Introduction
Immune-inflammatory cell response in the tumor
micro-environment is regulated by a heterogeneous family of tumor
infiltrating lymphocytes (TILs), which may have a dual function of
inhibiting or promoting tumor growth and progression (1,2). Many
reasons account for the failure of host immune systems to control
tumor growth, such as the development of tumor variants that escape
immune recognition or down-regulation of the major
histocompatibility complex class molecules. The latter involve
immune suppression mediated by T regulatory (Treg) cells and other
cells of the innate immune system with suppressor activities
(3–7).
Treg cells comprise 5–10% of the total population of
CD4+ T cells in mice and men and were primarily thought
to be critically involved in the repression of autoimmune disorders
(3–7). Treg cells are characterized by the
constitutive expression of a transmembrane protein, CD25 (the
α-chain of the receptor for interleukin-2), cytotoxic T lymphocyte
antigen-4 (CTLA-4) and forkhead transcription factor (FoxP3)
(3–9).
Previous results showed that Treg cells are potent
inhibitors of anti-tumor immune response and are associated with
poor prognosis in different types of cancer (6,10–23).
The depletion of CD4+ CD25+ Treg cells has
been shown to result in a slower tumor growth rate (13). A high prevalence of Treg cells has
been observed in the stroma of the pancreatic ductal
adenocarcinoma. These cells have also been closely correlated with
several malignant features, such as distant metastasis, high tumor
grade and advanced pathological tumor-node-metastasis stage
(18). Significant numbers of
regulatory T cells increase in the peripheral blood of patients
suffering from squamous cell carcinoma (SCC) of the head and neck
(12,24,25).
In contrast, a recent study showed a longer survival and better
locoregional control in patients with high numbers of
tumor-infiltrating CD25+ cells in head and neck SCC
(26).
CTLA-4 is a member of the immunoglobulin superfamily
and binds to the B7.1 and B7.2 costimulatory molecules (3–8). The
CTLA-4 gene encodes a receptor that is transiently expressed on
activated T cells and plays a pivotal role in immune regulation by
providing a negative feedback signal to the T cell once an immune
response has been initiated and completed (8). CTLA-4 plays a role in the suppressive
activity of CD4 and CD25 Treg against CD4 or CD8 T cells (6,8).
Specific antibodies that block CTLA-4 have been used as anti-tumor
agents, resulting in the enhancement of the anti-tumor immune
response (10,11,14,16,20,21).
On the other hand, CTLA-4 was previously demonstrated to play a
role in the destruction of tumor cells in vivo (27).
FoxP3 expression has been thought to be the most
specific marker of Treg cells. This protein is a member of the
forkhead family of transcription factors that are critically
involved in the development and function of CD25+
regulatory T cells (7,9,28,29).
In human cancer, FoxP3 expression is usually correlated with an
unfavorable course of disease and may even represent an independent
prognostic variable in terms of overall and progression-free
survival (15–18,23).
In contrast, FoxP3+ CD4+ T cells were
demonstrated to positively correlate with locoregional control in
head and neck cancer (30).
The significance of Treg cell markers in oral
squamous cell carcinoma (OSCC) and lip squamous cell carcinoma
(LSCC) has yet to be determined. This study aimed to investigate
the expression of CD4, CD25, CTLA-4 and FoxP3 in oral cavity
(OC)SCC and LSCC and their relationship with tumor aggressiveness
and prognosis.
Materials and methods
Patient population
Surgically excised specimens of primary OCSCC were
obtained from the files of the Anatomopathology and Cytopathology
Division of Araujo Jorge Hospital, Association of Cancer Combat of
Goias. The study was approved by the Ethics Committee of the
Universidade Federal de Minas Gerais and Araujo Jorge Hospital.
Our patient population consisted of 18 patients with
primary OCSCC, of which 7 were without cervical lymph node
metastasis and 11 presented cervical lymph node metastasis. Eight
other patients had LSCC. Patients with oral cavity tumors were
submitted to surgical treatment consisting of cervical lymph node
removal with microscopic evaluation. However, no patient with oral
cavity or lip tumors received radiotherapy, chemotherapy or any
other treatment prior to surgery. Clinical data (gender, age,
ethnic group, tobacco and alcohol consumption, tumor location,
extension, T and N stages) and follow-up information (clinical
outcome and survival time) were obtained from medical records.
Specimens were fixed in 10% buffered formalin (pH 7.4) and were
paraffin embedded. The microscopic features were evaluated from the
analysis of one 5 μm section of each sample, stained routinely with
hematoxylin and eosin. The sections were examined by light
microscopy to confirm the presence or absence of lymph node
metastasis, and to characterize OCSCC.
Immunohistochemistry
Sections (3 μm) from routinely processed
paraffin-embedded blocks were deparafinized and dehydrated. Dewaxed
sections were subjected to antigen retrieval (Table I). Endogenous peroxidase was blocked
by incubation with 3% hydrogen peroxide and methanol (1:1). The
slides were then incubated with the primary antibodies indicated in
Table I for 18 h at 4°C. After
washing in TBS, the sections were treated with the
EnVision®+ Dual Link System-HRP (Dako, Carpinteria, CA,
USA) or LSAB®+ system, HRP Peroxidase Kit (Dako). The
sections were then incubated in 3,3′-diaminobenzidine (DAB; Dako)
for 2–5 min. The sections were stained with Mayer’s hematoxylin and
then covered. Negative controls were obtained by the omission of
primary antibodies, which were substituted by 1% PBS-BSA and by
non-immune rabbit (X0902; Dako) or mouse (X501-1; Dako) serum.
 | Table IAntibodies and protocol of
immunohistochemical reaction. |
Table I
Antibodies and protocol of
immunohistochemical reaction.
| Antibody
(clone) | Dilution | Antigen
retrieval | Secondary
antibody |
|---|
| Anti-CD4a (0.N.52) | 1:100 | EDTA buffer (pH 8.0
for 30 min at 98°C) | EnVision |
| Anti-CD25a (N-19) | 1:50 | EDTA buffer (pH 8.0
for 30 min at 98°C) | EnVision |
| Anti-CTLA-4a (C-19) | 1:1200 | Citrate buffer (pH
6.0 for 30 min at 95°C) | Kit-LSAB |
| Anti-FoxP3a (236A/E7) | 1:400 | Citrate buffer (pH
6.0 for 30 min at 95°C) | Kit-LSAB |
| Ki67b (MM1) | 1:100 | Citrate buffer (pH
6.0 for 30 min at 95°C) | Kit-LSAB |
Cell counting and statistical
analysis
In primary OCSCC (lip and oral cavity) samples, the
densities of CD4, CD25, CTLA-4 and FoxP3 cells were determined in
relation to the total of inflammatory infiltrate adjacent to the
tumor front. To establish the proliferative tumor index, the number
of cells showing Ki67 staining was evaluated as a proportion of the
total epithelial cell population, but only the OCSCC samples were
considered. Counts were performed in 15 alternate microscopic high
power fields using an integration graticule
(4740680000000-Netzmikrometer x12.5, Carl Zeiss, Göttingen,
Germany). P<0.05 was considered to be statistically significant.
Comparative analyses between experimental groups were performed
using the non-parametric Kruskal-Wallis, followed by Dunn and/or
Mann-Whitney tests.
The influence of tumor-associated Treg markers on
the prognosis of OCSCC patients was evaluated by the Kaplan-Meier.
CD4, CD25, CTLA-4 and FoxP3 values were dichotomized by the median,
and differences in survival between groups were evaluated by the
log-rank test. Survival time was calculated from surgical resection
until the last follow-up appointment of the patient or until the
patient succumbed to the disease. Significance was set at 0.05.
Results
The main clinical features of our series of 18
patients with OCSCC and 8 patients with LSCC are summarized in
Table II. In OCSCC and LSCC,
CD4+, CD25+, CTLA-4+ and
FoxP3+ cells were distributed throughout the tumoral
stroma. The stained cells had a mononuclear appearance in the two
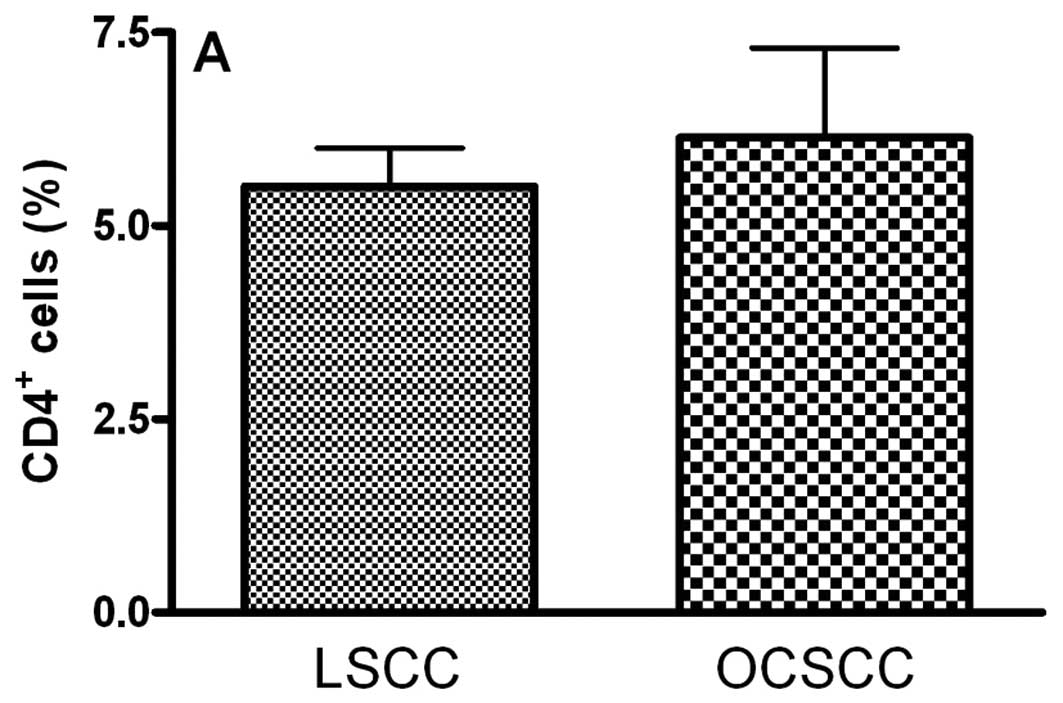
groups. We observed similar percentages of CD4- (5.51±0.50 and
6.14±1.16 for LSCC and OCSCC, respectively; P=0.826) (Fig. 1A), CD25- (6.08±0.50 and 6.79±0.22
for LSCC and OCSCC, respectively; P=0.159) (Fig. 1B) and FoxP3- (10.41±2.48 and
11.61±0.88 for LSCC and OCSCC, respectively; P=0.586) (Fig. 1D) positive cells in OCSCC and LSCC,
respectively. On the other hand, a lower percentage of
CTLA-4+ cells was observed in OCSCC (3.39±0.46) compared
with LSCC (13.12±0.26) (P=0.007; Fig.
1C).
 | Table IIMain clinical findings of patients
with OCSCC (oral cavity and lip). |
Table II
Main clinical findings of patients
with OCSCC (oral cavity and lip).
| Clinical
features | OCSCC (%) | LSCC (%) |
|---|
| Age |
| ≤60 years | 39.00 | 37.5 |
| >60 years | 61.00 | 62.5 |
| Gender |
| Male | 61.00 | 87.5 |
| Female | 39.00 | 12.5 |
| Ethnic group |
| Caucasian | 55.50 | 62.5 |
| Non-caucasian | 44.50 | 37.5 |
| Location |
| Tongue | 44.00 | 0 |
| Floor of the
mouth | 28.00 | 0 |
| Superior lip | 0 | 25.0 |
| Inferior lip | 0 | 75.0 |
| Others | 28.00 | 0 |
| Tobacco |
| Yes | 93.75 | 85.7 |
| Alcohol |
| Yes | 56.25 | 50.0 |
| T stage |
| T2 | 0 | 62.5 |
| T3–T4 | 100.0 | 37.5 |
| Clinical
outcome |
| Dead | 44.50 | 0 |
| Alive (overall
survival) | 22.62±16.52 | 69.0±43.5 |
| Survival time |
| ≥48 months | 0 | 75.0 |
| <48 months | 100.0 | 25.0 |
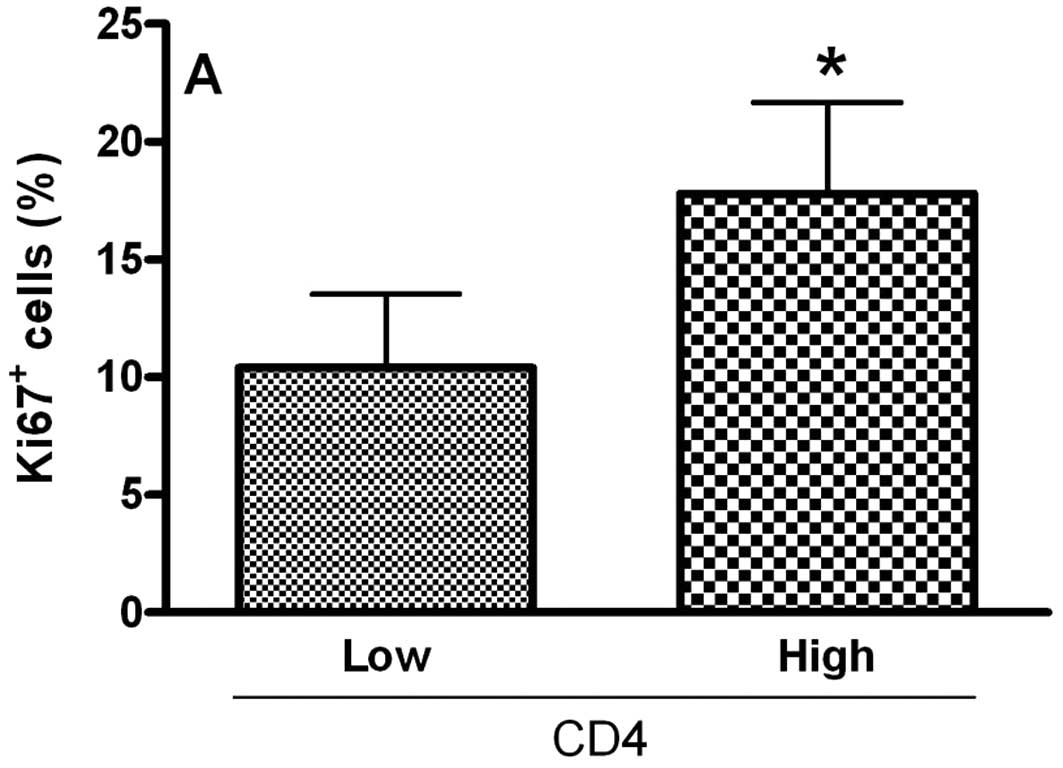
To analyze the relationship of Treg cell markers and
the proliferative index of tumoral cells in OCSCC, the values were
dichotomized into high and low CD4, CD25, CTLA-4 and FoxP3 groups
by using the median values. Samples with high counts of CD4 showed
significantly more Ki67-positive cells (P<0.05; Fig. 2A). In contrast, samples with high
counts of CTLA-4 exhibited significantly diminished proliferative
indices (P<0.05; Fig. 2C). No
significant association between the proliferative index and
CD25+ (Fig. 2B) and
FoxP3+ (Fig. 2D)
populations was achieved.
With regard to the last follow-up, the mean survival
time was 43.8 months (95% CI, 23.7–64) for patients with OCSCC
without lymph node metastasis and 34.5 months (95% CI, 17.5–51.5)
for patients with OCSCC with lymph node metastasis. The mean
follow-up was 49.75 months (95% CI, 37.61–61.89) for patients with
LSCC. For survival analysis, only patients with OCSCC were
considered; a log-rank test showed no difference in survival
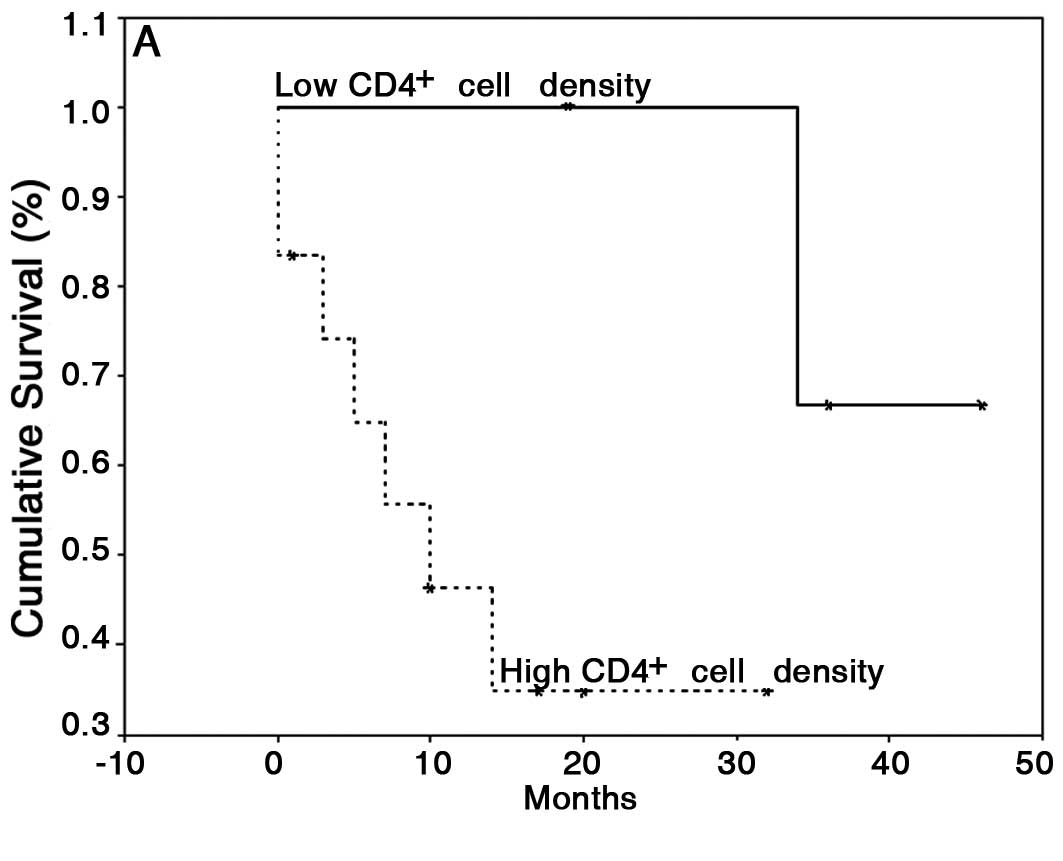
between the high and low CTLA-4 and FoxP3 groups. However, patients
with low counts of CD4+ cells showed a significant
increase in survival (42±3 months) compared with patients with high
CD4+ cell counts (15±4 months) (P=0.05; Fig. 3A). Furthermore, patients with low
counts of CD25+ cells showed a significantly lower
survival (4±2 months) compared with patients with high
CD25+ counts (33±5 months) (P=0.0008; Fig. 3B).
Discussion
Innate and adaptive immunity play important roles in
immunosurveillance and tumor destruction. Both types of effector
responses are regulated by a heterogeneous family of TILs. TILs are
present in the earlier stages of head and neck SCC with the
relative proportion of CD3+ CD4+ being higher
than or equal to CD3+ CD8+ (31). CD4+ T cells play a
central role in initiating and maintaining anticancer immune
responses (30). However,
regulatory CD4+ CD25+ T cells that express
FoxP3 have also been shown to inhibit anti-tumor effector T cells
(3–7) and may, therefore, contribute to the
growth of human tumors (6,10–23).
An increase in Treg cell populations has been observed in different
types of cancers, such as pancreas (18), ovarian tumors (17), metastatic melanoma (23) and head and neck cancer (12,24,25).
It has been reported that LSCC patients usually have
a favorable prognosis, and a low rate of regional lymph node
metastasis and mortality when compared with OCSCC (32,33).
Thus, the LSCC group was included in this study as a model of
non-metastatic and low aggressive SCC. Notably, we found a similar
percentage of CD4−, CD25− and
FoxP3+ cells in OCSCC and LSCC. In contrast, a
diminished percentage of CTLA-4+ cells was observed in
OCSCC compared with LSCC. In addition, a higher CTLA-4 expression
was significantly associated with a low tumor proliferative index.
These results may be related to the previously demonstrated
function of CTLA-4 in the destruction of tumor cells in vivo
via interaction with B7 (27). On
the other hand, much evidence indicates the importance of the
CTLA-4 blockade in the prevention of malignancy and the spread of
metastases (10,11,14,16,20,21).
Moreover, in OSCC, the A/A polymorphism of the CTLA-4 gene, which
results in a high producer phenotype, is associated with poor
survival (34). We found no
association between CTLA-4 expression and survival. These
discrepancies may be due to the significant variation in the
clinical outcome and methodologies used in the different studies.
Furthermore, the expression of CTLA-4 appears not to be exclusive
to Treg cells and may be associated with anti-tumor effector cells
(3,6,8).
The current model suggests that epithelial tumor
cells recruit Tregs to inhibit anti-tumor immunity in the tumor
microenvironment, thereby limiting the efficiency of anti-tumor
immune responses (35). On the
other hand, regulatory FoxP3+ CD4+ T cells
are positively correlated with locoregional control in head and
neck SCC (30). A possible
explanation is that Treg down-regulates the harmful inflammatory
reaction, which favors tumor progression. Furthermore, higher Treg
numbers in lymphomas predict improved survival and prognosis of
patients (35). We observed that
high numbers of CD25+ cells are positively correlated
with survival. In accordance, Loose et al verified a trend
towards longer survival and better locoregional control in patients
with a high level of tumor-infiltrating CD25+ cells
(26). It is important to consider
that the CD4+ CD25+ phenotype does not
discriminate between activated and regulatory T cells. A
significantly higher frequency of double-positive CD25+
FoxP3+ cells has recently been observed in OCSCC in
relation to control lymphoepithelial tissue, but no association
between these cells and clinical parameters was verified (36).
The clinical significance of CD4+ T cells
inside tumors is controversial. A large number of
tumor-infiltrating CD4+ cells was found to be an
independent favorable prognostic factor in esophageal squamous cell
carcinomas (37). On the other
hand, an increased level of tumor-infiltrating CD4+
cells is associated with poor outcome in renal cell carcinoma
(38). In the present study,
patients with low counts of CD4+ cells presented a
significantly increased survival in relation to patients with high
CD4+ cell counts. In accordance, samples with an
elevated CD4+ cell percentage exhibited a high tumor
proliferative index. Our results suggest a regulatory/suppressive
role for these cells in OCSCC as previously shown (39). However, considering the
heterogeneity of the CD4+ T cell phenotype, a further
definition of the role of these subsets in OCSCC (using double or
triple staining), as well as subsequent confirmation of activity
in vitro is needed.
FoxP3 expression is correlated with the development
and function of Treg. FoxP3 is a member of the forkhead family of
transcription factors that are critically involved in the
development and function of CD25+ regulatory T cells
(7,9,29). The
expression of FoxP3 can be transiently induced in human non-Treg
cells by activation through the T-cell receptor (28,29).
FoxP3 expression was thought to be restricted to the T-cell
lineage, but recently FoxP3 expression was detected in melanoma
(40) and other types of tumor
cells (41). We did not, however,
verify FoxP3 expression in neoplastic epithelial cells. FoxP3
expression can be influenced by different cytokines such as TGF-β,
IL-10 or IL-2 (7,9,29).
Notably, a slight increase in IL-10 concomitant with FoxP3
expression was observed in OCSCC samples (data not shown).
In conclusion, our results suggest an association of
CD4 expression with poor prognosis, while CD25 expression is
related to a favorable prognosis of OCSCC. These findings may
result from the heterogeneity of TIL subsets that display
antagonistic and complex roles in tumor immune cell response.
Acknowledgements
The authors thank the Anatomopathology and
Cytopathology Division of the Araujo Jorge Hospital and the
Association of Cancer Combat of Goias, Goiânia, Brazil. This work
was supported by grants from the Conselho Nacional de
Desenvolvimento Científico e Tecnológico (CNPq) (grants
401305/2005-8 and 471878/2006-5). T.A.S. is a research fellow of
CNPq.
References
|
1
|
Hanahan D, Lanzavecchia A and Mihich E:
Fourteenth Annual Pezcoller Symposium: the novel dichotomy of
immune interactions with tumors. Cancer Res. 63:3005–3008.
2003.PubMed/NCBI
|
|
2
|
Oliveira-Neto HH, Leite AF, Costa NL, et
al: Decrease in mast cells in oral squamous cell carcinoma:
possible failure in the migration of these cells. Oral Oncol.
43:484–490. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akbar AN, Taams LS, Salmon M and
Vukmanovic-Stejic M: The peripheral generation of CD4+
CD25+ regulatory T cells. Immunology. 109:319–325. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang HY, Lee DA, Peng G, et al:
Tumor-specific human CD4+ regulatory T cells and their ligands:
implications for immunotherapy. Immunity. 20:107–118. 2004.
|
|
5
|
Wei WZ, Morris GP and Kong YC: Anti-tumor
immunity and autoimmunity: a balancing act of regulatory T cells.
Cancer Immunol Immunother. 53:73–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang RF: Regulatory T cells and innate
immune regulation in tumor immunity. Springer Semin Immunopathol.
28:17–23. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamaguchi T and Sakaguchi S: Regulatory T
cells in immune surveillance and treatment of cancer. Semin Cancer
Biol. 16:115–123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen L: Co-inhibitory molecules of the
B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol.
4:336–347. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen W, Jen W, Hardegen N, et al:
Conversion of peripheral CD4+ CD25 naive T cells to
CD4+ CD25+ regulatory T cells by TGF-β
induction of transcription factor FoxP3. J Exp Med. 198:1875–1886.
2003.
|
|
10
|
Hurwitz AA, Yu TF, Leach DR and Allison
JP: CTLA-4 blockade synergizes with tumor-derived
granulocyte-macrophage colony-stimulating factor for treatment of
an experimental mammary carcinoma. Proc Natl Acad Sci USA.
95:10067–10071. 1998. View Article : Google Scholar
|
|
11
|
Hurwitz AA, Foster BA, Kwon ED, et al:
Combination immunotherapy of primary prostate cancer in a
transgenic mouse model using CTLA-4 blockade. Cancer Res.
60:2444–2448. 2000.PubMed/NCBI
|
|
12
|
Tartour E, Mosseri V, Jouffroy T, et al:
Serum soluble interleukin-2 receptor concentrations as an
independent prognostic marker in head and neck cancer. Lancet.
357:1263–1264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones E, Dahm-Vicker M, Simon AK, et al:
Depletion of CD25+ regulatory cells results in
suppression of melanoma growth and induction of autoreactivity in
mice. Cancer Immun. 2:1–12. 2002.
|
|
14
|
Phan GQ, Yang JC, Sherry RM, et al: Cancer
regression and autoimmunity induced by cytotoxic T
lymphocyte-associated antigen 4 blockade in patients with
metastatic melanoma. Proc Natl Acad Sci USA. 100:8372–8377. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Curiel TJ, Coukos G, Zou L, et al:
Specific recruitment of regulatory T cells in ovarian carcinoma
fosters immune privilege and predicts reduced survival. Nat Med.
10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghaderi A, Yeganeh F, Kalantari T, et al:
Cytotoxic T lymphocyte antigen-4 gene in breast cancer. Breast
Cancer Res Treat. 86:1–7. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolf D, Wolf AM, Rumpold H, et al: The
expression of the regulatory T cell-specific forkhead box
transcription factor FoxP3 is associated with poor prognosis in
ovarian cancer. Clin Cancer Res. 11:8326–8331. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hiraoka N, Onozato K, Kosuge T and
Hirohashi S: Prevalence of FoxP3+ regulatory T cells
increases during the progression of pancreatic ductal
adenocarcinoma and its premalignant lesions. Clin Cancer Res.
12:5423–5434. 2006.
|
|
19
|
Larkin J, Tangney M, Collins C, et al:
Oral immune tolerance mediated by suppressor T cells may be
responsible for the poorer prognosis of foregut cancers. Med
Hypotheses. 66:541–544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Downey SG, Klapper JA, Smith FO, et al:
Prognostic factors related to clinical response in patients with
metastatic melanoma treated by CTL-associated antigen-4 blockade.
Clin Cancer Res. 13:6681–6688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fecci PE, Ochiai H, Mitchell DA, et al:
Systemic CTLA-4 blockade ameliorates glioma-induced changes to the
CD4+ T cell compartment without affecting regulatory
T-cell function. Clin Cancer Res. 13:2158–2167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu J, Xu D, Liu Z, et al: Increased
regulatory T cells correlate with CD8 T-cell impairment and poor
survival in hepatocellular carcinoma patients. Gastroenterology.
132:2328–2339. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miracco C, Mourmouras V, Biagioli M, et
al: Utility of tumour-infiltrating CD25+
FoxP3+ regulatory T cell evaluation in predicting local
recurrence in vertical growth phase cutaneous melanoma. Oncol Rep.
18:1115–1122. 2007.PubMed/NCBI
|
|
24
|
Strauss L, Bergmann C and Whiteside TL:
Functional and phenotypic characteristics of D4+
CD25-high FoxP3+ Treg clones obtained from peripheral
blood of patients with cancer. Int J Cancer. 121:2473–2483.
2007.PubMed/NCBI
|
|
25
|
Chikamatsu K, Sakakura K, Whiteside TL and
Furuya N: Relationships between regulatory T cells and
CD8+ effector populations in patients with squamous cell
carcinoma of the head and neck. Head Neck. 29:120–127.
2007.PubMed/NCBI
|
|
26
|
Loose D, Signore A, Bonanno E, et al:
Prognostic value of CD25 expression on lymphocytes and tumor cells
in squamous-cell carcinoma of the head and neck. Cancer Biother
Radiopharm. 23:25–33. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bai XF, Liu J, May KF Jr, Guo Y, Zheng P
and Liu Y: B7-CTLA4 interaction promotes cognate destruction of
tumor cells by cytotoxic T lymphocytes in vivo. Blood.
99:2880–2889. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Ioan-Facsinay A, van der Voort EI,
Huizinga TW and Toes RE: Transient expression of FoxP3 in human
activated nonregulatory CD4+ T cells. Eur J Immunol.
37:129–138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zheng Y and Rudensky AY: Foxp3 in control
of the regulatory T cell lineage. Nat Immunol. 8:457–462. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Badoual C, Hans S, Rodriguez J, et al:
Prognostic value of tumor-infiltrating CD4+ T-cell
subpopulations in head and neck cancers. Clin Cancer Res.
12:465–472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Böheim K, Denz H, Böheim C, Glassl H and
Huber H: An immunohistologic study of the distribution and status
of activation of head and neck tumor infiltrating leukocytes. Arch
Otorhinolaryngol. 244:127–132. 1987.PubMed/NCBI
|
|
32
|
Antunes JLF, Biazevic MGH, de Araujo ME,
Tomita NE, Chinellato LEM and Narvai PC: Trends and spatial
distribution of oral cancer mortality in São Paulo, Brazil,
1980–1998. Oral Oncol. 37:345–350. 2001.
|
|
33
|
Vartanian JG, Carvalho AL, Filho MJA,
Júnior MH, Magrin J and Kowalski LP: Predictive factors and
distribution of lymph node metastasis in lip cancer patients and
their implications on the treatment of the neck. Oral Oncol.
40:223–227. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wong YK, Chang KW, Cheng CY and Liu CJ:
Association of CTLA-4 gene polymorphism with oral squamous cell
carcinoma. J Oral Pathol Med. 35:51–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ke X, Wang J, Li L, Chen IH, Wang H and
Yang XF: Roles of CD4+xCD25(high) FoxP3+
Tregs in lymphomas and tumors are complex. Front Biosci.
13:3986–4001. 2008.
|
|
36
|
Schwarz S, Butz M, Morsczeck C, Reichert
TE and Driemel O: Increased number of CD25 FoxP3 regulatory T cells
in oral squamous cell carcinomas detected by chromogenic
immunohistochemical double staining. J Oral Pathol Med. 37:485–489.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cho Y, Miyamoto M, Kato K, et al:
CD4+ and CD8+ T cells cooperate to improve
prognosis of patients with esophageal squamous cell carcinoma.
Cancer Res. 63:1555–1559. 2003.
|
|
38
|
Bromwich EJ, McArdle PA, Canna K, et al:
The relationship between T-lymphocyte infiltration, stage, tumour
grade and survival in patients undergoing curative surgery for
renal cell cancer. Br J Cancer. 89:1906–1908. 2003. View Article : Google Scholar
|
|
39
|
Agarwal A, Rani M, Saha GK, et al:
Disregulated expression of the Th2 cytokine gene in patients with
intraoral squamous cell carcinoma. Immunol Invest. 32:17–30. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ebert LM, Tan BS, Browning J, et al: The
regulatory T cell-associated transcription factor FoxP3 is
expressed by tumor cells. Cancer Res. 68:3001–3009. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Karanikas V, Speletas M, Zamanakou M, et
al: FoxP3 expression in human cancer cells. J Transl Med. 6:19–24.
2008. View Article : Google Scholar : PubMed/NCBI
|