Introduction
The term epigenetics defines the heritable changes
in gene expression that are not coded in the DNA sequence.
Epigenetic changes are characterized mainly by DNA methylation and
histone deacethylation, preventing transcription of the target
gene. DNA methylation occurs when methyl groups are added to
cytosine nucleotides in specific areas of the gene by the enzyme
DNA methyltransferase (DNMT) (1). A
number of DNMTs have been identified in mammals. DNMT3a and DNMT3b
are mainly involved in de novo methylation, whereas DNMT1
acts mainly as a maintenance methyltransferase (2–4).
Epigenetic silencing of the gene expression by promoter CpG island
hypermethylation was shown to be important in the formation of a
variety of cancer types including oral squamous cell carcinoma
(5). DNMTs were also found to be
overexpressed in tumorigenic cells (6) and in certain human tumours (7,8).
Genetic alterations were already observed in certain
odontogenic cysts and tumours (9,10).
Although a recent study showed the methylation of tumour suppressor
genes in odontogenic keratocysts (OKCs) (11), the expression of DNMTs has yet to be
investigated in odontogenic tumours. We investigated the expression
of DNMT3a and DNMT1 in radicular cysts, OKC, ameloblastomas and
adenomatoid odontogenic tumours (AOT).
Materials and methods
Tissue samples
Formalin-fixed and paraffin-embedded tissue samples
of eight radicular cysts, 10 OKC, eight AOT, 16 ameloblastomas
(eight plexiform and eight follicular histologic types) and eight
samples of normal mucosae were included in the study. The age,
gender and location of the lesions in each group are summarized in
Table I.
 | Table IClinical data of the cases included in
the study. |
Table I
Clinical data of the cases included in
the study.
| | Gender | Location |
|---|
| |
|
|
|---|
| Age (mean,
range) | Female | Male | Mandible | Maxilla |
|---|
| OKC (n=10) | 18 (06–36) | 05 | 05 | 06 | 04 |
| AOT (n=08) | 24 (13–53) | 06 | 02 | 04 | 04 |
| Radicular cysts
(n=08) | 25 (14–76) | 03 | 05 | 04 | 04 |
| Plexiform
ameloblastomas (n=08) | 30 (12–51) | 03 | 05 | 06 | 02 |
| Follicular
ameloblastomas (n=08) | 21 (08–27) | 04 | 04 | 08 | 00 |
Immunohistochemistry
Tissue sections (4 μm) were stained with DNMT1
antiserum (diluted 1:150, clone 60B1220.1, Imgenex, USA) and DNMT3a
(diluted 1:100, clone 64B1446, Imgenex). Heat-induced epitope
retrieval was performed with 10 mM citrate buffer, pH 6.0, for 30
min in a steamer at 96°C. After dilution in 0.5% BSA for 18 h at
4°C primary antiserum incubation was performed, followed by
conventional streptavidin peroxidase method (LSAB, Dako,
Carpinteria, CA, USA). Peroxidase activity was developed with DAB
(Sigma, St. Louis, MI, USA) with timed monitoring using a positive
control sample. For each reaction, a negative control was included.
The sections were counterstained with hematoxylin.
Analysis
Only sections containing sufficient epithelium to
assess the antibody reactivity were considered for this study. Two
experienced independent pathologists examined multiple fields and
made a descriptive analysis of each case. The samples were
individually scored as Grade 1 (<25% of positive cells) or Grade
2 (>25% of positive cells). The score of the nuclear staining
was performed separately from the cytoplasm.
Results
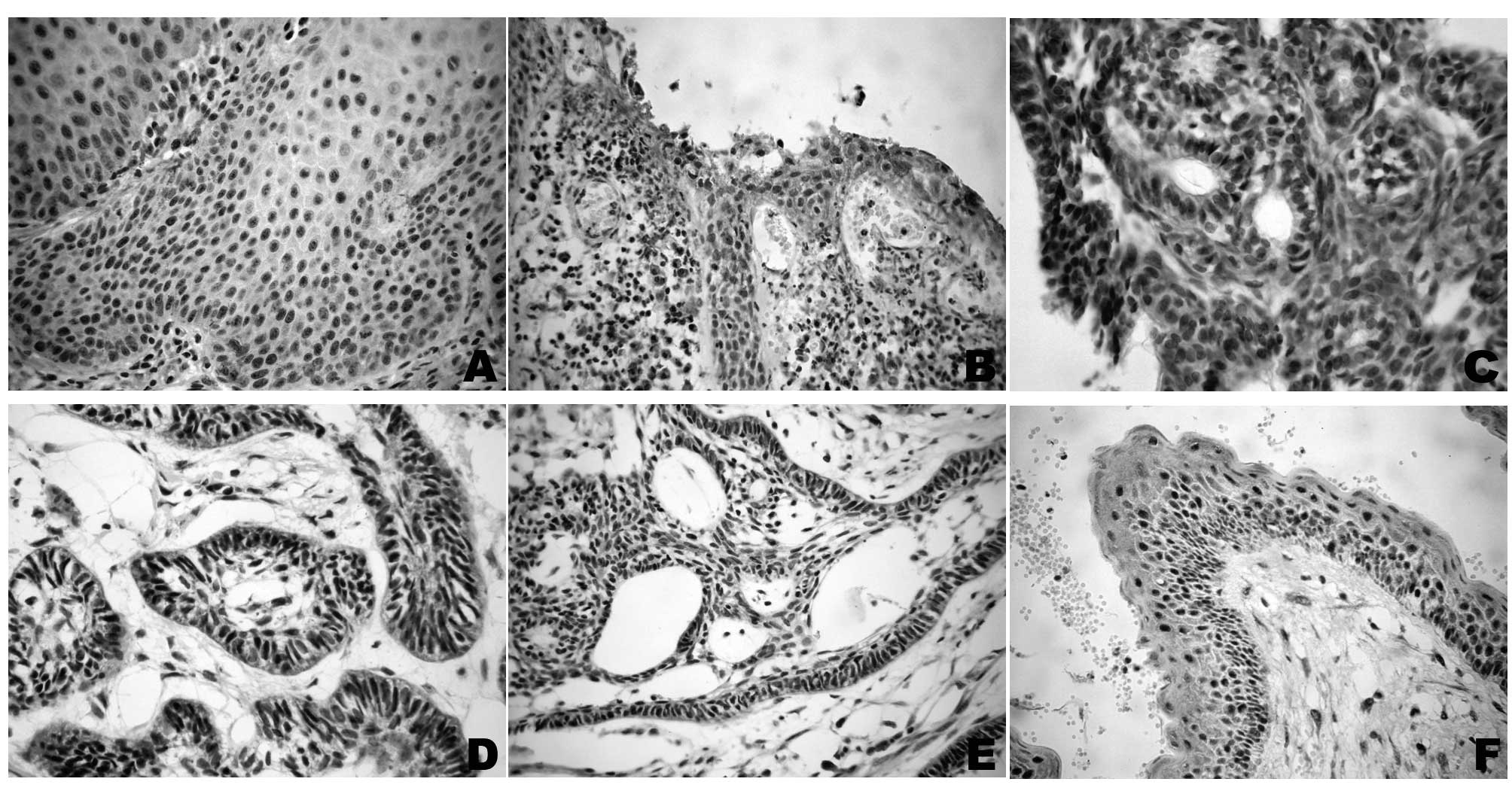
DNMT1 and DNMT3a immunopositivity was detected in
the nucleus and/or cytoplasm. The OKC, AOT, radicular cyst and
ameloblastoma cases showed a widespread nuclear and cytoplasmic
immunopositivity for DNMT1 in all cell layers, reproducing the
pattern observed in the normal oral mucosa samples (Grade 2)
(Fig. 1).
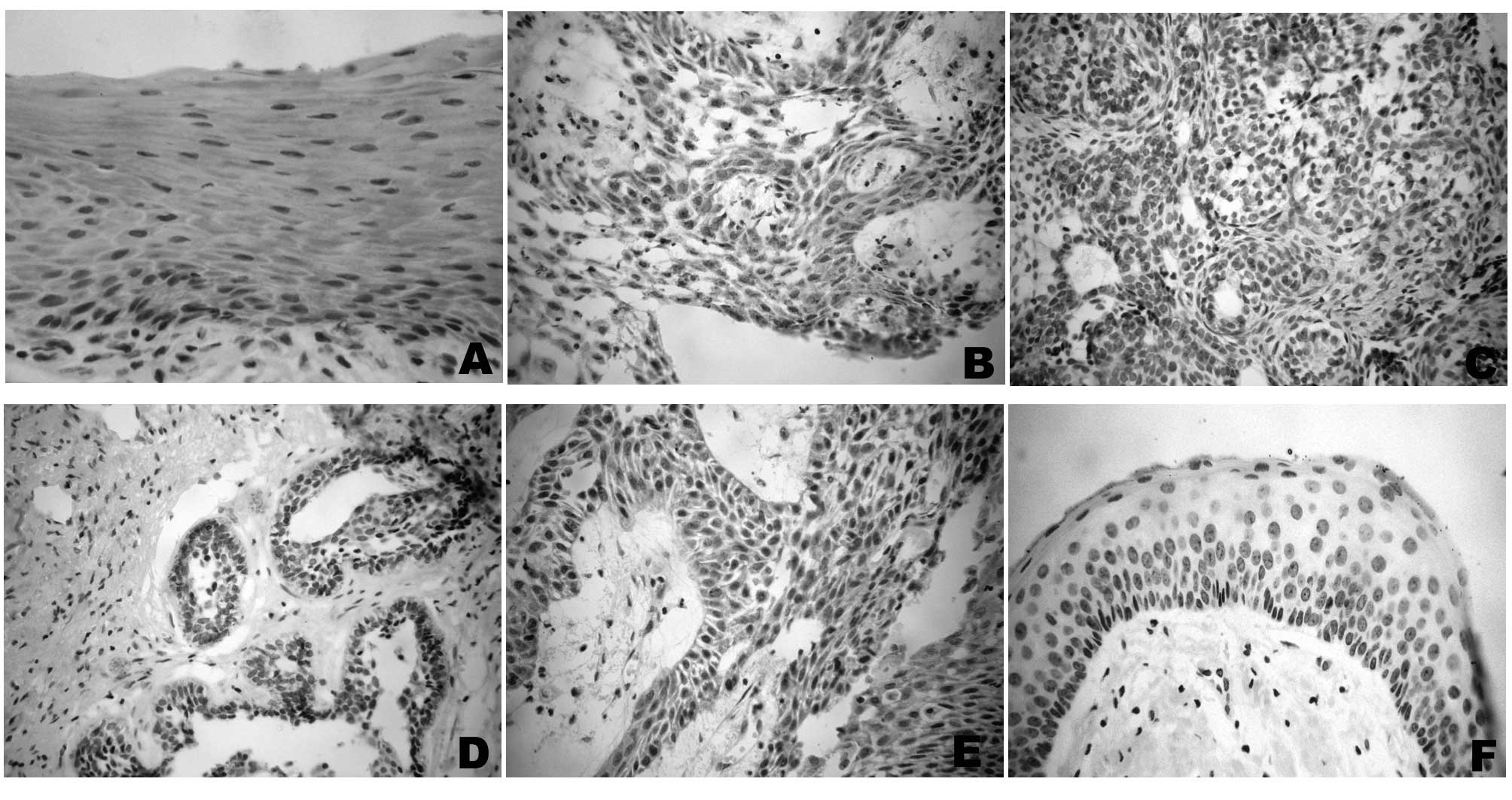
All of the radicular cyst samples, 13 out of 16
ameloblastoma cases and some of the OKC and AOT cases showed a
positive cytoplasmatic reaction for DNMT3a (Grade 2), but no
specific pattern of positivity was observed on peripheral, central,
basal or suprabasal layers (Fig.
2). Nuclear positivity for this antibody was found only in the
suprabasal cell layers of three OKC samples (Grade 2) (Table II). In total, 28 (66.6%) out of 42
odontogenic cysts and tumours showed positive nuclear and/or
cytoplasmic staining for DNMT3a. The normal oral mucosa samples
exhibited negative staining for DNMT3a.
 | Table IIImmunoreactivity for DNMT3a (no., %)
in odontogenic keratocysts, adenomatoid odontogenic tumours,
radicular cysts, plexiform ameloblastomas, follicular
ameloblastomas and normal oral mucosae. |
Table II
Immunoreactivity for DNMT3a (no., %)
in odontogenic keratocysts, adenomatoid odontogenic tumours,
radicular cysts, plexiform ameloblastomas, follicular
ameloblastomas and normal oral mucosae.
| Immunopositivity for
DNMT3a (no., %) | Nuclear and
cytoplasmic | Nuclear | Cytoplasmic | Absence of
immunoreaction |
|---|
| OKC (n=10) | 02 (20)a | 01 (10)a | 01 (10.0)a | 06 (60.0) |
| AOT (n=08) | 00 (0) | 00 (0) | 03 (37.5)a | 05 (62.5) |
| Radicular cysts
(n=08) | 00 (0) | 00 (0) | 08 (100.0)a | 00 (0.0) |
| Plexiform
ameloblastomas (n=08) | 00 (0) | 00 (0) | 07 (87.5)a | 01 (12.5) |
| Follicular
ameloblastomas (n=08) | 00 (0) | 00 (0) | 06 (75.0)a | 02 (25.0) |
| Normal oral mucosae
(n=08) | 00 (0) | 00 (0) | 00 (0.0) | 08 (100.0) |
Discussion
In the present report, we described the
immunoexpression pattern of two catalytically active DNMTs in a
group of odontogenic cysts and tumours, as well as in normal oral
mucosae. To the best of our knowledge, no previous report exists
about the DNMT1 and DNMT3a expression pattern in odontogenic cysts
and tumours.
Hypermethylation of tumour suppressor genes that are
normally unmethylated correlates with loss of expression in cancer
cell lines and primary tumours. Overexpression of DNMT1 and DNMT3a
was reported in human tumours (12). Previous studies a revealed
significant overexpression of DNMT1 in tumour tissues (6,13,14),
while others found little increase of this enzyme in tumours when
compared to matched normal tissue (12,15).
Moreover, murine DNMT1 levels were shown to vary during the cell
cycle and correlate with cell proliferation (15,16).
Although DNMT1 is considered to be a maintenance form of DNMT that
copies methylation patterns after DNA replication, it has been
proposed that in human cancers unknown factors target this enzyme
in unmethylated substrate DNA. Therefore, some authors believe that
DNMT1 protein overexpression results in de novo DNA
hypermethylation during carcinogenesis (14). The cases included in our study
showed a widespread nuclear and cytoplasmic immunopositivity for
DNMT1 in all cell layers, as did the normal oral mucosa samples.
Since DNMT1 was found in normal oral mucosa cells, it may not be
relevant to the development of odontogenic cysts and tumours.
DNMT3a expression was shown to be slightly increased
in tumours from bladder, colon, kidney and pancreas when compared
to normal tissue (12,15). We found four different expression
patterns of this protein: negative, cytoplasmic, both cytoplasmic
and nuclear, as well as only nuclear staining. The normal oral
mucosa fragments were DNMT3a-negative. Some cases of OKC, AOT,
radicular cyst and ameloblastoma showed a cytoplasmic reaction for
this protein. Although the exact meaning of this cytoplasmic
immunostaining is unknown, it suggests that epigenetic alterations
occur in odontogenic cysts and tumours. A previous study suggested
that P16 hypermethylation is involved in the malignant
transformation of ameloblastoma (17), but epigenetics has been the focus of
few studies regarding the pathogenesis of odontogenic tumours.
Nuclear expression of DNMT3a was observed in only
three samples of OKC, mainly in the suprabasal layers. It has been
demonstrated in hepatocarcinogenesis, the sequential decrease in
cytoplasmic immunoreactivity for DNMT3a, as well as the concurrent
increase in nuclear DNMT3a in high-grade dysplasia and carcinomas
when compared to non-neoplastic and low-grade dysplasia (18). The biological significance of
nuclear staining has yet to be established, but since methylation
occurs inside the nuclei, we hypothesize that the nuclear
expression of DNMT3a would mediate DNA hypermethylation events on
OKC epithelial cells. Interestingly, a recent study performed by
our group demonstrated the hypermethylation of some tumour
suppressor genes in OKC (11).
In conclusion, our study shows an increased
expression of DNMT3a in odontogenic cysts and tumours, supporting
the theory that epigenetic mechanisms are relevant to the
development of such lesions.
Acknowledgements
This study was supported by grants from Milênio
CNPq/MCT (Conselho Nacional de Desenvolvimento Científico e
Tecnológico) and FAPEMIG (Fundação de Amparo à Pesquisa do Estado
de Minas Gerais), Brazil. Dr R.S. Gomez is a research fellow of
CNPq.
References
|
1
|
Shaw R: The epigenetics of oral cancer.
Int J Oral Maxillofac Surg. 35:101–108. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bestor TH and Ingram VM: Two DNA
methyltransferases from murine erythroleukemia cells: purification,
sequence specificity, and mode of interaction with DNA. Proc Natl
Acad Sci USA. 80:5559–5563. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okano M, Xie S and Li E: Cloning and
characterization of a family of novel mammalian DNA (cytosine-5)
methyltransferases. Nat Genet. 19:219–220. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pradhan S, Bacolla A, Wells RD and Roberts
RJ: Recombinant human DNA (cytosine-5) methyltransferase. I
Expression, purification, and comparison of de novo and maintenance
methylation. J Biol Chem. 274:33002–33010. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ha PK and Califano JA: Promoter
methylation and inactivation of tumour-suppressor genes in oral
squamous-cell carcinoma. Lancet Oncol. 7:77–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kautiainen TL and Jones PA: DNA
methyltransferase levels in tumorigenic and nontumorigenic cells in
culture. J Biol Chem. 261:1594–1598. 1986.PubMed/NCBI
|
|
7
|
Sun L, Hui AM, Kanai Y, Sakamoto M and
Hirohashi S: Increased DNA methyltransferase expression is
associated with an early stage of human hepatocarcinogenesis. Jpn J
Cancer Res. 88:1165–1170. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Marzo AM, Marchi VL, Yang ES,
Veeraswamy R, Lin X and Nelson WG: Abnormal regulation of DNA
methyltransferase expressing during colorectal carcinogenesis.
Cancer Res. 59:3855–3860. 1999.PubMed/NCBI
|
|
9
|
Barreto DC, Gomez RS, Bale AE, Boson WL
and De Marco L: PTCH gene mutations in odontogenic keratocysts. J
Dent Res. 79:1418–1422. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Perdigão PF, Gomez RS, Pimenta FJGS and De
Marco L: Ameloblastin gene (AMBN) mutations associated with
epithelial odontogenic tumours. Oral Oncol. 40:841–846.
2004.PubMed/NCBI
|
|
11
|
Moreira PR, Guimarães MM, Guimarães ALS,
Diniz MG, Gomes CC, Brito JAR and Gomez RS: Methylation of P16,
P21, P27, RB1 and P53 genes in odontogenic keratocysts. J Oral
Pathol Med. 38:99–103. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Robertson KD, Uzvolgyi E, Liang G,
Talmadge C, Sumegi J, Gonzales FA and Jones PA: The human DNA
methyltransferases (DNMTs) 1, 3a and 3b: coordinate mRNA expression
in normal tissues and overexpression in neoplasms. Nucleic Acids
Res. 27:2291–2298. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Etoh T, Kanai Y, Ushijima S, Nakagawa T,
Nakanishi Y, Sasako M, Kitano S and Hirohashi S: Increased DNA
methyltransferase 1 (DNMT1) protein expression correlates
significantly with poorer tumour differentiation and frequent DNA
hypermethylation of multiple CpG islands in gastric cancers. Am J
Pathol. 164:689–699. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peng DF, Kanai Y, Sawada M, Ushijima S,
Hiraoka N, Kosuge T and Hirohashi S: Increased DNA
methyltransferase 1 (DNMT1) protein expression in precancerous
conditions and ductal carcinomas of the pancreas. Cancer Sci.
96:403–408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee PJ, Washer LL, Law DJ, Boland CR,
Horon IL and Feinberg AP: Limited up-regulation of DNA
methyltransferase in human colon cancer reflecting increased cell
proliferation. Proc Natl Acad Sci USA. 93:10366–10370. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szyf M, Bozovic V and Tanigawa G: Growth
regulation of mouse DNA methyltransferase gene expression. J Biol
Chem. 266:10027–10030. 1991.PubMed/NCBI
|
|
17
|
Abiko Y, Nagayasu H, Takeshima M, Yamazaki
M, Nishimura M, Kusano K, Kitajo H, Saitoh M, Kawakami T, Chiba I
and Kaku T: Ameloblastic carcinoma ex ameloblastoma: report of a
case-possible involvement of CpG island hypermethylation of the p16
gene in malignant transformation. Oral Surg Oral Med Oral Pathol
Oral Radiol Endod. 103:72–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi MS, Shim YH, Hwa JY, Lee SK, Ro JY,
Kim JS and Yu E: Expression of DNA methyltransferases in multistep
hepatocarcinogenesis. Hum Pathol. 34:11–17. 2003. View Article : Google Scholar : PubMed/NCBI
|