Introduction
Anthracyclines demonstrate significant disease
activity in breast cancer and are a key component of the therapy
regimen in both early and advanced disease (1,2).
Despite its excellent antitumor activity, conventional doxorubicin
has a relatively low therapeutic index and its use is limited due
to the development of myelosuppression, alopecia, acute nausea and
vomiting, stomatitis and cumulative cardiotoxicity (3). Cardiotoxicity occurs more commonly and
at lower cumulative doses than previously thought. In one
retrospective analysis involving 630 doxorubicin-treated patients,
cardiac events were observed in 7, 9, 18, 38 and 65% of patients at
a cumulative doxorubicin dose of 150, 250, 350, 450 and 550
mg/m2, respectively (4).
Several factors increase the risk of developing irreversible
cardiotoxicity. These include the extent of anthracycline exposure,
age (both the very young and elderly have greater risk), a history
of cardiac disease, diabetes and previous cancer therapies, such as
mediastinal radiotherapy (RT) and concurrent use of chemotherapy
that includes paclitaxel or trastuzumab (5–8).
In these cases, pegylated liposomal doxorubicin
(PLD) is an attractive option. PLD has demonstrated comparable
efficacy to doxorubicin with a favorable toxicity profile. It is
associated with less alopecia, myelotoxicity and cardiac toxicity
than free doxorubicin, but with higher rates of palmar-plantar
erythrodysesthesia (PPE) and mucositis. At least two factors may
contribute to the lower cardiotoxicity associated with PLD: i)
changes in tissue distribution with less drug exposure to sensitive
organs, such as the heart muscle, and ii) slow release of the drug,
which may avoid high peak plasma concentrations (9).
Investigators have evaluated whether dose- and
schedule-dependent adverse events of PLD, such as stomatitis and
PPE, are able to be minimized. Subsequently, an empiric dose
reduction of PLD 40 mg/m2 every 4 weeks has been
suggested (10). Moreover, the
results of a phase I study in patients with advanced solid tumors
indicate that alternative dosing regimens may improve the
tolerability of PLD (11). One
retrospective analysis was performed to test the hypothesis that
toxicities decrease when lower doses of PLD at less frequent
intervals are administered (12).
Combination therapy with PLD is potentially
attractive because of its non-overlapping toxicity profile with
other agents commonly used in these settings, such as gemcitabine
(13,14), vinorelbine (15,16),
cyclophosphamide (17,18), paclitaxel (19–22)
and docetaxel (23–26). Response rates range between 31 and
75%, with a low incidence of symptomatic cardiac events.
Paclitaxel is one of the most established and active
anticancer drugs (27). Phase II
studies of paclitaxel showed significant antitumor activity in
various types of solid tumors, including ovarian, non-small cell
lung and head and neck. In previously treated patients with
metastatic breast carcinoma (MBC), paclitaxel produced objective
responses in 30–60% of the cases (28).
Investigators have examined ‘dose-dense’
chemotherapy, with drugs being administered more frequently, such
as once a week or every 2 weeks (29–32).
The administration of taxanes on a weekly schedule, while
maintaining the dose intensity of a 21-day schedule, showed a
marked reduction in grade 3–4 leukopenia and increased activity in
terms of response rate and time-to-progression.
These results showed that there is a rational basis
for the use of weekly PLD and paclitaxel as front-line therapy in
patients with MBC at high risk of cardiotoxicity.
Patients and methods
Patient eligibility
Female patients with histologically confirmed MBC
and with a high risk of cardiotoxicity (previous adjuvant
doxorubicin-based chemotherapy or previous RT to mediastinal and/or
left chest wall and/or hypertension) were eligible. Patients who
had received prior adjuvant anthracyclines were considered eligible
if the relapse occurred 12 months after conclusion of the adjuvant
chemotherapy. An Eastern Cooperative Oncology Group (ECOG)
performance status of 0–2, age >65 (<65 if high risk of
cardiotoxicity), life expectancy >3 months, no concurrent
uncontrolled medical illness, no other malignancies (with the
exception of squamous cell carcinoma of the skin treated by
surgery), baseline left ventricular ejection fraction (LVEF)
>50% and sufficient hepatic and bone marrow function were also
required. Patients were excluded if they had cardiac diseases,
including congestive heart failure, atrial or ventricular
arrhythmia, were pregnant or breast-feeding. Participants provided
written informed consent prior to enrollment, and the study
protocol was approved by the institutional ethics committees.
Chemotherapy
PLD 10 mg/m2 in 250 ml of 5% glucose
solution was administered as a 30-min infusion on Days 1, 8 and 15
every 4 weeks.
Paclitaxel in 250 ml of normal saline was
administered as a 1-h infusion weekly at a dose of 70
mg/m2. The patients were pre-medicated with
dexamethasone (4 mg), diphenhydramine (25 mg) and ranitidine (50
mg) 1 h prior to paclitaxel infusion to prevent hypersensitivity
reaction. Patients received standard antiemetic treatment with a
5HT3 antagonist (before the administration of cytotoxic drugs and
for 1 day after chemotherapy). All 35 patients received vitamin B6
(pyridoxine, 300 mg) orally once daily after lunch to prevent
PPE.
Treatment was administered on an outpatient basis
and was repeated if the absolute neutrophil count (ANC) was
>1,500/mmc, platelet count >100,000/mmc and non-hematologic
toxicities were resolved. G-CSF was permitted when ANC was
<500/mmc.
Toxicity assessment and definition
Toxicity was graded according to the National Cancer
Institute Common Toxicity Criteria (NCI-CTC 3.0). Cardiac toxicity
(defined as a decrease in LEVF >10% from the baseline) was based
on echocardiographic LEVF measurements that were performed at the
baseline and every 12 weeks and on 12-lead electrocardiogram that
was performed at the baseline and every 4 weeks. These measurements
were performed only during the treatment.
Dose modification of PLD and paclitaxel were
permitted for hematological toxicity, increases in total bilirubin,
cardiac toxicity and other NCI-CTC grade 3–4 events.
Assessment of efficacy and
definition
Efficacy was measured as the overall response rate
(complete and partial response) and overall survival (OS).
Responses were classified according to World Health
Organization criteria. Computed tomography scans of lesions were
carried out within 4 weeks prior to treatment commencing and
repeated every 3 cycles. Patients who discontinued the study were
evaluated for survival. Patients were assessable for response if
they had early disease progression or had received at least 3
cycles of treatment with at least one tumor assessment. OS was
measured from the time treatment commenced until patients succumbed
to any cause. Distribution of time-to-event was estimated by the
Kaplan-Meier product limit methods.
Results
Between June 2003 and November 2007, 35 female
patients with MBC and a high risk of cardiotoxicity were enrolled
in the study. The median age of the patients was 60 years (range
30–78), but 19 out of the 35 patients were >65 years of age.
ECOG performance status ranged from 0 to 2 (Table I). The majority of patients (80%)
had two or more sites of disease, with the most common one being
the bone (48.5%), lung (37.1%) and liver (34.2%). A large number of
patients (82.8%) were previously treated with adjuvant chemotherapy
and 18 were treated with adjuvant anthracycline-based chemotherapy.
The median dose of adjuvant doxorubicin or epidoxorubicin received
was 240 or 600 mg/m2, respectively. No patients were
pre-treated for advanced disease. All 35 patients were evaluable
for toxicity, while 31 were evaluable for efficacy.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Enrolled
patients | 35 | |
| Evaluable for
toxicity | 35 | 100% |
| Evaluable for
efficacy | 31 | 88.5% |
| Age (years) | 60 (median) | 30–78 (range) |
| ≥65 | 19 | 54.2% |
| Performance status
(ECOG) |
| 0 | 29 | 82.8% |
| 1 | 5 | 14.2% |
| 2 | 1 | 2.8% |
| Previous
treatments |
| Surgery | 34 | 97.0% |
| Adjuvant
chemotherapy | 29 | 82.8% |
| Adjuvant
anthracycline chemotherapy | 18 | 51.4% |
| Doxorubicin (dose
mg/m2) | 240 (median) | 160–240 (range) |
| Epidoxorubicin
(dose mg/m2) | 600 (median) | 400–600 (range) |
| Oestrogen and/or
progesterone receptor-positive | 26 | 74.2% |
| HER 2-positive | 5 | 14.2% |
| Sites of disease |
| Bone | 17 | 48.5% |
| Lung | 13 | 37.1% |
| Nodes | 12 | 34.2% |
| Liver | 12 | 34.2% |
| Skin/soft
tissue | 7 | 20.0% |
| Pleura | 2 | 5.7% |
| Breast | 1 | 2.8% |
| Ovary | 1 | 2.8% |
| No. of sites of
disease |
| >2 | 28 | 80.0% |
Toxicity
All 35 patients evaluable for safety received a
total of 175 cycles. The median number of cycles was 4 (range 1–9).
The median cumulative dose of PLD was 150 mg/m2 (range
30–270) and the median cumulative dose of paclitaxel was 1,400
mg/m2 (range 280–2,520). No patients required a dose
reduction of PLD or paclitaxel. Treatment was discontinued in one
patient after 1 cycle for grade 3 liver toxicity, possibly related
to the study treatment. No treatment interruptions or
discontinuations for cardiac toxicity occurred, and no
treatment-related deaths were reported. The toxicities are listed
in Table II. Leukopenia (12.5%)
was the most frequently reported grade 3–4 toxicity and grade 4
anemia was recorded in 1 patient (2.8%). Among the
non-hematological adverse events, 1 case (2.8%) of grade 3 liver
toxicity was reported, as well as 1 case (2.8%) of grade 3
mucositis and 3 cases (8.5%) of grade 3 PPE G3. Grade 3 hair loss
occurred in only 2.8% of patients.
 | Table IIToxicity.a |
Table II
Toxicity.a
| Type of toxicity | Grade 1–2
(%)(n=35) | Grade 3–4
(%)(n=35) |
|---|
| Non-hematologic |
| PPE | 14.2 | 8.5 |
| Diarrhea | 8.5 | - |
| Mucositis | 2.8 | 2.8 |
| Nausea/vomiting | 10.0 | - |
| Asthenia | 7.8 | - |
| AST/ALT | - | 2.8 |
| Hair loss | 82.7 | 2.8 |
| Neurotoxicity | 17.1 | - |
| Hematologic |
| Leukopenia | 14.7 | 12.5 |
| Anemia | 13.5 | 2.8 |
|
Thrombocytopenia | 2.8 | - |
No abnormal electrocardiograms were documented
during treatment. No decrease in LEVF >10% from the baseline was
observed.
Efficacy
A total of 4 complete (12.9%; 95% CI 11–24.6) and 16
partial responses (51.6%; 95% CI 34.1–69) were achieved in 31
evaluable patients for an overall response rate of 64.5% (95% CI
47.7–81.3). The median duration of complete and partial response
was 13 months (range 1–14) and 15 months (range 4–36),
respectively. A total of 6 patients had stable disease (19.3%; 95%
CI 5–33) with a median duration of 7 months (range 6–18), and 5
patients had progressive disease (16.1%; 95% CI 4–29). The median
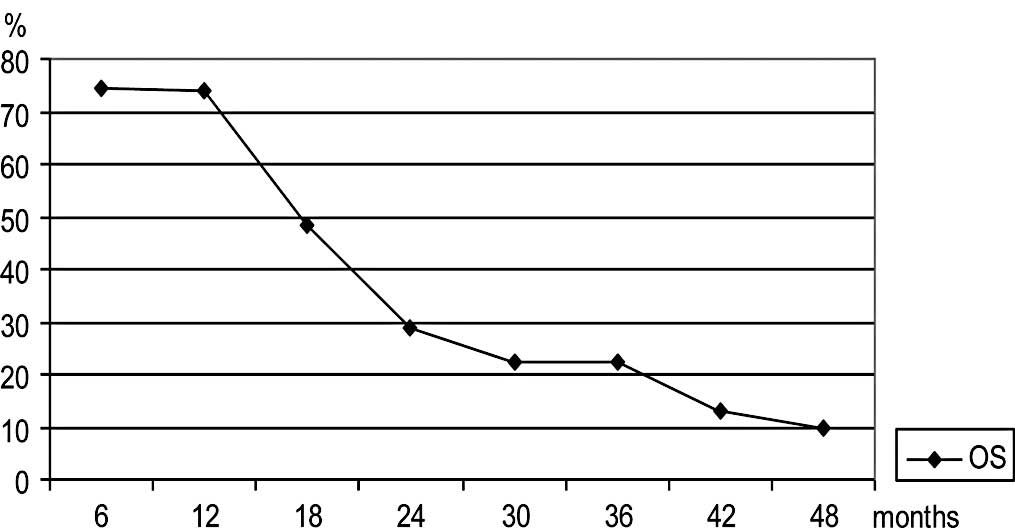
OS for all 31 evaluable patients was 18 months (range 5–64) with a
median OS of 24 months for patients with complete response (range
5–47 months) and median OS of 21 months for patients with partial
response (range 9–64 months). Patients with stable or progressive
disease had a median survival of 12 months (range 5–40) and 12
months (range 6–22), respectively. No relationship was noted
between patients pre-treated or not with anthracycline-based
chemotherapy. Response and survival are reported in Tables III and
IV and in Fig. 1.
Discussion
Despite advances in the early detection and adjuvant
treatment of early stage disease, breast cancer remains a
significant health problem. The outlook for patients with MBC is
generally poor, with only 10% expected to survive 10 years after
the diagnosis. The management of patients with MBC continues to
evolve, but there remains considerable room for improvement. In
recent years, many new chemotherapeutic agents have improved the
efficacy and tolerability of chemotherapy.
In this study, a weekly schedule of both paclitaxel
and PLD was selected to improve efficacy and tolerability. Weekly
administration of paclitaxel was evaluated in numerous studies, and
improvement in efficacy and tolerability was demonstrated (?).
Perez et al reported their findings of PLD
administered at less frequent intervals to improve tolerability
(12).
Moreover, the limited data on the safety and
efficacy of PLD in elderly women with MBC are thought-provoking.
Results of a phase II trial in MBC patients 65 years of age or
older were recently reported by the European Organisation for
Research and Treatment of Cancer. Efficacy and safety were
comparable to that reported in other phase II and III studies
suggesting that PLD is a useful alternative in elderly patients
with MBC (33).
Herein, we report the results of efficacy and
tolerability in a phase II study with weekly administration of both
paclitaxel and PLD as first-line treatment in women with MBC. The
enrolled patients were considered to be high risk for
cardiotoxicity due to their age (over 65 years) and/or previous
treatment with adjuvant anthracycline-based chemotherapy, previous
treatment with RT on the left chest wall and/or hypertension. In
this setting of patients we reported an overall response rate of
64.5%. The median duration of complete and partial response was 13
and 15 months, respectively. The median OS was 18 months. The
results achieved in this study are similar to those reported in the
literature, which range overall response between 31 and 75% in
combination therapy trials.
Regarding the safety profile, the combination of
drugs used in the present study appear to show a favorable profile
with low incidence of grade 3–4 toxicities. It is hypothesized that
the weekly administration of PLD and paclitaxel should reduce the
incidence and severity of adverse events. Notably, in patients that
were over 65 years of age (up to 78 years) and in those considered
to be high risk for cardiotoxicity, we observed no cardiac
toxicity, including LEVF declines over 10%. The improved cardiac
safety of PLD was confirmed by two larger scale studies. The first
of these, conducted by Safra et al, was a retrospective
review of patients with solid tumors who had received cumulative
doses of PLD ranging from 500 to 1,500 mg/m2 without a
significant decrease in LEVF (34).
A second large scale study evaluated the progression-free survival
and cardiac safety of patients receiving PLD or conventional
doxorubicin as first-line therapy for MBC. Although
progression-free survival was similar for the two treatment groups,
the incidence of cardiac toxicity was significantly lower with PLD
(35).
In conclusion, several clinical studies support the
use of PLD in patients with MBC (10–12).
Moreover, there is growing evidence supporting the use of PLD
monotherapy or combination regimens as first-line treatment for
women with MBC. In the population of women with MBC and with high
risk for cardiotoxicity, weekly paclitaxel plus PLD regimen appears
to be a well-tolerated and effective treatment modality.
References
|
1
|
Early Breast Cancer Trialist Group
(EBCTCG). Effect of chemotherapy and hormonal therapy for early
breast cancer on recurrence and 15-year survival: an overview of
the randomized trials. Lancet. 365:1687–1717. 2005.PubMed/NCBI
|
|
2
|
Hortobagyi GN: Anthracyclines in the
treatment of cancer: an overview. Drugs. 54(Suppl 4): 1–7.
1997.
|
|
3
|
Von Hoff D, Layard M, Basa P, Davcis HL,
von Hoff AL, Rozencweig M and Muggia FM: Risk factors for
doxorubicin-induced congestive heart failure. Ann Inter Med.
91:710–717. 1979.PubMed/NCBI
|
|
4
|
Swain S, Whaley FS and Ewer MS: Congestive
heart failure in patients treated with doxorubicin: a retrospective
analysis of three trials. Cancer. 97:2869–2879. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pai VB and Nahata MC: Cardiotoxicity of
chemotherapeutic agents: incidence, treatment and prevention. Drug
Saf. 22:263–302. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balmer C and Valley AW: Basic principles
of cancer treatment and cancer chemotherapy. Pharmacotherapy: A
Pathophysiology Approach. 3rd edition. Di Piro JT, Talbert RL, Yee
GC, et al: Appleton and Lange; Stanford: pp. 2403–2465. 1997
|
|
7
|
Waterhouse DN, Tardi PG, Mayer Ld and
Balli MB: A comparison of liposomal formulation of doxorubicin with
drug administered in free form: changing toxicity profiles. Drug
Saf. 24:903–920. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maluf FC and Spriggs D: Anthracyclines in
the treatment of gynecologic malignancies. Gynecol Oncol. 85:18–31.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gabizon AA: Liposomal anthracyclines.
Hematol Oncol Clin North Am. 8:431–450. 1994.PubMed/NCBI
|
|
10
|
Rivera E: Liposomal anthracyclines in
metastatic breast cancer: Clinical update. Oncologist. 8(Suppl 3):
3–9. 2003. View Article : Google Scholar
|
|
11
|
Androulakis N, Kouroussis C, Mavroudis D,
Kakolyris S, Souglakos J, Agelaki S, Kalbakis K, Malas K, Pallis A,
Samonis G and Georgoulias V: Phase I study of weekly paclitaxel and
liposomal doxorubicin in patients with advanced solid tumours. Eur
J Cancer. 38:1992–1997. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perez AT, Domenech GH, Frankel C and Vogel
CL: Pegylated liposomal doxorubicin (Doxil) for metastatic breast
cancer: the Cancer Research Network, Inc. experience Cancer Invest.
20(Suppl 2): 22–29. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rivera E, Valero V, Arun B, Royce M,
Adinin R, Hoelzer K, Walters R, Wade JL, Pusztai L and Hortobagyi
GN: Phase II study of pegylated liposomal doxorubicin in
combination with gemcitabine in patients with metastatic breast
cancer. J Clin Oncol. 21:3249–3254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fabi A, Ferretti G, Papaldo P, Salesi N,
Ciccaresi M, Lo russo V, Carlini P, Carpino A, Mottolese M,
Cianciulli AM, Giannarelli D, Sperduti I, Felici A and Cognetti F:
Pegylated liposomal doxorubicin in combination with gemcitabine: a
phase II study in anthracyclines-naïve and anthracyclines
pretreated metastatic breast cancer patients. Cancer Chemother
Pharmacol. 57:615–623. 2006.
|
|
15
|
Gebbia V, Mauceri G, Fallica G, Borsellino
N, Tirrito ML, Testa A, Varvara F, Colombo A and Ferrera P:
Pegylated liposomal doxorubicin in combination with vinorelbine in
metastatic breast carcinoma. Oncology. 63:23–30. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ardavanis A, Mavroudis D, Kalbakis K,
Malamos N, Syrigos K, Vamvakas L, Kotsakis A, Kentepozidis N,
Kouroussis C, Agelaki S and Geogoulias V: Pegylated liposomal
doxorubicin with vinorelbine as salvage treatment in pretreated
patients with advanced breast cancer: a multicentre phase phase II
study. Cancer Chemother Pharmacol. 58:742–748. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Overmoyer B, Silverman P, Holder LW,
Tripathy D and Henderson IC: Pegylated liposomal doxorubicin and
cyclophophamide as first line therapy for patients with metastatic
or recurrent breast cancer. Clin Cancer. 6:150–157. 2005.PubMed/NCBI
|
|
18
|
Vandenberg TA, Trudeau M, Provencher L,
Panasci LC, Yelle L, Rayson D, Latreille J, Clemons M, Giroux M and
Pouliot J: Pegylated liposomal doxorubicin (PLD) with
cyclophosphamide (c) as 1st-line chemotherapy for metatstatic
breast cancer (MBC) patients previously treated with adjuvant
anthracyclines. J Clin Oncol. 24:106272006.
|
|
19
|
Rigatos SK, Tsavdaridis D, Athananasiadis
A, Stathopoulos JG and Stathopoulos GP: Paclitaxel and liposomal
doxorubicin (Caelyx) combination in advanced breast cancer
patients: A phase II study. Oncol Rep. 10:1817–1819.
2003.PubMed/NCBI
|
|
20
|
Simoncini E, Ferrari VD, Amoroso V,
Valcamonico F, Grisanti S, Vassalli L, Marpicati P, Montini E,
Rangoni G and Marini G: Bi-weekly administration of pegylated
liposomal doxorubicin plus paclitaxel in metastatic breast cancer
(MBC) patients: a phase II study. J Clin Oncol. 23:8442005.
|
|
21
|
Vorobiof DA, Rapoport BL, Chasen MR,
Slabber C, McMichael G, Eek R and Mohammed C: First line therapy
with paclitaxel (Taxol) and pegylated liposomal doxorubicin
(Caelyx) in patients with metastatic breast cancer: a multicentre
phase II study. Breast. 13:219–226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rossi D, Baldelli AM, Casadei V, Fedeli
SL, Alessandroni P, Catalano V, Giordani P, Ceccolini M, Graziano F
and Catalano G: Neoadjuvant chemotherapy with low dose of pegylated
liposomal doxorubicin plus weekly paclitaxel in operable and
locally advanced breast cancer. Anti-Cancer Drugs. 19:733–737.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alexopoulos A, Karamouzis MV, Stavrinides
H, Ardavanis A, Kandilis K, Stavrakakis J, Georganta C and Rigatos
G: Phase II study of pegylated liposomal doxorubicin (Caelyx) and
docetaxel as first line treatment in metastatic breast cancer. Ann
Oncol. 15:891–895. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morabito A, Gattuso D, Stani SC, Fanelli
M, Ferraù F, De Sio L, Castellana MA, Lorusso V, Priolo D, Vitale
S, Mariani L and Gasperini G: Safety and activity of the
combination of pegylated liposomal doxorubicin and weekly docetaxel
in advanced breast cancer. Breast Cancer Res Treat. 86:249–257.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sparano JA, Makhson AN, Semiglazov VF, et
al: Pegylated liposomal doxorubicin plus docetaxel improves time to
progression without additive cardiotoxicity compared with docetaxel
monotherapy in patients with advanced breast cancer previously
treated with neoadjuvant-adjuvant anthracycline therapy: results
from randomized phase III study. J Clin Oncol. 27:4522–4529.
2009.
|
|
26
|
De la Fouchardiere, Largillier R, Goubely
Y, Hardy-Bessard AC, Slama B, Cretin J, Orfeuvre H, Paraiso D,
Bachelot T and Pujade-Lauraine E: Docetaxel and pegylated liposomal
doxorubicin combination as first-line therapy for metastatic breast
cancer patients: results of the phase II GINECO trial CAPYTTOLE.
Ann Oncol. 20:1959–1963. 2009.PubMed/NCBI
|
|
27
|
Vogel CL: Salvage chemotherapy of breast
cancer. Semin Oncol. 21:19–24. 1996.
|
|
28
|
Holmes FA, Walters RS, Theriault RL,
Buzdar AU, Frye DK, Hortobagyi GN, Forman AD, Newton LK and Raber
MN: Phase II trial of Taxol, an active drug in the treatment of
metastatic breast cancer. J Natl Cancer Inst. 83:1797–1805. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Seidman AD, Hudis C, Albanel J, Tong W,
Tepler I, Currie V, Moynahan ME, Theodoulou M, Gollub M, Baselga J
and Norton L: Dose-dense therapy with weekly-1-hour paclitaxel
infusion in the treatment of metastatic breast cancer. J Clin
Oncol. 16:3353–3361. 1998.PubMed/NCBI
|
|
30
|
Perez EA, Irwin DH, Patel R, Vogel CL and
Kirshner J: A large phase II trial of paclitaxel administered as a
weekly one hour infusion in patients with metastatic breast cancer.
Proc ASCO. 18:1261999.
|
|
31
|
Loffler TM: Is there a place for
‘dose-dense’ weekly schedules of the taxoids? Sem Oncol. 25(Suppl
12): 32–34. 1998.
|
|
32
|
Verrill MW, Lee J, Cameron DA, Agrawal R,
Cole Ferrigan E and Yellowlees A: Anglo-Celtic IV: first results of
a UK National Cancer Research Network randomized phase III
pharmacogenetic trial of weekly compared to 3 weekly paclitaxel in
patients with locally advanced or metastatic breast cancer (ABC). J
Clin Oncol. 25:LBA10052007.
|
|
33
|
Coleman RE, Biganzoli L, Canney P, Dirix
L, Mauriac L, Chollet P, Batter V, Ngalula-Kabanga E, Dittrich C
and Piccart M: A randomised phase II study of two different
schedules of pegylated liposomal doxorubicin in metastatic breast
cancer (EORTC 10993). Eur J Cancer. 42:882–887. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Safra T, Muggia F, Jeffers S, Tsao-Wei DD,
Groshen S, Lyass O, Henderson R, Berry G and Gabizon A: Pegylated
liposomal doxorubicin (Doxil): reduced clinical cardiotoxicity in
patients reaching or exceeding cumulative doses of 500
mg/m2. Ann Oncol. 11:1029–1033. 2000. View Article : Google Scholar
|
|
35
|
O’Brien MER, Wigler N, Inbar M, Rosso R,
Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP,
Orlandi F, Mellars L, Alland L and Tendler C: Reduced cardiac
toxicity and comparable efficacy in a phase III trial of pegylated
liposomal doxorubicin HCL (Caelyx/Doxil) versus conventional
doxorubicin for first-line treatment of metastatic breast cancer.
Ann Oncol. 15:440–449. 2004.
|