Introduction
Nuclear factor (NF)-κB is one of the most important
transcription factors that plays an essential role in the
regulation of the expression and function of a wide spectrum of
genes involved in the modulation of the cell cycle, apoptosis, cell
growth, angiogenesis, inflammation and tissue invasiveness of
highly malignant cells (1–3). NF-κB is a homodimeric or heterodimeric
complex comprising proteins of the Rel-family: p65 (RelA), RelB,
c-Rel, p50 (NF-κB1) and p52 (NF-κB2), all of which contain a Rel
homology domain in the N-terminal region that mediates dimerization
and DNA binding (4). The most
commonly detected dimers are p65/p50, p65/p65 and p50/p50. Due to
the presence of a strong transcriptional activation domain, p65 is
responsible for the majority of NF-κB transcriptional activity
(5). Moreover, the p65/p50
heterodimer comprises the major activated form of NF-κB in numerous
cell types (4). NF-κB proteins are
expressed in most cells, but are normally sequestered in the
cytoplasm through binding with the inhibitors of NF-κBs (IκBs)
(6). A number of pathways of cell
stimulation, such as pro-inflammatory, mutagenic and pro-apoptotic
stimuli, lead to the activation of the IκB kinase complex which
phosphorylates the IκBs, targeting them for ubiquitination and
degradation by the 26S proteosome according to the canonical NF-κB
signal transduction pathway (7,8). The
released and activated NF-κB complexes are then free to translocate
into the nucleus and engage transcriptional programs, binding to
the consensus enhancer sequence on the promoters of target
genes.
The activation of NF-κB, usually assessed by the
presence of nuclear p65, has been observed in a number of human
tumors from either a haematological or solid origin, such as
melanomas (1). Enhanced nuclear
translocation of p65/p50 was found in melanoma cell lines in
comparison to normal melanocytes (2). Moreover, other in vitro and
in vivo studies have shown that NF-κB activity is
up-regulated in dysplastic nevi and lesions of human melanoma when
compared to human nevi or melanocytes in normal skin (9–11).
Largely due to the central role that NF-κB plays in suppressing
apoptosis (7), NF-κB activation
appears to promote melanoma progression (12–14).
The anti-apoptotic mechanisms are essentially based on the ability
of NF-κB to activate the transcription of genes that are able to
suppress cell death, such as survivin (2), thus allowing the escape of tumor cells
from apoptosis and enhancing their metastatic potential.
Survivin is a member of the inhibitor of apoptosis
protein family (15), undetectable
in most differentiated normal tissues, but strongly expressed in
embryonic and fetal organs. It is implicated in cell division,
prevention of apoptosis, cellular stress response and checkpoint
mechanisms of genomic integrity (16). It is overexpressed in many human
malignancies, and such overexpression is associated with poor
prognosis (17–19). Transcription of the survivin gene is
inhibited by the p53 tumor suppressor (20), essential in the regulation of
cellular response to DNA damage. p53 regulates the expression of
various genes that contribute to cell cycle arrest, DNA repair or
apoptosis (21–26). Mutations of p53 occur in
approximately 50% of cancer types and are generally associated with
a worse prognosis as well as a higher resistance to treatment
(27). Loss or mutation of p53, in
addition to being a possible mechanism responsible for survivin
overexpression, appears to directly or indirectly lead to NF-κB
activation in melanoma cells (4,28–30).
In this study, the expression of NF-κB, survivin and
p53 was immunohistochemically investigated, and the relationship
among these factors was analyzed in primary cutaneous melanoma.
Since further improvements in melanoma prognosis are likely to come
from the development of novel molecular markers, the study aimed to
evaluate the prognostic prediction of melanoma by NF-κB expression.
The correlation between NF-κB expression and clinicopathological
factors of patients was also examined.
Materials and methods
Samples
Archival tissue blocks of sporadic primary cutaneous
melanoma were obtained from 70 patients. The patients underwent
observation at the Oncologic Hospital ‘Businco’, Cagliari, Italy,
and at the Department of Pathology, Cancer Center of Solca, Cuenca,
Ecuador, between November 1995 and April 2008, and were selected
for further study according to the following criteria: melanoma
with vertical growth phase and complete clinical data including
follow-up until July 2009. Lymph node status and the presence of
metastases were verified by a clinical and pathological
examination. This study included a total of 70 stage I–IV melanoma
patients, whose clinicopathological characteristics are shown in
Table I. The patients included 30
men and 40 women, ranging in age from 12 to 100 years (median 68).
The anatomic location of the primary tumor included 18 tumors
located in the head and neck, 13 in the trunk, 8 in the upper
extremities and 31 in the lower extremities. According to Clark’s
classification (31), 4 tumors were
level II, 11 level III, 23 level IV and 32 level V. According to
the American Joint Committee on Cancer (AJCC) staging system
(32), 51 tumors were stages I–II
and 19 were stages III–IV. Regarding tumor thickness, 17 tumors
were classified as T1–T2 and 53 as T3–T4.
 | Table IClinicopathological characteristics of
70 cutaneous melanoma patients. |
Table I
Clinicopathological characteristics of
70 cutaneous melanoma patients.
| Characteristics | Patients (n=70) |
|---|
| Age at diagnosis
(%) |
| ≤68a years | 37 (53) |
| >68 years | 33 (47) |
| Gender (%) |
| Men | 30 (43) |
| Women | 40 (57) |
| Tumor thickness
(%) |
| T1–T2 | 17 (24) |
| T3–T4 | 53 (76) |
| Clark level
(%) |
| II–III | 15 (21) |
| IV–V | 55 (79) |
| Stage (%) |
| I–II | 51 (73) |
| III–IV | 19 (27) |
| Anatomic location
(%) |
| Head and neck | 18 (26) |
| Trunk | 13 (19) |
| Upper
extremities | 8 (11) |
| Lower
extremities | 31 (44) |
Following surgical resection, each tumor was fixed
in formalin and completely embedded in multiple paraffin blocks.
The sections removed from the block with the largest tumor
thickness were evaluated. Tumoral areas were identified on
haematoxylin and eosin-stained sections and on adjacent sections
immunohistochemically stained for melanoma-associated antigens,
including S-100 protein, melan A and HMB-45. An independent
histopathological analysis was performed by two pathologists (M.P.
and J.U.) on separate occasions. The study protocol was approved by
the Research Ethics Committee of our institutions, and informed
consent was obtained from all of the patients involved in the
study.
Immunohistochemistry
Serial microtome sections (5-μm) were treated for
the immunohistochemical demonstration of NF-κB (p65), survivin, p53
and melanoma-associated antigens S-100, melan A and HMB-45, using
the alkaline phosphatase-streptavidin method.
Antigen retrieval was performed by heating at 95°C
for 40 min in 10 mM citrate buffer solution (pH 6.0), followed by
gradual cooling for 20 min for the demonstration of NF-κB (p65),
survivin, p53 and melan A, and by immersion in 0.1% trypsin
solution in phosphate-buffered saline at 37°C for 5 or 10 min for
the S-100 protein and the HMB-45 antigen, respectively.
Non-specific binding was blocked with 10% normal goat or normal
horse serum for 45 min. Mouse monoclonal antibodies to human p65
(clone F-6, 1:100 dilution; Santa Cruz Biotechnology Inc., Santa
Cruz, CA, USA), to human p53 (clone DO-7, 1:50 dilution; Dakopatts,
Glostrup, Denmark), to human melan A (clone A103, 1:100 dilution;
Dakopatts) and to human HMB-45 (clone HMB-45, 1:100 dilution;
Dakopatts), rabbit polyclonal antibodies to recombinant human
survivin protein (1:2,000 dilution; Novus Biologicals, Littleton,
CO, USA) and to bovine S-100 protein (1:1,000 dilution; Dakopatts)
were used as primary antisera. Biotinylated anti-mouse and
anti-rabbit immunoglobulins G (1:800 and 1:200 dilution,
respectively; Vector Laboratories, Burlingame, CA, USA) were used
as secondary antisera. The sections were further incubated in
alkaline phosphatase-streptavidin (1:1,000 dilution; Vector
Laboratories) for 30 min at room temperature, reacted with Fast Red
Substrate System (Dakopatts) and counterstained with Mayer’s
haematoxylin. Sections from human prostate gland were used as
positive controls for NF-κB, sections of rat testis as positive
controls for survivin and sections of melanoma strongly expressing
p53 as positive controls for p53. Negative controls were
established by replacing the primary antibodies with normal
serum.
Evaluation of immunoreactivity
To identify and count tumor cells positive for NF-κB
in the nucleus, cytoplasm or both, the entire tumor of each case
was microscopically examined through ×200 magnification fields with
a 144-intersection point square reticulum (0.78 mm2)
inserted in the eyepiece. The average of these counts per field was
considered. Similarly, adjacent sections from the same samples were
evaluated for survivin and p53 immunoreactivity.
The results were stratified according to a staining
score that considered the percentage of positive cells as well as
the staining intensity. Cases in which NF-κB cytoplasmic staining
was detected in >10% of tumor cells with a moderate/strong
staining intensity were considered to show NF-κB overexpression in
the cytoplasm. Regarding NF-κB nuclear staining, >5% positive
cells were used with staining intensity from weak to strong as a
cut-off, indicating NF-κB nuclear immunopositivity. The samples
were scored as positive for the expression of survivin or p53 when
>10% of tumor cells showed a moderate/strong staining intensity;
otherwise, the samples were scored as negative.
Statistical analysis
Data were computed with the Statistical Package for
the Social Sciences (SPSS) 15.0 software. Correlations between
NF-κB, survivin and p53 expression, and that of NF-κB expression
with clinicopathological parameters of stages I–IV melanoma
patients were assessed by Fisher’s exact or Pearson’s χ2
test.
Overall survival of patients with stages I and II
melanoma (51 samples) was calculated from the date of histological
diagnosis to the date the patients succumbed to melanoma or the
last follow-up, until July 2009. Data on patients who succumbed to
other causes were censored at the time of death. Survival curves
were obtained using the Kaplan-Meier method, and comparisons were
made using the log-rank test and adjusted for specific prognostic
factors. The 95% confidence intervals (95% CI) for survival were
calculated and reported. Multivariate analysis was performed using
the Cox proportional hazard model. The tests used were two-sided.
Differences were considered statistically significant at
P≤0.05.
Results
Immunohistochemistry
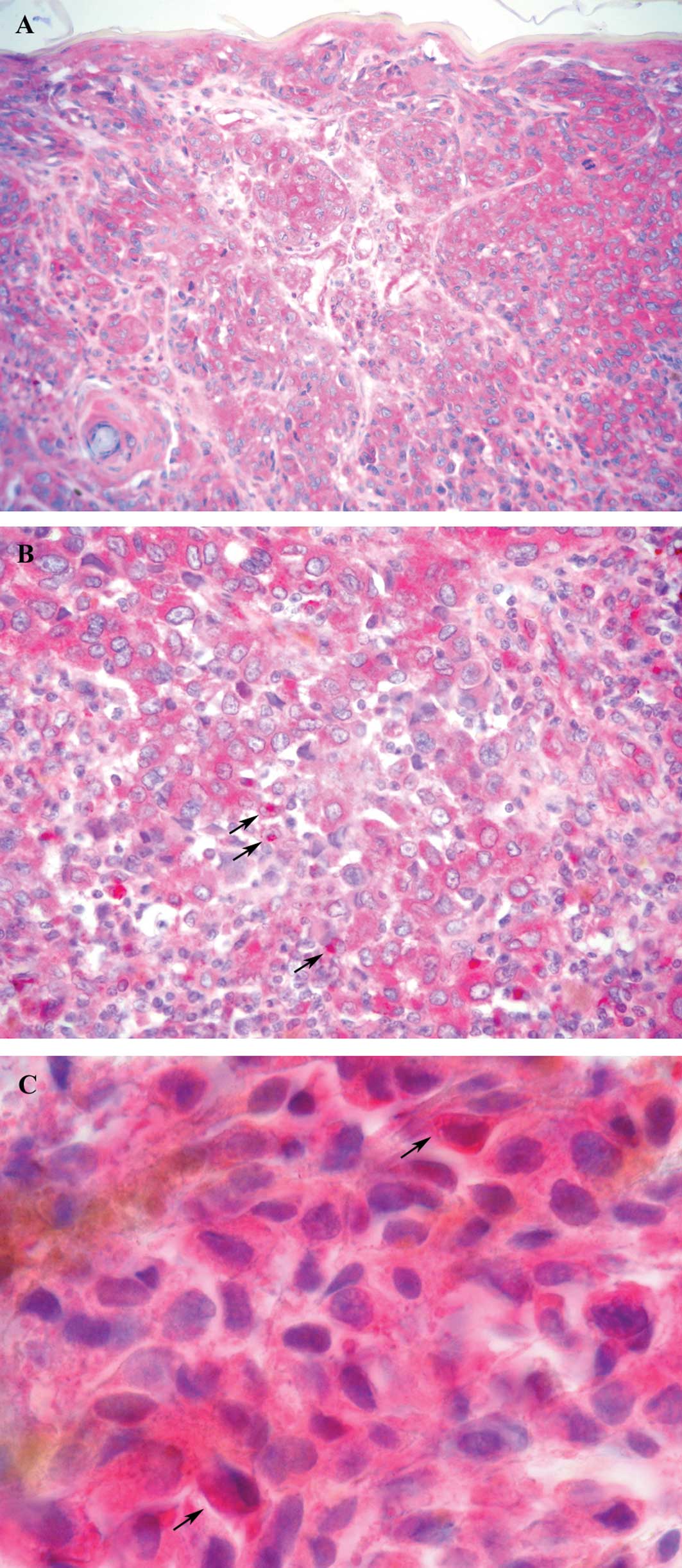
Immunoreactivity for NF-κB was observed in the
nuclear and cytoplasmic compartment of the tumor cells, with
nuclear staining localized in a small amount of cells with respect
to cytoplasmic staining. The immunostaining pattern in the nuclei
and cytoplasm was heterogeneous, with the cell clusters showing a
stronger staining intensity than other cells in some cases since in
other samples positive cells were homogeneously distributed
throughout the tumor with an intense immunostaining. In normal
epidermis, NF-κB was expressed in a cytoplasmic as opposed to a
nuclear pattern. In some cases, the percentage of positive cells
decreased from the edge towards the central area of tumor nodules.
A total of 29% of the samples showed immunoreactivity for NF-κB in
the nuclei, and 54% exhibited NF-κB overexpression in the cytoplasm
of tumor cells. An intense immunoreactivity adjacent to the
nucleus, leaning against the nuclear envelope, was observed in a
number of tumors (46%). A total of 50% of the samples showed this
staining and/or the immunoreaction localized inside the nucleus and
were scored as positive for nuclear NF-κB (Fig. 1).
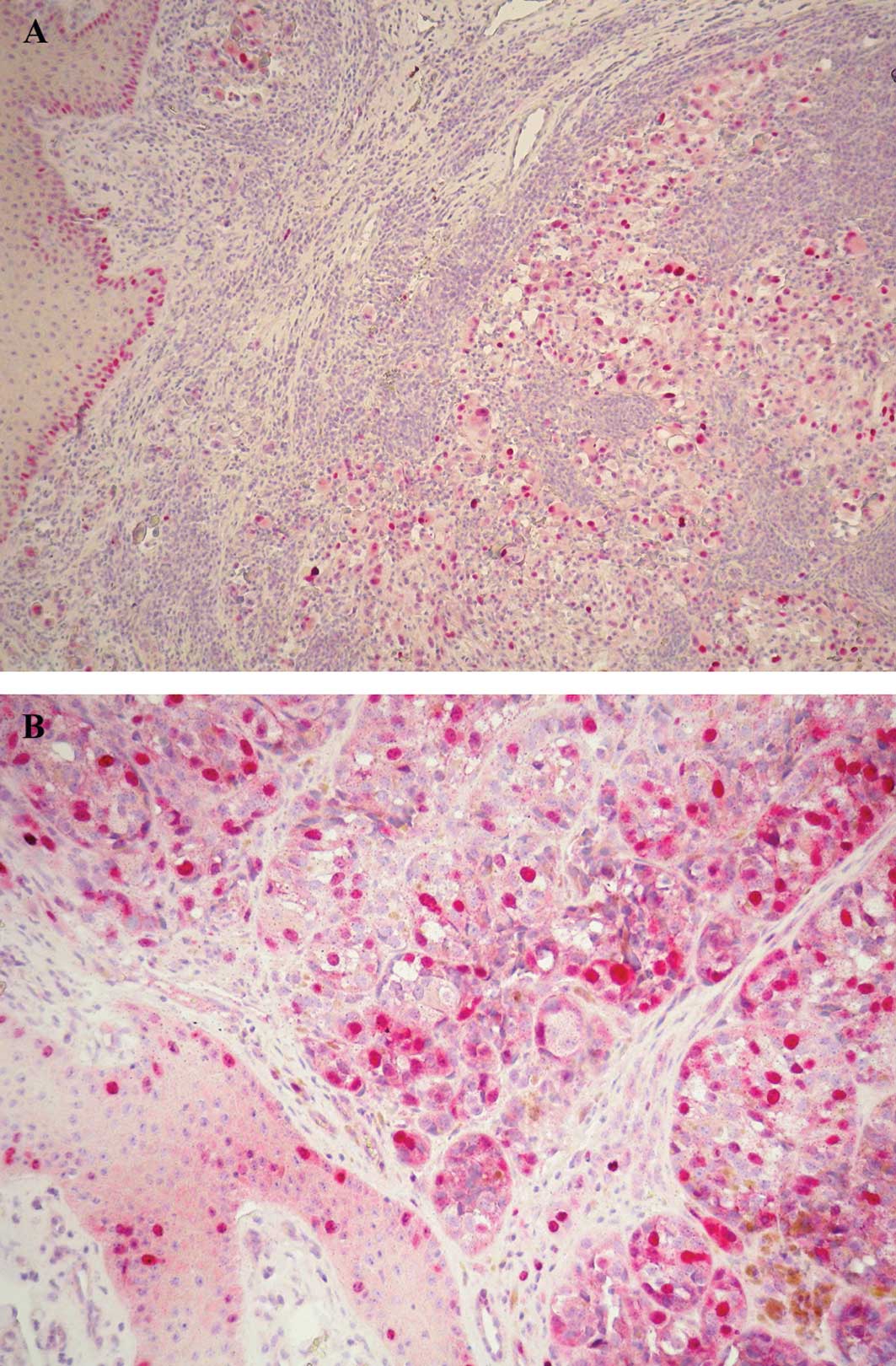
p53 expression, restricted to the nuclei of tumor
cells, was found in 63% of cases. p53-positive cells were
distributed homogeneously within the tumor with a staining
intensity from moderate to strong. Positive cells were also
observable in the basal epidermal layers (Fig. 2A).
Survivin immunoreaction was found in the nucleus and
cytoplasm, with the nuclear staining being more intense. Survivin
immunopositivity was noted in 59% of the cases in nuclear and/or
cytoplasmic staining with positive cells distributed homogeneously
throughout the tumor in some cases or with a heterogeneous staining
pattern in other cases. A number of mitotic figures were intensely
stained, and the epidermis surrounding the tumor also showed
survivin-positive cells (Fig.
2B).
Statistical analysis
Positive immunoreactivity for NF-κB in the nuclei or
cytoplasm of tumor cells was more frequent in cases positive for
p53 and survivin. When analyzed by Fisher’s exact test, NF-κB
positivity in the nuclei was significantly associated with p53
overexpression in tumor cells (P=0.025), but not with survivin
immunoreactivity (P>0.05). Although tumors with NF-κB
overexpression in the cytoplasm were positive for p53 and survivin,
the differences were not statistically significant (P>0.05).
When a combined nuclear and cytoplasmic NF-κB staining was
evaluated, i.e., tumor cells were considered positive if they
exhibited nuclear and/or a cytoplasmic immunoreaction, total NF-κB
showed a statistical association with p53 (P=0.004) and survivin
overexpression (P=0.045). Table II
shows NF-κB expression in relation to p53 and survivin.
 | Table IINF-κB expression in relation to p53
and survivin. |
Table II
NF-κB expression in relation to p53
and survivin.
| Nuclear NF-κB (no.
of cases) | Cytoplasmic NF-κB
(no. of cases) | Total NF-κB (no. of
cases) |
|---|
|
|
|
|
|---|
| Negative | Positive | P-valuea | Negative | Positive | P-valuea | Negative | Positive | P-valuea |
|---|
| p53 | | | 0.025 | | | 0.143 | | | 0.004 |
| Negative | 18 | 8 | | 15 | 11 | | 15 | 11 | |
| Positive | 17 | 27 | | 17 | 27 | | 9 | 35 | |
| Survivin | | | 0.145 | | | 0.469 | | | 0.045 |
| Negative | 18 | 11 | | 15 | 14 | | 14 | 15 | |
| Positive | 17 | 24 | | 17 | 24 | | 10 | 31 | |
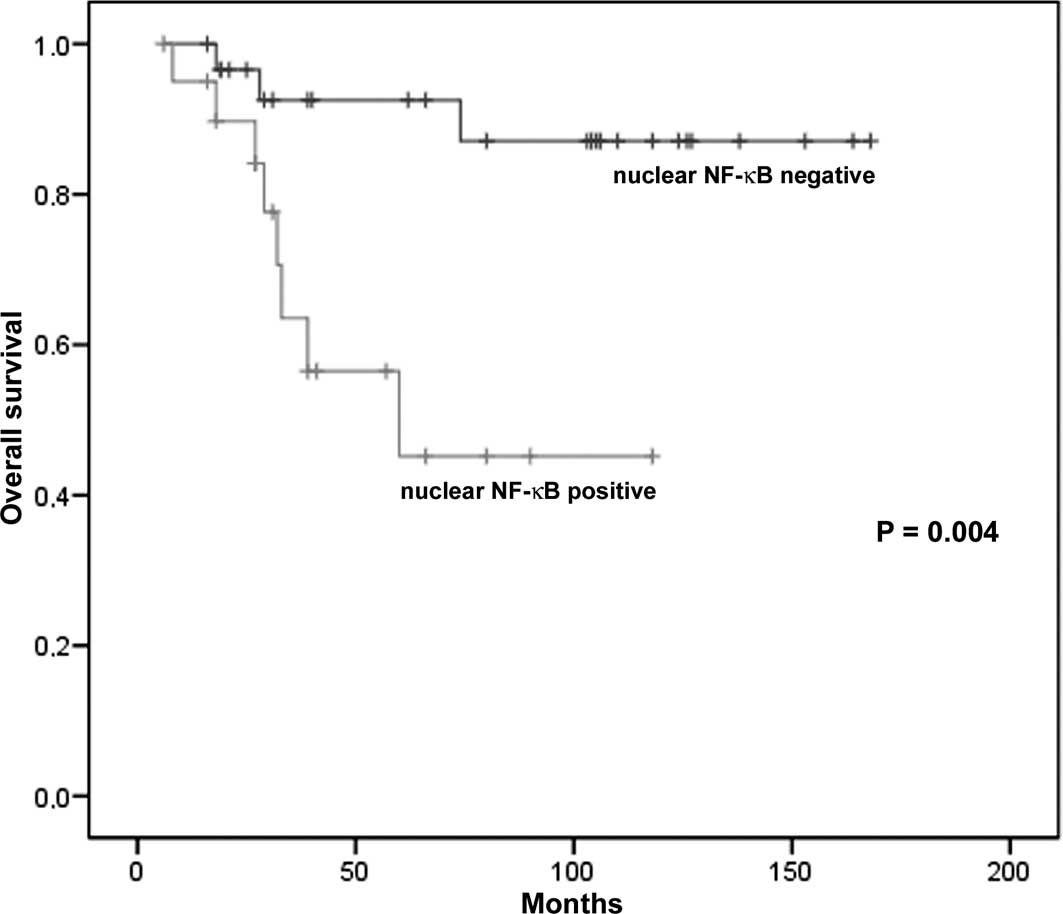
Kaplan-Meier univariate analysis showed that
patients with low levels of NF-κB in the nuclei of tumor cells
(nuclear NF-κB-negative) had a significantly longer survival
compared to those with high levels (nuclear NF-κB-positive)
(P=0.004; Fig. 3). The Kaplan-Meier
estimates of overall survival probabilities at 60 months were 0.92
(95% CI 0.83–1.00) for patients with nuclear NF-κB-negative tumors
(low nuclear NF-κB expression) and 0.45 (95% CI 0.24–0.66) for
those with nuclear NF-κB-positive tumors (high expression)
(Table III). Multivariate Cox
regression analysis showed that the predictive value of nuclear
NF-κB expression maintained significance after the model was
adjusted using clinicopathological factors, such as age, gender,
anatomic location, tumor thickness, Clark level and AJCC stage
(P<0.05). Patient survival was also associated with total NF-κB
expression (P=0.04) since the Kaplan-Meier estimates of overall
survival probabilities at 60 months were 0.90 (95% CI 0.78–1.00)
for patients with a low total NF-κB expression and 0.62 (95% CI
0.45–0.80) for those with a high expression (Table III). This predictive value
maintained significance even after adjustment with the
clinicopathological factors (P<0.05). In the correlation
analysis, nuclear and total NF-κB positivity was significantly
associated with advanced tumor stage (P=0.03 and 0.002,
respectively), but not with other clinicopathological factors, such
as gender, age, tumor thickness, Clark level and anatomic site
(P>0.05).
 | Table IIIUnivariate analysis of NF-κB
expression for 5-year survival in melanoma patients. |
Table III
Univariate analysis of NF-κB
expression for 5-year survival in melanoma patients.
| Variable | No. of
patients | No. of events | 5-year
survival | SEa | P-valueb |
|---|
| Nuclear NF-κB | | | | | 0.004 |
| Negative | 30 | 3 | 0.92 | 0.05 | |
| Positive | 21 | 8 | 0.45 | 0.14 | |
| Total NF-κB | | | | | 0.040 |
| Negative | 23 | 2 | 0.90 | 0.06 | |
| Positive | 28 | 9 | 0.62 | 0.10 | |
Discussion
NF-κB plays an important role in cancer development
and progression. Numerous studies have demonstrated that NF-κB is
activated in a variety of cancer types, including breast, lung,
gastric, esophageal, pancreatic, prostate cancer (33–38)
and melanoma (2,8).
Our study aimed to evaluate whether NF-κB expression
in tumor tissues has an effect on the survival of melanoma
patients. We used immunohistochemistry, a simple reproducible
method for pathologists to conduct the investigation or for routine
use in the estimation of NF-κB. Intense immunoreactivity was found
adjacent to the nucleus, leaning against the nuclear envelope. This
immunopositivity may reflect the presence of NF-κB dimers activated
in the cytoplasm and travelling to the nucleus. For this reason,
immunopositivity was considered to indicate NF-κB activation in the
tumor tissue, together with nuclear staining and cytoplasmic
overexpression. In particular, it was included in nuclear
positivity as an index of NF-κB activation towards its
transcriptional activity. The Kaplan-Meier univariate analysis
showed that patients with low levels of NF-κB in the nuclei of
tumor cells (nuclear NF-κB-negative) had a significantly longer
survival compared to those with high levels (nuclear
NF-κB-positive). In order to confirm the prognostic role of nuclear
NF-κB expression, we conducted a multivariate Cox regression
analysis for patient survival, including known prognostic factors
such as age, gender, anatomic location, tumor thickness, Clark
level and AJCC stage. The predictive value of nuclear NF-κB
expression was found to maintain significance after the model was
adjusted using the aforementioned clinicopathological factors,
thereby confirming the value of nuclear NF-κB as an independent
prognostic variable in this patient population. This result is
consistent with previously reported immunohistochemical studies
showing the association between high nuclear NF-κB p65 expression
and progression-free survival of patients with prostate cancer
(39,40) and metastatic serous ovarian
carcinoma (41). In numerous other
studies, NF-κB activation was universally verified to be an adverse
prognostic factor (42), and in
melanoma it was proposed as an event that promotes tumor
progression (12–14). Our findings, which show that the
nuclear staining of NF-κB p65 is correlated with disease-specific
5-year survival of melanoma patients, confirm that an increased
nuclear expression of NF-κB p65 is a crucial activity during
melanoma progression.
Inhibition of apoptosis is the most likely pathway
through which NF-κB signaling promotes the development of cancer.
Moreover, NF-κB activation is known to suppress apoptosis (7). NF-κB may facilitate invasion and
metastasis by inducing the expression of molecules that are
mediators of the migration of cancerous cells, crossing of vessel
walls and invasion at sites of metastasis (2). NF-κB activity is involved in the
regulation of angiogenesis, the process by which tumor cells
promote neo-vascularization, an essential step for their growth and
invasiveness. Vascular endothelial growth factor, the main member
of the angiogenic factor family, is under the transcriptional
regulation of NF-κB (43). It has
been reported that tumor vascularity is an early requirement for
melanoma progression and that NF-κB plays a mediating role between
melanoma cells and tumor vasculature (44).
Statistical analysis showed that a positive
immunoreaction for NF-κB in the nuclei or cytoplasm of tumor cells
was more frequent in cases positive for p53 and survivin and that a
complete NF-κB expression was statistically associated with p53 and
survivin overexpression. Our results show the concomitant presence
of activated forms of NF-κB and p53 overexpression in melanoma
cells, an association that may be explained by the functional
relationship between the two proteins, since they both play a
central role in the control of proliferation and apoptosis.
Normally, p53 is able to block the activity of other transcription
factors, including NF-κB, in order to promote apoptosis. When p53
is mutated or has lost its activity, a condition detectable through
its accumulation and pronounced immunohistochemical expression, its
functional loss leads to the activation of NF-κB, observable as an
enhanced nuclear localization, as noted in our melanoma samples.
Moreover, p53 and NF-κB (p65) mutually inhibit the transcriptional
activity, establishing a functional relationship between the two
pathways (1). The concomitant
expression of activated NF-κB and survivin is in agreement with the
role of NF-κB in enhancing the expression of anti-apoptotic
proteins, such as survivin, as a mechanism for protection from
apoptosis in melanoma (2). Our
findings confirm the presence of a constitutive activation of NF-κB
in melanoma cells, showing in particular a significantly elevated
expression of RelA (p65) and its increased nuclear translocation,
suggesting NF-κB activation through the canonical pathway. Notably,
the majority of the studies concerning the association between
NF-κB and tumor development usually involved the p65 subunit
(39–41).
The present study indicates that the interaction of
NF-κB with p53 and survivin is a potential key signaling pathway in
the process of melanoma pathogenesis. Moreover, it suggests that
patients with tumors that are strongly positive for nuclear NF-κB
p65 expression should be regarded as being at high risk of
mortality, and that nuclear NF-κB p65 may be a promising early
independent prognostic factor in patients with primary cutaneous
melanoma. The evaluation of nuclear NF-κB expression, either alone
or in combination with other routinely available clinical and
histological prognostic markers, may be useful in improving the
prediction of outcome of melanoma patients. NF-κB inhibition is
considered to be promising in the fight against cancer, and many
efforts are now concentrated on the ability to identify novel NF-κB
targets specifically activated in tumors. For this reason, we
believe that targeting NF-κB may be useful in developing a
risk-adjusted approach to adjuvant therapies in melanoma
patients.
Acknowledgements
This study was supported by grants from the
Ministero Istruzione Università Ricerca – MIUR, Ministero Affari
Esteri – MAE and Fondazione Banco di Sardegna. Particular thanks
are due to Mrs. Itala Mosso and Mr. Massimo Annis for the expert
technical assistance.
References
|
1
|
Pacifico F and Leonardi A: NF-κB in solid
tumors. Biochem Pharmacol. 72:1142–1152. 2006.
|
|
2
|
Amiri KI and Richmond A: Role of nuclear
factor-κB in melanoma. Cancer Metastasis Rev. 24:301–313. 2005.
|
|
3
|
Meteoglu I, Erdogdu IH, Meydan N, Erkus M
and Barutca S: NF-kappaB expression correlates with apoptosis and
angiogenesis in clear cell renal cell carcinoma tissues. J Exp Clin
Cancer Res. 27:532008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ueda Y and Richmond A: NF-κB activation in
melanoma. Pigment Cell Res. 19:112–124. 2006.
|
|
5
|
Poser I and Bosserhoff AK: Transcription
factors involved in development and progression of malignant
melanoma. Histol Histopathol. 19:173–188. 2004.PubMed/NCBI
|
|
6
|
Ghosh S and Karin M: Missing pieces in the
NF-kappaB puzzle. Cell. 109:S81–S96. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naugler WE and Karin M: NF-κB and
cancer-identifying targets and mechanisms. Curr Opin Genet Dev.
18:19–26. 2008.
|
|
8
|
Yang J, Pan WH, Clawson GA and Richmond A:
Systemic targeting inhibitor of kappaB kinase inhibits melanoma
tumor growth. Cancer Res. 67:3127–3134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dhawan P and Richmond A: A novel NF-kappa
B-inducing kinase-MAPK signaling pathway up-regulates NF-kappa B
activity in melanoma cells. J Biol Chem. 277:7920–7928. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McNulty SE, Tohidian NB and Meyskens FL:
RelA, p50 and inhibitor of kappa B alpha are elevated in human
metastatic melanoma cells and respond aberrantly to ultraviolet
light B. Pigment Cell Res. 14:456–465. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McNulty SE, Del Rosario R, Cen D, Meyskens
FL Jr and Yang S: Comparative expression of NF-kappaB proteins in
melanocytes of normal skin vs. benign intradermal naevus and human
metastatic melanoma biopsies. Pigment Cell Res. 17:173–180. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang S, DeGuzman A, Bucana CD and Fidler
IJ: Level of interleukin-8 expression by metastatic human melanoma
cells directly correlates with constitutive NF-kappaB activity.
Cytokines Cell Mol Ther. 6:9–17. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Payne AS and Cornelius LA: The role of
chemokines in melanoma tumor growth and metastasis. J Invest
Dermatol. 118:915–922. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Richmond A: NF-kappa B, chemokine gene
transcription and tumour growth. Nat Rev Immunol. 2:664–674. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salvesen GS and Duckett CS: Apoptosis: IAP
proteins: blocking the road to death’s road. Nat Rev Mol Cell Biol.
3:401–410. 2002.
|
|
16
|
Altieri DC: The case for survivin as a
regulator of microtubule dynamics and cell-death decisions. Curr
Opin Cell Biol. 18:609–615. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Altieri DC: Validating survivin as a
cancer therapeutic target. Nat Rev Cancer. 3:46–54. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zaffaroni N, Pennati M and Daidone MG:
Survivin as a target for new anticancer interventions. J Cell Mol
Med. 9:360–372. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Piras F, Perra MT, Murtas D, Minerba L,
Floris C, Maxia C, Demurtas P, Ugalde J, Ribatti D and Sirigu P:
Combinations of apoptosis and cell-cycle control biomarkers predict
the outcome of human melanoma. Oncol Rep. 20:271–277.
2008.PubMed/NCBI
|
|
20
|
Mirza A, McGuirk M and Hockenberry TN:
Human survivin is negatively regulated by wild-type p53 and
participate in p53-dependent apoptotic pathway. Oncogene.
21:2613–2622. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mees C, Nemunaitis J and Senzer N:
Transcription factors: their potential as targets for an
individualized therapeutic approach to cancer. Cancer Gene Ther.
16:103–112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iyer NG, Chin SF, Ozdag H, Daigo Y, Hu DE,
Cariati M, Brindle K, Aparicio S and Caldas C: p300 regulates
p53-dependent apoptosis after DNA damage in colorectal cancer cells
by modulation of PUMA/p21 levels. Proc Natl Acad Sci USA.
101:7386–7391. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sirigu P, Piras F, Minerba L, Murtas D,
Maxia C, Colombari R, Corbu A, Perra MT and Ugalde J: Prognostic
prediction of the immunohistochemical expression of p16 and p53 in
cutaneous melanoma: a comparison of two populations from different
geographical regions. Eur J Histochem. 50:191–198. 2006.
|
|
24
|
Ryan KM, O’Prey J and Vousden KH: Loss of
nuclear factor-κB is tumor promoting but does not substitute for
loss of p53. Cancer Res. 64:4415–4418. 2004.
|
|
25
|
Fridman JS and Lowe SW: Control of
apoptosis by p53. Oncogene. 22:9030–9040. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zuckerman V, Wolyniec K, Sionov RV, Haupt
S and Haupt Y: Tumour suppression by p53: the importance of
apoptosis and cellular senescence. J Pathol. 219:3–15.
2009.PubMed/NCBI
|
|
27
|
Olivier M, Hussain SP, Caron de Fromentel
C, Hainaut P and Harris CC: TP53 mutation spectra and load: a tool
for generating hypotheses on the etiology of cancer. IARC Sci Publ.
157:247–270. 2004.PubMed/NCBI
|
|
28
|
Culmsee C, Siewe J, Junker V, Retiounskaia
M, Schwarz S, Camandola S, El-Metainy S, Behnke H, Mattson MP and
Krieglstein J: Reciprocal inhibition of p53 and nuclear
factor-kappaB transcriptional activities determines cell survival
or death in neurons. J Neurosci. 23:8586–8595. 2003.PubMed/NCBI
|
|
29
|
Wang J, Ouyang W, Li J, Wei L, Ma Q, Zhang
Z, Tong Q, He J and Huang C: Loss of tumor suppressor p53 decreases
PTEN expression and enhances signaling pathways leading to
activation of activator protein 1 and nuclear factor-kappaB induced
by UV radiation. Cancer Res. 65:6601–6611. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shao J, Fujiwara T, Kadowaki Y, Fukazawa
T, Waku T, Itoshima T, Yamatsuji T, Nishizaki M, Roth JA and Tanaka
N: Overexpression of the wild-type p53 gene inhibits NF-kappaB
activity and synergizes with aspirin to induce apoptosis in human
colon cancer cells. Oncogene. 19:726–736. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Clark WH Jr, Elder DE, Guerry D IV,
Braitman LE, Trock BJ, Schultz D, Synnestvedt M and Halpern AC:
Model predicting survival in stage I melanoma based on tumor
progression. J Natl Cancer Inst. 81:1893–1904. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Greene FL, Page DL, Fleming ID, Fritz A,
Balch CM, Haller DG and Morrow M: American Joint Committee on
Cancer Staging Manual. 6th edition. Springer; Philadelphia: 2002,
View Article : Google Scholar
|
|
33
|
Montagut C, Tusquets I, Ferrer B,
Corominas JM, Bellosillo B, Campas C, Suarez M, Fabregat X, Campo
E, Gascon P, Serrano S, Fernandez PL, Rovira A and Albanell J:
Activation of nuclear factor-kappa B is linked to resistance to
neoadjuvant chemotherapy in breast cancer patients. Endocr Relat
Cancer. 13:607–616. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Z, Ma J, Li N, Sun N and Wang C:
Expression of nuclear factor-kappaB and its clinical significance
in non-small-cell lung cancer. Ann Thorac Surg. 82:243–248. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Levidou G, Korkolopoulou P, Nikiteas N,
Tzanakis N, Thymara I, Saetta AA, Tsigris C, Rallis G, Vlasis K and
Patsouris E: Expression of nuclear factor kappaB in human gastric
carcinoma: relationship with I kappaB a and prognostic
significance. Virchows Arch. 450:519–527. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Izzo JG, Malhotra U, Wu TT, Luthra R,
Correa AM, Swisher SG, Hofstetter W, Chao KS, Hung MC and Ajani JA:
Clinical biology of esophageal adenocarcinoma after surgery is
influenced by nuclear factor-kappaB expression. Cancer Epidemiol
Biomarkers Prev. 16:1200–1205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weichert W, Boehm M, Gekeler V, Bahra M,
Langrehr J, Neuhaus P, Denkert C, Imre G, Weller C, Hofmann HP,
Niesporek S, Jacob J, Dietel M, Scheidereit C and Kristiansen G:
High expression of RelA/p65 is associated with activation of
nuclear factor-kappaB-dependent signaling in pancreatic cancer and
marks a patient population with poor prognosis. Br J Cancer.
97:523–530. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lessard L, Karakiewicz PI, Bellon-Gagnon
P, Alam-Fahmy M, Ismail HA, Mes-Masson AM and Saad F: Nuclear
localization of nuclear factor-kappaB p65 in primary prostate
tumors is highly predictive of pelvic lymph node metastases. Clin
Cancer Res. 12:5741–5745. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fradet V, Lessard L, Bégin LR, Karakiewicz
P, Masson AM and Saad F: Nuclear factor-kappaB nuclear localization
is predictive of biochemical recurrence in patients with positive
margin prostate cancer. Clin Cancer Res. 10:8460–8464. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ross JS, Kallakury BV, Sheehan CE, Fisher
HA, Kaufman RP Jr, Kaur P, Gray K and Stringer B: Expression of
nuclear factor-kappa B and I kappa B alpha proteins in prostatic
adenocarcinomas: correlation of nuclear factor-kappa B
immunoreactivity with disease recurrence. Clin Cancer Res.
10:2466–2472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kleinberg L, Dong HP, Holth A, Risberg B,
Trope’ CG, Nesland JM, Flørenes VA and Davidson B: Cleaved
caspase-3 and nuclear factor-kappaB p65 are prognostic factors in
metastatic serous ovarian carcinoma. Hum Pathol. 40:795–806. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu X, Wang B, Ma X and Guo Y: NF-kappaB
activation through the alternative pathway correlates with
chemoresistance and poor survival in extranodal NK/T-cell lymphoma,
nasal type. Jpn J Clin Oncol. 39:418–424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kiriakidis S, Andreakos E, Monaco C,
Foxwell B, Feldmann M and Paleolog E: VEGF expression in human
macrophages is NF-kappaB-dependent: studies using adenoviruses
expressing the endogenous NF-kappaB inhibitor IkappaBalpha and a
kinase-defective form of the IkappaB kinase 2. J Cell Sci.
116:665–674. 2003. View Article : Google Scholar
|
|
44
|
Kashani-Sabet M, Shaikh L, Miller JR III,
Nosrati M, Ferreira CM, Debs RJ and Sagebiel RW: NF-kappa B in the
vascular progression of melanoma. J Clin Oncol. 22:617–623. 2004.
View Article : Google Scholar : PubMed/NCBI
|