Introduction
The effectiveness of chemotherapy directly affects
the prognosis of advanced lung cancer. Adjuvant chemotherapy is
expected to contribute to the improvement of the prognosis of
patients who undergo curative surgical treatments. The clinical
outcome of chemotherapy against non-small cell lung cancer (NSCLC)
has improved since new drugs have been developed and their
combination therapies have been established. However, these drugs
are not always equally efficacious in all NSCLC patients. A drug
that has curative effects on some patients has sometimes been
observed to cause only side effects in other patients. These
findings suggest the need for the appropriate selection of drugs
separately for each individual patient. Certain in vitro
chemosensitivity tests have been established for the identification
of an effective anticancer drug for individual cases (1–3), and
the efficacy of these assays has been described in many clinical
reports on lung cancer (4–9) and other malignancies (10–15).
Anticancer drugs are expected to be effective not
only against primary but also against metastatic lesions.
Specifically, adjuvant chemotherapy, after complete resection of
lung cancer, targets putative dormant metastasis. However, in
certain clinical cases the chemosensitivity of metastatic lesions
differs from that of primary lesions. This appears to be normal
since the pathological features of the primary lesion and lymph
node metastasis (LM) of lung cancer are frequently different from
each other. In such cases, chemosensitivity testing for the primary
lesion may be useless in controlling dormant metastatic
lesions.
We performed chemosensitivity tests for both primary
lesions and LMs obtained during the surgery of NSCLC patients and
compared them in order to evaluate the test for surgically resected
metastatic lesions.
Materials and methods
Patients and samples
Operative specimens were obtained from 13 patients
with NSCLC [6 with squamous cell carcinoma (SQ) and 7 with
adenocarcinoma (AD)] whose lymph nodes were confirmed to be
positive for metastasis by frozen section. All of the patients
consented to participate in this study.
A block of cancer tissue, 5–10 mm in diameter, was
obtained with aseptic manipulation immediately following resection,
and stored at 4°C (primary lesion; PL). A lymph node obtained from
the same patient was swollen and metastasis was suspected. The
lymph node was divided into three sections; one was stored in the
frozen section and was confirmed to be cancerous, and the two
remaining sections, which were >0.5 mm3 in size, were
stored at 4°C (LM).
In vitro chemosensitivity test
We initiated the collagen gel droplet-embedded
culture drug sensitivity test (CD-DST) within 36 h after resection.
This is one of the growth assays described by Kobayashi et
al (3) as a chemosensitivity
test. Each sample was minced finely using a scalpel or razor blade
and digested in a cell dispersion enzyme solution (EZ; Nitta
Gelatin Inc., Osaka, Japan) for 2 h. The dispersed cancer cells
were treated with ethylene glycol tetra-acetic acid (EGTA)-trypsin
and filtered through a 200-μm nylon mesh. The cells were then
incubated in a collagen gel-coated flask (CG-flask; Nitta Gelatin
Inc.) containing preculture medium (PCM-1; Nitta Gelatin Inc.) with
10% fetal bovine serum (FBS) at 37°C in 5% CO2
overnight.
Only the viable cancer cells that adhered to the
collagen gel were collected, treated again with EGTA-trypsin and
filtered through a 125-μm nylon mesh.
Type I collagen (Cell Matrix Type CD; Nitta Gelatin
Inc.), 10X F-12 medium and reconstruction buffer were added to ice
water at a ratio of 8:1:1. The prepared cancer cell suspension was
added to the mixed collagen solution with a final density of
1×105 cells/ml. Three drops of the collagen-cell mixture
(30 μl/drop) were placed in each well of 6-well plates and in a
35-mm dish and were left to set at 37°C in a CO2
incubator. The final concentration was 3×103
cells/droplet. One hour later, 3 ml of DF medium containing 10% FBS
(DF-10) was overlaid on each well and 6 ml on the 35-mm dish and
incubated in a CO2 incubator at 37°C overnight.
At the 0-time control, the drops in the 35-mm dish
were stained with neutral red and fixed with 10% formalin and
dried. In each well of the 6-well plates, the anticancer drugs were
added at final concentrations (Table
I) and incubated for 24 h. Following the removal of the medium
containing the anticancer drugs, each well was rinsed twice,
overlaid with serum-free culture medium (PCM-2; Nitta Gelatin Inc.)
and incubated further for 7 days. Only 5-fluorouracil (5-FU) was
left in the culture media for 7 days. Control drops were also
cultured for 7 days without the drugs under the same condition.
 | Table IThe exposure condition of anticancer
drugs. |
Table I
The exposure condition of anticancer
drugs.
| Drugs | Concentration
(μg/ml) | Exposure time |
|---|
| 5-FU | 1.00 | 7 days |
| CDDP | 0.20 | 24 h |
| GEM | 0.03 | 24 h |
| TXT | 0.10 | 24 h |
| VNR | 0.05 | 24 h |
| SN38 | 0.10 | 24 h |
After the 7-day culture period, neutral red was
added to each well at a final concentration of 50 μg/ml, and viable
colonies in the droplets were stained for 1–2 h. Each droplet was
fixed with 10% formalin, washed in water and dried. A video
microscope (Pico Scopeman; Moritex, Tokyo, Japan), a grayscale
image digitizer (LG-3; Scion Corp., MD, USA), a personal computer
(Apple Power Macintosh G3; Tokyo, Japan) and modification of the
NIH Image Macro-program (Primage; Toshiba Tech Corp., Tokyo, Japan)
were used to measure and quantify the amount of neutral red dye
taken up by the viable cells in the droplets.
When the ratio of control (7-day culture without
drug) to the 0-time control was >0.8, the case was regarded as
assessable. When the growth rate, which was determined by the T/C
ratio (T, the signal for the viable cells in the treated group and
C, the signal in the control on day 7) was <50%, we regarded the
case as sensitive to the drug.
Statistical analysis
Statistical significance was determined using the
paired Student’s t-test. Test analyses were performed on SPSS 14.0,
statistical software (SPSS Japan Inc., Tokyo, Japan).
Results
Differences in the chemosensitivity
between PLs and LMs
In 3 of the 13 cases, differences in the
chemosensitivity against the drugs were observed between the PL and
LM, while the chemosensitivity was exactly the same for all
anticancer drugs in the remaining 4 cases (2 cases of SQ, 2 of AD;
Table II). For docetaxel,
vinorelbine and SN38 (active metabolite of irinotecan), the
chemosensitivity between the PL and LM was approximately the same.
Specifically, the chemosensitivity to vinorelbine in the AD cases
was identical (Table IIB).
 | Table IIChemosensitivity of the squamous cell
carcinoma and adenocarcinoma cases. |
Table II
Chemosensitivity of the squamous cell
carcinoma and adenocarcinoma cases.
| A, Six cases of
squamous cell carcinoma |
|---|
|
|---|
| Age | Gender | Stage | Origin of cells | 5-FU | CDDP | GEM | TXT | VNR | SN38 |
|---|
| 75 | M | IIIA | PL | + | − | + | + | + | + |
| | | LM | − | − | − | − | + | − |
| 73 | M | IIIA | PL | + | + | + | + | + | + |
| | | LM | + | − | − | + | − | + |
| 62 | M | IIIB | PL | − | − | − | − | − | − |
| | | LM | − | + | − | − | + | − |
| 78 | M | IIA | PL | + | − | − | − | − | + |
| | | LM | + | − | − | − | + | + |
| 66 | F | IIIA | PL | + | + | + | + | + | + |
| | | LM | + | + | + | + | + | + |
| 62 | M | IIIB | PL | − | − | − | − | − | − |
| | | LM | − | − | − | − | − | − |
|
| B, Seven cases of
adenocarcinoma |
|
| Age | Gender | Stage | Origin of cells | 5-FU | CDDP | GEM | TXT | VNR | SN38 |
|
| 74 | F | IIIA | PL | − | − | + | − | − | + |
| | | LM | + | − | − | − | − | − |
| 72 | M | IIIB | PL | − | − | − | − | − | − |
| | | LM | + | − | − | + | − | + |
| 60 | M | IIIA | PL | − | + | − | − | + | + |
| | | LM | + | − | − | − | + | + |
| 57 | M | IIIA | PL | + | + | + | + | + | + |
| | | LM | + | − | − | + | + | + |
| 49 | F | IIIA | PL | − | − | − | + | + | + |
| | | LM | + | − | − | + | + | + |
| 64 | M | IIIB | PL | − | − | − | − | − | − |
| | | LM | − | − | − | − | − | − |
| 63 | M | IIIA | PL | − | − | − | − | − | − |
| | | LM | − | − | − | − | − | − |
Higher sensitivity of LM in
adenocarcinoma to 5-FU
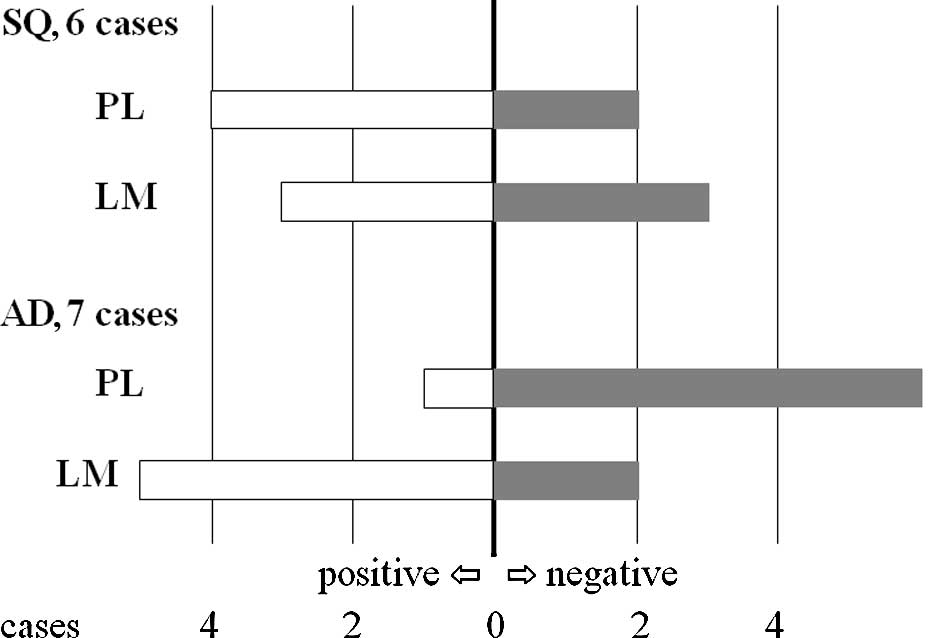
In 5 of the 6 SQ cases, chemosensitivity to 5-FU was
the same (Fig. 1 and Table IIA). In contrast, chemosensitivity
to 5-FU differed between 2 lesions in 4 of the 7 cases with AD in
which the PLs were considered to be negative with respect to
sensitivity and the LMs positive (Fig.
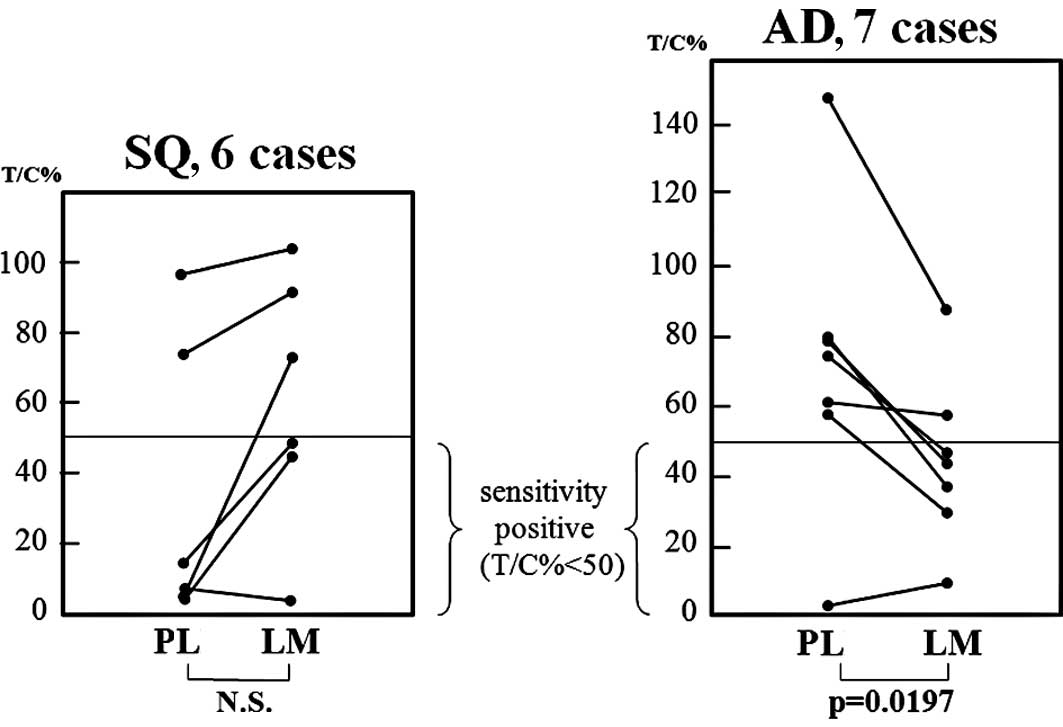
1 and Table IIB). When the
growth rates (T/C%) of the 2 lesions in the AD patients were
compared, it was observed that the growth rates of the LM lesions
were significantly lower than those of the primary lesions in the
AD cases (Student’s t-test) (Fig.
2).
Discussion
In the 1990s, five new agents, including two taxanes
(paclitaxel and docetaxel), gemcitabine, navelbine and irinotecan,
were applied in the treatment of NSCLC and were shown to produce
higher response rates and longer survival (16). A randomized study of 1,207 patients
showed that four platinum-based combination regimens (gemcitabine,
paclitaxel, docetaxel and vinorelbine) were similarly effective
with a response rate of 17–21% and a 1-year survival rate of 31–36%
in previously untreated patients with stage IIIB or IV NSCLC
(17). In the event that one of
these new reagents can be selected, we seek the optimal choice for
each patient. In vitro drug sensitivity tests have been
employed to select effective chemotherapeutic agents for a given
patient (1–3), and CD-DST has been established as a
simple and objective in vitro chemosensitivity assay
(3). The CD-DST technique has
certain advantages. Chemosensitivity can be measured directly by
the shrinking of tumor size in vitro, and this method of
estimating chemosensitivity can yield results in a short time.
Thus, this assay has been employed in clinical investigations
(7–9,11–14).
The Japanese Joint Committee of the Lung Cancer
Registry compiled clinicopathological data for the NSCLC Patient
Japanese Lung Cancer Registry Study for 6,644 NSCLC patients who
underwent resection. In their report, the 5-year survival rate of
the entire group was 52.6%, and the 5-year survival rates by
pathological stage were: 79.5% for IA (n=2,009), 60.1% for IB
(n=1,418), 59.9% for IIA (n=232), 42.2% for IIB (n=757), 29.8% for
IIIA (n=1,250), 19.3% for IIIB (n=719) and 20.0% for IV (n=259)
(18). These results indicated the
need for adjuvant therapies in order to improve the outcomes for
each stage. Many adjuvant chemotherapies have been administered
after complete resection to target putative dormant metastases. The
International Adjuvant Lung Cancer Trial Collaborative Group
reported that cisplatin-based adjuvant chemotherapy improved the
5-year survival rate of patients with completely resected NSCLC
(stages I–III) (19). Adjuvant
chemotherapy with uracil-tegafur improves survival among patients
with completely resected pathological stage I adenocarcinoma of the
lung (20). In contrast, it was
reported that postoperative chemotherapy using cisplatin with
vindesine was not shown to be efficacious in cases of completely
resected n2 NSCLC (21).
Furthermore, adjuvant radiotherapy and chemotherapy with cisplatin
and etoposide did not decrease the risk of intrathoracic recurrence
or prolong survival in patients with completely resected stage II
or IIIA NSCLC (22). These
unsatisfactory results of postoperative adjuvant chemotherapies
that target the dormant disease appear to be caused by the
diversity of chemosensitivity of metastatic lesions. For this
reason, the drug response assay for resected specimens from PLs
appears to be inadequate for the control of dormant metastatic
lesions. This may be one of the reasons that in vitro
chemosensitivity testing has yet to come into wide use.
Chemosensitivity of the metastatic lesions as well as that of the
primary lesions should be considered in order to decide upon
effective agents. To evaluate this hypothesis, we compared the
chemosensitivities between PLs and metastatic lesions in individual
patients.
In the present study, when LM was pathologically
confirmed by examination of the frozen section during surgery,
specimens were obtained from the PL and LM, and in vitro
drug sensitivity testing was performed. The chemosensitivities for
six drugs were successfully compared in 13 NSCLC patients. As a
result, the chemosensitivity of the PLs differed from that of the
metastatic lymph nodes for each anticancer drug in many of the
cases.
The new generation chemotherapeutic agents,
gemcitabine, docetaxel, vinorelbine and SN-38, yielded the same
results between the 2 lesions in most of the AD cases.
Specifically, the chemosensitivity for vinorelbine was found to be
concomitant in all 7 cases. In contrast, the discrepancy was more
striking in the cases of SQ. In these cases, the selection of
anticancer drugs based on chemosensitivity testing for the primary
lesion may be insufficient for adjuvant chemotherapy targeting
dormant metastatic lesions.
In AD cases, the chemosensitivities of the
metastatic lymph nodes to 5-FU were significantly higher than those
of the primary lesions. 5-FU and its derivatives may be effective
for adjuvant chemotherapy after complete resection of AD. This
result appears to be consistent with a study involving the efficacy
of uracil-tegafur for adjuvant chemotherapy after complete
resection of lung AD (20). Further
investigation regarding the relationship between chemosensitivity
against 5-FU and the metastatic activity of AD cells is
required.
In conclusion, both primary and metastatic tumors
should be examined in order to ensure maximum clinical efficacy of
in vitro drug sensitivity testing for adjuvant chemotherapy
after complete resection of n1 and n2 NSCLC.
References
|
1
|
Freeman AE and Hoffman RM: In vivo-like
growth of human tumors in vitro. Proc Natl Acad Sci USA.
83:2694–2698. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cole SP: Rapid chemosensitivity testing of
human lung tumor cells using the MTT assay. Cancer Chemother
Pharmacol. 17:259–263. 1986.PubMed/NCBI
|
|
3
|
Kobayashi H, Tanisaka K, Doi O, et al: An
in vitro chemosensitivity test for solid human tumors using
collagen gel droplet embedded cultures. Int J Oncol. 11:449–455.
1997.
|
|
4
|
Shaw GL, Gazdar AF, Phelps R, et al:
Individualized chemotherapy for patients with non-small cell lung
cancer determined by prospective identification of neuroendocrine
markers and in vitro drug sensitivity testing. Cancer Res.
53:5181–5187. 1993.PubMed/NCBI
|
|
5
|
Gazdar AF, Steinberg SM, Russell EK, et
al: Correlation of in vitro drug-sensitivity testing with response
to chemotherapy and survival in extensive-stage small cell lung
cancer: a prospective clinical trial. J Natl Cancer Inst.
82:117–124. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cortazar P, Gazdar AF, Woods E, et al:
Survival of patients with limited-stage small cell lung cancer
treated with individualized chemotherapy selected by in vitro drug
sensitivity testing. Clin Cancer Res. 3:741–747. 1997.PubMed/NCBI
|
|
7
|
Higashiyama M, Kodama K, Yokouchi H, et
al: Cisplatin-based chemotherapy for postoperative recurrence in
non-small cell lung cancer patients: Relation of the in
vitro chemosensitive test to clinical response. Oncol Rep.
8:279–283. 2001.PubMed/NCBI
|
|
8
|
Takamura Y, Kobayashi H, Taguchi T, et al:
Prediction of chemotherapeutic response by collagen gel droplet
embedded culture-drug sensitivity test in human breast cancers. Int
J Cancer. 98:450–455. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kikuchi T, Daigo Y, Katagiri T, et al:
Expression profiles of non-small cell lung cancers on cDNA
microarrays: identification of genes for prediction of lymph-node
metastasis and sensitivity to anti-cancer drugs. Oncogene.
22:2192–2205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamaue H, Tanimura H, Noguchi K, et al:
Chemosensitivity testing of fresh human gastric cancer with highly
purified tumour cells using the MTT assay. Br J Cancer. 66:794–799.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takamura Y, Kobayashi H, Taguchi T,
Motomura K, Inaji H and Noguchi S: Prediction of chemotherapeutic
response by collagen gel droplet embedded culture-drug sensitivity
test in human breast cancers. Int J Cancer. 98:450–455. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakahara T, Sakaeda T, Nakamura T, et al:
Chemosensitivity assessed by collagen gel droplet embedded culture
drug sensitivity test, and MDR1, MRP1 and MRP2 mRNA expression in
human colorectal adenocarcinomas. Pharm Res. 21:406–412. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagai N, Minamikawa K, Mukai K, Hirata E,
Komatsu M and Kobayashi H: Predicting the chemosensitivity of
ovarian and uterine cancers with the collagen gel droplet culture
drug-sensitivity test. Anticancer Drugs. 16:525–531. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimizu T, Murata S, Mekata E, et al:
Clinical potential of an antitumor drug sensitivity test and
diffusion-weighted MRI in a patient with a recurrent solid
pseudopapillary tumor of the pancreas. J Gastroenterol. 42:918–922.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cartazar P and Johnson BE: Review of the
efficacy of individualized chemotherapy selected by in vitro drug
sensitivity testing for patients with cancer. J Clin Oncol.
17:1625–1631. 1999.PubMed/NCBI
|
|
16
|
Bunn PA and Kelly K: New chemotherapeutic
agents prolong survival and improve quality of life in non-small
cell lung cancer: a review of the literature and future directions.
Clin Cancer Res. 5:1087–1100. 1998.PubMed/NCBI
|
|
17
|
Schiller JH, Harrington D, Belani CP, et
al; Eastern Cooperative Oncology Group. Comparison of four
chemotherapy regimens for advanced non-small cell lung cancer. N
Engl J Med. 346:92–98. 2002. View Article : Google Scholar
|
|
18
|
Goya T, Asamura H, Yoshimura H, et al: The
Japanese Joint Committee of Lung Cancer Registry. Prognosis of 6644
resected non-small cell lung cancers in Japan: a Japanese Lung
Cancer Registry Study. Lung Cancer. 50:227–234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
The International Adjuvant Lung Cancer
Trial Collaborative Group. Cisplatin-based adjuvant chemotherapy in
patients with completely resected non-small cell lung cancer. N
Engl J Med. 350:350–360. 2004.PubMed/NCBI
|
|
20
|
Kato H, Ichinose Y, Ohta M, et al: A
randomized trial of adjuvant chemotherapy with uracil-tegafur for
adenocarcinoma of the lung. N Engl J Med. 350:1713–1721. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tada H, Tsuchiya R, Ichinose Y, et al: A
randomized trial comparing adjuvant chemotherapy versus surgery
alone for completely resected pN2 non-small cell lung cancer. Lung
Cancer. 43:167–173. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Keller SM, Adak S, Wagner H, et al: A
randomized trial of postoperative adjuvant therapy in patients with
completely resected stage II or IIIA non-small cell lung cancer. N
Eng J Med. 343:1217–1222. 2000. View Article : Google Scholar : PubMed/NCBI
|