Introduction
Hepatocellular carcinoma (HCC) is the major cause of
cancer-related death in Japan. Approximately 70–80% of HCCs in
Japanese patients are associated with hepatitis C virus (HCV)
infection (1). HCV causes chronic
infection in more than 70% of cases, and liver disease gradually
progresses to liver cirrhosis and finally to HCC.
Interferon (IFN) has been used for the treatment of
chronic hepatitis C (CHC) patients. Many investigators have
reported that IFN treatment is effective in the reduction of the
serum alanine amino transferase (ALT) level, eliminating HCV RNA
from the circulation and improving liver histology in CHC patients
(2–6). In certain patients, IFN therapy
normalizes the serum ALT levels and leads to sustained eradication
of HCV. These patients are commonly referred to as having achieved
a sustained viral response (SVR) (7), and it has been noted that the
cumulative incidence of HCC is significantly lower in SVR patients
than in those with a non-response (NR) to IFN therapy (8,9),
suggesting that the success of treatment for HCV infection is
expected to significantly reduce the risk of developing HCC.
However, the development of HCC among CHC patients
with SVR to IFN therapy has been reported (10–16).
In most cases, HCCs occurred within 5 years after the termination
of IFN treatment. The risk factors for developing HCC after
achieving SVR were suggested in these reports; however, the
associated significant factors remain unknown. Moreover, the risk
factors for the development of HCC in patients who have achieved
SVR for more than 10 years are not fully understood. Therefore, it
remains undetermined which patient groups should undergo long-term
follow-up after SVR to IFN therapy for the risk of HCC. Recently,
we identified 5 patients who developed HCC more than 10 years after
SVR to IFN therapy. In this study, we investigated the
characteristics of these patients.
Patients and methods
Patients
Between 1992 and 2000, a total of 674 patients with
chronic HCV infection were treated with IFN (6–10 million units of
IFN-α or -β daily for 2–4 weeks, followed by 6–10 million units of
IFN three times a week for 20–22 weeks). We were able to follow 464
of the 674 patients; 142 of these 469 patients attained continuous
normalization of the serum ALT level. Moreover, the disappearance
of HCV RNA from the serum was determined by a nested reverse
transcription-polymerase chain reaction (RT-PCR) assay or the
Amplicor HCV monitor assay (Roche Molecular System, Pleasanton, CA,
USA) at 6 months after termination of IFN therapy. HCV was
considered to be eradicated in these patients, and they achieved a
sustained viral response (SVR). Ninety-two patients presented with
a positive serum HCV RNA, but with a normal serum ALT level at the
end of treatment. They were defined as achieving a biological
response (BR). In 230 patients, the serum HCV RNA was positive
while the serum ALT level was elevated after termination of the IFN
therapy. These patients demonstrated non-response (NR).
Follow-up and diagnosis of HCC
Follow-up of the patients consisted of blood
examinations including ALT, AST, PLT and α-fetoprotein (AFP) at
regular intervals of 1–3 months. To detect HCC, diagnostic imaging
was performed every 6 months by ultrasonography (US). Computed
tomography (CT) was performed once a year. The diagnosis of HCC was
made using liver imaging (US, CT or magnetic resonance imaging)
and/or angiography. In patients whose angiogram did not demonstrate
a typical hypervascular image of HCC, a microscopic examination of
liver specimens obtained by echo-guided needle biopsy was
performed. Liver biopsy was performed before IFN induction and HCC
onset. Histological diagnosis was carried out according to the
Metavir scoring system.
Results
A total of 464 patients underwent IFN therapy
between 1992 and 2000. Of these, 142 (30.7%) achieved SVR, 92
(19.9%) ended the therapy with BR and 230 patients (49.8%)
demonstrated NR. Eleven patients with SVR developed HCC during
follow-up. In 5 patients, HCC was detected more than 10 years after
the end of the IFN therapy.
The clinical characteristics of the 5 patients at
the induction of IFN therapy are listed in Table I. All patients were male and their
mean age was 51.6±9.1 years. Two patients were moderate alcohol
drinkers with an intake of 43.2 g ethanol per day. Four patients
had a history of previous blood transfusion. None of the 5 patients
were positive for either HBs Ag or anti-HBc Ab. A histological
examination showed the activity scores to be A2 in all cases, and
the fibrosis scores at least F2.
 | Table IClinical characteristics of 5 patients
at the INF induction. |
Table I
Clinical characteristics of 5 patients
at the INF induction.
| Case no. | Gender | Age | Blood
transfusion | Alcohol intake
(g/day) | HBs-Ag | HBs-Ab | AST (IU/l) | ALT (IU/l) | PLT
(104/mm3) | T-Cho (mg/dl) | AFP (ng/ml) | Histology |
|---|
| 1 | Male | 62 | (+) | (−) | (−) | (−) | 90 | 123 | 21.3 | 162 | 6.6 | A2/F2 |
| 2 | Male | 59 | (−) | 43.2 | (−) | (−) | 244 | 153 | 10.8 | 169 | 22.5 | A2/F2 |
| 3 | Male | 44 | (+) | 43.2 | (−) | (−) | 56 | 97 | 13.2 | N/A | N/A | A2/F3 |
| 4 | Male | 52 | (+) | (−) | (−) | (−) | 194 | 172 | 15.5 | 179 | N/A | A2/F3 |
| 5 | Male | 41 | (+) | N/A | (−) | (−) | 106 | 114 | 9.6 | 164 | 21.8 | A2/F3 |
Table II shows the
clinical parameters of the 5 patients at the diagnosis of HCC. The
mean interval from the end of therapy to the detection of HCC was
15.4±2.9 years. The transaminase levels fluctuated in all 5 cases,
and scarcely fell below the upper limits even after SVR was
achieved. The PLT levels improved in 3 patients. In 3 patients,
liver tissues were obtained during treatment of HCC. A histological
examination of these 3 patients showed marked improvement in both
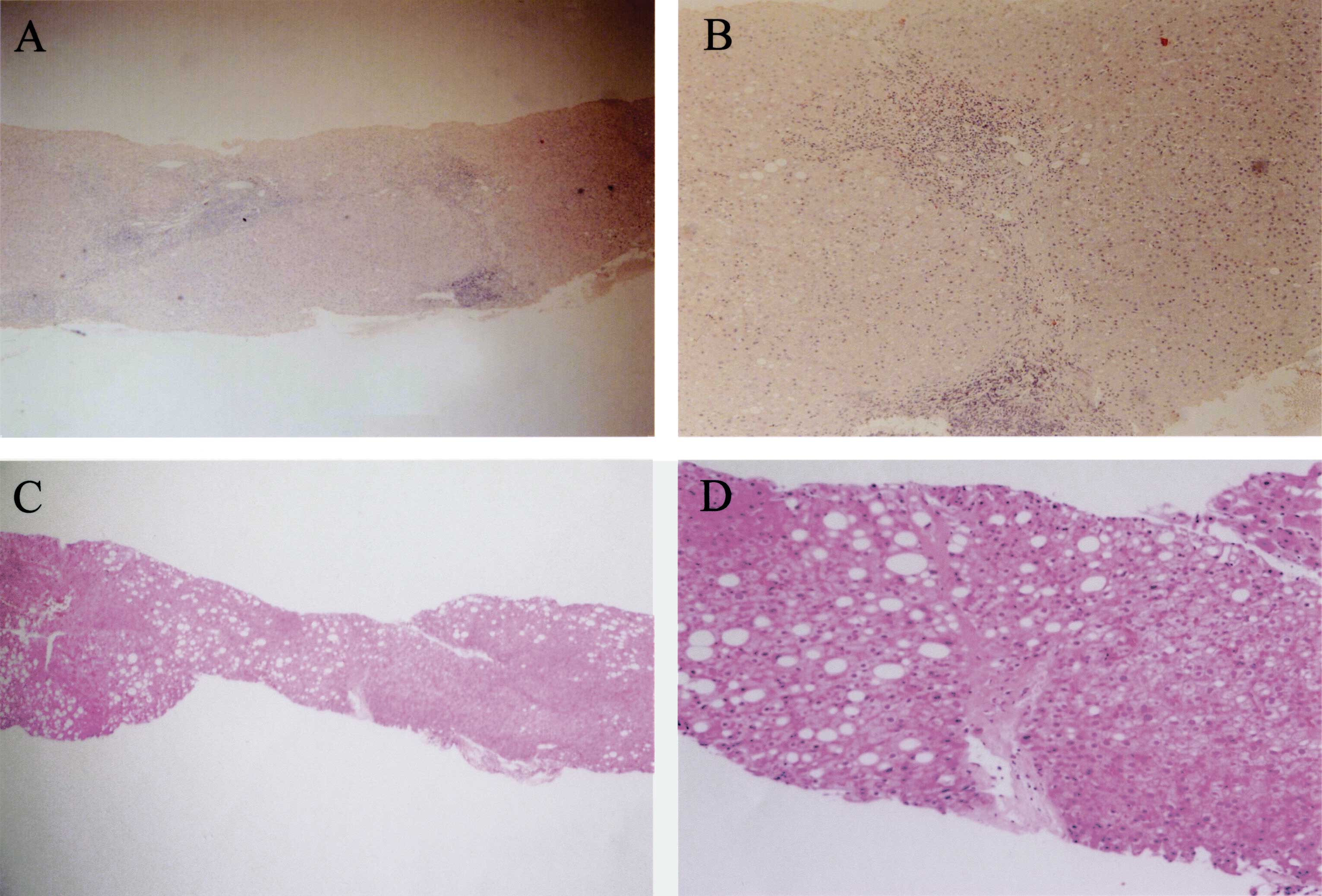
activities and fibroses. The histological findings in case no. 2
are shown in Fig. 1. In this case,
the diagnosis of A2/F2 was made histologically before IFN therapy
(Fig. 1A and B), and the scores
significantly decreased to A0/F1 upon detection of HCC 18 years
after IFN therapy (Fig. 1C and D).
Moreover, notable macrovesicular fat depositions were observed in
the hepatocytes of the second biopsy specimen.
 | Table IIClinical characteristics of 5 patients
at the HCC onset. |
Table II
Clinical characteristics of 5 patients
at the HCC onset.
| Case no. | Years after IFN | AST (IU/l) | ALT (IU/l) | PLT
(104/mm3) | AFP (ng/ml) | Histology | Tumor size (mm) | No. |
|---|
| 1 | 18 | 35 | 27 | 21.5 | 4.4 | A0/F2 | 25 | 2 |
| 2 | 18 | 83 | 66 | 9.5 | 2.0 | A0/F1 | 21 | 1 |
| 3 | 11 | 35 | 40 | 10.9 | 4.9 | N/A | 25 | 1 |
| 4 | 15 | 40 | 21 | 17.8 | 1,931.1 | N/A | N/A | Multiple |
Four patients underwent successful transcatheter
arterial chemoembolization (TACE) and radiofrequency ablation for
the therapy of HCCs. Only case no. 5 succumbed to the disease due
to the progression of HCC and metastasis to the lung and bones.
Discussion
The cumulative incidences of HCC in IFN-treated
patients are significantly low compared to non-treated patients,
particularly in the F3 and F4 groups (5). This suggests that IFN reduces the risk
of HCC even in the non-SVR patient group. In the present study, 5
cases developed HCC more than 10 years after the eradication of HCV
by IFN therapy. The clinicopathological findings of these patients
included male gender and age at treatment of over 40 years. In
addition, the histological examination showed the fibrosis score of
each case to be at least F2, and the serum ALT levels were elevated
in all cases even after SVRs were achieved. Previous studies
suggest that older age, male gender and advanced hepatic fibrosis
are linked to an increased risk for the development of HCC among
patients with SVR. These factors are consistent with our findings.
We did not determine the accurate incidence of HCC in the SVR group
as many patients were unable to be followed up after the end of the
treatment. One retrospective study reported that 3.5% (13) of 373 SVR cases developed HCC. The
mean interval from IFN therapy to the detection of HCC in this
study was 5.8 years, which did not differ significantly from that
in the non-SVR patient group (17).
One of the most important findings of the present
study is that all 5 cases presented elevated ALT levels after SVR
to IFN therapy, which was already suggested as a risk factor for
HCC by a previous study. Although the reasons why ALT levels did
not decrease below the normal range in these cases were not fully
defined, we can speculate several possibilities. First, HCV may
remain in the hepatocytes at a very low level causing persistent
hepatitis. Maylin et al revealed that HCV RNA was detectable
in 2 (1.7%) out of 114 liver specimens after SVR, though serum HCV
RNA remained undetectable in all cases (18). However, HCV RNA is not integrated in
host genome DNA and is not a carcinogen by itself without
inflammation or fibrosis. Therefore, the scenario that HCV RNA
remained in the hepatocytes to sustain hepatitis which ultimately
caused HCC appears to be unlikely in our cases, since the
histological examination showed that both activity and fibrosis
significantly improved in the 3 cases in which liver biopsies at
therapy for HCC were performed. A second possibility for the
increase in ALT levels is steatosis due to alcohol, diabetes
mellitus (DM) or obesity. In the present study, 2 patients had a
history of alcohol intake, 3 patients had DM and, the histological
findings revealed that 2 patients had moderate steatosis in the
liver cells. Therefore, we cannot eliminate the possibility that
steatosis affected the occurrence of HCC to a certain degree in
these cases.
In addition, HCC is associated with occult HBV
infection. Although the anti-HBc antibody was negative in all of
our cases, Tamori et al reported the integration of HBV DNA
into the host genome in 4 out of 7 patients, 2 of whom tested
negative for both anti-HBs Ab and anti-HBc Ab (20). Since we did
not evaluate the HBV DNA in HCC cells or integration, whether HBV
was related to the carcinogenesis in our cases needs to be
ascertained. Further investigation is thus required to elucidate
this issue.
Furthermore, the transformation of normal
hepatocytes to cancer cells may occur before IFN therapy when the
activities of inflammation are high. This may have occurred in the
cases in which HCC developed within 5 years after SVR. Since HCC
generally progresses very slowly, particularly at an early stage,
and all of our cases were histologically well-differentiated HCCs
(data not shown), it is possible that IFN had an effect on the
differentiation of HCC cells and inhibited cell growth. This
resulted in the HCCs being undetectable by diagnostic imaging for
more than 10 years after carcinogenesis.
Although we did not define the accurate mechanism of
the occurrence of HCC after a long period of SVR, we conclude that
male gender, advanced fibrosis, older age at treatment and
sustained elevation of ALT are potential risk factors for HCC.
Moreover, in our cases, HCC may have developed even 18 years after
SVR had been achieved. Therefore, CHC patients who respond to IFN
monotherapy or combination therapy should undergo long-term
follow-up, even after the eradication of HCV, with particular
attention to patients who exhibit the above-mentioned risk factors,
in order to detect small and controllable HCCs.
References
|
1
|
Kiyosawa K, Tanaka E and Sodeyama T:
Hepatitis C virus and hepatocellular carcinoma. Curr Stud Hematol
Blood Transfus. 62:161–180. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marcellin P, Boyer N, Gervais A, Martinot
M, Pouteau M, Castelnau C, Kilani A, Areias J, Auperin A, Benhamou
JP, Degott C and Erlinger S: Long-term histologic improvement and
loss of detectable intrahepatic HCV RNA in patients with chronic
hepatitis C and sustained response to interferon-alpha therapy. Ann
Intern Med. 127:875–881. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reichard O, Glaumann H, Frydén A, Norkrans
G, Wejstål R and Weiland O: Long-term follow-up of chronic
hepatitis C patients with sustained virological response to
alpha-interferon. J Hepatol. 30:783–787. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poynard T, Moussalli J, Ratziu V,
Regimbeau C and Opolon P: Effect of interferon therapy on the
natural history of hepatitis C virus-related cirrhosis and
hepatocellular carcinoma. Clin Liver Dis. 3:869–881. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshida H, Shiratori Y, Moriyama M, et al:
Interferon therapy reduces the risk for hepatocellular carcinoma:
national surveillance program of cirrhotic and noncirrhotic
patients with chronic hepatitis C in Japan. IHIT Study Group.
Inhibition of Hepatocarcinogenesis by Interferon Therapy. Ann
Intern Med. 131:174–181. 1999. View Article : Google Scholar
|
|
6
|
Ikeda K, Saitoh S, Arase Y, Chayama K,
Suzuki Y, Kobayashi M, Tsubota A, Nakamura I, Murashima N, Kumada H
and Kawanishi M: Effect of interferon therapy on hepatocellular
carcinogenesis in patients with chronic hepatitis type C: a
long-term observation study of 1,643 patients using statistical
bias correction with proportional hazard analysis. Hepatology.
29:1124–1130. 1999. View Article : Google Scholar
|
|
7
|
Fried MW and Hoofnagle JH: Therapy of
hepatitis C. Semin Liver Dis. 15:82–91. 1995. View Article : Google Scholar
|
|
8
|
Kasahara A, Hayashi N, Mochizuki K,
Takayanagi M, Yoshioka K, Kakumu S, Iijima A, Urushihara A,
Kiyosawa K, Okuda M, Hino K and Okita K: Risk factors for
hepatocellular carcinoma and its incidence after interferon
treatment in patients with chronic hepatitis C. Osaka Liver Disease
Study Group. Hepatology. 27:1394–1402. 1998. View Article : Google Scholar
|
|
9
|
Kurokawa M, Hiramatsu N, Oze T, et al:
Effect of interferon alpha-2b plus ribavirin therapy on incidence
of hepatocellular carcinoma in patients with chronic hepatitis.
Hepatol Res. 39:432–438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tamori A, Kuroki T, Nishiguchi S, Morimoto
H, Morimoto M, Hirohashi K, Kinoshita AH and Kobayashi K: Case of
small hepatocellular carcinoma in the caudate lobe detected after
interferon caused disappearance of hepatitis C virus.
Hepatogastroenterology. 43:1079–1083. 1996.PubMed/NCBI
|
|
11
|
Hirashima N, Mizokami M, Orito E, Koide T,
Itazu I, Kumada K, Sakakibara K, Kano H and Lau JY: Case report:
development of hepatocellular carcinoma in a patient with chronic
hepatitis C infection after a complete and sustained response to
interferon-alpha. J Gastroenterol Hepatol. 11:955–958.
1996.PubMed/NCBI
|
|
12
|
Tong MJ, Lai LP and Murakami-Mori K:
Development of hepatocellular carcinoma after clearance of
hepatitis C virus with interferon therapy. West J Med. 167:103–105.
1997.PubMed/NCBI
|
|
13
|
Yamaguchi K, Omagari K, Kinoshita H,
Yoshioka S, Furusu H, Takeshima F, Nanashima A, Yamaguchi H and
Kohno S: Development of hepatocellular carcinoma in a patient with
chronic hepatitis C after 6 years of a sustained and complete
response to IFN-alpha. J Clin Gastroenterol. 29:207–209.
1999.PubMed/NCBI
|
|
14
|
Miyano S, Togashi H, Shinzawa H, Sugahara
K, Matsuo T, Takeda Y, Saito K, Saito T, Ishiyama S, Kaneko M and
Takahashi T: Case report: occurrence of hepatocellular carcinoma
4.5 years after successful treatment with virus clearance for
chronic hepatitis C. J Gastroenterol Hepatol. 14:928–930.
1999.PubMed/NCBI
|
|
15
|
Yamada M, Ichikawa M, Matsubara A,
Ishiguro Y, Yamada M and Yokoi S: Development of small
hepatocellular carcinoma 80 months after clearance of hepatitis C
virus with interferon therapy. Eur J Gastroenterol Hepatol.
12:1029–1032. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Enokimura N, Shiraki K, Kawakita T, Saitou
Y, Inoue H, Okano H, Yamamoto N, Deguchi M, Sakai T, Ohmori S,
Fujikawa K, Murata K, Niki Y and Nakano T: Hepatocellular carcinoma
development in sustained viral responders to interferon therapy in
patients with chronic hepatitis C. Anticancer Res. 23:593–596.
2003.PubMed/NCBI
|
|
17
|
Kobayashi S, Takeda T, Enomoto M, Tamori
A, Kawada N, Habu D, Sakaguchi H, Kuroda T, Kioka K, Kim SR, Kanno
T, Ueda T, Hirano M, Fujimoto S, Jomura H, Nishiguchi S and Seki S:
Development of hepatocellular carcinoma in patients with chronic
hepatitis C who had a sustained virological response to interferon
therapy: a multicenter, retrospective cohort study of 1124
patients. Liver Int. 27:186–191. 2007. View Article : Google Scholar
|
|
18
|
Maylin S, Martinot-Peignoux M, Moucari R,
et al: Eradication of hepatitis C virus in patients successfully
treated for chronic hepatitis C. Gastroenterology. 135:821–829.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tamori A, Nishiguchi S, Shiomi S, Hayashi
T, Kobayashi S, Habu D, Takeda T, Seki S, Hirohashi K, Tanaka H and
Kubo S: Hepatitis B virus DNA integration in hepatocellular
carcinoma after interferon-induced disappearance of hepatitis C
virus. Am J Gastroenterol. 100:1748–1753. 2005. View Article : Google Scholar : PubMed/NCBI
|