Introduction
Leukemia is one of the causes of cancer-related
death in humans (1,2). According to the Department of Health,
Executive Yuan, Taiwan R.O.C. (2010), approximately 4.2 per 100,000
people in Taiwan succumb to leukemia each year (3,4).
Currently, treatment of leukemia in clinics includes hematopoietic
stem cell transplantation, radiotherapy and chemotherapy agents;
however, these outcomes are not fully satisfactory (5–7). Thus,
numerous studies have been focused on discovering a novel compound
from natural products that blocks the development of cancer,
including leukemia. The most effective strategy for killing cancer
cells is to induce apoptosis. Evidence has shown that increased
consumption of a plant-based diet may reduce the risk of cancer
(8,9).
For a number of years, Glycyrrhiza glabra
(Licorice) has been used as a traditional Chinese medicine for the
treatment of liver disease. It was reported that glycyrrhizic acid
(GA) and 18β-glycyrrhetinic acid are the biologically active
compounds in Licorice (10). GA,
one of the triterpenoid saponin glycoside, was found to protect
PC12 cells from 1-methyl-4-phenylpyridinium-induced cytotoxicity
(11). GA alters inflammatory
processes by modulating NF-κB activities (12) and by blocking the activation of
NF-κB in primary neurons (13).
Furthermore, it was reported that the neuroprotective effects of GA
in PC12 cells is via modulation of the PI3K/Akt pathway (14). GA was found to modulate critical end
points of oxidative stress-induced apoptosis and may be beneficial
against liver diseases (15).
Recently, it was reported that GA exerts an anti-inflammatory
effect, at least in part, by inhibiting HMGB1 secretion (16). However, there is no available
evidence demonstrating that GA induces apoptosis in leukemia cells.
Therefore, the present study investigated the effects of GA on the
cytotoxicity of mouse leukemia cells (WEHI-3). Our findings
indicated that GA induced apoptosis in WEHI-3 cells through
caspase- and mitochondria-dependent pathways.
Materials and methods
Chemicals and reagents
GA, agarose, 4,6-diamidino-2-phenylindole
dihydrochloride (DAPI), dimethyl sulfoxide (DMSO), propidium iodide
(PI), Triton X-100, Tris-HCl and ribonuclease A were purchased from
Sigma-Aldrich Corp. (St. Louis, MO, USA).
2′,7′-Dichlorodihydrofluorescein diacetate (H2DCF-DA),
3,3′-dihexyloxacarbocyanine iodide (DiOC6), RPMI-1640
medium, fetal bovine serum (FBS), L-glutamine, trypsin-EDTA and
penicillin/streptomycin were purchased from Invitrogen/Life
Technologies (Carlsbad, CA, USA). The caspase-3 activity assay kit
and the caspase-3-specific inhibitor
(z-Asp-Met-Gln-Asp-fluoromethyl ketone; z-DEVD-fmk) were obtained
from R&D Systems (Minneapolis, MN, USA). Primary and secondary
antibodies used for western blotting were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell morphology and viability determined
in WEHI-3 cells
WEHI-3 cells were placed in 24-well plates at a
density of 2×105 cells/well overnight and were then
incubated with 0, 200, 250, 300, 350 and 400 μM of GA at 37°C, in
5% CO2 and 95% air for 24 and 48 h. In order to examine
morphological changes, cells from each treatment were examined and
images were captured under a phase-contrast microscope at a
magnification of ×200. Cells in each well were harvested, stained
with PI (5 μg/ml) and then the total number of viable cells for all
samples was determined using flow cytometry (Becton-Dickinson, San
Jose, CA, USA) as previously described (17,18).
Assay of cell cycle distribution using
flow cytometry
WEHI-3 cells (2×105 cells/ml) in 24-well
plates were incubated with 200, 250, 300, 350 and 400 μM of GA and
were incubated for 0, 24 and 48 h. Cells from each treatment were
collected, fixed in 70% ethanol overnight, washed twice with PBS
and re-suspended in 500 μl of 192 mM Na2HPO4,
4 mM citric acid and pH 7.8 at 25°C for 30 min. Then all samples
were individually stained with 0.5 ml of PBS containing 1 mg/ml
RNase and 10 μg/ml PI for 30 min in the dark and were directly
analyzed using flow cytometry as previously described (18).
DAPI staining and comet assay for
examining the DNA damage in WEHI-3 cells
Approximately 5×104 cells/ml of WEHI-3
cells in 24-well plates were individually treated with 0, 200, 250,
300, 350 and 400 μM of GA for 24 and 48 h. After incubation, all
samples from each treatment were harvested. For DAPI staining,
cells were stained with DAPI (4,6-diamidino-2-phenylindole
dihydrochloride), examined and images were captured using a
fluorescence microscope as previously described (9,19). For
comet assay, cells were harvested, isolated and examined for DNA
damage by using the comet assay as previously described (9,17).
DNA gel electrophoresis for DNA
fragmentation of WEHI-3 cells
Approximately 1×106 cells/ml of WEHI-3
cells on 10-cm dishes were individually treated with 0, 200, 250,
300, 350 and 400 μM of GA for 24 and 48 h. At the end of
incubation, all samples from each treatment were harvested for DNA
isolation (Genomic DNA Purification kit; Genemark Technology Co.,
Ltd., Tainan, Taiwan) and DNA gel electrophoresis was performed to
examine the DNA fragmentation as previously described (20,21).
Flow cytometric detection of reactive
oxygen species (ROS), mitochondrial membrane potential (ΔΨm) and
caspase-3 activity
WEHI-3 cells were placed in 24-well plates at the
density of 2×105 cells/ml and were then exposed to 300
μM of GA for indicated intervals of time. ROS and ΔΨm were assessed
by cell permeable probes H2DCF-DA (10 μM) and
DiOC6 (500 nM) using a flow cytometer, respectively, as
previously described (9,22). Cells were pretreated with or without
NAC (5 mM) or Z-VAD-FMK (5 μM), were incubated with 300 μM GA for
various time periods and were then harvested and lysed in a lysis
buffer [50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 10 mM EGTA, 10 mM
digitonin and 2 mM DTT]. Cell lysates (50 μg protein) were
incubated with caspase-3 specific substrates (DEVD-pNA) for 1 h at
37°C and the caspase-3 activity was determined by measuring OD405
of the released pNA as previously described (23,24).
Western blot analysis for examining the
associated protein levels in WEHI-3 cells
WEHI-3 cells were placed in 10-cm dishes at the
density of 5×105 cells/ml and were exposed to 300 μM of
GA for 0, 6, 12, 24 and 48 h. Then cells from each treatment were
harvested and were dissolved in the PRO-PREP™ protein extraction
solution (iNtRON Biotechnology, Seongnam-si, Gyeonggi-do, Korea).
All lysed samples were boiled at 100°C for 10 min with 4X protein
loading dye. All samples were individually subjected to
SDS-polyacrylamide gel electrophoresis as previously described
(9,17). All proteins in the gel were
transferred onto an Immobilon-P PVDF membrane (Merck Millipore,
Bedford, MA, USA) and incubated with the primary antibodies Fas,
FasL, caspase-3, Bid, Bax, apoptosis-inducing factor (AIF),
cytochrome c, Endo G, IRE-1α, calpain 1, caspase-12, GRP 78,
SOD (Cu/Zn), SOD (Mn) and catalase overnight (1:1,000 dilution),
were then washed and then were incubated with horseradish
peroxide-linked secondary antibody (1:8,000 dilution) and analyzed
using the Immobilon Western Chemiluminescent HRP substrate
(Millipore) as previously described (9,25).
Confocal laser microscopy
WEHI-3 cells at the density of 5×104
cells/well were plated on 4-well chamber slides and were then
treated without (control) or with 300 μM of GA for 24 h. At the end
of incubation, cells were washed with PBS and fixed with 4%
formaldehyde in PBS for 15 min, followed by permeabilization for 1
h using 0.3% Triton X-100 in PBS containing 2% BSA for blocking
non-specific binding sites. Cell samples were then stained by
anti-AIF, anti-Endo G or anti-cytochrome c antibodies (1:100
dilution, respectively) for 24 h, washed twice with PBS and then
stained with a secondary antibody (FITC-conjugated goat anti-mouse
IgG at 1:100 dilution) for 40 min, followed by PI staining for DNA
analysis. Examinations and photomicrographs were captured using a
Leica TCS SP2 Confocal Spectral Microscope, as previously described
(26,27).
Statistical analyses
The data from individual experiments are presented
as the means ± SD. The differences between the GA-treated and
-untreated (control) groups were analyzed using the Student's
t-test; a probability of P<0.05 indicated a statistically
significant difference.
Results
GA induces cell morphological changes and
decreases the percentage of viable WEHI-3 mouse leukemia cells
WEHI-3 cells were treated with various
concentrations (0, 200, 250, 300, 350 and 400 μM) of GA or DMSO for
24 and 48 h. Cells were examined and images were captured using a
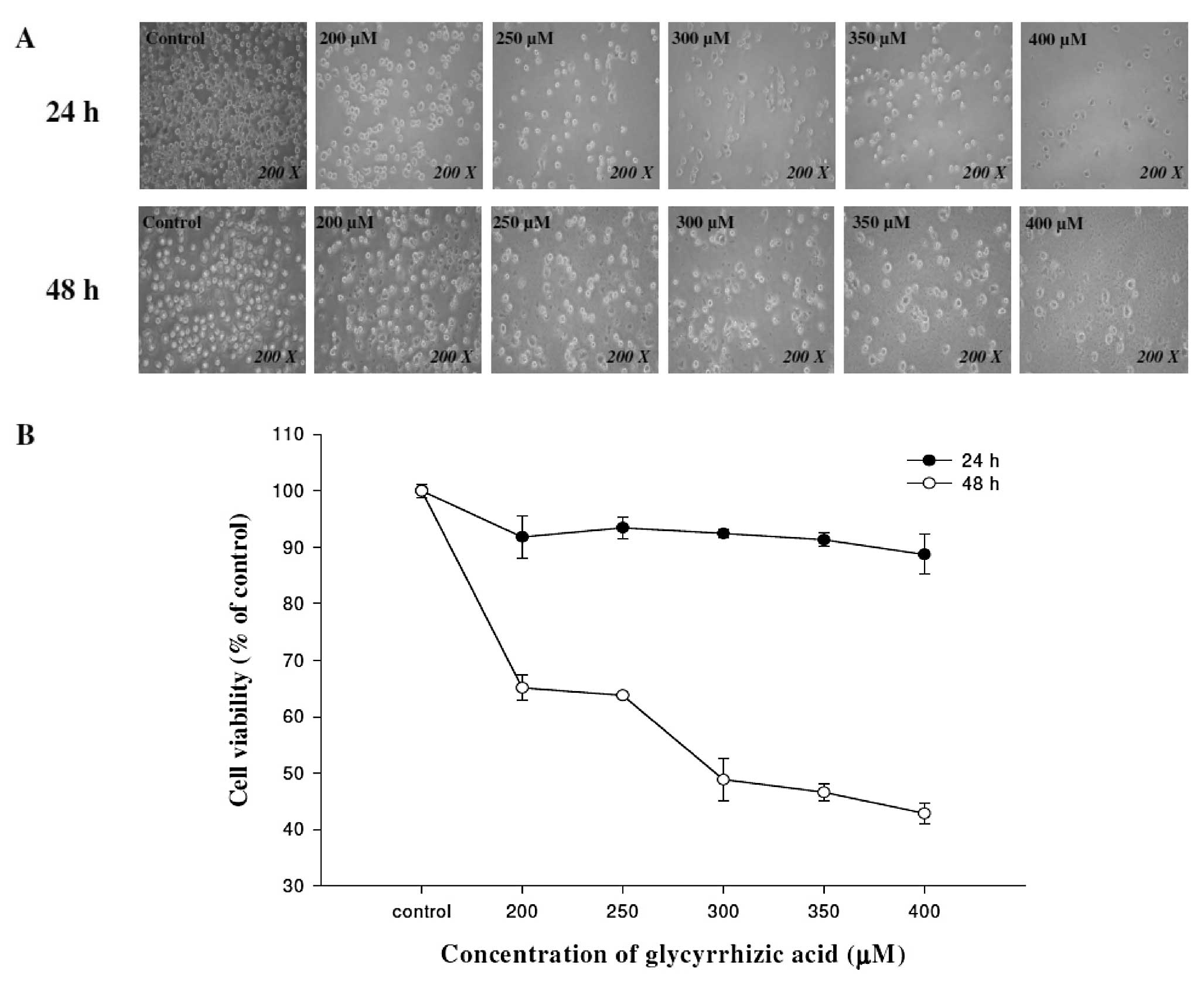
contrast-phase microscope at a magnification of ×200. The results
showed that GA induced cell morphological changes in a
dose-dependent manner (Fig. 1A).
The percentage of total viable cells in each treatment was
determined by flow cytometric assay. Results demonstrated that GA
concentrations of 200–400 μM decreased the cell number (inhibited
cell growth) in a dose- and time-dependent manner (Fig. 1B).
GA induces G0/G1
phase arrest in WEHI-3 cells
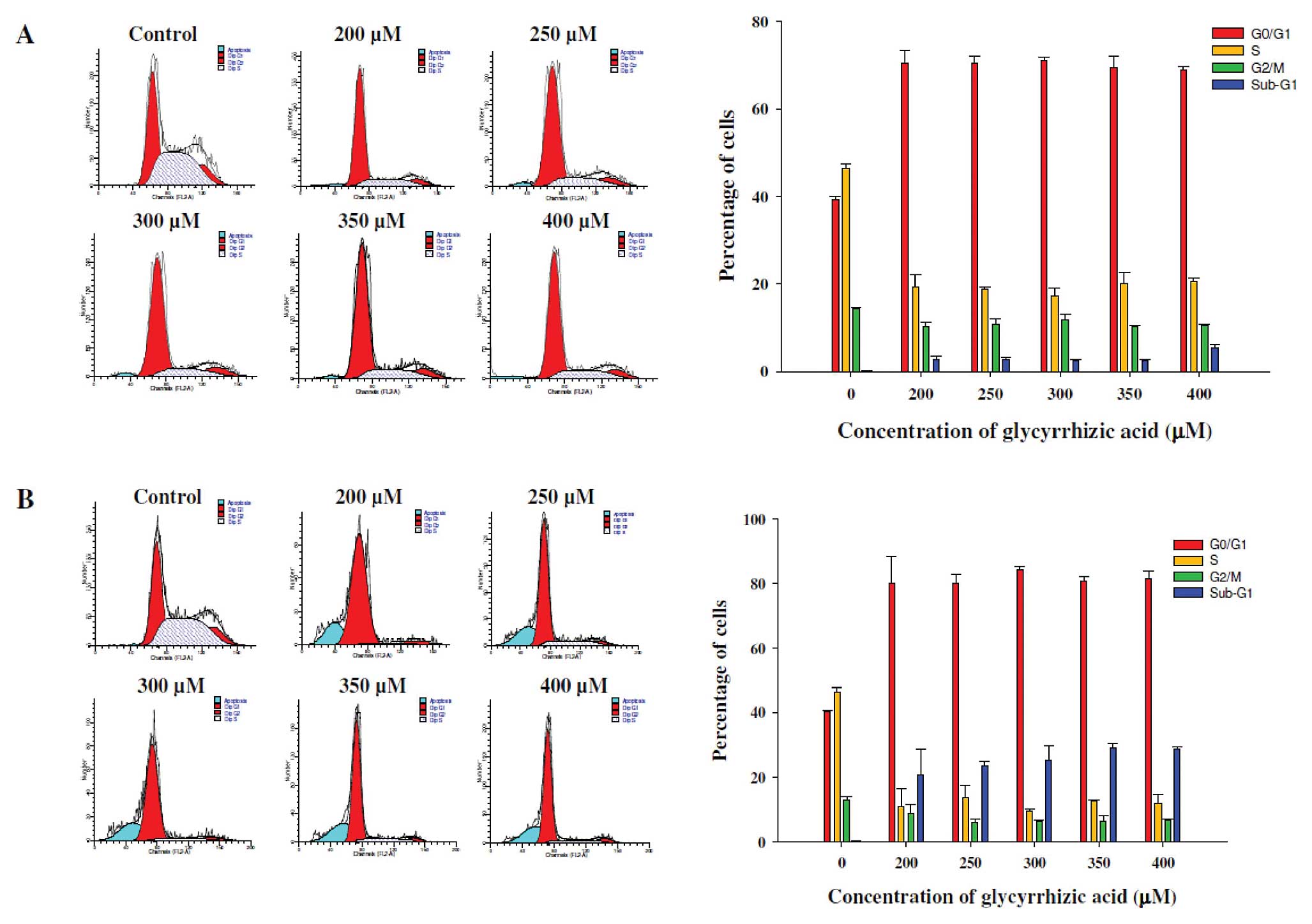
Flow cytometry was used for evaluating the cell
cycle distribution of WEHI-3 cells with or without GA treatment for
24 and 48 h. As shown in Fig. 2A and
B, exposure to 200–400 μM GA caused an increase in the
G0/G1 phase fraction from 39.2 to 72.3% and
from 41.1 to 80.3%, as compared to the control samples following 24
and 48 h of treatment, respectively. These effects of GA on
G0/G1 phase arrest were exhibited in a dose-
and time-dependent manner. These data suggest that GA-induced
G0/G1 phase arrest accounts for the decrease
in the percentage of viable WEHI-3 cells by GA.
GA induces DNA damage and apoptosis in
WEHI-3 cells
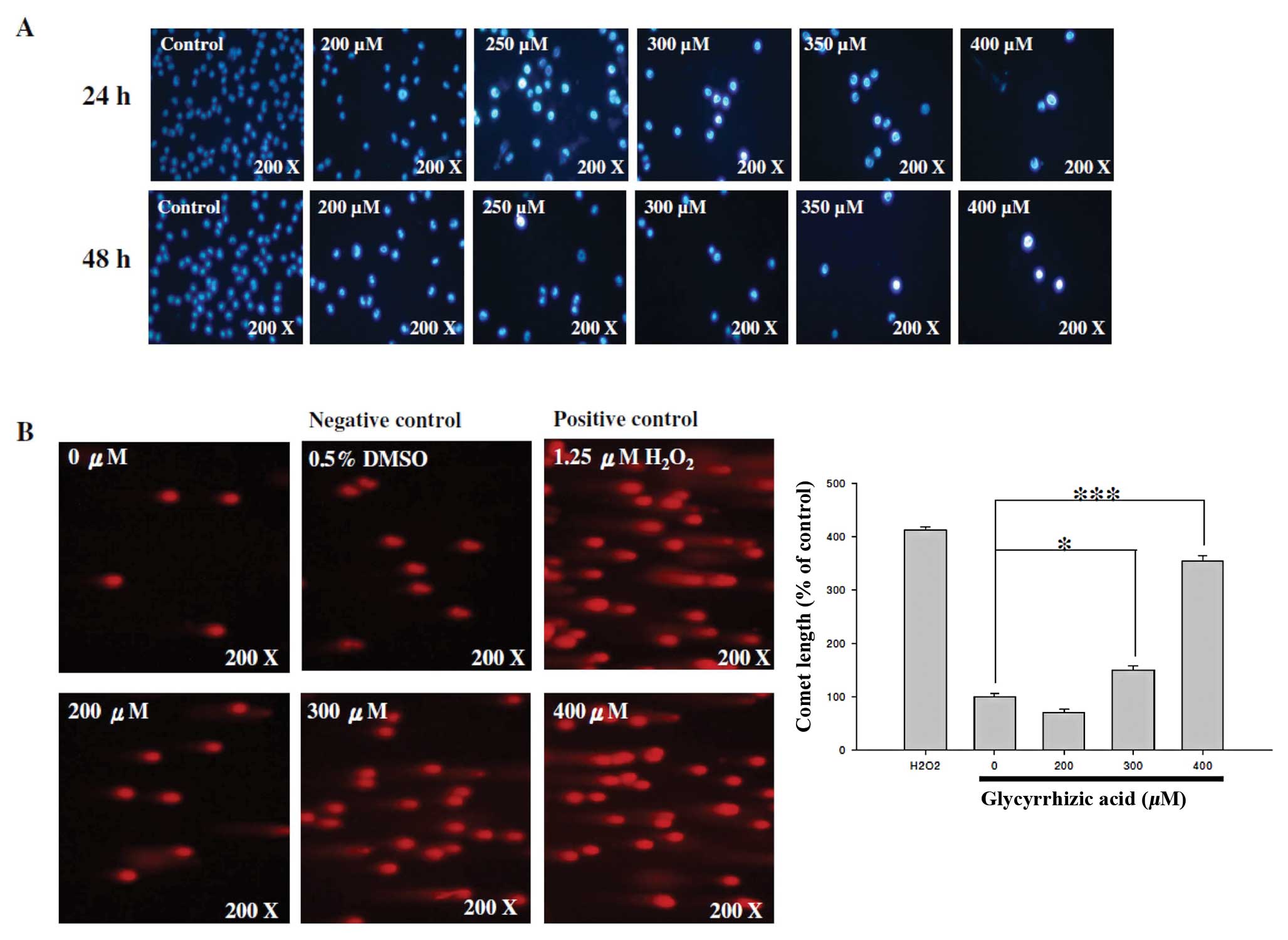
DAPI staining and comet assay were used for
investigating GA-induced DNA damage and chromatin condensation
(apoptosis) of WEHI-3 cells after exposure to various
concentrations of GA. GA induced DNA condensation as shown by an
increased fluorescent intensity (Fig.
3A) indicating that GA induced apoptosis. GA induced DNA damage
as noted by a longer comet tail (Fig.
3B). The higher the GA concentration, the longer the comet tail
and the lower the number of cells (Fig.
3B).
GA induces apoptotic death of WEHI-3
cells
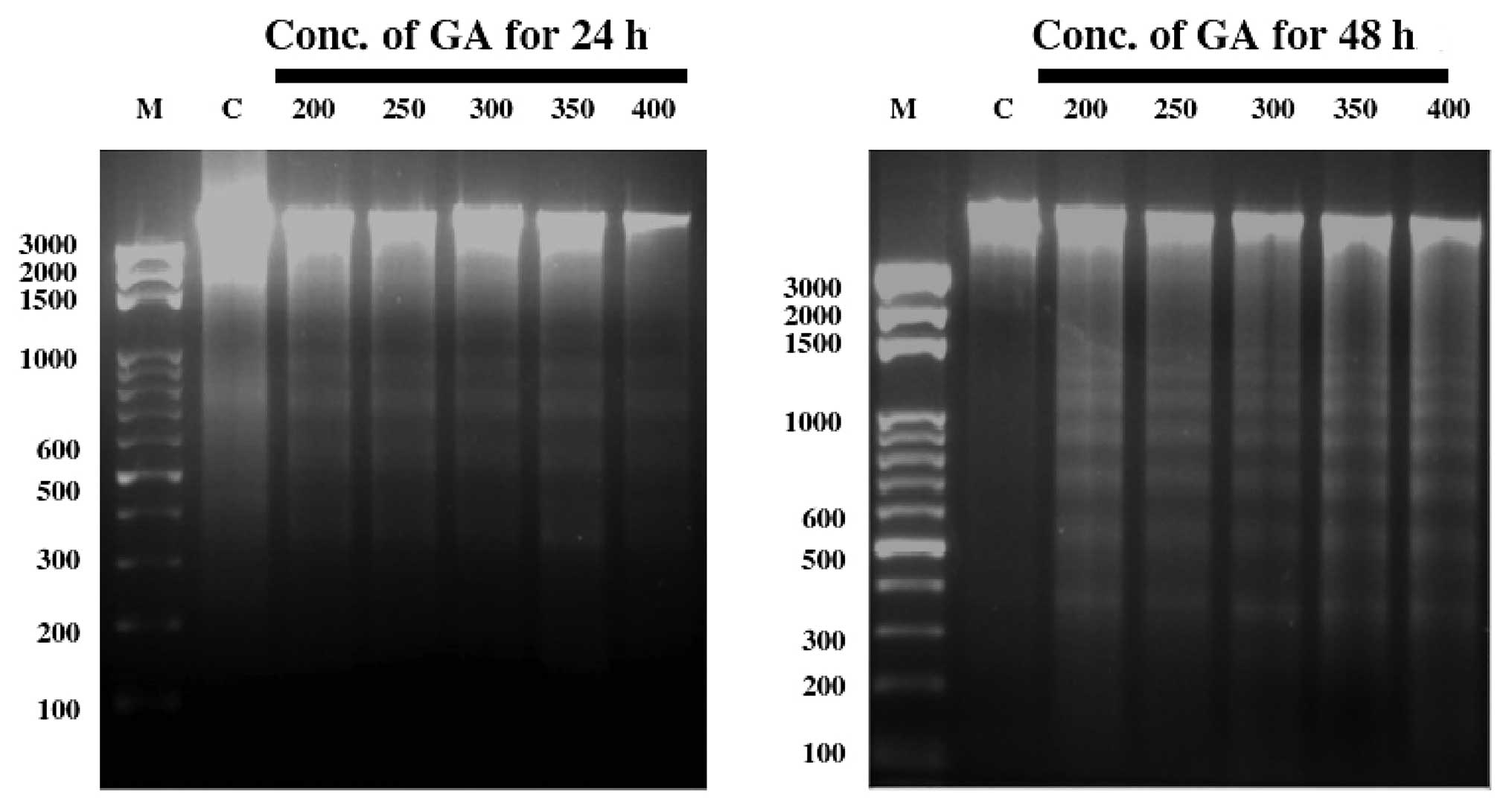
After treatment with GA for 24 and 48 h, WEHI-3
cells were harvested for DNA isolation followed by DNA gel
electrophoresis (Fig. 4). GA
induced DNA fragmentation (DNA ladder) in WEHI-3 cells at all
examined concentrations.
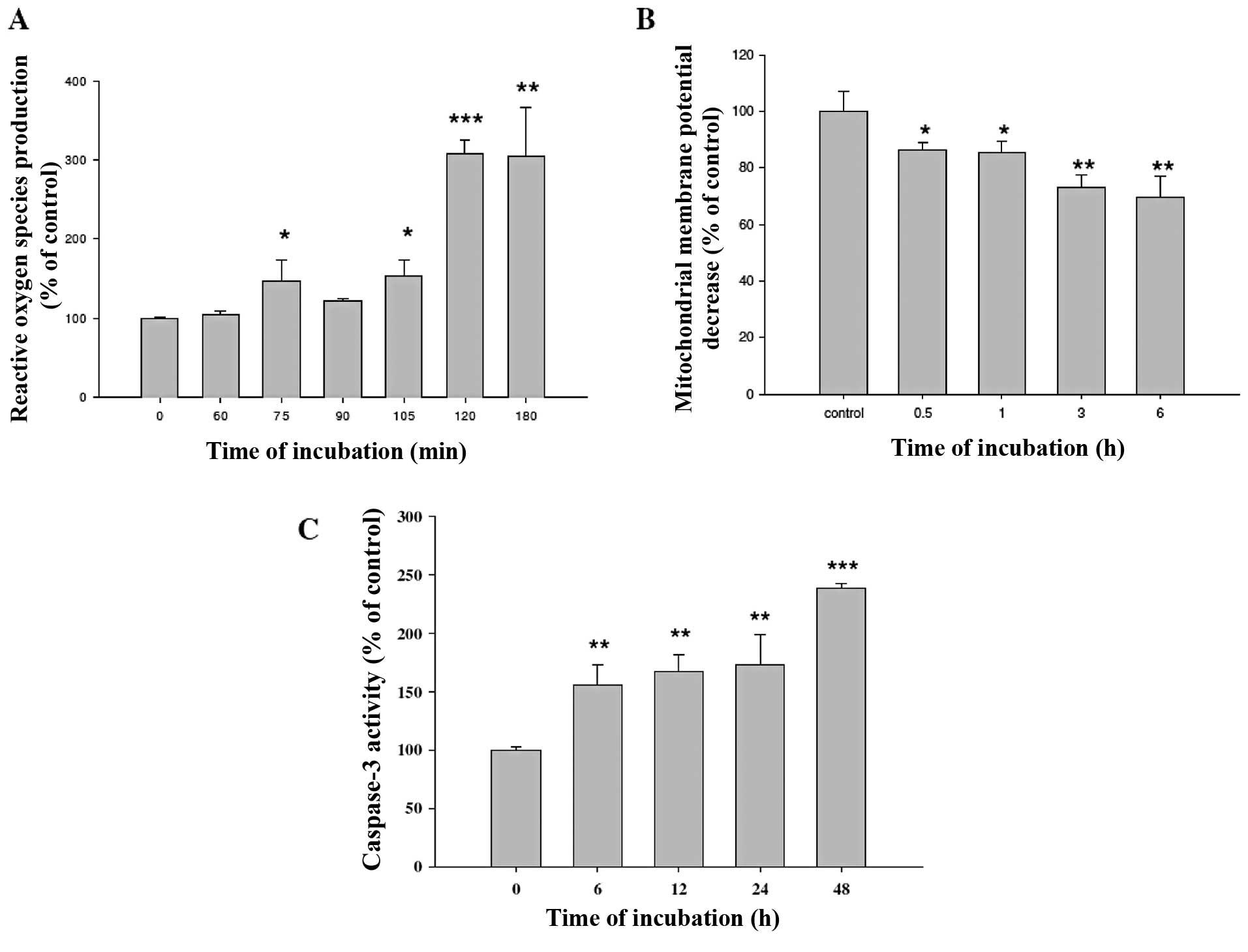
GA affects reactive oxygen species (ROS)
production, decreases the level of ΔΨm and promotes caspase-3
activity in WEHI-3 cells
WEHI-3 cells were treated with 300 μM GA for various
time periods and were then harvested for the measurements of ROS
production, the levels of ΔΨm and caspase-3 activity. GA promoted
the production of ROS (Fig. 5A) and
caspase-3 activities (Fig. 5C) but
decreased the levels of ΔΨm (Fig.
5B) in WEHI-3 cells.
GA affects levels of apoptosis-associated
proteins in WEHI-3 cells
Cells treated with GA were then harvested for
determination of apoptotic-associated protein levels by using
western blotting. Results indicated that GA promoted the protein
expression of Fas, FasL and caspase-3 (Fig. 6A); Bid, Bax, AIF, cytochrome
c and Endo G (Fig. 6B);
IRE-1-α, calpain-1, caspase-12 and GRP 78 (Fig. 6C); catalase, SOD (Cu/Zn) and SOD
(Mn) (Fig. 6D). This indicated that
GA induced apoptosis in WEHI-3 cells through caspase-dependent, ER
stress and mitochondria-dependent pathways.
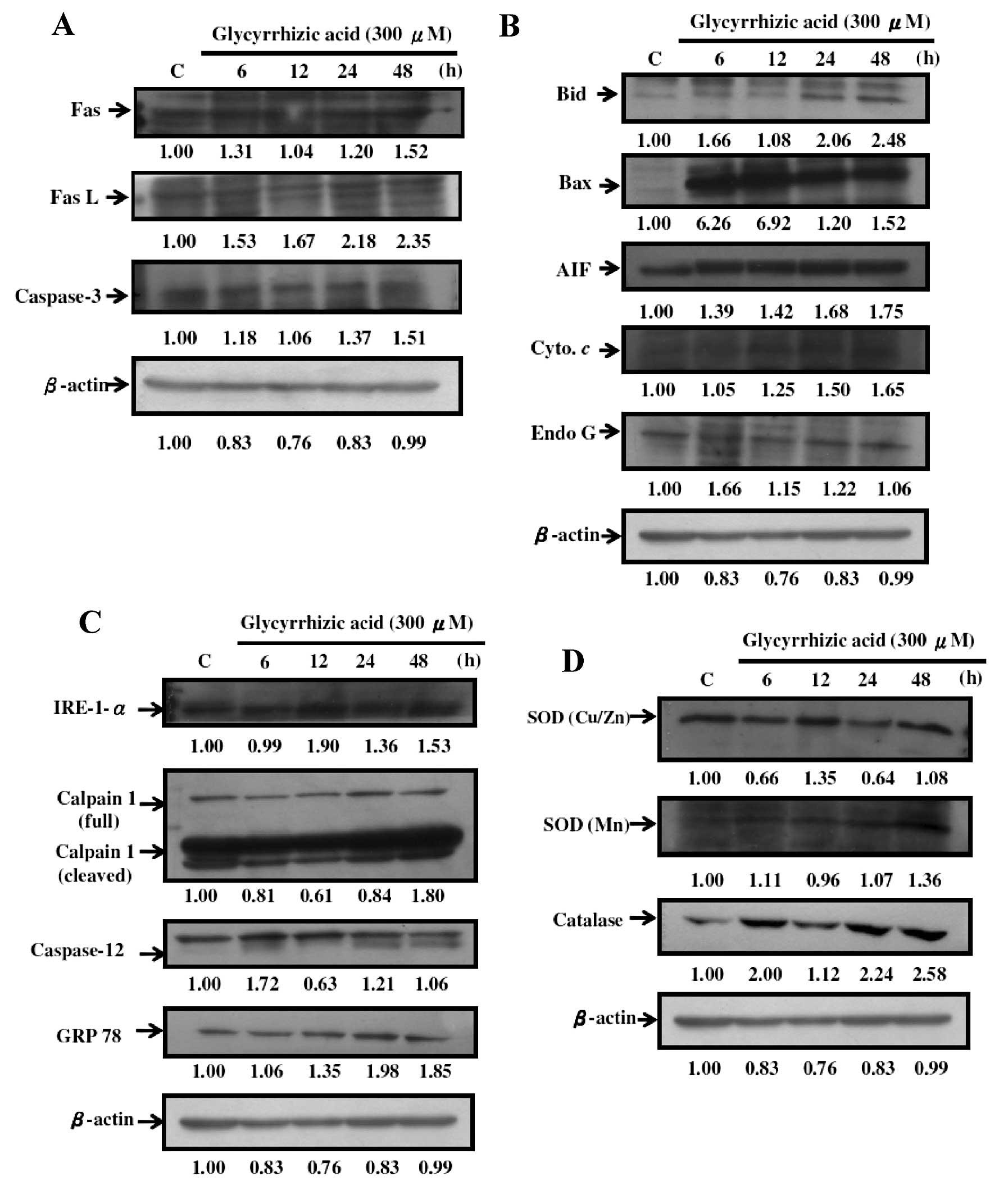 | Figure 6GA affects apoptosis-associated
proteins in WEHI-3 cells. Cells (1×106 cells/dish)
seeded in 10-cm dishes were then treated with 300 μM of GA and were
incubated for 0, 6, 12, 24, 36 and 48 h. Cells were harvested for
western blotting to examine the protein levels of Fas, Fas L and
caspase-3 (A); Bid, Bax, AIF, cytochrome c and Endo G (B);
IRE-1-α, calpain-1 (full and cleaved), caspase-12 and GRP 78 (C);
catalase, SOD (Cu/Zn) and SOD (Mn) (D) as described in Materials
and methods. |
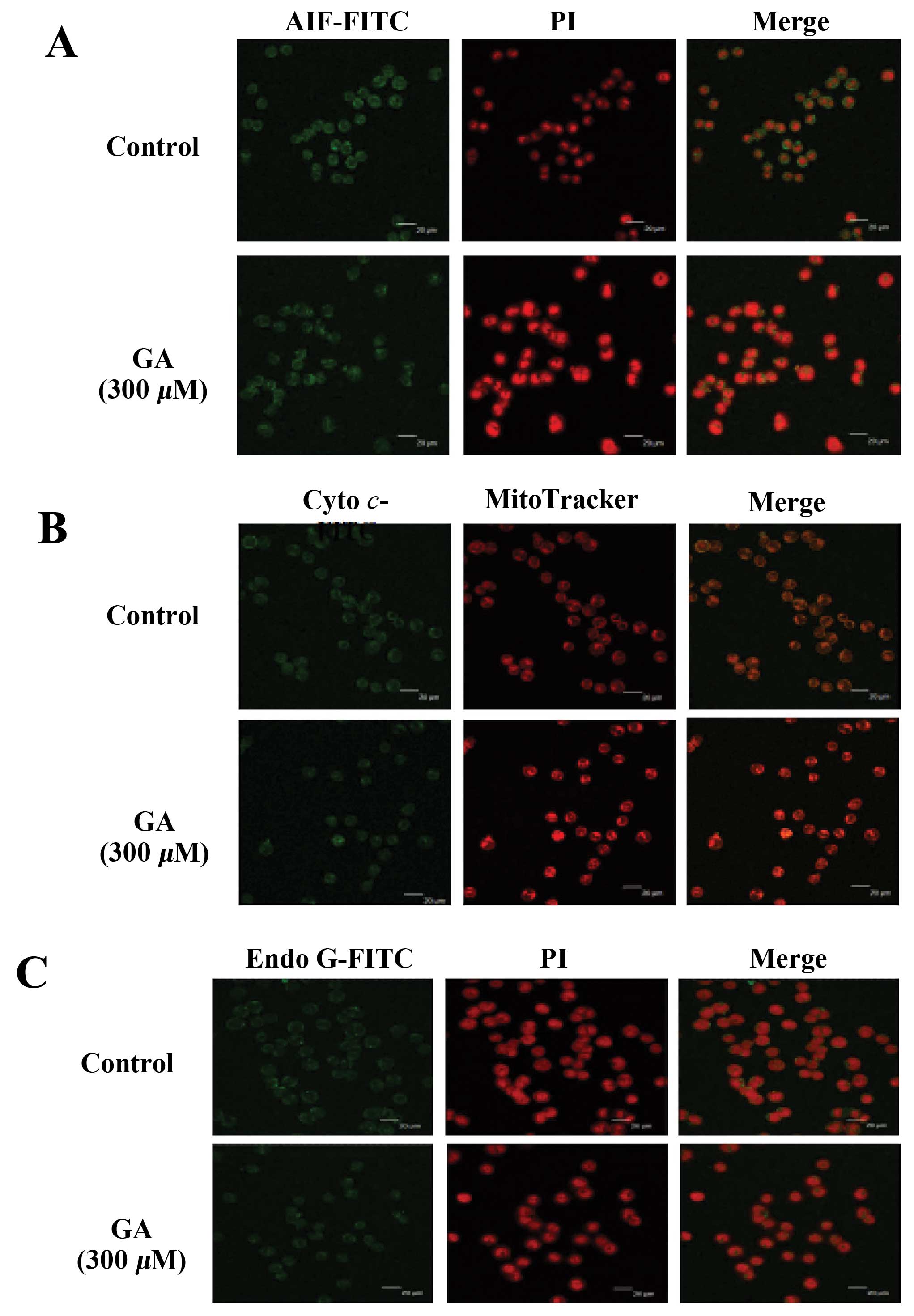
GA affects the AIF, cytochrome c and Endo
G expression in WEHI-3 cells
Cells were treated with GA for 24 h and the
expression levels of AIF, cytochrome c and Endo G were
examined (Fig. 7). GA promoted the
release of AIF, cytochrome c and Endo G from mitochondria.
These results indicated that GA induced apoptosis in WEHI-3 cells
via a mitochondria-dependent pathway.
Discussion
Although numerous studies have shown that GA induces
cytotoxic effects in many type of cancer cells (10,15,28,29),
there is no report demonstrating that GA induces apoptosis in mouse
leukemia cells. In the present study, we investigated the effects
of GA on mouse leukemia WEHI-3 cells and the results indicated that
GA decreased the percentage of viable cells and induced apoptosis
in WEHI-3 cells. Furthermore, we also demonstrated that GA induced
ROS production (Fig. 5A) and
decreased the levels of ΔΨm (Fig.
5B) which was assayed by flow cytometry.
It is well documented that apoptosis may be divided
into caspase-dependent and -independent pathways (30,31).
The results in the present study indicate that GA decreased the
percentage of viable cells through the induction of apoptosis based
on the observations of DAPI staining (Fig. 3A) and flow cytometric assay
(Fig. 2). Additionally, ROS is
known to be involved in the induction of apoptosis after cells are
exposed to various compounds (32,33).
Our results (Fig. 5A) demonstrated
that GA promoted ROS production in WEHI-3 cells. These results
indicated that GA induced apoptosis via ROS production. The ER
stress pathway is also another possible signaling pathway involved
in agent-induced apoptosis in cancer cells (34,35).
The hallmarkers of ER stress, such as the expression of GRP 78 and
GADD153 are able to activate caspase-12 and IRE-1α (34–36).
In the present study, we also found that GA promoted the expression
of GRP78 (Fig. 6C) and GADD153
(data not shown) which was measured by western blotting. We
suggested that GA induced apoptosis in part through ER stress.
It is well documented that agent-induced cancer cell
apoptosis occurs through a mitochondria-dependent pathway (31). We also observed that GA decreased
the levels of ΔΨm in WEHI-3 cells (Fig.
5B) in a time-dependent manner. Furthermore, results from
western blotting also showed that GA promoted the expression of
cytochrome c, AIF and Endo G which are released from
mitochondria (Fig. 6). Thus, we
suggest that GA induces apoptosis, in part, through the
caspase-independent and mitochondria-dependent pathways. These
findings also were confirmed using a confocal laser systems
microscope which demonstrated that GA promoted the release of AIF,
cytochrome c and Endo G (Fig.
6B).
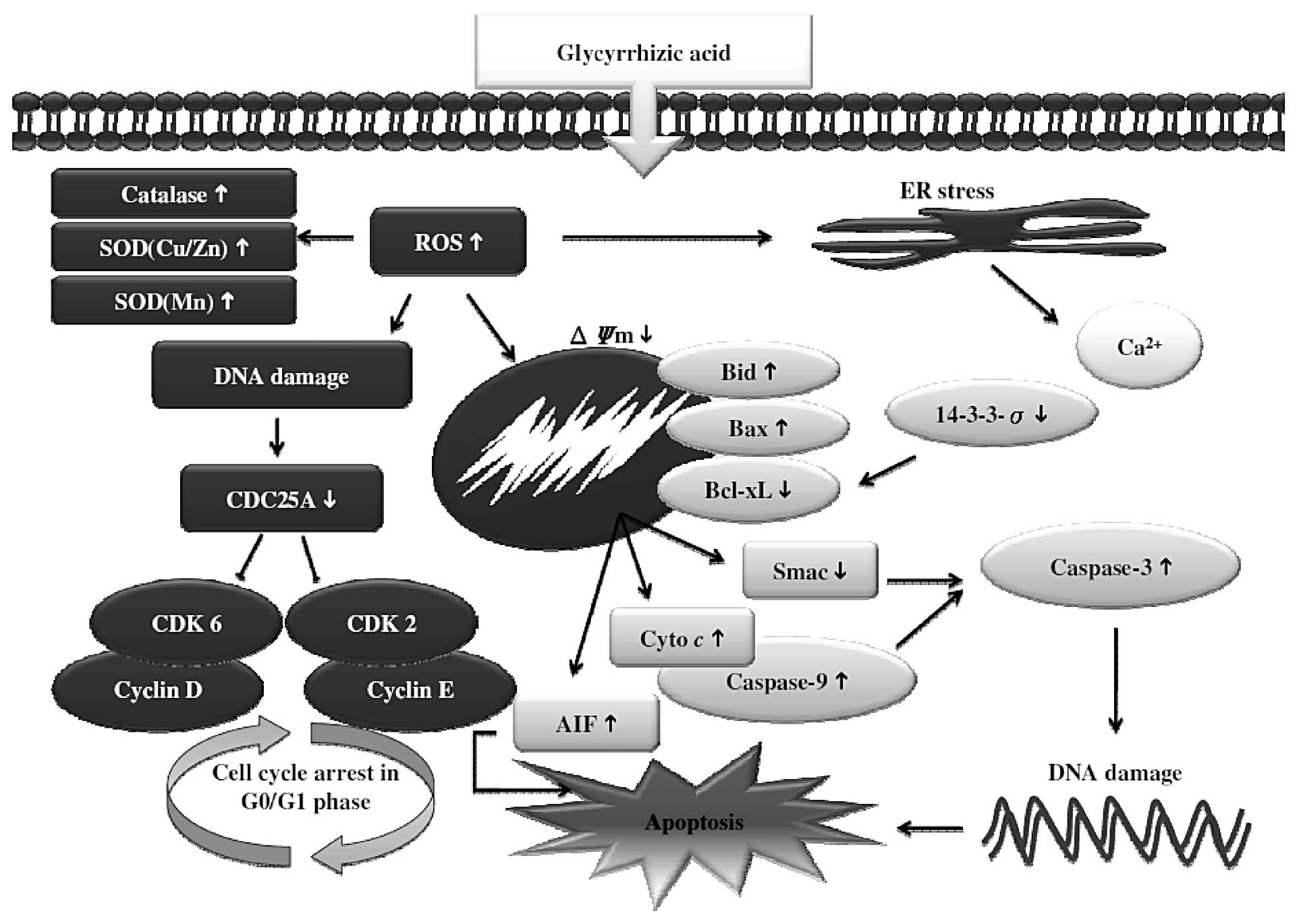
Overall, our study showed that the natural compound
GA acts as an apoptosis-inducing agent against mouse leukemia cells
in vitro. In our WEHI-3 mouse leukemia cell study, GA not
only induced cytotoxic effects, but also suppressed cell growth and
induced apoptosis (Fig. 8). This
highly correlates with the inhibition of numerous biomarkers linked
to apoptosis via caspase-dependent and -independent, ER stress and
mitochondria-dependent pathways.
Acknowledgements
This study was supported by the grant CMU100-ASIA-4
from the China Medical University and by the Taiwan Department of
Health, China Medical University Hospital Cancer Research Center of
Excellence (DOH101-TD-C-111-005).
References
|
1
|
Lee SJ, Kim KH, Park JS, et al:
Comparative analysis of cell surface proteins in chronic and acute
leukemia cell lines. Biochem Biophys Res Commun. 357:620–626. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stahnke K, Eckhoff S, Mohr A, Meyer LH and
Debatin KM: Apoptosis induction in peripheral leukemia cells by
remission induction treatment in vivo: selective depletion and
apoptosis in a CD34+ subpopulation of leukemia cells.
Leukemia. 17:2130–2139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu CC, Yang JS, Chiang JH, et al: Novel
quinazolinone MJ-29 triggers endoplasmic reticulum stress and
intrinsic apoptosis in murine leukemia WEHI-3 cells and inhibits
leukemic mice. PLoS One. 7:e368312012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin JP, Yang JS, Lin JJ, et al: Rutin
inhibits human leukemia tumor growth in a murine xenograft model in
vivo. Environ Toxicol. 27:480–484. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sack H: Leukemia in patients with breast
carcinoma after adjuvant chemotherapy and/or postoperative
radiotherapy. Strahlenther Onkol. 171:420–421. 1995.PubMed/NCBI
|
|
6
|
Liu W, Lee HW, Liu Y, Wang R and Rodgers
GP: Olfactomedin 4 is a novel target gene of retinoic acids and
5-aza-2′-deoxycytidine involved in human myeloid leukemia cell
growth, differentiation, and apoptosis. Blood. 116:4938–4947.
2010.PubMed/NCBI
|
|
7
|
Sakoe Y, Sakoe K, Kirito K, Ozawa K and
Komatsu N: FOXO3A as a key molecule for all-trans retinoic
acid-induced granulocytic differentiation and apoptosis in acute
promyelocytic leukemia. Blood. 115:3787–3795. 2010.PubMed/NCBI
|
|
8
|
Evan G and Littlewood T: A matter of life
and cell death. Science. 281:1317–1322. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chiang JH, Yang JS, Ma CY, et al:
Danthron, an anthraquinone derivative, induces DNA damage and
caspase cascade-mediated apoptosis in SNU-1 human gastric cancer
cells through mitochondrial permeability transition pores and
Bax-triggered pathways. Chem Res Toxicol. 24:20–29. 2011.
View Article : Google Scholar
|
|
10
|
Ploeger B, Mensinga T, Sips A, Seinen W,
Meulenbelt J and DeJongh J: The pharmacokinetics of glycyrrhizic
acid evaluated by physiologically based pharmacokinetic modeling.
Drug Metab Rev. 33:125–147. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yim SB, Park SE and Lee CS: Protective
effect of glycyrrhizin on 1-methyl-4-phenylpyridinium-induced
mitochondrial damage and cell death in differentiated PC12 cells. J
Pharmacol Exp Ther. 321:816–822. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schrofelbauer B, Raffetseder J, Hauner M,
Wolkerstorfer A, Ernst W and Szolar OH: Glycyrrhizin, the main
active compound in liquorice, attenuates pro-inflammatory responses
by interfering with membrane-dependent receptor signalling. Biochem
J. 421:473–482. 2009. View Article : Google Scholar
|
|
13
|
Cherng JM, Lin HJ, Hung MS, Lin YR, Chan
MH and Lin JC: Inhibition of nuclear factor kappaB is associated
with neuroprotective effects of glycyrrhizic acid on
glutamate-induced excitotoxicity in primary neurons. Eur J
Pharmacol. 547:10–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kao TC, Shyu MH and Yen GC:
Neuroprotective effects of glycyrrhizic acid and
18beta-glycyrrhetinic acid in PC12 cells via modulation of the
PI3K/Akt pathway. J Agric Food Chem. 57:754–761. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tripathi M, Singh BK and Kakkar P:
Glycyrrhizic acid modulates t-BHP induced apoptosis in primary rat
hepatocytes. Food Chem Toxicol. 47:339–347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SW, Jin Y, Shin JH, et al:
Glycyrrhizic acid affords robust neuroprotection in the
postischemic brain via anti-inflammatory effect by inhibiting HMGB1
phosphorylation and secretion. Neurobiol Dis. 46:147–156. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu CC, Yang JS, Huang AC, et al:
Chrysophanol induces necrosis through the production of ROS and
alteration of ATP levels in J5 human liver cancer cells. Mol Nutr
Food Res. 54:967–976. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ji BC, Hsu WH, Yang JS, et al: Gallic acid
induces apoptosis via caspase-3 and mitochondrion-dependent
pathways in vitro and suppresses lung xenograft tumor growth in
vivo. J Agric Food Chem. 57:7596–7604. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu FS, Yang JS, Yu CS, et al: Safrole
induces apoptosis in human oral cancer HSC-3 cells. J Dent Res.
90:168–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuo CL, Wu SY, Ip SW, et al: Apoptotic
death in curcumin-treated NPC-TW 076 human nasopharyngeal carcinoma
cells is mediated through the ROS, mitochondrial depolarization and
caspase-3-dependent signaling responses. Int J Oncol. 39:319–328.
2011.
|
|
21
|
Chung JG, Yang JS, Huang LJ, et al:
Proteomic approach to studying the cytotoxicity of YC-1 on U937
leukemia cells and antileukemia activity in orthotopic model of
leukemia mice. Proteomics. 7:3305–3317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Petronilli V, Miotto G, Canton M, et al:
Transient and long-lasting openings of the mitochondrial
permeability transition pore can be monitored directly in intact
cells by changes in mitochondrial calcein fluorescence. Biophys J.
76:725–734. 1999. View Article : Google Scholar
|
|
23
|
Huang WW, Chiu YJ, Fan MJ, et al:
Kaempferol induced apoptosis via endoplasmic reticulum stress and
mitochondria-dependent pathway in human osteosarcoma U-2 OS cells.
Mol Nutr Food Res. 54:1585–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang JS, Hour MJ, Huang WW, Lin KL, Kuo SC
and Chung JG: MJ-29 inhibits tubulin polymerization, induces
mitotic arrest, and triggers apoptosis via cyclin-dependent kinase
1-mediated Bcl-2 phosphorylation in human leukemia U937 cells. J
Pharmacol Exp Ther. 334:477–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lai TY, Yang JS, Wu PP, et al: The
quinolone derivative CHM-1 inhibits murine WEHI-3 leukemia in
BALB/c mice in vivo. Leuk Lymphoma. 51:2098–2102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu HF, Lai KC, Hsu SC, et al: Curcumin
induces apoptosis through FAS and FADD, in caspase-3-dependent and
-independent pathways in the N18 mouse-rat hybrid retina ganglion
cells. Oncol Rep. 22:97–104. 2009.PubMed/NCBI
|
|
27
|
Wu SH, Hang LW, Yang JS, et al: Curcumin
induces apoptosis in human non-small cell lung cancer NCI-H460
cells through ER stress and caspase cascade- and
mitochondria-dependent pathways. Anticancer Res. 30:2125–2133.
2010.PubMed/NCBI
|
|
28
|
Zhao MX, Ji LN and Mao ZW:
β-Cyclodextrin/glycyrrhizic acid functionalised quantum dots
selectively enter hepatic cells and induce apoptosis. Chemistry.
18:1650–1658. 2012.
|
|
29
|
Curreli F, Friedman-Kien AE and Flore O:
Glycyrrhizic acid alters Kaposi sarcoma-associated herpesvirus
latency, triggering p53-mediated apoptosis in transformed B
lymphocytes. J Clin Invest. 115:642–652. 2005. View Article : Google Scholar
|
|
30
|
Kelloff GJ, Crowell JA, Steele VE, et al:
Progress in cancer chemoprevention: development of diet-derived
chemopreventive agents. J Nutr. 130:S467–S471. 2000.PubMed/NCBI
|
|
31
|
Lavrik IN, Golks A and Krammer PH:
Caspases: pharmacological manipulation of cell death. J Clin
Invest. 115:2665–2672. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Orrenius S: Reactive oxygen species in
mitochondria-mediated cell death. Drug Metab Rev. 39:443–455. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kadowaki H, Nishitoh H and Ichijo H:
Survival and apoptosis signals in ER stress: the role of protein
kinases. J Chem Neuroanat. 28:93–100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rao RV, Ellerby HM and Bredesen DE:
Coupling endoplasmic reticulum stress to the cell death program.
Cell Death Differ. 11:372–380. 2004. View Article : Google Scholar : PubMed/NCBI
|