero V, Regalia E, Doci R, et al:
Liver transplantation for the treatment of small hepatocellular
carcinomas in patients with cirrhosis. N Engl J Med. 334:693–699.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Watanabe R, Matsumura M, Chen CJ, et al:
Characterization of tumor imaging with microbubble-based ultrasound
contrast agent, Sonazoid, in rabbit liver. Biol Pharm Bull.
28:972–977. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Watanabe R, Matsumura M, Munemasa T, et
al: Mechanism of hepatic parenchyma-specific contrast of
microbubble-based contrast agent for ultrasonography: microscopic
studies in rat liver. Invest Radiol. 42:643–651. 2007. View Article : Google Scholar
|
|
21
|
Kindberg GM, Tolleshaug H, Roos N, et al:
Hepatic clearance of Sonazoid perfluorobutane microbubbles by
Kupffer cells does not reduce the ability of liver to phagocytose
or degrade albumin microspheres. Cell Tissue Res. 312:49–54.
2003.
|
|
22
|
Uehara T, Hirooka M, Ishida K, et al:
Percutaneous ultrasound-guided radiofrequency ablation of
hepatocellular carcinoma with artificially induced pleural effusion
and ascites. J Gastroenterol. 42:306–311. 2007. View Article : Google Scholar
|
|
23
|
Minami Y, Kudo M, Kawasaki T, et al:
Percutaneous radiofrequency ablation guided by contrast-enhanced
harmonic sonography with artificial pleural effusion for
hepatocellular carcinoma in the hepatic dome. Am J Roentgenol.
182:1224–1226. 2004. View Article : Google Scholar
|
|
24
|
Wang JH, Lu SN, Hung CH, et al: Small
hepatic nodules (≤2 cm) in cirrhosis patients: characterization
with contrast-enhanced ultrasonography. Liver Int. 26:928–934.
2006.
|
|
25
|
Livraghi T, Meloni F, Di Stasi M, et al:
Sustained complete response and complications rates after
radiofrequency ablation of very early hepatocellular carcinoma in
cirrhosis: Is resection still the treatment of choice? Hepatology.
47:82–89. 2008. View Article : Google Scholar
|