Introduction
Prostate cancer is the most common urological
malignancy in elderly individuals and is one of the leading causes
of male malignancy-related death worldwide. In the normal prostate
gland, cell proliferation equilibrates with cell death, in order
that disproportionate overgrowth does not occur. On the other hand,
many studies have demonstrated that an imbalance in the growth
equation is one of the etiological factors of tumor progression. In
other words, the up-regulation of cancer cell proliferation and/or
deregulation of apoptosis have been reported to play crucial roles
in carcinogenesis and malignant activities in various malignancies,
including prostate cancer (1,2). In
addition, angiogenesis is an important regulator of malignant
aggressiveness, while dissemination via blood vessels is an
important process for prognosis (3). Consequently, investigations regarding
the regulator of these activities are important for determining
treatment strategies in patients with prostate cancer.
Matrix metalloproteinases (MMPs) are zinc-dependent
proteolytic enzymes capable of cleaving extracellular matrix and
basement membrane macromolecules. Since the degradation of such
components is an important process in cancer cell invasion, much
interest has centered on the relationship between MMPs and tumor
progression in various malignancies (4,5). In
addition to such invasive activity, certain MMPs exhibit various
pathological functions, such as cell proliferation, apoptosis and
angiogenesis (4–6). Thus, we hypothesized that a variety of
MMPs modulate cancer cell progression and survival in patients with
prostate cancer. Although MMP-2 and -9 are the most representative
and well-studied members of the MMP family (7), the clinical and pathological
significance of other MMPs in patients with prostate cancer is not
fully understood.
With regard to the therapeutic as well as the side
effects of MMP inhibitors in cancer patients, disappointing results
have been shown against expectations, probably due to the broad
specificity of these effects (8).
However, specific MMP inhibitors were previously developed, and
several investigators examined their effects on the prevention and
treatment of various malignancies (9,10).
Thus, understanding the detailed clinical significance and
pathological roles of MMPs is important in the determination of
treatment strategies for cancer patients. MMP-10 (stromelysin-2) is
overexpressed in various cancer cells, and its expression level
correlates with malignant aggressiveness, including invasion
(11,12). Moreover, several investigators
reported that MMP-10 plays a role in cell proliferation, apoptosis
and angiogenesis under certain physiological and pathological
conditions (13,14). However, to the best of our
knowledge, little is known as regards the clinical significance and
role of MMP-10 in human prostate cancer tissues.
In prostate cancer, when the cells are localized in
the prostate, the majority of patients present few symptoms.
Conversely, clinical symptoms that affect quality of life, such as
bone pain and urination disorders, are observed in prostate cancer
patients with distant metastasis. In addition, most prostate
cancer-related deaths are not the result of the primary tumor but
the spread of cancer cells into surrounding tissues and distant
organs. In general, up-staging of the T stage precedes the
formation of metastatic tumor. Consequently, investigations
concerning the mechanism of early stages in cancer cell invasion
and tumor growth are important in the planning of effective
treatment strategies in patients with prostate cancer.
The present study aimed to clarify the clinical and
pathological significance of MMP-10 in human non-metastatic
prostate cancer. The relationship between MMP-10 expression and
cancer cell proliferation, apoptosis and angiogenesis were also
investigated.
Materials and methods
Patients
We retrospectively evaluated the medical records of
prostate cancer patients who underwent radical surgery between
March 1994 and November 2007. To evaluate the preoperative status
of metastasis, patients underwent ultrasonography, computed
tomography (CT) of the abdomen and pelvis, bone scanning and lung
X-ray photography. Pathological diagnoses, including Gleason’s
score (GS) and pT and pN stage, were determined using specimens
obtained during surgery, and staging was assessed by the 2002
tumor-node-metastasis classification. In addition to the
pathological diagnosis, vascular invasion was judged by one
pathologist (T.H.). Patients with metastatic cancers were excluded.
None of the patients had received neoadjuvant therapy. After
excluding the patients to whom the above criteria were applicable,
63 patients were enrolled in the study; there was no patient with
stage pT4. In addition, control samples (n=40) of normal prostate
tissues obtained by transurethral resection were also examined. For
statistical analyses, GS was divided into the groups: low (≤6),
middle (7) and high (≥8). The study
protocol complied with the regulations of the Human Ethics Review
Committee of Nagasaki University Graduate School of Biomedical
Science.
Immunohistochemistry
Sections (5-μm) cut from formalin-fixed and
paraffin-embedded tissue samples were deparaffinized and
rehydrated. Antigen retrieval was performed at 95°C for 40 min (for
MMP-10 and CD34) and at 121°C for 15 min (for Ki-67) in 0.01 M
sodium citrate buffer (pH 6.0). Sections were then immersed in 3%
hydrogen peroxide for 30 min. Primary antibodies were obtained from
Lab Vision Corporation, Freemont, CA, USA (MMP-10) and Dako Corp.,
Glostrup, Denmark (Ki-67 and CD34). The sections were then
incubated with the primary antibody at 4°C overnight. Following
incubation, the sections were treated with peroxidase using the
labeled polymer method with EnVision+™ Peroxidase (Dako Corp.) for
60 min. The peroxidase reaction was visualized with the liquid DAB
substrate kit (Zymed Laboratories Inc., San Francisco, CA, USA).
Sections were counterstained with Mayer’s hematoxylin. A
consecutive section from each sample processed without the primary
antibody was used as a negative control. The positive control
consisted of a human breast cancer tissue for MMP-10, tonsil tissue
for Ki-67 and kidney tissue for CD34. In situ labeling for
apoptosis was performed as described previously (14). We used the Apop Tag In Situ
Apoptosis Detection kit (Intergen Company, Purchase, NY, USA),
which is based on the terminal deoxynucleotidyl
transferase-mediated nick end-labeling (TUNEL) method.
Evaluation
To evaluate immunohistochemical staining for MMP-10,
the staining intensity was graded as none, weak, moderate or
strong. However, it was difficult to distinguish between weak and
moderate staining. Subsequently, carcinoma cells with strong
staining intensity were considered as positively stained cells. The
proliferation index (PI) was estimated by counting the number of
cells with nuclei positively stained for the anti-Ki-67 antibody.
The apoptotic index (AI) was estimated by the percentage of
TUNEL-positive cells. Semi-quantitative analyses were performed on
at least 500 cancer cells in 3–6 different fields per section. From
the PI and AI values, we calculated the cell renewal index
(CRI=PI/AI). This index was used in previous reports (3,13).
To analyze microvessel density (MVD), tumor sections
stained with the anti-CD34 antibody were examined under an E-400
microscope (Nikon, Tokyo, Japan). The digital images were captured
using a digital camera (model DU100; Nikon) at ×200 magnification.
For each tumor section, 3–5 fields with the highest blood vessel
density (hot spots) were evaluated. To determine the MVD, defined
as the number of vessels per field (x200), we used a computer-aided
image analysis system (Win ROOF, version 5.0; Mitani Corp., Fukui,
Japan).
To perform logistic regression analyses for the
above variables, we divided the tissue samples into two groups:
those with values higher than the median value for the entire
group, and those with values lower than the group median value.
Slides were evaluated twice at different times by two investigators
(S.M. and Y.S.) who were blinded to the clinical characteristics
and pathological data.
Statistical analysis
Results are expressed as median and interquartile
range (IQR) values. The Mann-Whitney U test was performed for
continuous variables of the data. The Scheffé test was used for
multiple comparisons of the data. Pearson’s correlation was used to
evaluate the relationship between continuous variables. The
correlation coefficient (r) and corresponding P-values were also
reported. Spearman’s rank correlation coefficient was calculated to
confirm Pearson’s correlation. Variables that achieved statistical
significance (P<0.050) in the univariate analysis were
subsequently entered into a multivariate analysis using logistic
regression analysis [described as odds ratios (OR) with 95%
confidence intervals (95% CI), together with the P-values].
Statistical tests were two-sided, and significance was defined as
P<0.050. Statistical analyses were performed on a personal
computer with the statistical package StatView for Windows (version
5.0).
Results
Clinicopathological features
The mean age of patients at surgery was 62 years
(range 44–77). Based on a histopathological examination, 39 cases
(61.9%) in this cohort were diagnosed as pT2 and 24 (38.1%) as pT3.
With regard to GS, 2 cases (3.2%) had a score of 5, 18 cases
(28.6%) of 6, 21 cases (33.3%) of 7, 9 cases (14.3%) of 8 and 13
cases (20.6%) had a score of 9. Thus, the low GS group consisted of
20 patients (31.7%), the middle of 21 (33.3%) and the high GS group
of 22 patients (34.9%).
MMP-10 expression
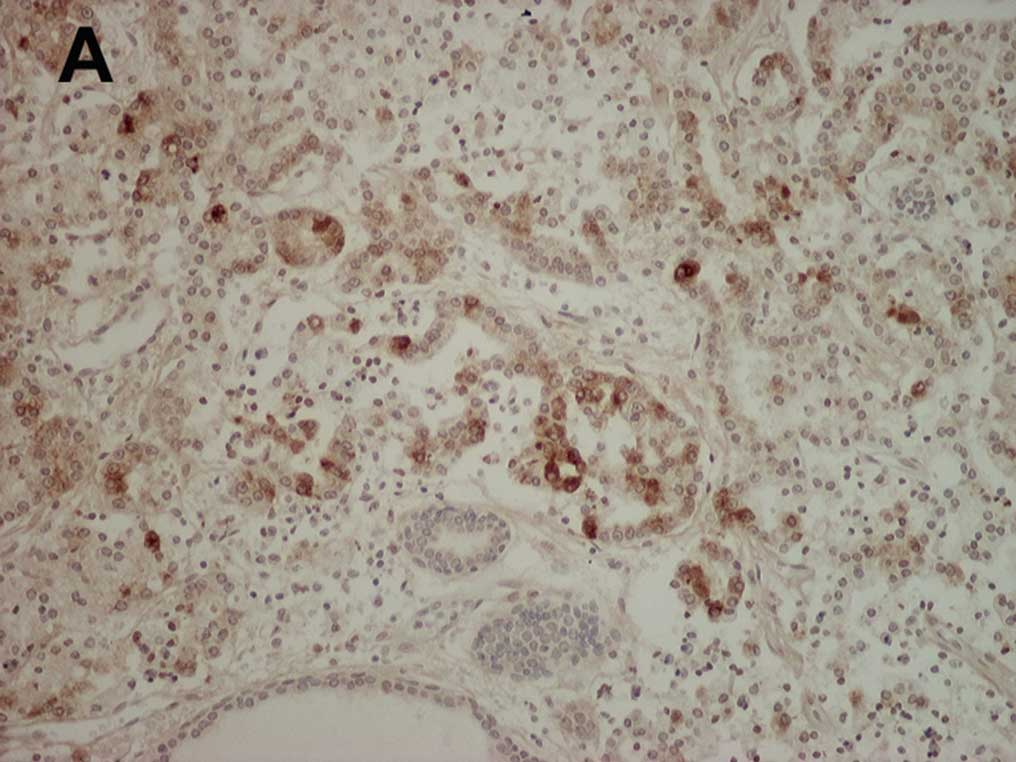
A representative example of cancer cells positively
stained for MMP-10 is shown in Fig.
1A. MMP-10 immunostaining was mainly detected in the cell
cytoplasm, and weak staining was noted in some stromal tissues.
MMP-10-positive cancer cells were uniformly present throughout the
cancer tissue but no unique distribution was noted. On the other
hand, in prostate glands that were free of tumors, cells strongly
stained for MMP-10 were rare (Fig.
1B). The proportion of MMP-10-stained cancer cells (median
13.8%, IQR 8.8–25.5%) was significantly higher (P<0.001) than
the non-tumoral gland cells (median 2.4%, IQR 0–3.7%).
Correlation with clinicopathological
features
As shown in Table I,
the proportion of MMP-10-stained cancer cells was significantly
higher in pT3 (P=0.007) than in pT2 tumors. Furthermore, the
proportion of MMP-10-stained cancer cells in high GS tumors was
significantly higher (P=0.043) than in low GS tumors. To test the
independent significance of MMP-10 expression for a high pT stage,
a multivariate analysis model including MMP-10 and GS was examined.
This analysis showed that MMP-10 expression correlated closely and
positively with a high pT stage (OR 3.63, 95% CI 1.14–11.58,
P=0.029). We then investigated the relationship between MMP-10
expression and vascular invasion. As shown in Table I, MMP-10 expression in specimens
with vascular invasion was significantly higher (P=0.025) than
those without vascular invasion.
 | Table IRelationship between MMP-10 expression
and pathological features. |
Table I
Relationship between MMP-10 expression
and pathological features.
| | MMP-10-stained cancer
cells | |
|---|
| |
| |
|---|
| No. of patients | Median (interquartile
range) | P-value |
|---|
| Pathological
features |
| pT stage | | | 0.007 |
| T2 | 39 | 11.3 (7.6–20.6) | |
| T3 | 24 | 22.3 (13.4–30.2) | |
| Venous invasion | | | 0.025 |
| Absence | 40 | 11.2 (7.9–22.1) | |
| Presence | 23 | 21.8 (13.2–26.9) | |
| Gleason’s score | | | 0.043 |
| Low (<7) | 20 | 10.0 (7.1–13.6) | |
| Middle (=7) | 21 | 17.9 (9.2–31.2) | |
| High (>7) | 22 | 21.6 (11.5–28.0) | |
Correlation with proliferation, apoptosis
and angiogenesis
We examined the correlation between MMP-10 and PI,
AI, MVD and CRI (Table II).
Although MMP-10 expression tended to correlate with PI and MVD, the
correlations were not statistically significant (P=0.068 and 0.100,
respectively). In contrast, MMP-10 expression correlated negatively
with AI, although this correlation showed borderline significance
(P=0.057). On the other hand, MMP-10 expression correlated
significantly with CRI (=PI/AI) (r=0.34, P=0.001). When the
relationship between pT stage and these parameters was examined,
CRI in pT3 (median 5.2, IQR 3.3–8.2) was significantly higher
(P=0.048) than in pT2 (median 3.9, IQR 2.2–5.3) tumors. Thus, both
MMP-10 and CRI were significantly associated with pT stage.
 | Table IIThe relationship between MMP-10 and
parameters. |
Table II
The relationship between MMP-10 and
parameters.
| r | P-value |
|---|
| Proliferation
index | 0.230 | 0.068 |
| Apoptotic index | −0.240 | 0.057 |
| Microvessel
density | 0.210 | 0.100 |
| Cell renewal
index | 0.340 | 0.001 |
Discussion
The present study found that MMP-10 was
overexpressed in prostate cancer cells, and its positively stained
ratio in pT3 was significantly higher than in pT2 tumors. In
several studies using prostate cancer cell lines, MMP-10 expression
levels in cancer cells were significantly higher than those in
normal epithelial cells (16,17).
Our results, based on the study of human cancer tissues, support
the conclusion of the above studies. Therefore, we speculated that
MMP-10 is up-regulated during the carcinogenic process, and plays
an important role in tumor development in patients with prostate
cancer.
Special attention was paid to the pathological roles
of MMP-10 at the early stages of tumor progression. To examine the
characteristics and distribution patterns of MMP-10 expression in
human prostate cancer tissues, we only examined specimens obtained
through radical surgery rather than from needle biopsy. In previous
reports, a strong expression of certain MMP members was detected at
the invasive front of tumors (18,19).
However, such unique features were not found in almost all of the
specimens in this study. On the other hand, our results
demonstrated that MMP-10 was closely associated with pT stage in
our multivariate analysis model. Thus, while MMP-10 plays an
important role in tumor growth, further detailed examination is
necessary regarding its role in prostate cancer. In addition, our
results showed that MMP-10 expression was associated with vessel
invasion. To the best of our knowledge, this is the first report on
such an association. Moreover, this finding confirms that MMP-10
has important pathological significance in the early stages of
prostate cancer progression.
To clarify the pathological roles of MMP-10 in human
prostate cancer, we investigated the relationship between its
expression and PI, AI, MVD and CRI. In other types of malignancies,
MMP-10 has been reported to play crucial roles in cancer cell
proliferation, apoptosis and angiogenesis (12,13).
However, in contrast to our expectation, although some correlation
was found between the proportion of MMP-10-stained cells and PI, AI
or MVD in prostate cancer, this correlation was not statistically
significant. Our results cannot explain such a disparity between
the studies. We believe, however, that these differences are due to
variations in pathological stage and malignant potential. In
particular, our study population did not include patients with
local invasion or metastasis. In general, the pathological
significance and activity of cell proliferation, apoptosis and
angiogenesis in the early stages of tumor development is relatively
lower compared to those in advanced cancer types. In addition, it
is known that the malignant potential and aggressiveness of
prostate cancer, especially in low stage tumors, is relatively
lower compared to that of other cancer types. Although PI, AI and
MVD in advanced stages of prostate cancer are significantly
different from those in the early stages, such differences were not
found between pT3 and pT2 tumors (1,3).
Therefore, it was difficult to detect a statistically significant
correlation between MMP-10 and these parameters in our study
population. Thus, we suggest that MMP-10 expression does not
correlate significantly with PI, AI and MVD in patients with
organ-confined prostate cancer. On the other hand, MMP-10
expression correlated positively with CRI (=PI/AI). The balance of
cancer cell proliferation and apoptosis is one of the strongest
determinants of tumor growth. Thus, we presume that CRI reflects
cancer cell growth more precisely than PI or AI alone, especially
in organ-confined prostate cancer. Several investigators described
CRI as a useful marker of true growth activity under physiological
and pathological conditions, including malignancy (15,20).
Our results showed that there was a significant difference between
CRI in pT2 compared to that in pT3 tumors. We speculate that MMP-10
modulates the balance between cell proliferation and the apoptosis
of cancer cells in non-metastatic prostate cancer tissues, and this
mechanism may play a role in the growth and up-staging of this
disease.
In conclusion, our results demonstrated that MMP-10
was up-regulated during the process of carcinogenesis. Moreover,
the expression of MMP-10 was significantly and positively
associated with pT stage and GS in patients with non-metastatic
prostate cancer. In addition, we speculated that MMP-10 modulates
tumor growth and up-staging via the regulation of the balance of
cell proliferation and apoptosis. Our results provide important
findings for planning observation and treatment strategies for
patients with non-metastatic prostate cancer.
Acknowledgements
We are grateful to Mr. Yoshikazu Tsuji and Mr.
Takumi Shimogama for the outstanding support. This study was
supported by a Grant-in-Aid for Scientific Research from the
Ministry of Education, Science, Technology and Culture of Japan
(grant no. 17791080).
References
|
1
|
Matsushima H, Goto T, Hosaka Y, Kitamura T
and Kawabe K: Correlation between proliferation, apoptosis and
angiogenesis in prostate carcinoma and their relation to androgen
ablation. Cancer. 85:1822–1827. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Visakorpi T, Kallioniemi O-P, Koivula T
and Isola J: New prognostic factors in prostatic carcinoma. Eur
Urol. 24:438–449. 1993.PubMed/NCBI
|
|
3
|
Weidner N, Carroll PR, Flax J, Blumenfeld
W and Folkman J: Tumor angiogenesis correlates with metastasis in
invasive prostate carcinoma. Am J Pathol. 143:401–409.
1993.PubMed/NCBI
|
|
4
|
Egelbald M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar
|
|
5
|
Ray JM and Stetler-Stevenson WG: The role
of matrix metalloproteinases and their inhibitors in tumor
invasion, metastasis and angiogenesis. Eur Respir J. 7:2062–2072.
1994.PubMed/NCBI
|
|
6
|
Miyata Y, Iwata T, Ohba K, Kanda S,
Nishikido M, Koga S and Kanetake H: Expression of matrix
metalloproteinase-7 on cancer cells and tissue endothelial cells in
renal cell carcinoma: prognostic implication and clinical
significance for invasion and metastasis. Clin Cancer Res.
15:6998–7003. 2006. View Article : Google Scholar
|
|
7
|
Wood M, Fudge K, Mohler AR, Frost AR,
Garcia F, Wang M and Stearns ME: In situ hybridization studies of
metalloproteinase 2 and 9 and TIMP-1 and TIMP-2 expression in human
prostate cancer. Clin Exp Metastasis. 15:246–258. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pavlaki M and Zucker S: Matrix
metalloproteinase inhibitors (MMPIs): the beginning of the phase I
or the termination of phase III clinical trials. Cancer Metastasis
Rev. 22:177–203. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arlt M, Kopitz C, Pennington KL, Watson
KL, Krell HW, Bode W, Gansbacher B, Khokha R, Edward DR and Krüger
A: Increase in gelatinase-specificity of matrix metalloproteinase
inhibitors correlated with antimetastatic efficacy in a T-cell
lymphoma model. Cancer Res. 62:5543–5550. 2002.PubMed/NCBI
|
|
10
|
Miyazaki K, Koshikawa N, Hasegawa S,
Momiyama N, Nagashima Y, Moriyama K, Ichikawa Y, Ishikawa T,
Mitsuhashi M and Shimada H: Matrilysin as target for chemotherapy
for colon cancer: use of antisense oligonucleotides as
antimetastatic agents. Cancer Chemother Pharmacol. 43:S52–S55.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mathew R, Khanna R, Kumar R, Mathur M,
Shukla NK and Ralhan R: Stromelysis-2 overexpression in human
esophageal squamous cell carcinoma: potential clinical
implications. Cancer Detect Prev. 26:222–228. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miyata Y, Iwata T, Maruta S, Kanda S,
Nishikido M and Kanetake H: Expression of matrix
metalloproteinase-10 in renal cell carcinoma. Eur Urol. 52:791–797.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meyer E, Vollmer J-Y, Bovey R and
Stamenkovic I: Matrix metalloproteinase 9 and 10 inhibit protein
kinase C-potentiated, p-53-mediated apoptosis. Cancer Res.
65:4261–4272. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miyata Y, Koga S, Kanda S, Nishikido M,
Hayashi T and Kanetake H: Expression of cyclooxygenase-2 in renal
cell carcinoma: correlation with tumor cell proliferation,
apoptosis, angiogenesis, expression of matrix metalloproteinase-2
and survival. Clin Cancer Res. 9:1741–1749. 2003.
|
|
15
|
Bai M, Agnantis N, Kamina S, Demou A,
Zagorianakou P, Katsaraki A and Kanavaros P: In vivo cell kinetics
in breast carcinogenesis. Breast Cancer Res. 3:276–283. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singh S, Singh UP, Grizzle WE and Lillard
JW Jr: CXCL12-CXCR4 interactions modulate prostate cancer cell
migration, metalloproteinase expression and invasion. Lab Invest.
84:1666–1676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh S, Singh UP, Stiles JK and Lillard
JW Jr: Expression and functional role of CCR9 in prostate cancer
cell migration and invasion. Clin Cancer Res. 10:8743–8750. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adachi Y, Yamamoto H, Itoh F, Arimura Y,
Nishi M, Endo T and Imai K: Clinicopathologic and prognostic
significance of matrilysin expression at the invasive front in
human colorectal cancers. Int J Cancer. 95:290–294. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gu ZD, Li JY, Li M, Gu J, Shi XT, Ke Y and
Chen KY: Matrix metalloproteinase expression correlates with
survival in patients with esophageal squamous cell carcinoma. Am J
Gastroenterol. 100:1835–1843. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Navarrete MA, Maier CM, Falzoni R, Gerk de
Azevedo Quadros L, Kima GR, Baracat EC and Nazário AC: Assessment
of the proliferative, apoptotic and cellular renovation indices of
the human mammary epithelium during the follicular and luteal
phases of the menstrual cycle. Breast Cancer Res. 7:R306–R313.
2005. View
Article : Google Scholar
|