Introduction
It has been reported that there is a relationship
between the prognosis of endometrial carcinoma and the extent of
tumor differentiation. Poorly differentiated adenocarcinoma shows a
faster progression and is more refractory to therapy than
well-differentiated adenocarcinoma, resulting in poor prognosis.
However, little research has been conducted on the process of
differentiation of endometrial cancer. Research on endometrial
cancer usually employs tumor cell lines. When established, such
cell lines possess the characteristics of the original tumor, but
due to an increasing number of passages the original morphological
and functional features are gradually lost. For example, a cell
line derived from well-differentiated endometrial cancer that forms
glands in transplanted tumors shortly after establishment may be
transformed to poorly differentiated cancer without any gland
formation after ≥50 passages.
A number of reports on the role of the extracellular
matrix (ECM) in the proliferation, differentiation, metastasis and
infiltration of cancer are available. It has been reported that the
differentiation of endometrial carcinoma and gland formation can be
achieved by culturing tumor cells in or on ECM
(MatrigelTM) produced by Engelbreth-Holm-Swam mouse
sarcoma (1). However, Matrigel is
ECM produced by a tumor, and has an unknown composition; thus, it
remains unclear which of its components are involved in gland
formation.
Our studies of human endometrial tissue have
demonstrated that sulfolipids are expressed during the secretory
rather than the proliferative phase, i.e., sulfolipid expression,
which is controlled by estrogen, is reduced during the
proliferative phase (2). We also
analyzed the composition of sulfolipids expressed in cultured
gynecological cell lines such as those derived from cervical,
ovarian or endometrial cancer, and showed that sulfolipids are
particularly expressed in cell lines originating from endometrial
cancer (3,4). Results suggest that sulfolipids are
involved in the development of cancer as well as in the
differentiation of the endometrium.
The objective of the present study was to induce the
differentiation of poorly differentiated endometrial cancer into
well-differentiated cancer. Cell lines derived from endometrial
cancer that showed a poorly differentiated morphology after
transplantation into nude mice were cultured with type I collagen
to induce morphological differentiation (i.e., gland formation).
Consequently, the role of sulfolipids in the induction of
differentiation was investigated.
Materials and methods
Cell lines and culture
The six cell lines shown in Table I were provided courtesy of the
sources mentioned. Cells were cultured in Ham’s F-12 medium
containing 10% fetal calf serum and antibiotics (100 U/ml
penicillin and 100 mg/ml streptomycin) at 37°C in an atmosphere of
5% CO2, and 100% humidity. The cells were then detached
with 0.05% EDTA-trypsin for passaging. The number of passages for
each cell line used in these experiments is shown in Table I.
 | Table ICharacteristics of endometrial
adenocarcinoma cell lines used in this experiment. |
Table I
Characteristics of endometrial
adenocarcinoma cell lines used in this experiment.
| Cell line | Refs. | Passage no. | Histology of the
original tumor |
|---|
| SNG-II | (5) | >100 | G1 |
| SNG-M | (6) | >100 | G2 |
| Ishikawa | (7) | >100 | G1 |
| HEC108 | (8) | >100 | G3 |
| HHUA | (9) | >100 | G1 |
| HOOUA | (10) | >100 | Undifferentiated |
Tumor transplantation into nude mice
Female BALB/c nude mice were used at 6–8 weeks of
age. Tumor cells (1×107) were injected subcutaneously
into the back of each mouse. After a mass measuring ~2 cm formed,
each animal was sacrificed and the tumor was resected
immediately.
Floating collagen gel culture
After 1 ml of type I collagen gel (Cellmatrix I-A,
Nitta Gelatin Inc., Japan) was placed into a 35-mm dish,
2×106 tumor cells were cultured on it for 2 days. The
gel was then detached from the dish, allowed to float in the medium
and culture was continued for 28 days. When sufficient cells had
developed, another layer of collagen gel was placed on top, and
culture was continued for 3 more days with the cells sandwiched
between the gel layers. This floating sandwich culture using two
layers of type I collagen gel was performed in Ham’s F-12 medium
containing 10% fetal calf serum and antibiotics (100 U/ml
penicillin and 100 mg/ml streptomycin) at 37°C in an atmosphere of
5% CO2 and 100% humidity. The medium was changed every
other day.
Light and electron microscopy
Following completion of the culture, the tumor cell
colonies were fixed with 10% neutral formalin, embedded in paraffin
and cut into sections perpendicular to the gel surface. Then the
sections were stained with hematoxylin and eosin (H&E) and
alcian blue for light microscopy. Tumors grown in nude mice were
also stained using the same methods. Other tumor specimens were
fixed by the addition of 4% sucrose/60 mM cacodylate buffer (pH
7.4) containing 4% paraformaldehyde and 5% glutaraldehyde to the
medium in equal proportions, followed by post fixation with 1%
osmium tetroxide, dehydration and embedding in Epon 812. Ultrathin
sections were cut perpendicular to the surface of the culture dish,
double-stained with uranyl and lead acetate, and observed under a
transmission electron microscope (Hitachi H-7000).
Analysis of sulfolipids
Cell lines derived from the endometrial cancers
listed in Table I were cultured for
72 h in Ham’s F-12 medium containing 10% fetal calf serum and
antibiotics (100 U/ml penicillin and 100 mg/ml streptomycin).
35S-labelled Na2SO4 (ICN
Biomedicals, USA) was added at 20 μCi/ml. After culture for 24 h,
the medium was removed, the cells were washed three times with
phosphate-buffered saline (pH 7.2) and harvested with a rubber
policeman. Then the cells were homogenized and the homogenate was
lyophilized. Lipids were extracted from the lyophilized powder with
1 ml of chloroform-methanol (2:1 and 1:2 vol/vol) for 20 min at
40°C. The combined extracts were washed by the Folch method to
remove water-soluble radioactive materials and an extract
corresponding to 2×106 cells was subjected to
chromatography on an HPTLC plate (0.25 mm thick, Merck, FRG) with
chloroform/methanol/acetone/acetic acid/water (8:2:4:2:1 vol/vol).
Autoradiograms of the TLC plate were made with X-ray film (X-Omat,
Kodak).
Results
Transplanted tumors in nude mice
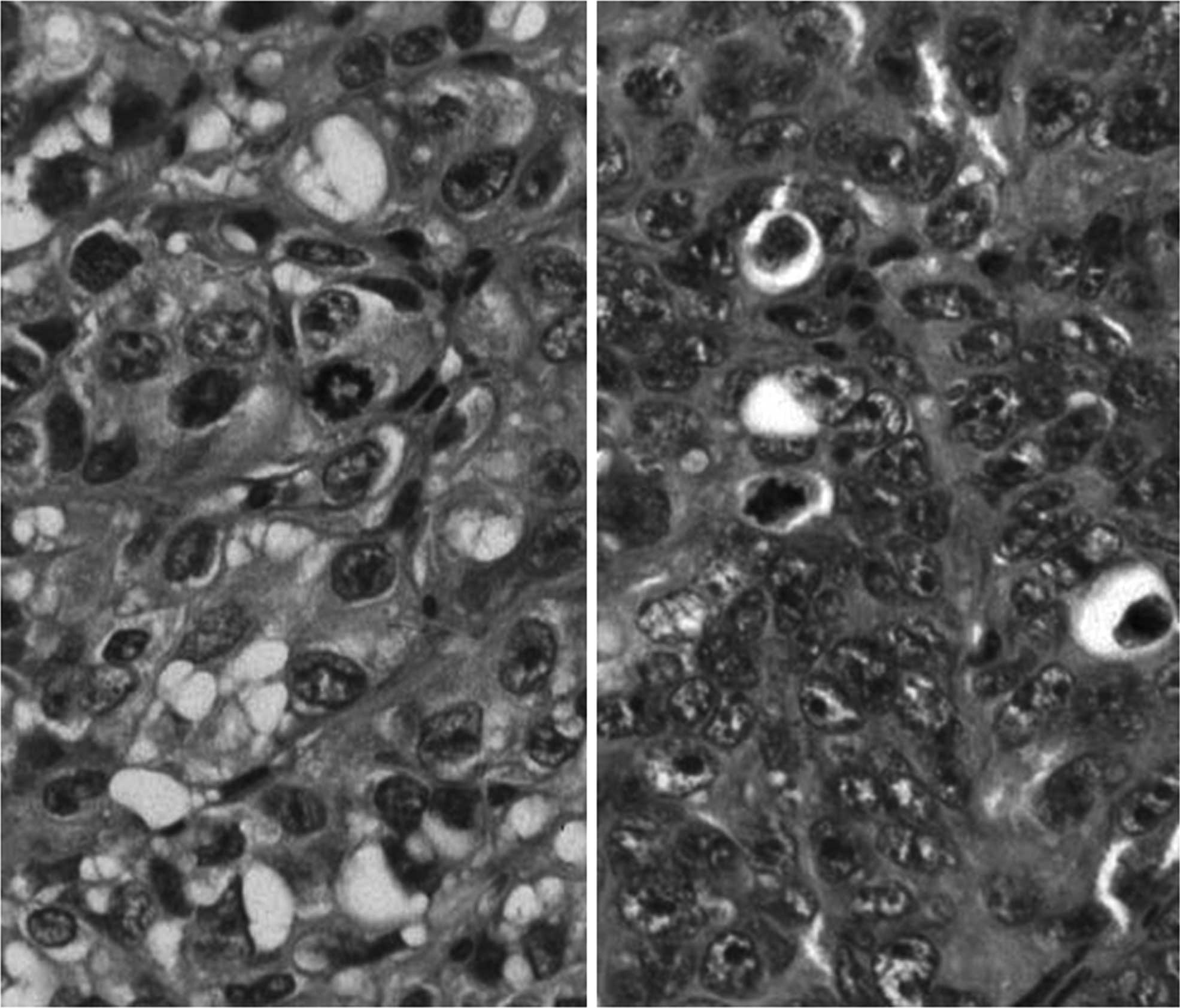
Fig. 1 shows
H&E-stained specimens of the tumors formed in nude mice by the
transplanted SNG-M (left panel) and HHUA (right panel) cell lines.
The primary tumors from which these cell lines were isolated were
moderate to well-differentiated, but the tumors that grew in mice
injected with the cells were solid and did not show any gland
formation or other signs of differentiation. The cell lines derived
from the SNG-II, HEC108 and HOOUA tumors also showed a poorly
differentiated morphology.
Floating collagen gel culture
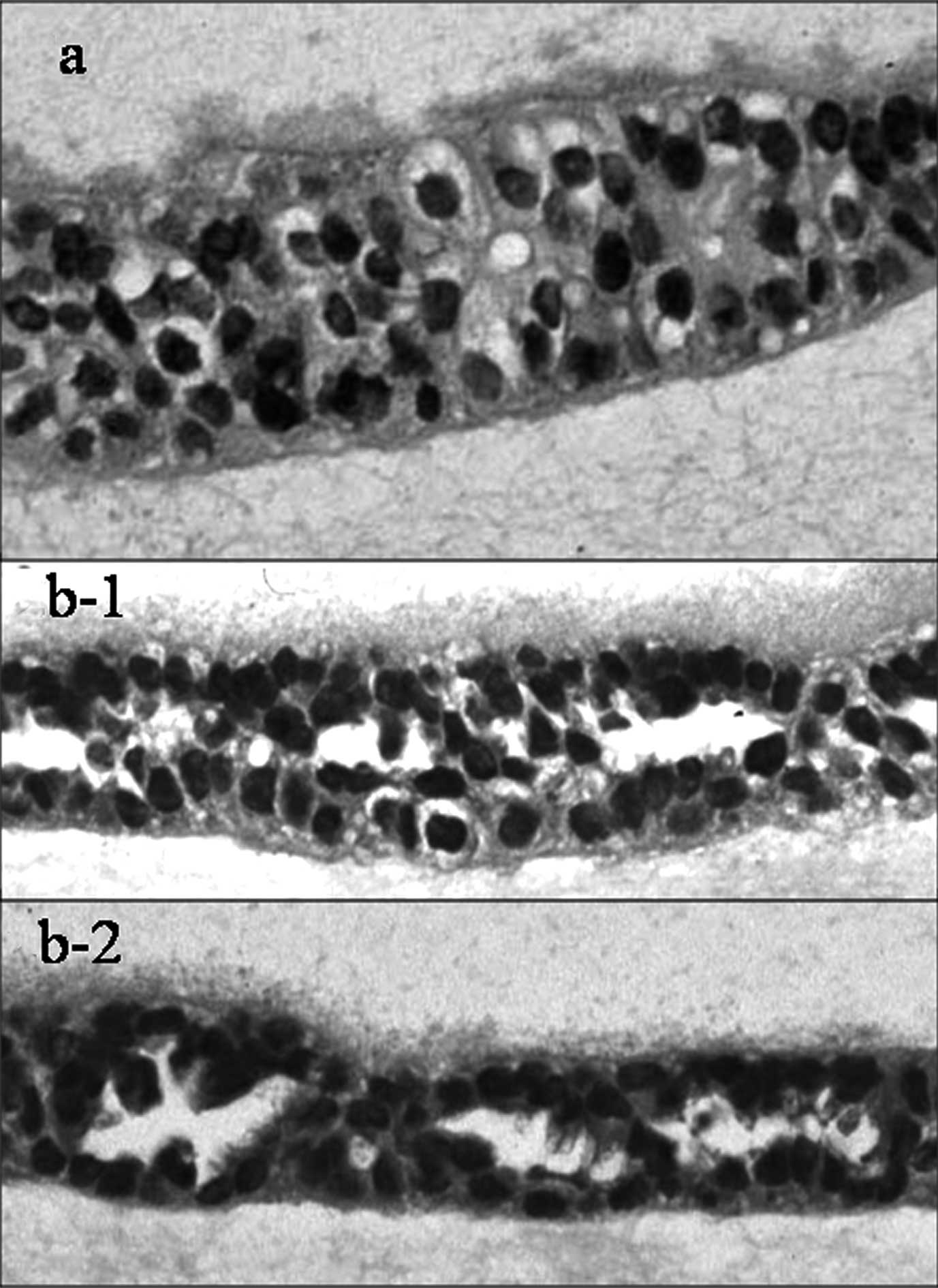
Fig. 2a shows the
H&E-stained specimens of the Ishikawa cell line cultured by the
sandwich collagen gel method. While some of the cells are slightly
polarized, there are no obvious signs of differentiation, such as
gland formation, in most parts of the specimen. Similarly, the
SNG-II, HEC108 and HOOUA cell lines cultured by this collagen gel
method showed no differentiation. However, in the H&E-stained
specimens of the SNG-M (Fig. 2b-1)
and HHUA (Fig. 2b-2) cell lines
cultured by the collagen gel method, glandular structures were
observed. The cell nuclei were lined up along the basement
membrane, showing obvious polarity. Secretion of substances inside
the lumens of glands was also observed. Alcian blue (acid
mucopolysaccharide)-stained specimens of the SNG-M cell line
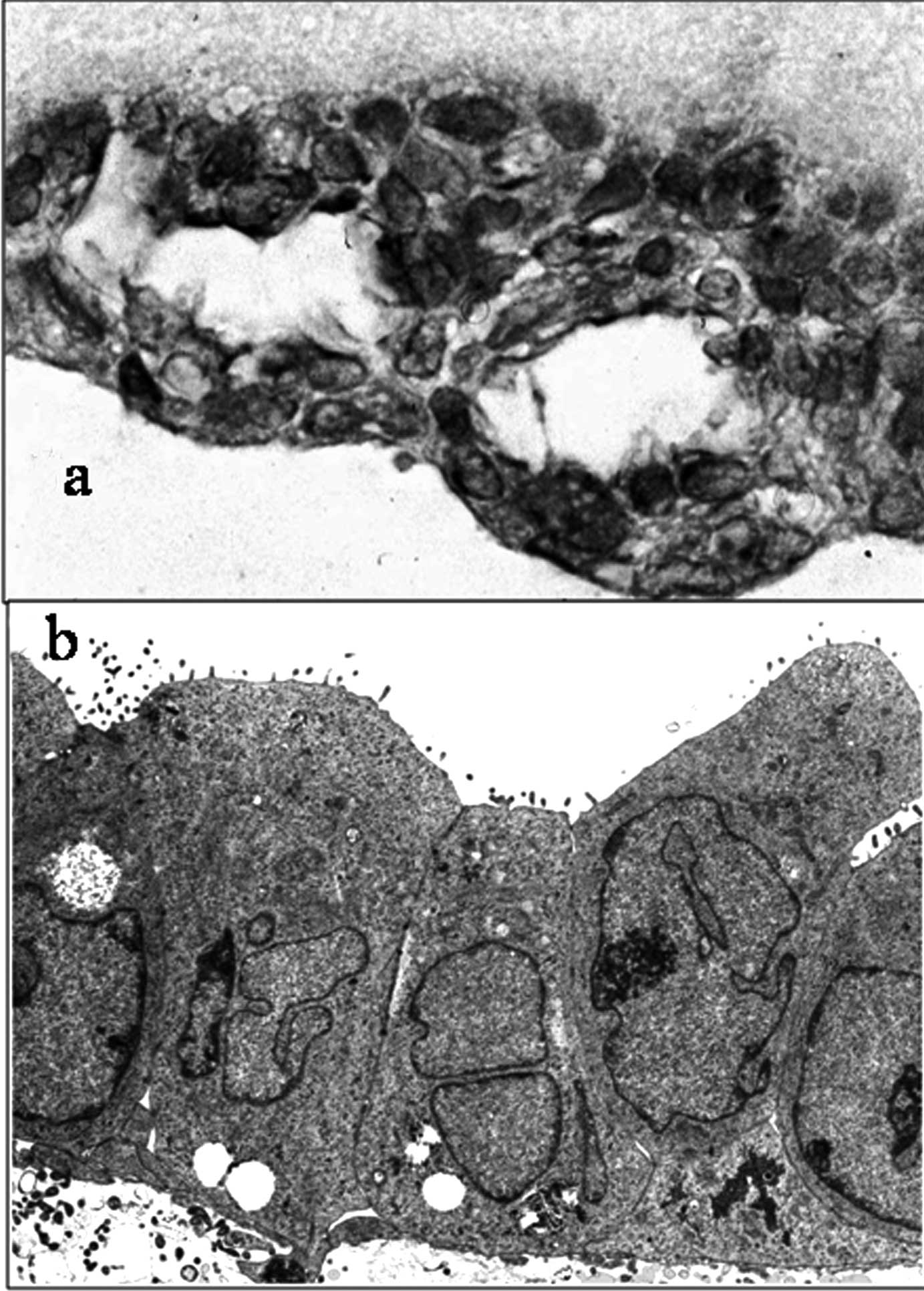
cultured by the collagen gel method are shown in Fig. 3a. The lumens of the glandular
structure were positive for staining. Electron microscopy of the
HHUA cell line after collagen gel culture (Fig. 3b) showed microvilli protruding into
the lumens of the glandular structures and the presence of
junctional complexes and desmosomes on the lateral surface.
Sulfolipids in the cell lines
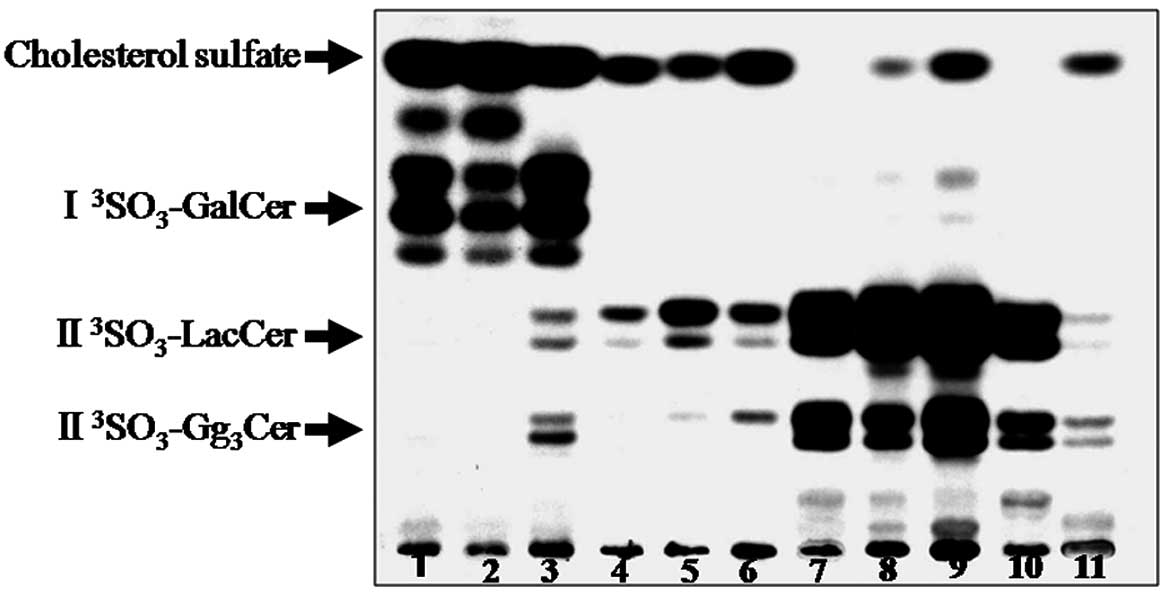
Fig. 4 shows a TLC
autoradiogram of the sulfolipids expressed by various cell lines
derived from endometrial cancer. The four sulfolipids found on the
TLC plate (I3SO3-GalCer,
II3SO3-LacCer and
II3SO3-Gg3Cer) were identical to
those of cholesterol sulfate. The cell lines were found to express
glycolipids containing one of the three sulfate groups
(I3SO3-GalCer,
II3SO3-LacCer or
II3SO3-Gg3Cer), while the SNG-M
and HHUA cell lines showed no expression of cholesterol sulfate.
The results are summarized in Table
II.
 | Table IIResults. |
Table II
Results.
| Cell line | Histological grading
of xenografts | Glandular
differentiation by embedded collagen culture | Cholesterol
sulfate | Sulfoglycolipids |
|---|
| SNG-II | G3 | − | + | + |
| SNG-M | G3 | + | − | ++ |
| Ishikawa | G3 | − | ++ | ++ |
| HEC108 | G3 | − | ++ | ++ |
| HHUA | G3 | + | − | ++ |
| HOOUA | G3 | − | + | + |
Discussion
The extent of differentiation is reported to be an
important determinant of the behavior of endometrial cancer. The
survival rate of patients with poorly differentiated adenocarcinoma
is low as these tumors are often advanced at the time of detection
and metastasize easily. However, little is known about the reasons
for such tumor behavior.
The present study aimed to elucidate the mechanism
of differentiation of endometrial cancer. A culture method was
developed to induce differentiation of the cultured cell lines
derived from primary tumors. The tumors exhibited gland formation
that had become poorly differentiated to anaplastic after a number
of passages.
Induction of the differentiation of cultured cells
using ECM was previously reported (1). The addition of ECM to cultures causes
cells to produce a basement membrane and maintain their original
morphology and volume. Subsequently, the cells are cultured under
‘physiological’ conditions. Matrigel is often used for the
induction of differentiation. However, Matrigel contains various
biologically active molecules, including ECM, cytokines, proteases
and inhibitors, making it difficult to relate a certain effect to a
particular agent. Accordingly, using type I collagen, we induced
gland formation by a simple culture method. After a monolayer of
cells had formed on a sheet of collagen gel, it was allowed to
either float freely in the medium or the cells were suspended in
collagen and cultured. However, neither method achieved gland
formation.
In most of the studies reported thus far,
differentiation was induced in cell lines that formed
well-differentiated transplanted tumors (1). The present study differs in that we
succeeded in inducing the differentiation of adenocarcinoma cell
lines which formed poorly differentiated tumors only in mice. This
induction was achieved by culture using type I collagen and without
any special factors. Furthermore, the cell lines in which
differentiation was induced expressed sulfolipids but not
cholesterol sulfate (Table II). It
has been reported that the induction of differentiation in
endometrial cancer using Matrigel can be blocked by anti-laminin
antibodies (10), and that laminin
binds to sulfolipids to transmit intracellular signals (11,12).
This suggests that the induction of differentiation in endometrial
cancer cells grown on Matrigel is based on an interaction between
sulfolipids and laminin in ECM. The cells used in the present study
expressed glycolipids containing one of three sulfate groups:
I3SO3-GalCer,
II3SO3-LacCer or
II3SO3Gg3Cer, while
differentiation was only induced among cells that did not express
cholesterol sulfate. These results lead to the hypothesis that
gland formation (differentiation of adenocarcinoma) is induced
through an interaction between the type I collagen substrate and
cellular glycolipids containing sulfate groups. With an increasing
expression of cholesterol sulfate on cell membranes, glycolipids
containing sulfate groups would be masked and the interaction with
type I collagen would be weakened leading the cells to become
poorly differentiated. In previous studies, cholesterol sulfate in
keratinocytes has been shown to play a role in the differentiation
of squamous epithelial cells through the finding that protein
kinase C is activated by cholesterol sulfate (13,14).
Moreover, although differentiation of the squamous and glandular
epithelia are two completely different processes, both types of
cells are of epithelial origin. Therefore, it is possible that
cholesterol sulfate is also involved in the differentiation of
glandular epithelium.
Inducing the differentiation of poorly
differentiated endometrial cancer in vivo, would result in
an improvement in the prognosis of this type of tumor. Thus, based
on the results obtained in the present study, we aim to further
investigate the induction of differentiation in endometrial cancer
cells in vivo and develop differentiation inducers for
clinical use.
Acknowledgements
This work was supported in part by Grants-in-aid for
scientific research from the Ministry of Education, Japan (No.
20591962).
References
|
1
|
Hopfer H, Rinehart CA, Kaufman DG and
Vollmer G: Basement membrane induced differentiation of HEC-1B(L)
endometrial adenocarcinoma cells affect both morphology and gene
expression. Biochem Cell Biol. 74:165–177. 1996. View Article : Google Scholar
|
|
2
|
Kubushiro K, Kojima K, Mikami M, Nozawa S,
Iizuka R, Iwamori M and Nagai Y: Menstrual cycle-associated
alteration of sulfogalactosylceramide in human uterine endometrium.
Arch Biochem Biophys. 268:129–136. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kiguchi K, Takamastu K, Tanaka J, Nozawa
S, Iwamori M and Nagai Y: Glycosphingoplipids of various human
ovarian tumors: a significantly high expression of
I3SO3 GalCer and Lewis antigen in mucinous
cyst adenocarcinoma. Cancer Res. 52:416–421. 1992.PubMed/NCBI
|
|
4
|
Kubushiro K, Tsukazaki K, Tanaka J,
Takamastu K, Kuguchi K, Mikami M, Nozawa S, Nagai Y and Iwamori M:
Human uterine endometrial adenocarcinoma: characteristic
acquirement of synthetic potentials for
II3SO3-LacCer and Ganglio series
sulfoglycosphingolipids after transfer of the cancer cells to
culture. Cancer Res. 52:803–809. 1992.
|
|
5
|
Nozawa S, Sakayori M, Ohta K, Iizuka R,
Mochizuki H, Soma M, Fujimoto J, Hata J, Iwamori M and Nagai Y: A
monoclonal antibody (MSN-1) against a newly established uterine
endometrial cancer cell lines (SNG-II) and its application to
immunohistochemıstry and flow cytometry. Am J Obstet Gynecol.
161:1079–1086. 1989.PubMed/NCBI
|
|
6
|
Ishiwata I, Nozawa S, Inoue T and Okumura
H: Development and characterization of established cell lines from
primary and metastatic region of human endometrial adenocarcinoma.
Cancer Res. 37:1777–1785. 1977.PubMed/NCBI
|
|
7
|
Nishida M, Kasahara K, Kaneko K, Iwasaki H
and Hayashi K: Establishment of a new human endometrial
adenocarcinoma cell line, Ishikawa cells, containing estrogen and
progesterone receptors. Acta Obstet Gynecol Jpn. 37:1103–1111.
1985.
|
|
8
|
Morizawa T: Establishment and
characterization of a endometrial cancer cell line. J Jpn Soc Clin
Cytol. 26:433–442. 1987. View Article : Google Scholar
|
|
9
|
Shimizu H, Inoue M and Tanizawa O:
Adoptive cellular immunotherapy to the endometrial cancer cell line
xenografts in nude mice. Gynecol Oncol. 34:195–199. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Behrens P, Meissner C, Hopfer H, Schümann
J, Tan MI, Ellerbrake N, Strunck E and Vollmer G: Laminin mediates
basement membrane induced differrentiation of HEC 1B endometrial
adenocarcinoma cells. Biochem Cell Biol. 74:875–886. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taraboletti G, Rao CN, Krutzsch HC, Liotta
LA and Roberts DD: Sulfatide-binding domain of the laminin A chain.
J Biol Chem. 265:12253–12258. 1990.PubMed/NCBI
|
|
12
|
Kawakita M, Tsuji Y, Nakata Y, Ogasawara
T, Takemura T, Isojima S and Koyama K: Progesterone treatment
decreases sulfate carbohydrate antigen on endometrial carcinoma
cells and inhibits the cell binding to laminin. Gynecol Oncol.
57:313–320. 1995. View Article : Google Scholar
|
|
13
|
Kuroki T, Ikuta T, Kashiwagi M, Kawabe S,
Ohba M, Huh N, Mizuno K, Ohno S, Yamada E and Chida K: Cholesterol
sulfate, an activator of protein kinase C mediating squamous cell
differentiation: a review. Mutat Res. 462:189–195. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kashiwagi M, Ohba M, Chida K and Kuroki T:
Protein kinase C eta (PKC eta): its involvement in keratinocyte
differentiation. J Biochem. 132:853–857. 2002. View Article : Google Scholar : PubMed/NCBI
|