Introduction
Gastric cancer is still a major health problem and a
leading cause of cancer-related death although its incidence is
decreased worldwide (1). In spite
of the standardization of surgical techniques and multimodal
therapy, the postoperative survival of patients with advanced
gastric cancer still remains very low. It was recently reported
that the understanding of the molecular and genetic factors
involved in gastric cancer progression may identify novel gastric
biomarkers and highlight potential avenues of investigation for
targeted therapies.
Recent evidence shows that PIAS1, a downstream
target protein of STAT signaling pathway inhibitor, is related to
anti-inflammatory response through negative regulation of
NF-kB/STAT1 signaling, which mediates inflammatory cell adhesion
and inhibits inflammatory injury (2). Consistently, PIAS1 null mice show
increased protection against pathogenic infection and are
hypersensitive to LPS-induced septic shock (3). This finding provides a theoretical
basis for revealing the mechanism of reversing inflammation by
PIAS1. However, recent studies demonstrated that the expression of
PIAS1 in some cancer cells is significantly downregulated or lost
(4). Several studies showed that
PIAS1 negatively regulates Bcl2 expression and thus
reverses the cancer growth. Therefore, PIAS1 not only plays
functions of inflammatory inhibitor but also plays a role in the
reversal of cancer cell growth (4).
Therefore, in this study, the PIAS1 expression was first observed
the expression of gastric cancer tissue with patient to prove that
PIAS1 may be involved in the pathogenesis of cancer and to verify
whether PIAS1 acts as a marker for the preclinical detection and
clinical assessment of patients with gastric cancer.
The cascade reaction of mitogen-activated protein
kinases (MAPKs) is one of the vital intracellular signal
transduction systems, participating in many physiological
progressions, such as cell growth, proliferation, differentiation
and apoptosis. Three major subclasses of MAPKs, namely,
extracellular signal-regulated kinase (ERK), c-Jun NH2-terminal
kinase (JNK), and P38MAPK have been identified (5). Related studies indicated that MAPKs
are proposed to be a critical integrator of various signaling
transduction systems and involved in various cellular processes
including cell proliferation, differentiation, apoptosis, and
transformation (6). Constitutive
activation of these signaling cascades has been noted in the
malignant transformation of various cell lines and implicated in
carcinogenesis and metastatic potential of human cancers (7).
Therefore, we hypothesize that the regulation of
PIAS1 is a key step in the multiple biological regulations in
gastric cancer by regulating the MAPK signaling pathway. To test
this hypothesis, we used gastric cancer SGC7901 cells and studied
the migration response of the cells by upregulating administration
of PIAS1. Additionally, to gain a better understanding of the
mechanism of action of PIAS1, this study investigated further the
effect of PIAS1 on the levels of the MAPK signaling pathway, tumor
migratory factors including MMP-9, MMP-2, and ICAM-1, then
providing a possible effective mechanism for gastric cancer
treatment.
Materials and methods
Reagents
Patients and tissue samples
Tumor samples were obtained from gastric cancer
patients who underwent surgery between 2002 and 2010 at RuiJin
Hospital (Shanghai Jiaotong University School of Medicine,
Shanghai, China). All cases were staged according the guidelines of
the International Union Against Cancer (2002). There were 18 males
and 13 females with ages ranging from 20 to 92 years (mean 60.9
years). All available clinicopathological data were collected
(Table I). The study was approved
by the ethical review board of Ruijin Hospital.
 | Table IRelationship of PIAS1 expression with
clinicopathological parameters in gastric cancer with patients. |
Table I
Relationship of PIAS1 expression with
clinicopathological parameters in gastric cancer with patients.
| Total | Positive | P-value |
|---|
| Total cases | 31 | | |
| Age (years) |
| High (>50) | 25 | 11 | 0.318 |
| Low (<50) | 6 | 4 | |
| Gender |
| Female | 13 | 5 | 0.524 |
| Male | 18 | 9 | |
| Tumor extent |
| T2 | 10 | 8 | 0.00 |
| T3, T4 | 21 | 6 | |
| Size (cm) |
| Large (>5) | 24 | 3 | 0.971 |
| Small (<5) | 7 | 10 | |
Tissue microarray construction and
immunohistochemistry
Formalin-fixed paraffin-embedded samples were
reviewed for tissue array construction by a pathologist. At least
two core tissue biopsies were taken from morphologically
representative regions of each gastric tumor. The samples of 31
primary tumor cases and 31 adjacent normal tissues samples were
arranged in rows and columns to construct a tissue microarray. For
staining, the sections (4 μm) were transferred to glass
slides using an adhesive slide system (PSA-CS 4; Instrumedics,
Hackensack, NJ, USA) to support cohesion of the array elements. The
slides were de-waxed in xylene, and rehydrated in ethanol with
descending concentration. Anti-PIAS1 antibody (Santa Cruz
Biotechnology, Santa Cruz, CA, USA, diluted to 1:50) was added
after blocking of endogenous peroxidase and proteins, and each
section was incubated with HRP-labeled anti-goat IgG antibody. The
immunostained specimens were assessed by two independent observers
without prior knowledge of the clinicopathologic characteristics.
PIAS1 expression was scored using two measures: staining intensity
was graded as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong);
the percentage of positive cells was scored as 0 (negative), 1
(<10%), 2 (11–50%), 3 (51–80%), or 4 (>80%). The two scores
were multiplied and two categories of expression levels were set
up: negative expression (meaning 0–2); and positive expression
(meaning 3–12).
Construction of expression
plasmid
We used replication-defective adenovirus serotype
5/F35 (Ad5/F35) as the vector. Ad5/F35-PIAS1 was constructed by
Benyuanzhengyang Bio. (Shanghai, China) using the previously
described method (8). PIAS1 cDNA,
containing the full-length translated regions, were sub-cloned into
the PDC316-MCMV-EGFP transfer plasmid. This plasmid was
cotransfected into 293 cells, along with a fragment of the plasmid
containing the Ad5/F35 adenoviral vector. Additionally, an Ad5/F35
containing an empty expression cassette was constructed for use as
a control (Ad5/F35-vector). All of the viral constructs were
similar with the exception of the trans-gene, and the production
and purification procedures were identical.
Cell culture and gene
transduction
The culture of human gastric cancer cell SGC7901
cells (American Type Culture Collection, Rockville, MD, USA) was
conducted in the medium, containing RPMI-1640 medium (Gibco BRL,
Gaithersburg, MD, USA) and 10% fetal bovine (FBS, head-inactivated;
Gibco BRL) in the ratio 1:1 and with addition of 100 U/ml
penicillin and 100 μg/ml streptomycin (Sigma, St. Louis, MO,
USA) in the standard condition (37°C and 5% CO2).
In the study, when the SGC7901 cells reached 70%
confluence, the transfection of Ad5/F35-PIAS1 process began as
Ad5/F35-PIAS1 treated cells (MOI 10). Other SGC-7901 cells were
also transfected with the empty Ad5/F35 vector (MOI 10)
(Ad5/F35-vector) as Ad5/F35 vector treated cells. Cells with PBS
treatment serving as control cells. The samples were harvested at
24 or 72 h after the onset of induction for further
experiments.
Cell viability assay and PIAS1
expression
A total of 2×103 SGC7901 cells were
seeded in a 96-well plate. After 24 h, the cells were treated with
Ad5/F35-PIAS1 (MOI 10). These cells were transfected with the empty
Ad5/F35 vector (MOI 10). After 24 and 72 h, 20 μl
3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT)
(5 mg/ml) was added to each well. Four hours later, 100 μl
of dimethyl sulfoxide was added to each well after the medium was
removed. Finally, the absorbance was detected with an enzyme
calibrator at 570 nm and the cell viability = (A of study group/A
of control group) × 100%.
PIAS1 expression in cells by
RT-PCR
Total RNA was isolated from cells using TRIzol
reagent kits (Gibco BRL, Rockville, MD, USA). The cDNA obtained
from this reaction was mixed with PCR buffer, MgCl2,
dNTPs, Taq DNA polymerase, and human PIAS1 gene-specific
primers (the primer sequence of PIAS1, the forward primer: 5′-CCA
CGC CTT CCT GCT GTA GA-3′; the reverse primer: 5′-TAT CAC ACA GGC
AGT CTT AGA T-3′) and amplified in an automated thermal cycler. The
conditions of RT-PCR were as follows: 1 cycle for 5 min at 95°C; 35
cycles for 45 sec at 94°C, for 45 sec at 55°C, for 1 min at 72°C; 1
cycle for 10 min at 72°C. The PCR products were separated by
electrophoresis in 1.2% agarose gels and stained with ethidium
bromide. The densities of cDNA bands were analyzed by scanning
densitometry using GelDoc 2000 (Bio-Rad, Baltimore, MD, USA). The
band densities were normalized to GAPDH (the primer sequence of
GAPDH, the forward primer: 5′-GGC TGA GAA CGG GAA GCT TGT C-3′; the
reverse primer: 5′-CAG CCT TCT CCA TGG TGG TGA AGA-3′) band
densities and the results were expressed as ratio.
Scratch wound-healing assay
To measure cell motility, 4×105 cells
were seeded in 6-well plates. A central linear wound was created by
scraping the cell monolayer with a 200-μl sterile pipette
tip. The media were carefully changed to remove any floating cells
and cultured at 5% CO2 and 37°C. The migration of cells
into the denuded areas in the scraped region was calculated at 48
h, respectively. The wound at 0 h was considered 100% of the
average gap.
Cell invasion
For the determination of invading cells, the
transwell chambers (Corning Inc., NY) were used. The transwell
chamber membranes were covered with 75 μl Matrigel™ (2
mg/ml, BD Biosciences, NJ, USA) and incubated for 2 h at 37°C. The
cell lines were grown in RPMI-1640 (10% FBS) medium, thus were
trypsinized and suspended at a density of 1×106/ml in
serum-free RPMI-1640. Suspended cells (200 μl) were placed
into the upper compartment of the Transwell chambers. The lower
compartment of the chambers was filled with 600 μl RPMI-1640
containing 10% FBS. The transwell chamber systems were incubated in
the humidified incubator (37°C, 5% CO2) for 24 h. After
incubation, the non-invaded cells and the matrigel were removed
with a cotton toothpick at the top of the chamber. At the bottom of
the cell swab, the invaded cells were washed with PBS and fixed
with 4% paraformaldehyde and then were stained with Giemsa for 15
min. Five random fields were counted and the invasion rate was
calculated to find the number of the invaded cells under the
inverted microscope at magnification ×200. The data representing
the average cells of five fields was compared between the
experimental groups and control group. Each experiment was repeated
three times.
Western blot analysis
The levels of P38MAPK, P-P38MAPK, JNK/SAPK,
P-JNK/SAPK, ERK, P-ERK, MMP-9, MMP-2, and ICAM-1 proteins were
investigated in each group using western blot analysis. The cells
were washed twice with PBS and then homogenized in RIPA buffer.
Following centrifugation at 12,000 g at 4°C for 10 min, the
supernatant was collected and stored at -80°C. Protein
concentration of each sample was determined by BCA protein assay.
Each sample was adjusted up to a desired protein content of 40
μg, thus denatured in loading buffer and separated by
electrophoresis on 9% SDS polyacrylamide gel at 100 V for 120 min.
The separated proteins were transferred to polyvinylidene
difluoride membrane by using transfer buffer at 200 mA for 90 min.
The membranes were blocked with 5% non-fat dry milk in
Tris-buffered saline (TBS)-0.1% Tween for 1 h at room temperature,
washed three times for 10 min each in TBS-0.1% Tween, and incubated
with a primary antibody including P38MAPK, P-P38MAPK, JNK/SAPK,
P-JNK/SAPK, ERK, P-ERK, MMP-9, MMP-2, and ICAM-1 with 1:1000
dilution in TBS-0.1% Tween overnight at 4°C, respectively. After
three 10 min washes in TBS-0.1% Tween, the membranes were incubated
with a second antibody, horseradish peroxidase-conjugated goat
anti-rabbit or rabbit anti-mouse immunoglobulin G (Kangcheng Inc.,
Shanghai, China) for 1 h at room temperature. After washing, the
membranes were detected by the enhanced chemiluminescence methods
(Amersham Biosciences, Piscataway, NJ, USA), and then scanned by
densitometry using bio-image analysis system (Bio-Rad) for
quantification. GAPDH was determined in the similar manner to
anti-GAPDH antibody (diluted to 1:500, Santa Cruz) as an endogenous
control for other proteins.
Statistical analysis
All data were expressed as the mean ± standard
deviation (SD). Statistics were done by SPSS program at 10.5
versions. The χ2 test was used to evaluate the
correlation between PIAS1 expression and clinicopathological
characteristics. The one-way analysis of variance (ANOVA) with
Dunnett’s multiple comparison tests was used for comparisons. A
P<0.05 was considered as statistically significant.
Results
Expression of PIAS1 in gastric cancer
tissue
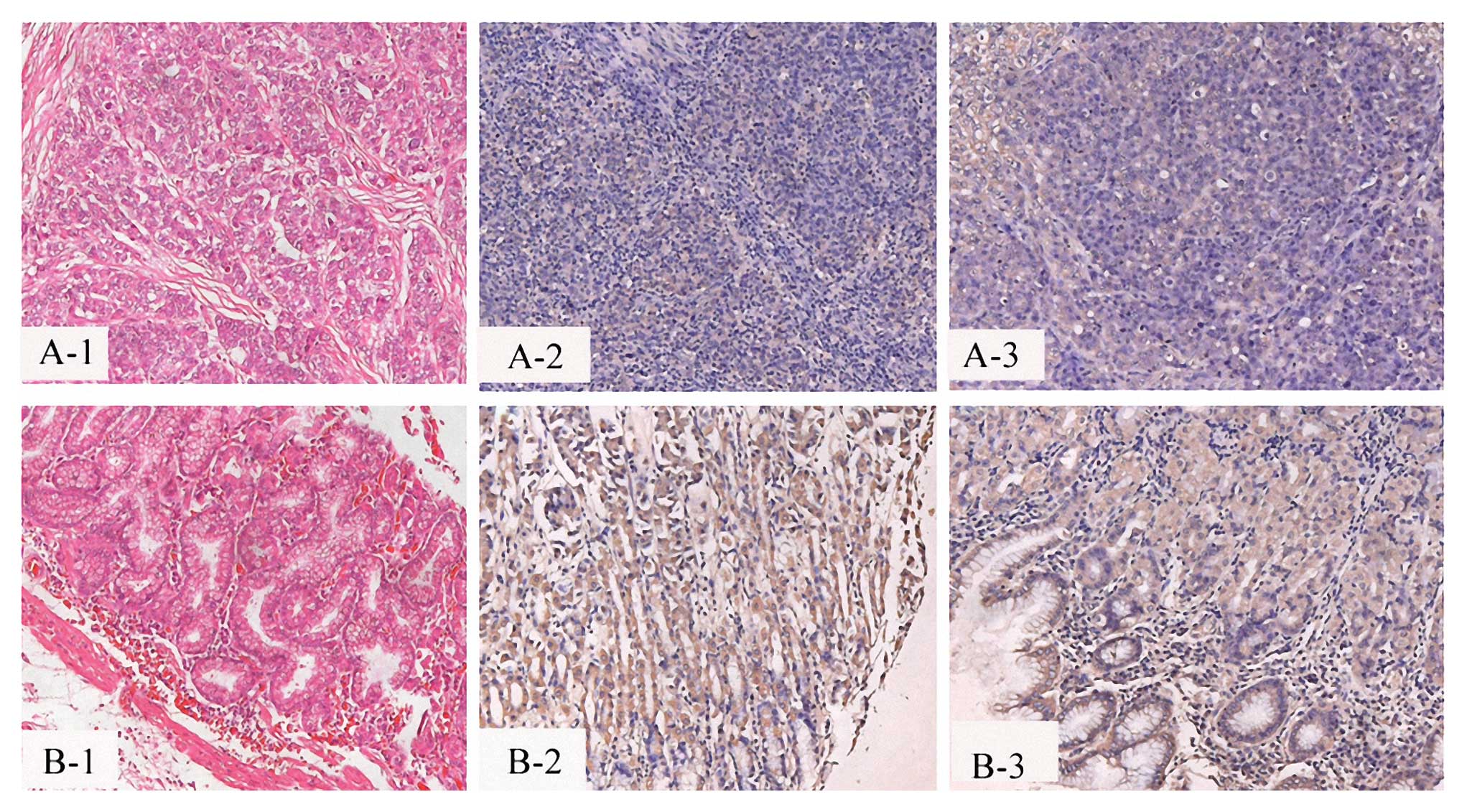
We investigated the expression of PIAS1 in 31 cases
of gastric cancer tissues and 31 cases of adjacent normal tissues
of gastric cancer (Fig. 1). The
results showed that PIAS1 levels were significantly lower in
gastric cancer than in the adjacent normal tissues of gastric
cancer in all 31 cases of patients. These results suggest that
PIAS1 expression was significantly decreased in human gastric
cancer.
Furthermore, the clinical relevance was confirmed by
the observation that PIAS1 expression correlated with prognosis
(Table I). Tumor staging was
significantly associated with low expression of PIAS1 protein
(P<0.05). However, a non-significant correlation was observed
between PIAS1 protein expression and age with patients. No
correlation was observed between PIAS1 protein expression and tumor
size with patients or between PIAS1 protein expression and gender
with patients (P>0.05).
Expression of PIAS1 in gastric cancer
cells
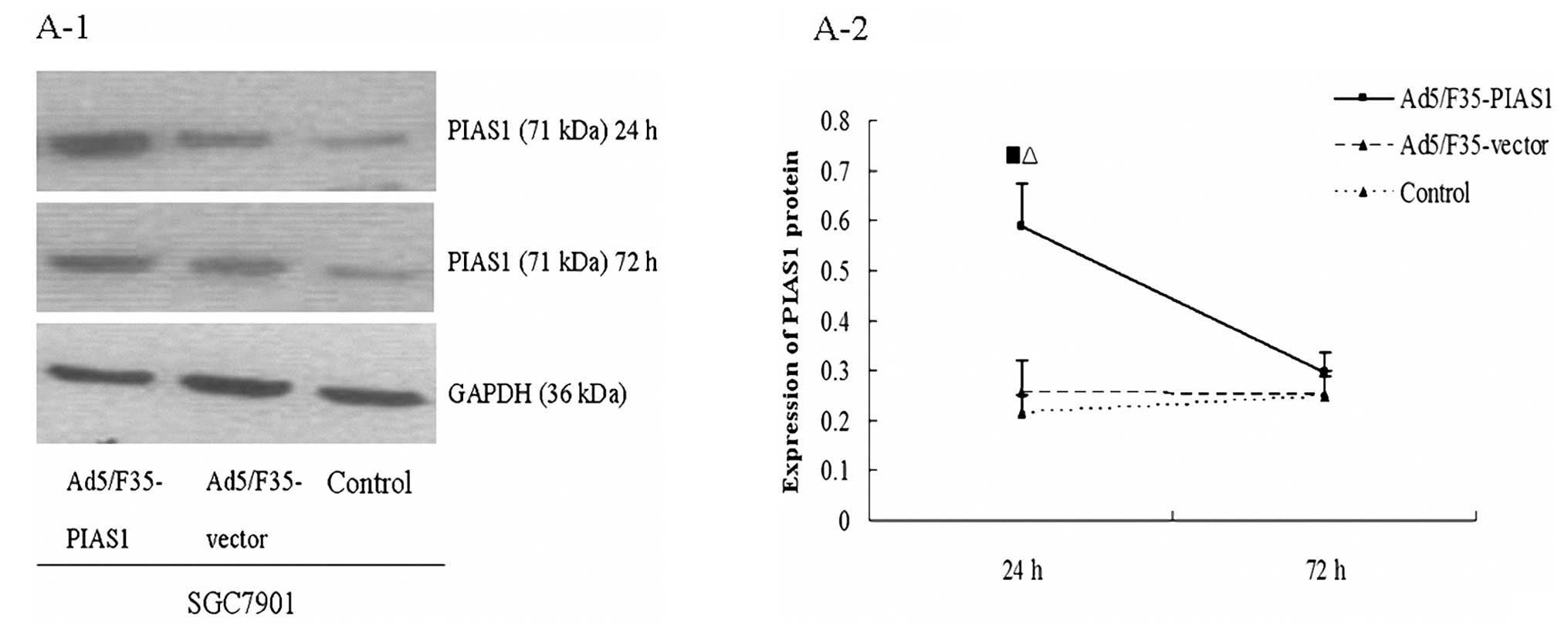
We monitored the levels of PIAS1 transcripts in
SGC7901 cells by RT-PCR assays (Fig.
2B). The data showed that at 6 h following Ad5/F35-PIAS1
treatment, PIAS1 mRNA levels were increased in SGC7901 cells but
not in the cells with Ad5/F35-vector treatment. The enhanced PIAS1
gene expression returned to basal level within 72 h after
stimulation. The protein expression of PIAS1 was monitored using
western blot analysis (Fig. 2A).
Similar to the RT-PCR data, the SGC7901 cells with Ad5/F35-PIAS1
treatment expressed a higher level of PIAS1 protein compared with
SGC7901 cells with Ad5/F35-vector treatment. SGC7901 cells at 72 h
of Ad5/F35-PIAS1 treatment did not show significant increase in
PIAS1 expression compared with the cells with Ad5/F35-vector
treatment and control.
The proliferation, invasion and migration
of gastric cancer cells
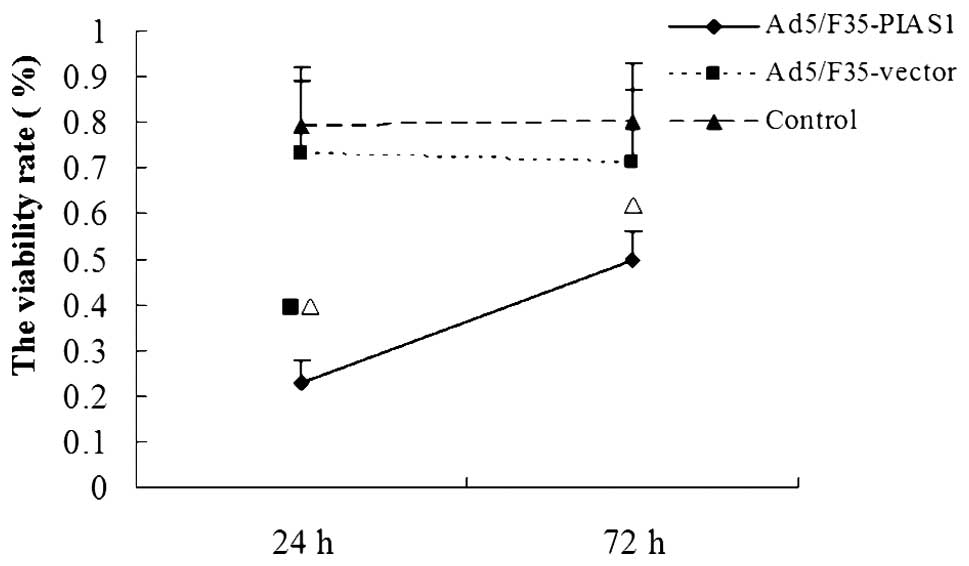
We investigated the effect of PIAS1 on proliferation
of gastric cancer SGC7901 cells using in vitro MTT assay
(Fig. 3). We found that the
increased PIAS1 expression by transfection with Ad5/F35-PIAS1
significantly decreased the proliferative ability of SGC7901
cells.
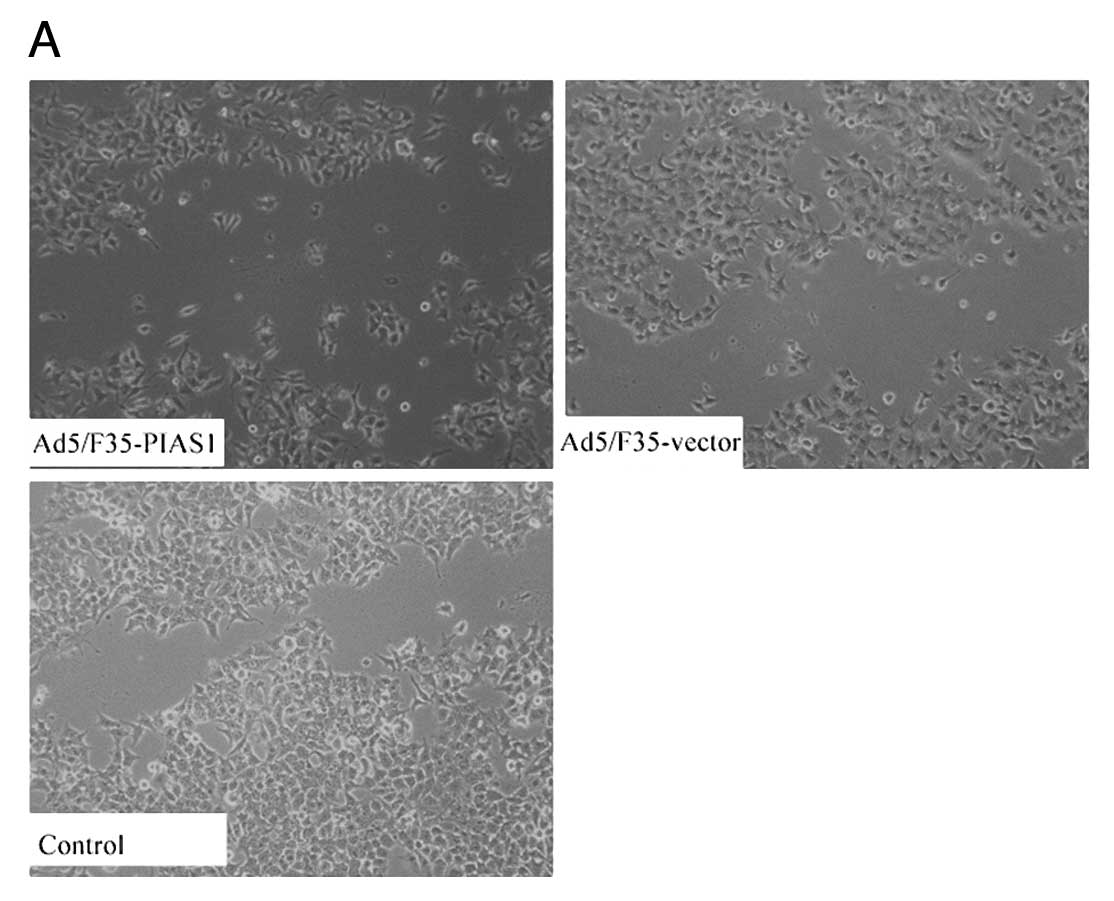
To examine the effect of Ad5/F35-PIAS1 on cell
motility, an in vitro scratch wound-healing assay was
performed (Fig. 4). The results
showed that the cells transfected with Ad5/F35-PIAS1 had a reduced
migration rate compared with the control cells at 24 h (P<0.01).
To further investigate the effect of Ad5/F35-PIAS1 on cell
invasion, we determined the invasion ability of the three groups of
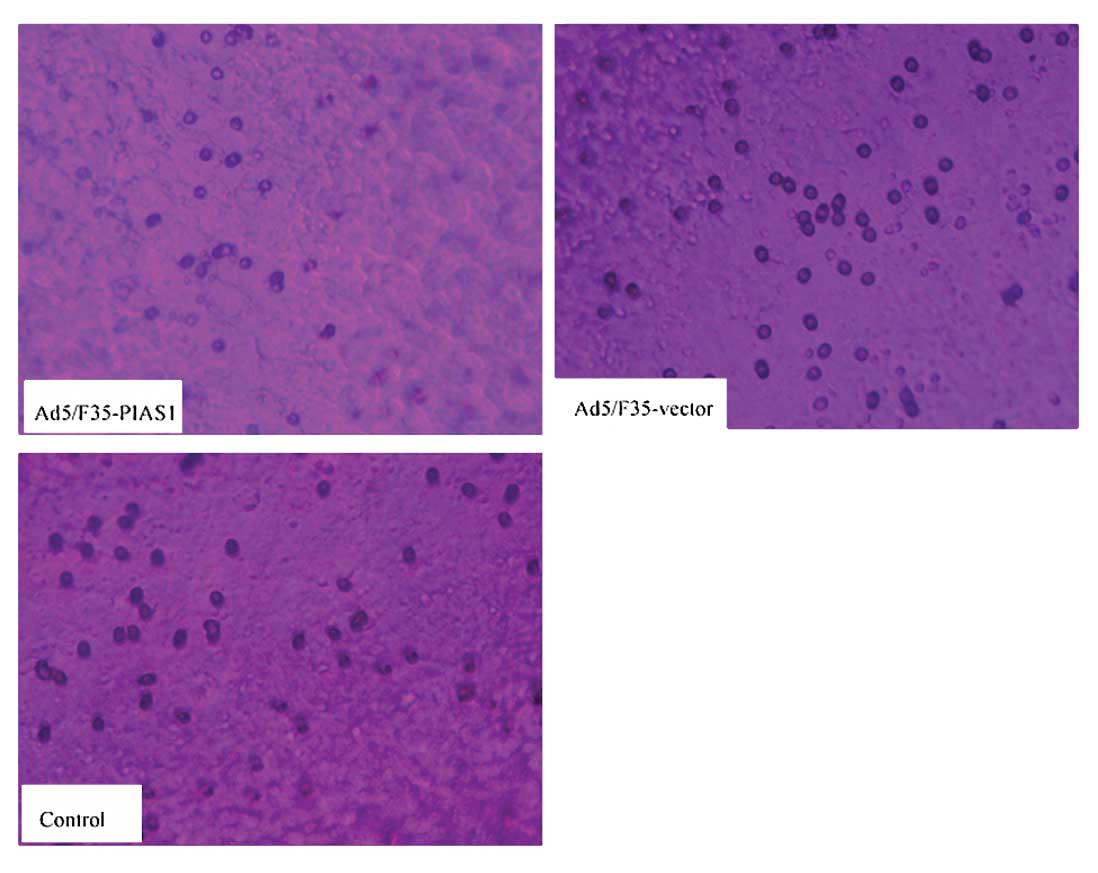
cells using the transwell chambers assay (Fig. 5). After incubation for 24 h, the
number of control group and Ad5/F35-PIAS1-vector treated cells
which had invaded the polycarbonate membrane of the matrigel
chamber was ~3.7- and 3.3-fold greater than that of the
Ad5/F35-PIAS1 treated group, respectively [(21.25±1.51) and
(19.35±1.33) vs (5.70±1.56)] (P<0.05). These results provide
strong evidence that PIAS1 plays a role in decreasing metastasis of
gastric cancer cells.
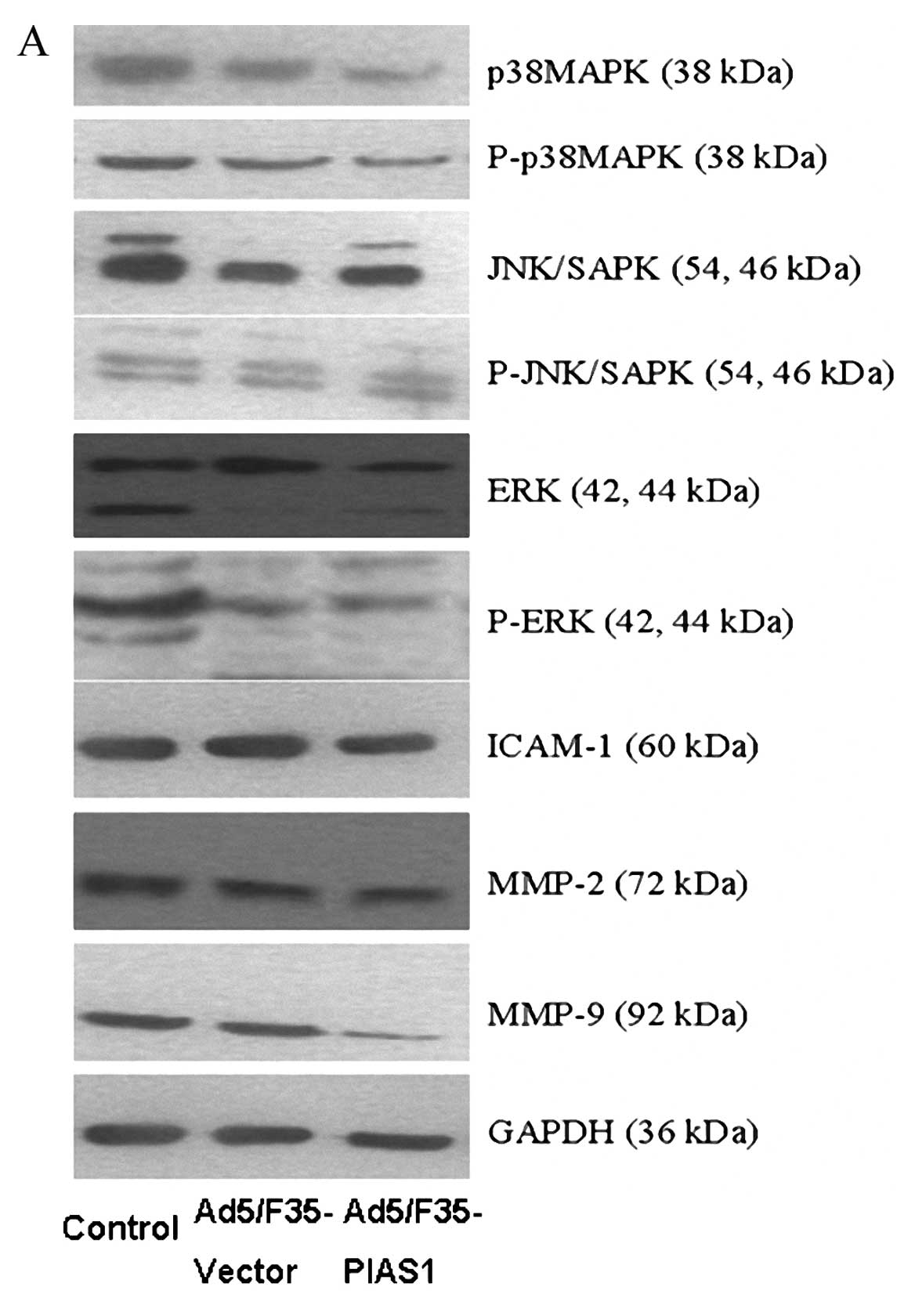
Expression of P38MAPK, P-P38MAPK,
JNK/SAPK, P-JNK/SAPK, ERK, P-ERK, MMP-9, MMP-2, and ICAM-1 in
gastric cancer cells
The proteins of P38MAPK, P-P38MAPK, JNK/SAPK,
P-JNK/SAPK, ERK, and P-ERK were expressed in each group by western
blot analysis (Fig. 6A and B). The
expression levels of P38MAPK, P-P38MAPK, ERK, and P-ERK proteins
were decreased in gastric cancer cells SGC7901 with Ad5/F35-PIAS1
treatment compared with these cells by transfection with
Ad5/F35-vector and control cells. The expression levels of
JNK/SAPK, P-JNK/SAPK proteins in gastric cancer with control had no
different when compared with those of Ad5/F35-PIAS1 and
Ad5/F35-vector treated cells, respectively. After Ad5/F35-PIAS1
treatment, the expressions of MMP-9, MMP-2, and ICAM-1 were
detected in gastric cancers cells, which were decreased compared
with those of cells with Ad5/F35-vector treatment and control,
respectively. The average protein levels of MMP-9, MMP-2, and
ICAM-1 in gastric cancer cells with control were no higher than
those of Ad5/F35-vector treated cells (Fig. 6A and C).
Discussion
Gastric cancer is the most frequent malignancy of
the gastrointestinal tract in Chinese populations and the second
most common cause of cancer related death in the world. The
prognosis of gastric cancer has been improving owing to progress in
diagnostic techniques and treatment methods for gastric cancer, but
the prognosis of gastric cancer which has invaded is still poor
with a 5-year survival of <35% (9). Among these malignant characteristics
of gastric cancer cells, metastasis is an especially complex
phenomenon, which requires the involvement of many different genes
in multiple steps. The adhesion molecules, metastatic chemotaxis
related genes, and others have been reported to play an important
role in metastasis of gastric cancers (10,11),
although many aspects of gastric cancer metastasis await further
clarification.
Gene activation analyses show that PIAS1 selectively
regulates a subset of STAT dependent genes, with a notable
preference for inflammatory cytokines (11,12).
In this study, we aimed to investigated the expression of PIAS1 on
gastric cancer and evaluated its relationship with development of
gastric cancer. Further the study elucidated the upregulating
effect of PIAS1 on gastric cancer by a series of studies including
cell viability assay and cell invasion, as well as the effective
mechanisms of PIAS1, in order to explore a new therapeutic agent
for gastric cancer. The PIAS1 expression was significantly
decreased in human gastric cancer, which was positively correlated
with the development of gastric cancer, but which showed no
correlation with tumor size, gender or age with patients. Our
results demonstrate that the decreasing expression of PIAS1 was
found in TNM stage III and IV tumors. PIAS1 might play an important
role in a certain stage. The exact role of PIAS1 in gastric cancer
has not been identified. Its upstream and downstream molecular
targets are also unidentified. Future investigation will provide
the necessary information to shed lights on these points.
Recently, the increasing evidence indicates that
anticancer drugs activated many pro-inflammatory signal pathways,
some of which were connected to the development of metastasis of
tumor cells. The MAPK signaling is an important signaling
transduction pathway activated by many different stimuli. Previous
reports have shown that the modulators of MAPK signaling pathway
can affect cell growth (13,14).
With regard to human gastric carcinoma, the investigations of
alterations in expression and activity of components of the MAPK
cascade will help to understand the mechanisms of malignant
behaviors of gastric cancer cells. The P38MAPK is a member of MAPK
signaling pathway, mediating many cell reactions induced by stress,
inflammatory cytokines or bacterial products and playing a key role
in the regulation of cell cycle. For the different cell lines of
gastric cell (15), the similar
phenomenon can be seen when these cells are presented under P38MAPK
activity. Therefore, P38MAPK pathway may be one candidate target of
cancer therapy for further study. The activation of signal
transduction pathways by growth factors, hormones and
neurotransmitters is mediated through JNK/SAPK signaling pathway.
It is the basic signal transduction pathway regulating cell
proliferation and differentiation (16). Recent research data indicate that
the activation of ERK cascade is involved in H.
pylori-induced proliferation of gastric epithelial cells
(17). Thus, these results suggest
that the MAPK signaling pathway is involved in regulation of the
tumor migratory factors in cancer cells, and may contribute to cell
growth and cell invasion. Furthermore, this study investigated the
relationship of the MAPK signaling pathway and PIAS1 overexpression
in gastric cancer. The results of the study showed that the
incubation with Ad5/F35-PIAS1 for 24 h in cells could block ERK and
P38MAPK proteins activity. We used Ad5/F35-vector to detect the
MAPKS signaling pathway in whole gastric cancer cells, which showed
that the administration of Ad5/F35-vector had no effect on the
expression and activation of MAPK signaling pathway including ERK,
JNK/SAPK, and P38MAPK proteins.
Local invasion and metastasis are hallmark features
in cancer progression. The modulation of the adhesive and migratory
properties of the disseminating tumor cells play an essential role
in the process of invasion and metastasis. The ability of tumor
cells to adhere and migrate across the endothelium and
extracellular matrix (ECM) is considered one of the most important
prerequisites for the formation of invasion and metastasis
(18). ICAM-1 has been found
overexpress in gastric cancer and it is correlated with cancer
progression (19). The MMPs
constitute a large family of endopeptidases, which are responsible
for degrading almost all ECM components, with each ECM element
being cleaved by a specific MMP. Consistent with their role in
tumor progression, the high levels of MMP-2 and MMP-9 have been
shown to correlate with poor prognosis (20). We took this experiments further by
examining whether MMP-2, MMP-9 and ICAM-1 regulate the invasion of
gastric cancer. We further observed the administration of
Ad5/F35-PIAS1 in gastric cancer cells markedly decreased the levels
of MMP-2, MMP-9 and ICAM-1 proteins. One of the mechanisms by which
P38 MAPK may promote tumor cell migration and invasion is by the
upregulation of MMPs. Our studies have also demonstrated that
MMP-2, MMP-9 and ICAM-1 can directly stimulate cell invasion and
invasion via MAPK-dependent mechanism. The low MMP-2, MMP-9 and
ICAM-1 protein expression was shown in SGC7901 cells with
Ad5/F35-PIAS1 treatment. Therefore, these findings provide a
possible explanation how the upregulation of PIAS1 expression could
inhibit the invasion of gastric cancer.
In conclusion, the downregulation of PIAS1
expression shows potential to be used as a valuable assessment
marker for the progression of gastric cancer. The upregulation of
PIAS1 expression inhibited the activation of P38MAPK and ERK
proteins and downregulated the levels of downstream cytokines
(MMP-2, MMP-9 and ICAM-1), relieving cell invasion. It might be
helpful to use PIAS1 in gastric cancer for therapeutic
strategy.
References
|
1
|
Bornschein J, Rokkas T, Selgrad M, et al:
Gastric cancer: clinical aspects, epidemiology and molecular
background. Helicobacter. 16(Suppl 1): 45–52. 2011. View Article : Google Scholar
|
|
2
|
Liu B, Yang R, Wong KA, et al: Negative
regulation of NF-kappaB signaling by PIAS1. Mol Cell Biol.
25:1113–1123. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tahk S, Liu B, Chernishof V, et al:
Control of specificity and magnitude of NF-kappa B and
STAT1-mediated gene activation through PIASy and PIAS1 cooperation.
Proc Natl Acad Sci USA. 104:11643–11648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coppola D, Parikh V, Boulware D, et al:
Substantially reduced expression of PIAS1 is associated with colon
cancer development. J Cancer Res Clin Oncol. 135:1287–1291. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seger R and Krebs EG: The MAPK signaling
cascade. FASEB J. 9:726–735. 1995.PubMed/NCBI
|
|
6
|
Whitmarsh AJ and Davis RJ: Transcription
factor AP-1 regulation by mitogen-activated protein kinase signal
transduction pathways. J Mol Med. 74:589–607. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Graziosi L, Mencarelli A, Santorelli C, et
al: Mechanistic role of p38 MAPK in gastric cancer dissemination in
a rodent model peritoneal metastasis. Eur J Pharmacol. 674:143–152.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang J, Yao WY, Zhu Q, et al: XAF1 as a
prognostic biomarker and therapeutic target in pancreatic cancer.
Cancer Sci. 101:559–567. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu J and Chen L: Current status and
progress in gastric cancer with liver metastasis. Chin Med J.
124:445–456. 2011.PubMed/NCBI
|
|
10
|
Tian MM, Sun Y, Li ZW, et al:
Polymorphisms of ICAM-1 are associated with gastric cancer risk and
prognosis. World J Gastroenterol. 18:368–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jang BG and Kim WH: Molecular pathology of
gastric carcinoma. Pathobiology. 78:302–310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tai DJ, Hsu WL, Liu YC, et al: Novel role
and mechanism of protein inhibitor of activated STAT1 in spatial
learning. EMBO J. 30:205–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu LM, Yan MG, Yang DH, et al: The
expression of protein inhibitor of activated signal transducers and
activators of transcription 3 in the evolutionary process of
gastric cancer. Eur J Intern Med. 22:e31–e35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dhillon AS, Hagan S, Rath O, et al: MAP
kinase signalling pathways in cancer. Oncogene. 26:3279–3290. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang JJ, Cho LY, Ma SH, et al: Oncogenic
CagA promotes gastric cancer risk via activating ERK signaling
pathways: a nested case-control study. PLoS One. 6:e211552011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Sun Y, Liu S, et al: Upregulation
of progranulin by Helicobacter pylori in human gastric
epithelial cells via p38MAPK and MEK1/2 signaling pathway: role in
epithelial cell proliferation and migration. FEMS Immunol Med
Microbiol. 63:82–92. 2011.
|
|
17
|
Mitsuno Y, Yoshida H, Maeda S, et al:
Helicobacter pylori induced transactivation of SRE and AP-1 through
the ERK signalling pathway in gastric cancer cells. Gut. 49:18–22.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsuoka T, Adair JE, Lih FB, et al:
Elevated dietary linoleic acid increases gastric carcinoma cell
invasion and metastasis in mice. Br J Cancer. 103:1182–1191. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haier J, Nasralla M and Nicolson GL: Cell
surface molecules and their prognostic values in assessing
colorectal carcinomas. Ann Surg. 231:11–24. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yashiro M, Sunami T and Hirakawa K: CD54
expression is predictive for lymphatic spread in human gastric
carcinoma. Dig Dis Sci. 50:2224–2230. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hara I, Miyake H, Hara S, et al:
Significance of matrix metalloproteinases and tissue inhibitors of
metalloproteinase expression in the recurrence of superficial
transitional cell carcinoma of the bladder. J Urol. 165:1769–1772.
2001. View Article : Google Scholar : PubMed/NCBI
|