Introduction
Thymoma is a rare tumor derived from epithelial
cells of the thymus. It is considered to be malignant due to the
occasional invasion to surrounding organs and dissemination.
Surgery remains the treatment of choice for thymoma, and
postoperative radiation has been used as adjuvant therapy (1–3).
Induction chemotherapy or chemoradiotherapy was suggested to
improve outcomes, especially in advanced disease (4–7).
However, the benefits of adjuvant radiotherapy in patients with
Masaoka stage II disease (1,2,8)
are controversial. The histological classification of thymoma
remained controversial for a long period of time (9), but in 1999 the World Health
Organization (WHO) Consensus Committee published a histological
typing system for tumors of the thymus (10). Thymomas are now identified as A, AB,
B1, B2 and B3 on the basis of the morphology of epithelial cells
and the ratio of lymphocytes to epithelial cells. Numerous studies
suggested that the WHO histological classification is useful for
the prediction of outcomes (11–13).
The addition of this new prognostic factor has therefore created
the need to reevaluate treatment strategies for thymomas.
Patients and methods
Between 1985 and 2001, 73 thymomas, unassociated
with myasthenia gravis (MG), were completely resected at the
Kanagawa Cancer Center. Patients who had thymic cancer, carcinoids
or non-curative surgery were excluded from this study. The extent
of resection was determined by the operating surgeons on the basis
of the patients’ condition and extent of tumor. Thymomas were
categorized according to the WHO classification (10). The histological diagnosis was based
on the most significant component of each tumor. Pathological
staging was performed according to the Masaoka staging system
(8). In this retrospective study,
the clinicopathological and prognostic relevance of the WHO
histological classification for thymomas were examined, including
the variables of age, gender, operation procedure, Masaoka staging
system (I and II vs. III and IV) and WHO histological
classification (A and AB vs. B1, B2 and B3; A, AB and B1 vs. B2 and
B3). The surgical procedure for thymoma was classified into 3
groups: extended thymectomy (resection of the thymus, including the
thymoma and the anterior mediastinal adipose tissue), thymectomy
(resection of the thymus, including the thymoma) and tumor
resection (resection of the tumor and a portion of the thymus)
(14). Kaplan-Meier curves were
plotted to evaluate overall and disease-free survival, and the
log-rank test was used to compare survival between the groups. Each
variable was tested by the Chi-square and Fisher’s exact tests. The
Cox regression analysis was used for multivariate analysis of
survival, performed with Stat View for Windows (version 5.0; SAS
Institute Inc., Cary, NC, USA). Significance was defined as a
p<0.05. Our institutional internal review board approved this
retrospective study.
Results
The study group comprised 30 men and 43 women. Their
median age was 59 years (range 18–86). Median follow-up was 80
months. Table I shows the clinical
characteristics of the 73 patients. The overall survival rate was
89.8% at 5 years and 66.2% at 10 years. Median survival time (MST)
was 169 months. A total of 16 patients succumbed to the disease,
with only 3 patients deceased from tumor recurrence. The
recurrences were locoregional or intrathoracic. Overall survival
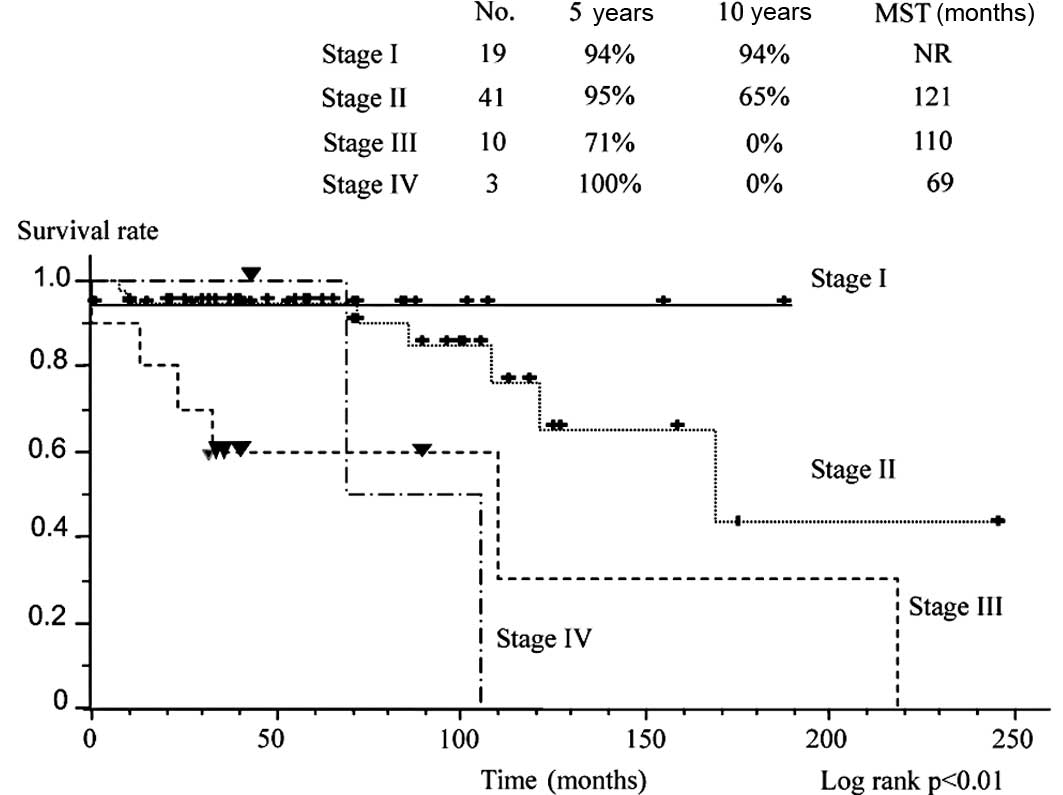
curves are plotted according to stage (Fig. 1). Survival was significantly better
in patients with stage I and II disease than in those with stage
III and IV disease. Fig. 2 shows
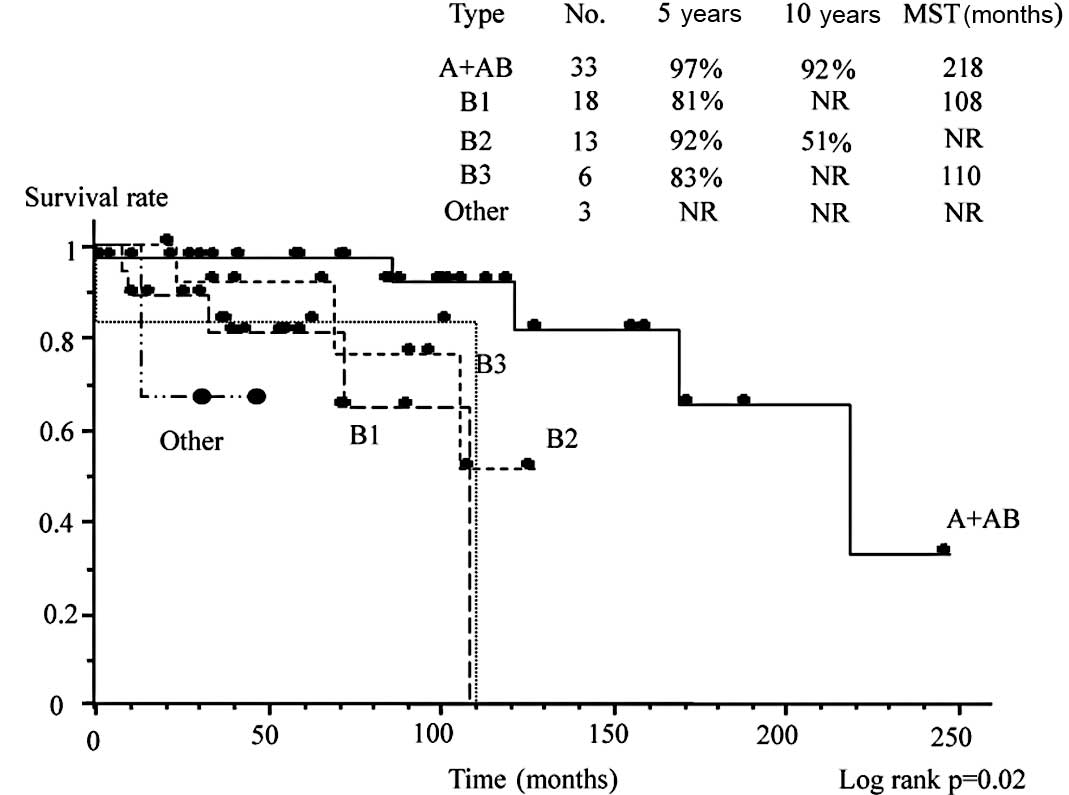
survival according to the WHO classification. WHO type A and AB
disease was associated with a more favorable survival than types
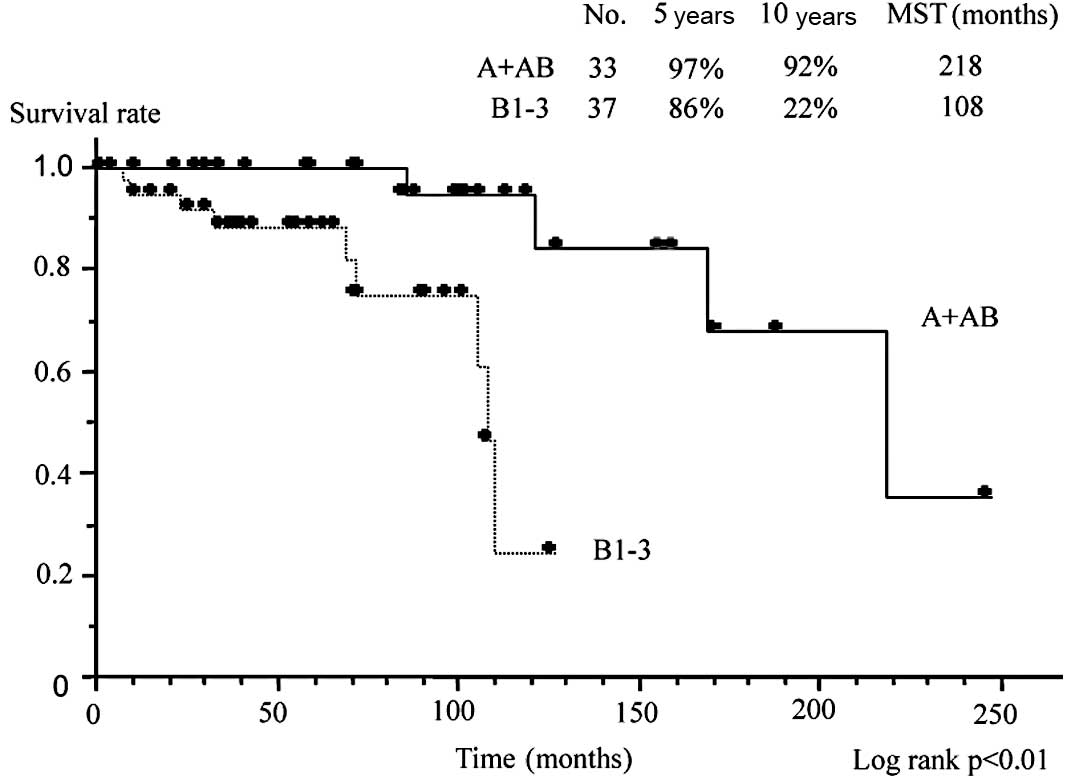
B1, B2 and B3. Fig. 3 compares
survival between patients with type A or AB disease compared to
those with type B1, B2 or B3 disease. In the former group, the
overall survival rate was 97% at 5 years and 92% at 10 years, with
an MST of 218 months. By contrast, the overall survival rate in the
latter group was only 86% at 5 years and 22% at 10 years, with an
MST of 108 months. Survival was significantly worse in type B1, B2
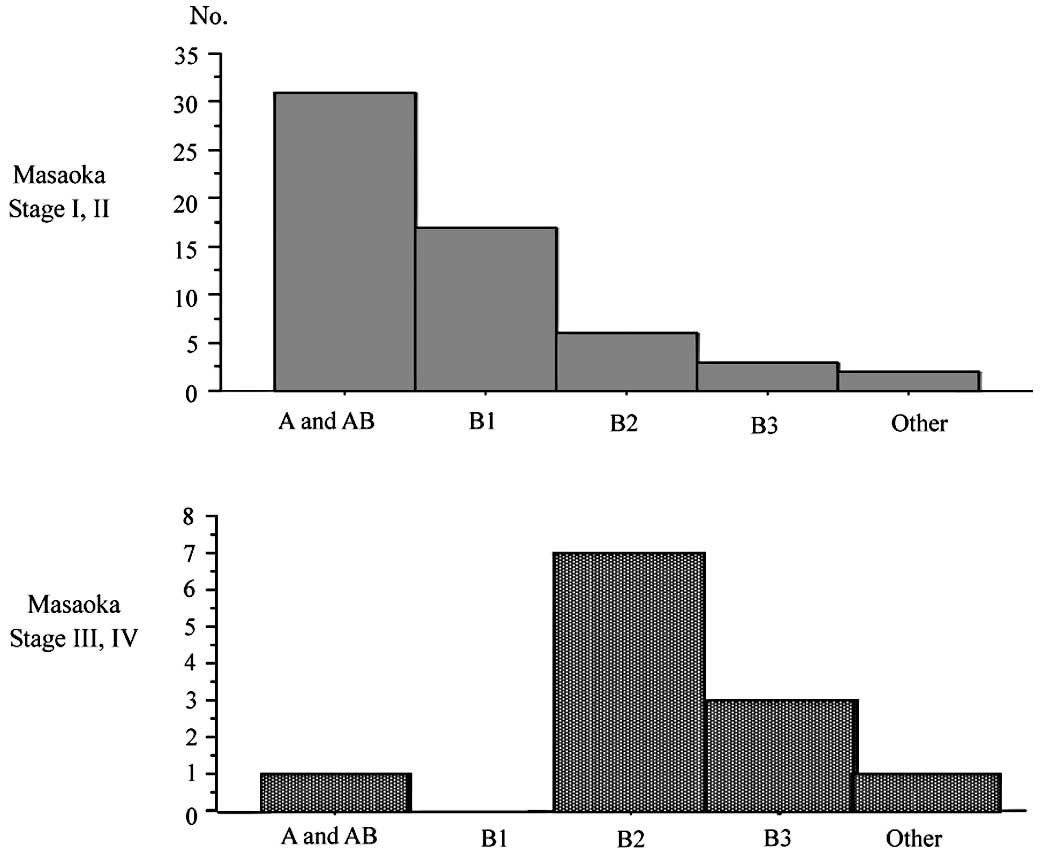
and B3 disease than in type A and AB (p<0.01). Fig. 4 shows the distribution of patients
according to the Masaoka staging system and WHO classification. A
total of 81% of patients (48 cases) with stage I or II disease
according to the Masaoka staging system had type A or AB disease
according to WHO classification, and 83% of patients (10 cases)
with stage III or IV disease were classified as having type B2 or
B3 disease. Histologically, advanced thymomas were significantly
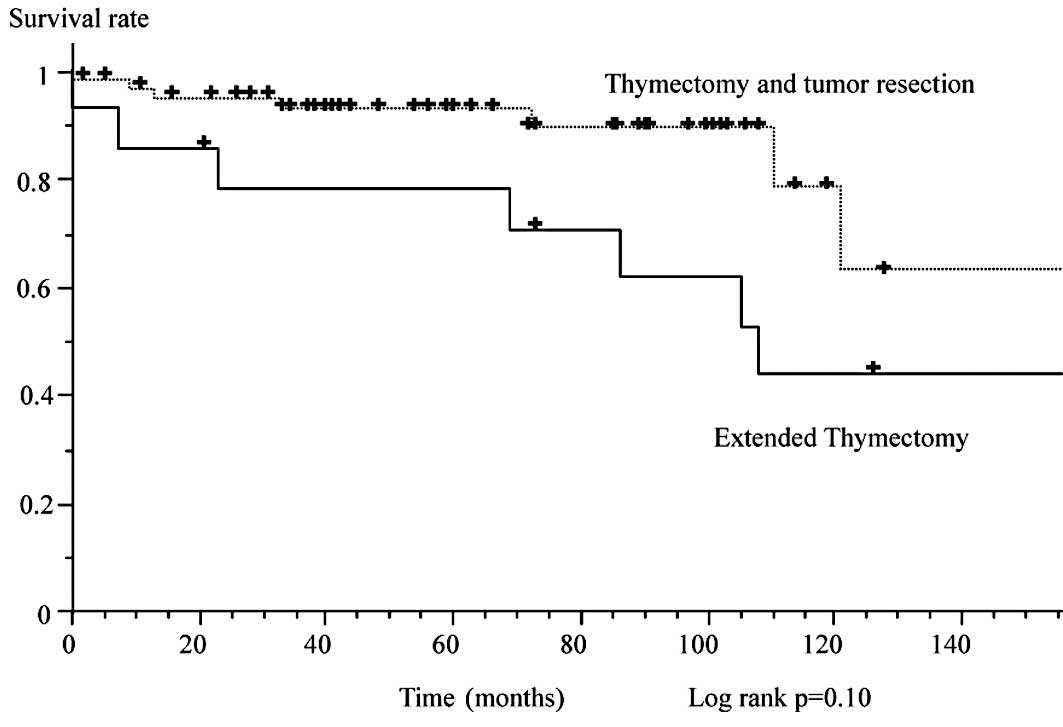
associated with type B2 and B3 disease (p<0.01). Fig. 5 shows survival according to the
extent of surgery. Extended thymectomy was associated with
significantly worse survival than thymectomy and tumor resection.
No differences were noted between total thymectomy and tumor
resection. On multivariate analysis, stage III and IV disease and
extended thymectomy were adverse, independent risk factors for
survival (p<0.01). No recurrence occurred among patients with
type A, AB or B1 disease (Fig.
6).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Age | 59.0 (18–86) |
| Gender | male 30, female
43 |
| Masaoka stage |
| I | 19 |
| II | 41 |
| III | 10 |
| IV | 3 |
| WHO
classification |
| Type A | 3 |
| Type AB | 30 |
| Type B1 | 18 |
| Type B2 | 13 |
| Type B3 | 6 |
| Others | 3 |
| Extent of
surgery |
| Extended
thymectomy | 14 |
| Thymectomy | 47 |
| Tumor resection | 12 |
Adjuvant radiotherapy in stage II disease conferred
no survival advantage as compared to no adjuvant radiotherapy
(survival rate at 5 years, 95 vs. 75%, p=0.17). In stage III and IV
disease, however, adjuvant or neoadjuvant therapy was associated
with significantly better survival (100% at 5 years) than no
adjuvant therapy (6 patients, 66.7%, p=0.07). Complete remission
was observed in two patients in response to induction chemotherapy
(cisplatin, vincristine, doxorubicin and etoposide). No viable
tumor cells were noted in the resected specimens.
MG developed postoperatively in 5 (6.8%) of the 73
patients. One patient each had stage I, III and IV disease, and 2
had stage II disease. According to the WHO classification, 1
patient had type AB disease, 2 had B1 and 2 patients had B2
disease. The mean time to the onset of MG was 16.4 months after
surgery (range 5–31), with no recurrence of thymoma. The incidence
of postoperative MG did not differ among the operative procedures
(extended thymectomy, thymectomy and tumor resection; p>0.99).
In 36 patients, anti-acetylcholine receptor antibodies were
measured pre-operatively and were abnormally elevated in only 1
(50%) of 2 patients with postoperative MG and 7 (21%) of 34
patients without postoperative MG; a difference that was not
significant (Fisher’s exact test, p=0.40). The incidence of
postoperative complications did not differ significantly among
extended thymectomy, thymectomy and tumor resection (15.4 vs.
12.8%, p>0.99), but the operation time of extended thymectomy
was significantly longer than with the other procedures (average
240 vs. 178 min, p=0.01).
Discussion
A number of thymomas are clinically asymptomatic
(1) and difficult to detect on
routine radiographic examinations at medical checkups due to their
location. At the time of diagnosis, thymomas are often large. Tumor
size is an important variable for staging many solid tumors
(14), but not thymoma. In the
Masaoka staging system, which is used internationally, staging is
based primarily on the extent of tumor cells, i.e., invasion of the
capsule and surrounding organs, dissemination of tumor cells and
metastasis are the most important variables (8). This staging system is well known for
its satisfactory relation to outcomes (1,2,8). In
general, stage I and II disease exhibit favorable outcomes, where
stage III and IV disease are occasionally associated with local
recurrence, leading to the patient succumbing to the disease
(2,8). Histological classifications were
controversial (9) until the
establishment of the WHO classification. Various studies have shown
that the WHO classification is strongly related to outcomes,
similar to the Masaoka staging system (8). In this series, we confirmed that type
A and AB disease, according to the WHO classification was
associated with a more favorable overall survival than was type B1,
B2 and B3 disease. None of the patients with type B1 disease
succumbed. Type A, AB and B1 disease, thus, had more favorable
outcomes than type B2 and B3, consistent with the results of
previous studies (11–13,15,16).
Surgery remains the mainstay of treatment for
thymomas, with thymectomy as the standard procedure (1). Thymomas can be accompanied by
autoimmune disease, such as MG. Extended thymectomy is one surgical
procedure for the treatment of MG (17). In previous studies, the incidence of
postoperative MG in patients with thymoma without MG ranged from
1.5 to 28% (18–20). We previously performed extended
thymectomy in patients with thymoma to prevent the postoperative
development of MG. In this series, however, extended thymectomy was
not more effective than standard procedures. Data from Japan
indicate that resection of the thymus gland does not prevent
postoperative MG (20). In our
series, the results of pre-operative tests for anti-acetylcholine
receptor antibodies did not predict the postoperative risk of MG.
Thus, the use of extended thymectomy for thymomas remains
controversial. No differences in postoperative complications among
surgical procedures occurred, but prolonged surgery time may lead
to increased costs and surgical stress on patients. Therefore, to
ensure a definite surgical margin, total thymectomy appears to be
the most appropriate procedure for thymomas.
The main site of recurrence of thymomas is the
thoracic cavity. Adjuvant radiotherapy of the mediastinum were
suggested to be useful for the decrease of the risk of local
recurrence, even in stage II disease (8,21). In
our study, adjuvant or neoadjuvant therapy did not improve overall
or disease-free survival in patients with stage II thymoma,
suggesting that adjuvant radiotherapy is not therapeutically useful
for stage II disease. By contrast, adjuvant or neoadjuvant therapy
was associated with more favorable outcomes in patients with stage
III and IV thymomas. Numerous studies reported that induction
therapy produces favorable results in advanced thymoma (2,4–7). In
our series, 2 patients had complete pathological responses to
induction chemotherapy. Induction therapy is thus anticipated to
play a more significant role in the treatment of advanced thymomas.
However, whether induction therapy should be indicated for the
treatment of resectable stage III thymomas remains a matter of
debate. In patients with unresectable thymomas, induction therapy
can produce favorable outcomes. Nonetheless, prospective studies
are required to confirm whether induction therapy is
therapeutically useful.
In conclusion, the outcomes of surgery for thymomas
are satisfactory. Patients with Masaoka stage I or II disease or
WHO type A or AB disease have favorable prognoses and do not
require adjuvant therapy. Patients with stage III or IV thymomas
should receive adjuvant therapy in addition to surgery. As for the
extent of surgery, thymectomy is the procedure of choice for the
management of thymomas.
References
|
1
|
Shields TW: Thymic tumors. Tymic Tumors.
Shields TW, Locicero J III and Ponn RB: General thoracic surgery.
fifth edition. Lippincott Williams and Wilkins; Philadelphia: pp.
2181–2205. 2000
|
|
2
|
Cowen D, Richaud P, Mornex F, et al:
Thymoma. Results of a multicentric retrospective series of 149
non-metastatic irradiated patients and review of the literature
FNCLCC trialists Federation Nationale des Centres de Lutte Contre
le Cancer. Radiother Oncol. 34:9–16. 1995. View Article : Google Scholar
|
|
3
|
Hejna M, Haberl I and Raderer M:
Nonsurgical management of malignant thymoma. Cancer. 85:1871–1884.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Igawa S, Murakami H, Takahashi T, et al:
Efficacy of chemotherapy with carboplatin and paclitaxel for
unresectable thymic carcinoma. Lung Cancer. 67:194–197. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim ES, Putnam JB, Komaki R, et al: Phase
II study of a multidisciplinary approach with induction
chemotherapy, followed by surgical resection, radiation therapy,
and consolidation chemotherapy for unresectable malignant thymomas:
final report. Lung Cancer. 44:369–379. 2005. View Article : Google Scholar
|
|
6
|
Rea F, Sartori F, Loy M, Calabrò F,
Fornasiero A, Daniele O and Altavilla G: Chemotherapy and operation
for invasive thymoma. J Thorac Cardiovasc Surg. 106:543–549.
1993.PubMed/NCBI
|
|
7
|
Berruti A, Borasio P, Gerbino A, et al:
Primary chemotherapy with adriamycin, cisplatin, vincristine and
cyclophosphamide in locally advanced thymomas: a single institution
experience. Br J Cancer. 81:841–845. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Masaoka A, Monden Y, Nakahara K and
Tanioka T: Follow-up study of thymomas with special reference to
their clinical stages. Cancer. 48:2485–2492. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimosato Y: Controversies surrounding the
subclassification of thymoma. Cancer. 74:542–544. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosai J: World Health Organization
International Histological Classification of Tumours. Histological
Typing of Tumours of the Thymus. 2nd edition. Springer-Verlag; New
York: 1999, View Article : Google Scholar
|
|
11
|
Rena O, Papalia E, Maggi G, et al: World
Health Organization histologic classification: an independent
prognostic factor in resected thymomas. Lung Cancer. 50:59–66.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kondo K, Yoshizawa K, Tsuyuguchi M, et al:
WHO histologic classification is a prognostic indicator in thymoma.
Ann Thorac Surg. 77:1183–1188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okumura M, Ohta M, Tateyama H, et al: The
World Health Organization histologic classification system reflects
the oncologic behavior of thymoma: a clinical study of 273
patients. Cancer. 94:624–632. 2002. View Article : Google Scholar
|
|
14
|
Pollock RE, Doroshow JH, Khayat D, et al:
UICC Manual of Clinical Oncology. 8th edition. Wiley-Liss; NY:
2004
|
|
15
|
Zisis C, Rontogianni D, Tzavara C, et al:
Prognostic factors in thymic epithelial tumors undergoing complete
resection. Ann Thorac Surg. 80:1056–1062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Detterbeck FC: Clinical value of the WHO
classification system of thymoma. Ann Thorac Surg. 81:2328–2334.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Masaoka A, Yamakawa Y, Niwa H, et al:
Extended thymectomy for myasthenia gravis patients: a 20-year
review. Ann Thorac Surg. 62:853–859. 1996.PubMed/NCBI
|
|
18
|
Namba T, Brunner NG and Grob D: Myasthenia
gravis in patients with thymoma, with particular reference to onset
after thymectomy. Medicine. 57:411–433. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosenow EC III and Hurley BT: Disorders of
the thymus. A review. Arch Intern Med. 144:763–770. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kondo K and Monden Y: Myasthenia gravis
appearing after thymectomy for thymoma. Eur J Cardiothorac Surg.
28:22–25. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakahara K, Ohno K, Hashimoto J, et al:
Thymoma: results with complete resection and adjuvant postoperative
irradiation in 141 consecutive patients. J Thorac Cardiovasc Surg.
95:1041–1047. 1988.PubMed/NCBI
|