Introduction
Endometrial carcinoma is one of the most common
malignancies in women, and its incidence has recently increased in
both developed and developing countries (1). Approximately 70–80% of human
endometrial cancers begin as hormone-dependent, and long-term
estrogen stimulation without progesterone counteraction plays an
important role in tumorigenesis (2,3).
However, the molecular basis of endometrial carcinoma remains
poorly understood.
The biological effects of estrogen are mediated by
the estrogen receptor (ER), which has 2 subtypes, ERα and ERβ
(4,5). Emerging evidence suggests that ER is a
key transcriptional regulator in hormone-associated cancer biology,
including endometrial cancer. The mechanisms of ER are complex and
involve genomic as well as non-genomic signaling events (6,7). Upon
binding to E2, ligand-activated ERs bind to ER-responsive elements
and modulate the respective gene expression (genomic signaling),
and ERs also participate in transcription-independent functions
(non-genomic action) through the activation of cytosolic signaling
pathways, including the Src, MAPK and protein kinase B (AKT)
pathways (7–9). ER function is modulated by
co-regulators (10,11). Advances in research during the past
decade have identified several novel proteins as being ER
co-regulators, with each co-regulator playing an important and a
non-overlapping function (10–18).
Proline-, glutamic acid- and leucine-rich protein-1;
also known as modulator of non-genomic activity of ER (PELP1/MNAR),
is a novel ER co-activator that plays an essential role in the
mechanisms of ER (19). Compared
with other ER co-regulators, PELP1/MNAR is unique, as it modulates
the function of ERα and ERβ and is involved in genomic as well as
non-genomic signaling events (20–22).
PELP1 is also a general co-regulator for a number of nuclear
receptors (NRs), including ER, ER-related receptor (ERR),
progesterone receptor (PR), glucocorticoid receptor (GR), androgen
receptor (AR) and transcription factors, such as E2F, four and a
half LIM domain protein 2 (FHL2) and signal transducer and
activator of transcription 3 (STAT3) (23,24).
Emerging findings suggest that PELP1/MNAR is a novel
proto-oncogene. Its expression is deregulated in several
hormone-responsive cancers, including breast, ovary, endometrial
and prostate cancer (24–28). The overexpression of PELP1/MNAR in
fibroblasts and epithelial model cells results in cellular
transformation and breast cancer cells stably overexpressing
PELP1/MNAR have shown rapid tumor growth in xenograft studies
(25). Endogenous PELP1/MNAR is
also required for optimal ligand-mediated transcription and
proliferation responses in endometrial cancer cells (27). However, the molecular mechanism(s)
responsible for its oncogenic function in endometrial carcinoma
remains unclear.
In this study, we examined PELP1/MNAR expression in
various endometrial cancer cell lines, and used a lentiviral
vector-based RNA interference expression system targeting
PELP1/MNAR to obtain a stable transcript knockdown and a high
efficiency of RNAi delivery. We aimed to determine the effect of
the downregulation of PELP1/MNAR on the proliferation, migration
and invasive potential of endometrial cancer cells with or without
estrogen stimulation in an attempt to provide a new strategy for
hormonal carcinogenesis prevention and new therapy opportunities
for the treatment of endometrial carcinoma.
Materials and methods
Reagents
PELP1/MNAR antibodies for western blot analysis were
purchased from Abicam (Beverly, MA, USA), PELP1/MNAR antibodies for
immunocytochemistry were purchased from Novus Biologicals
(Littleton, CO, USA), horseradish, peroxidase (HRP)-conjugated goat
anti-rabbit secondary antibodies were purchased from Santa Cruz
Biotechnology Inc., (Santa Cruz, CA, USA) and GAPDH was purchased
from Biosynthesis Technology (Beijing, China). ERα and ERβ
antibodies were purchased from Dako (Glostrup, Denmark).
17-Estradiol (purity ≥98%; cat. no. E8875) was purchased from Sigma
Chemicals (St. Louis, MO, USA).
HEC-1A and HEC-1B human endometrial carcinoma cells
were obtained from the American Type Culture Collection (Manassas,
VA, USA). AN3CA and RL-952 cells were provided by the Shanghai Cell
Collection, Chinese Academy of Sciences (Shanghai, China). Ishikawa
human endometrial carcinoma cells were kindly provided by Professor
Zehua Wang (Department of Obstetrics and Gynecology, Union
Hospital, Tongji Medical College, Huazhong University of Science
and Technology, Wuhan, China). The cells were grown in Dulbecco's
modified Eagle's medium with essential amino acid solution (Gibco,
Carlsbad, CA, USA) without phenol red and supplemented with 10%
fetal calf serum (HyClone, South Logan, UT, USA).
Western blot analysis
To detect the expression of PELP1/MNAR protein in
different endometrial carcinoma cell lines, we performed western
blot analysis, and used cervical cancer HeLa cell as the control.
Ishikawa, HEC-1A, HEC-1B, AN3CA, RL-952 and HeLa cells were lysed
using the KeyGen Total Protein Extraction kit (KeyGen, Nanjing,
China) according to the manufacturer's instructions. The lysates
were centrifuged at 4°C 14,000 rpm for 20 min. The supernatants
were collected and the concentrations of the supernatants were
measured with a BCA protein assay kit (Pierce, Rockford, IL, USA).
Protein (25–30 μg) was applied per lane and separated by 8–12% SDS
polyacrylamide gel electrophoresis, then transferred onto
polyvinylidene difluoride membranes (Millipore, Billerica, MA,
USA). The membranes were incubated with blocking buffer for 60 min
at room temperature. The blocking buffer consisted of 5% non-fat
dry milk dissolved in Tris-buffered saline containing 0.1% Tween-20
(TBS-T). After washing the membranes with TBS-T, they were
immunoblotted overnight at 4°C and were incubated with rabbit
anti-human PELP1/MNAR (1:300), at 4°C overnight. The membranes were
washed with TBS-T 3 times, and subjected to HRP-conjugated
secondary antibody for 60 min at room temperature. Protein-antibody
complexes were visualized with an ECL western blot detection system
(Pierce). Western blot films were digitized, net band intensities
were quantified using the Quantity One Image System and GAPDH
expression levels were used to further normalize the loading
amount. Each experiment was repeated 3 times to assess the
consistency of the results.
Immunocytochemistry
For immunocytochemical staining, the Ishikawa,
HEC-1A and AN3CA cells were plated on coverslips and cultured for
48 h. The coverslips were fixed in 4% paraformaldehyde for 30 min
at room temperature, washed in PBS, and permeabilized for 10 min
with 0.25% Triton X-100 in PBS. The cell coverslips were then
incubated with 0.3% H2O2 in PBS for 15 min.
After washing in PBS, the samples were then incubated with rabbit
polyclonal anti-human PELP1 antibody (1:500), mouse anti-human ERα
antibody (1:60), or rabbit anti ERβ (1:60) for 1 h in a humid
chamber. After washing with PBS, the sections and coverslips were
overlaid with Dako REAL EnVision HRP rabbit/mouse antibody at 37°C
for 30 min. The sections and coverslips were then counterstained
with hematoxylin. Positive reactions were detected by incubating
the slides with 3,3′-diaminobenzidine tetrahydrochloride for 1 min.
The immunocytochemical results were evaluated by a pathologist.
Construction and transfection of short
hairpin (shRNA) lentivirus vectors
DNA template oligonucleotides corresponding to the
PELP1/MNAR gene (GenBank NM_014389) were designed (28) and synthesized. The targeted
sequences were: PELP1/MNAR-shRNA, 5′-GGACCAAGGTGTATGCG ATAT-3′; the
sequence was inserted into the AgeI and EcoRI enzyme
sites of the pGCSIL-GFP vector. The negative control (NC) sequences
were 5′-TTCTCCGAACGTGTCACGT-3′. The lentiviral particles were
produced using a Lentiviral Vector Expression System (Auragene,
Changsha, China). Briefly, the vectors with helper plasmids were
transfected into 293T cells using the calcium phosphate
transfection method. The supernatant containing lentiviral
particles was collected and concentrated by ultracentrifugation.
The condensed lentiviral particle solution was tittered on 293T
cells with the final titer ~5×108 TU/ml.
For transfection, 2×105 cells/well were
seeded in a 6-well plate for 24 h. Cells were then transfected with
lentivirus-mediated shRNA targeting PELP1/MNAR or negative control
lentivirus vector. The multiplicity of infection (MOI) was 50.
Cells transfected with lentivirus-mediated shRNA targeting
PELP1/MNAR, negative control and with no transfection were named
Ishikawa-KD, Ishikawa-NC and Ishikawa-CON cells, respectively. The
transfection efficiency was evaluated by fluorescence microscopy
and flow cytometry.
Real-time PCR
Total RNA was isolated from the cells using TRIzol
(Invitrogen) according to the manufacturer's instructions.
First-strand cDNA was synthesized using a PrimeScript™ RT reagent
kit (Perfect Real-Time; Takara, Beijing, China). The reverse
transcription (RT) reaction mixture for first-strand cDNA synthesis
included 5X PrimeScript™ Buffer 2 μl, PrimeScript™ RT Enzyme mix I
0.5 μl, Oligo(dT) primer 25 pmol, random 6 mers 50 pmol, total RNA
500 ng and RNase free dH2O in a final volume of 10 μl.
The samples were placed on ice before reverse transcription. Then
tubes were incubated for 15 min at 37°C, 5 sec at 85°C and then
chilled to 4°C.
PCR was carried out in a 20 μl final volume
containing the following: 2 μl cDNA diluted in RNase-free water; 10
μl SYBR premix Ex Taq; the antisense and sense primers 0.5 μl
separately; ROX Reference dye II (50X) 0.4 μl and dH2O
in order to reach a 20 μl final volume. After an initial
denaturation step at 95°C for 30 sec, temperature cycling was
initiated. Each cycle consisted of denaturation at 95°C for 3 sec
and elongation at 60°C for 30 sec. A total of 40 cycles was
performed. The fluorescent signal was acquired at the end of the
elongation step. Real-time PCR was performed in a ABI 7500 Fast
Real-Time PCR System (Applied Biosystems, Foster City, CA, USA)
with specific real-time PCR primers for each gene (Table I). Results were normalized to the
actin transcript levels and the difference in fold-expression was
calculated using the ΔΔCT method. The results were representative
of 3 independent experiments. Data are shown as the means ± SD. The
primers for PELP1/MNAR, β-actin, and MMPs are shown in Table I.
 | Table IPrimer sequences for real-time
PCR. |
Table I
Primer sequences for real-time
PCR.
| mRNA | Primer sequence |
|---|
| β-actin | Sense:
5′-CAGCCATGTACGTTGCTATCCAGG-3′ |
| Antisense:
5′-CAGCCATGTACGTTGCTATCCAGG-3′ |
| PELP1 | Sense:
5′-CTCAGTAATGCACGTCTCAGTTCCA-3′ |
| Antisense:
5′-GAATGCTCCGAAGCCAAGACA-3′ |
| MMP-9 | Sense:
5′-ACGCACGACGTCTTCCAGTA-3′ |
| Antisense:
5-CCACCTGGTTCAACTCACTCC-3′ |
| MMP-2 | Sense:
5′-CTTCCAAGTCTGGAGCGATGTG-3′ |
| Antisense:
5′-ATGAGCCAGGAGTCCGTCCTTA-3′ |
Cell proliferation assays
The cell viability and proliferation were measured
by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) assay (Sigma, USA). Seven days after the transfection the 3
types of Ishikawa cells were seeded into 5 96-well culture plates
at 3.0×103/well, with each group consisting of 5
parallel wells. After 24 h, the cells were treated with or without
E2 (10−9 mol/l). For the following 5 days, 20 μl MTT (5
mg/ml) were added to each well and the cells were incubated at 37°C
for an additional 4 h. The reaction was terminated by lysing the
cells with 150 μl DMSO for 10 min. Absorbance was measured at 490
nm using a microplate reader (Bio-Tek, Winooski, VT, USA). Cell
growth curves were drawn by the mean optical density (OD)
values.
In vitro anchorage-dependent
colony-forming assay
Five days after the transfection, the 3 types of
Ishikawa cells were plated in 10-cm culture dishes at a density of
3×103/dish, respectively. After 24 h, the cells were
treated with or without E2 (10−9 mol/l). The cells were
fed every 2 days, and the foci of the transformed cells were
counted after 14 days. The cells were stained with crystal violet
and visible colonies were manually counted. The data are
representative of 3 independent experiments performed in
triplicate.
Cell migration and invasion
Cell migration and invasion assays were all
performed in a 24-well, Transwell chamber with a 8.0-μm pore
membrane (Corning, Corning, NY, USA). For the cell migration assay,
3 types of Ishikawa cells (5×104) were seeded in the
upper chamber with serum-free medium, and 0.75 ml of complete
growth medium containing 10% fetal bovine serum with or without E2
(10−9 mol/l) was added to each well in the lower chamber
(21). For the invasion assays, the
upper chambers were coated with 20% Matrigel (100 μl/well) (BD
Biosciences, San Diego, CA, USA). The cells were then seeded in the
chamber at 1×105/well and treated as described above.
After 24 h of incubation at 37°C in a 5% CO2 atmosphere,
cells on the upper side of the chamber were removed and cells
invaded to the lower side were fixed, stained and counted in 10
random fields at magnification, ×100.
Statistics analysis
All assays were conducted 3 times and found to be
reproducible. Data are expressed as the means ± SD. Comparisons
among the groups were performed with the one-way analysis of
variance (ANOVA) test with Bonferroni's correction for multiple
comparisons. All statistical analyses were performed by using SPSS
13.0. A P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Expression profile of PELP1/MNAR in
endometrial cancer cell lines
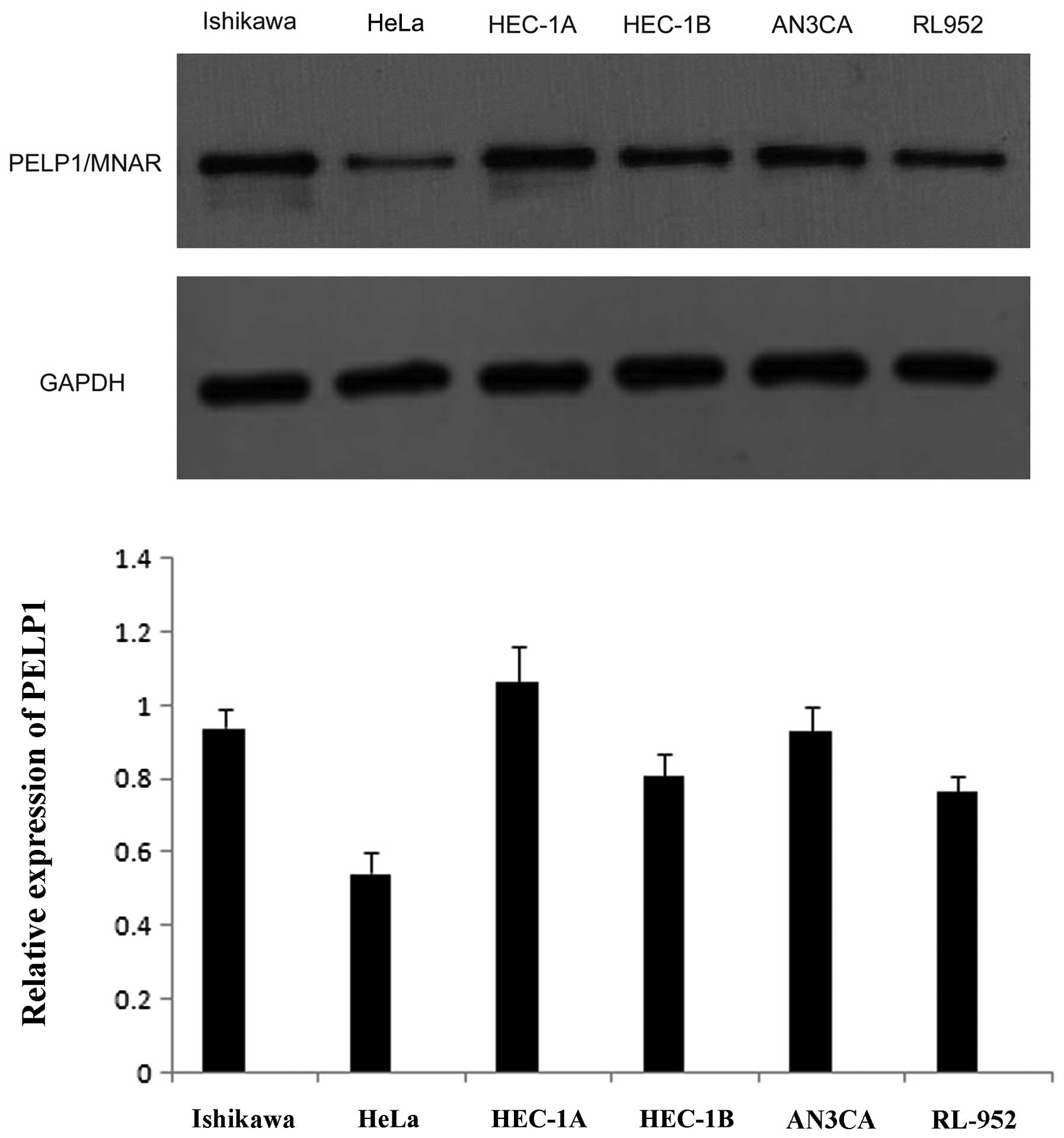
To determine whether PELP1/MNAR plays a role in
endometrial cancer, we first measured the PELP1/MNAR expression in
5 commonly used endometrial cancer cell lines (Ishikawa, HEC-1A,
AN3CA, HEC-1B and RL-952) and cervical cancer HeLa cells using
western blot analysis. The results revealed that PELP1/MNAR was
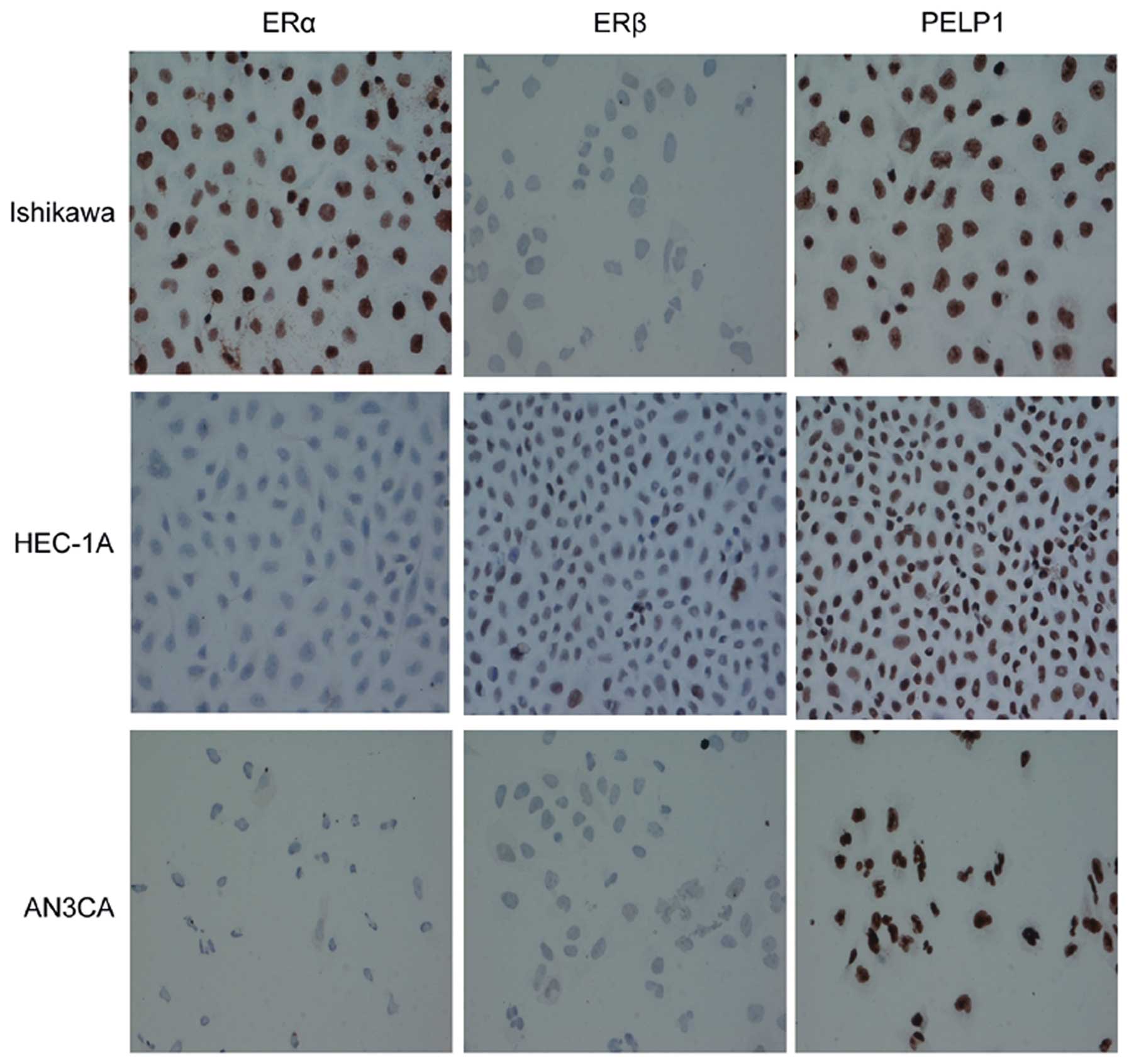
widely expressed in all the cell lines (Fig. 1). We then used immunocytochemistry
to detect PELP1/MNAR as well as ERα and ERβ expression and location
in the Ishikawa, HEC-1A and AN3CA endometrial cancer cells, and
found that the expression of ERα and ERβ varied among the cells.
Ishikawa cells were ERα-positive, HEC-1A cells were ERβ-positive,
whereas ERα and ERβ expression was low/undetectable in AN3CA cells
(Fig. 2). However, all the cell
lines were stained with PELP1/MNAR and the protein was mostly
located in the nucleus, which implies a potentially important role
for PELP1/MNAR in endometrial cancer. Therefore, we further
investigated the effects of PELP1/MNAR suppression on the Ishikawa
endometrial cancer cell line and the signaling pathway
involved.
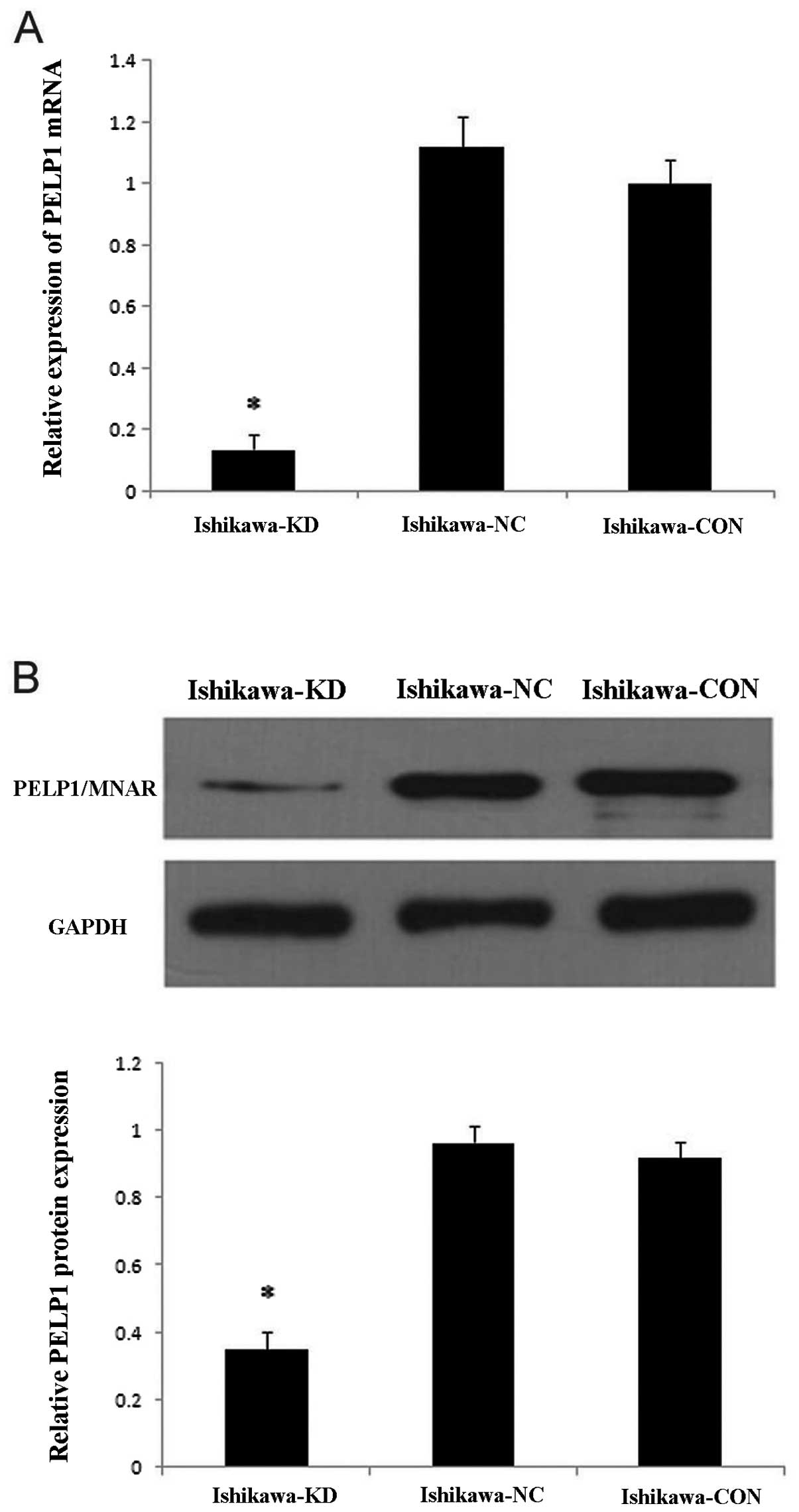
Downregulation of PELP1/MNAR mRNA and
protein expression in Ishikawa cells after lentivirus
transfection
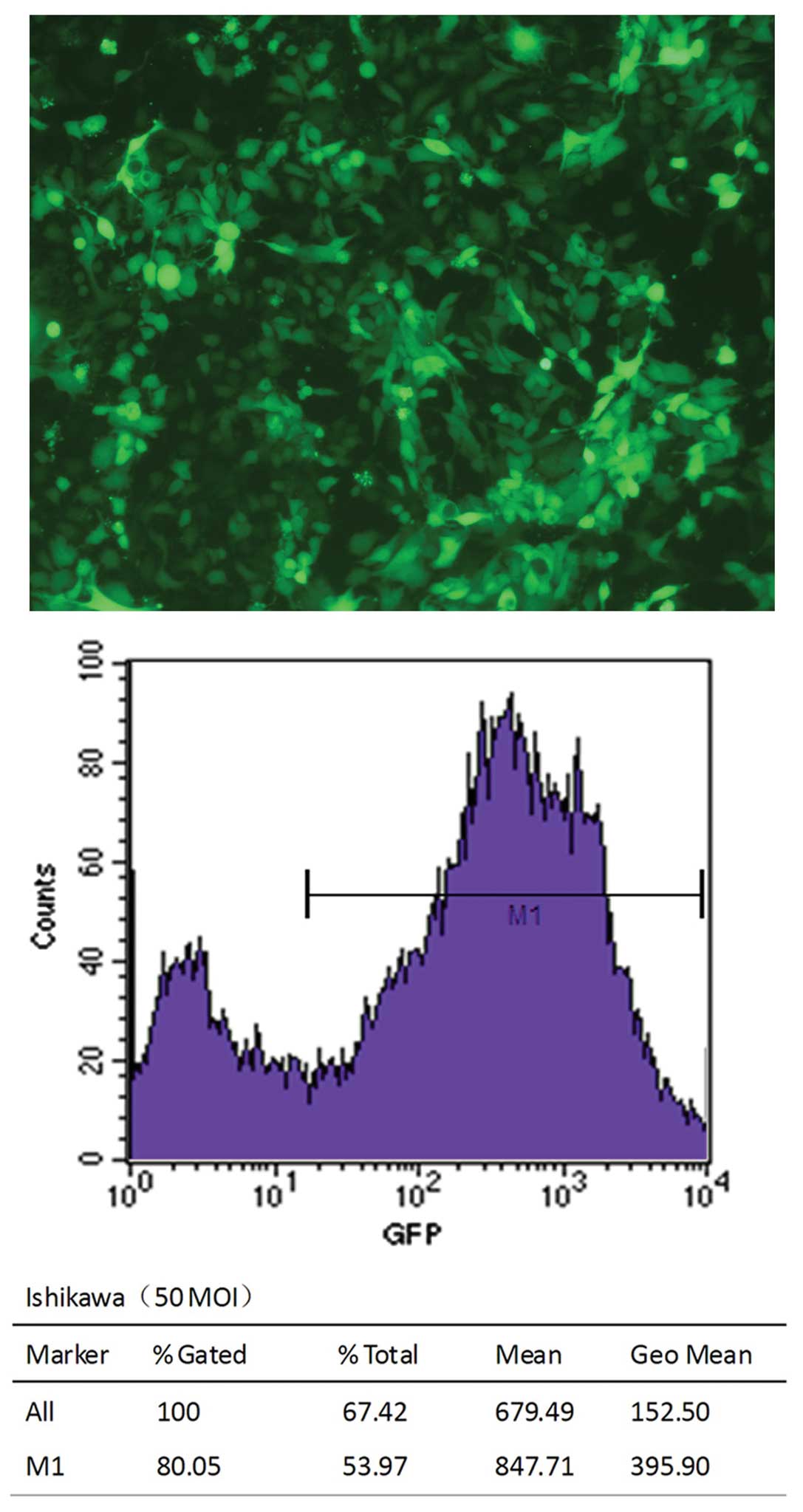
To examine the function of endogenous PELP1/MNAR in
endometrial cancer cells, we selectively knocked down the
endogenous PELP1/MNAR expression using shRNA methodology. The
fluorescent microphoto indicated that transfection was successful
(Fig. 3). Additionally, flow
cytometry analysis showed that the transfection efficiency was
above 80%. After 5 or 7 days of tranfection, the silencing effect
was examined by real-time PCR and western blot analysis in the mock
and stably transfected Ishikawa cells. As shown in Fig. 4, compared with the mock-transfected
Ishikawa cells, the levels of PELP1/MNAR mRNA and protein in
Ishikawa-KD cells were significantly reduced by 86 and 65%,
respectively (P<0.05); however, there was no significant
difference between the Ishikawa-NC and Ishikawa-CON cells. These
results confirmed the efficacy of lentivirus-mediated
PELP1/MNAR-specific shRNA in downregulating endogenous PELP1/MNAR
expression.
PELP1/MNAR downregulation inhibits cell
proliferation
To further analyze the effect of PELP1/MNAR
downregulation on the proliferation of Ishikawa cells, we measured
the proliferation rate of Ishikawa, Ishikawa-NC and Ishikawa-KD
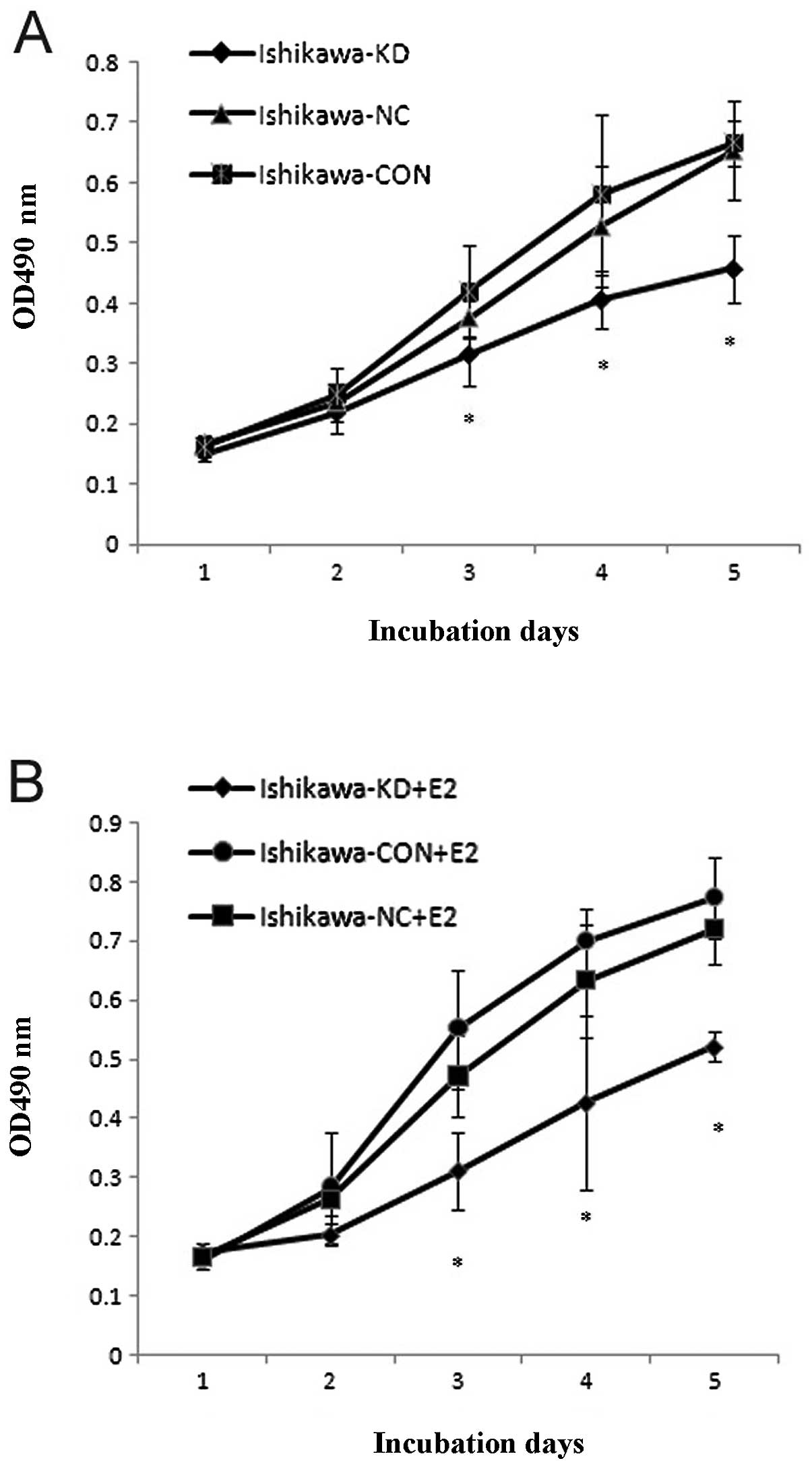
cells with or without 17β-E2 by MTT. The results showed that the
proliferation of the Ishikawa-KD cells decreased in a
time-dependent manner compared with the parental cells and that the
effect of PELP1/MNAR downregulation on cell proliferation was more
pronounced when 17β-E2 was added (Fig.
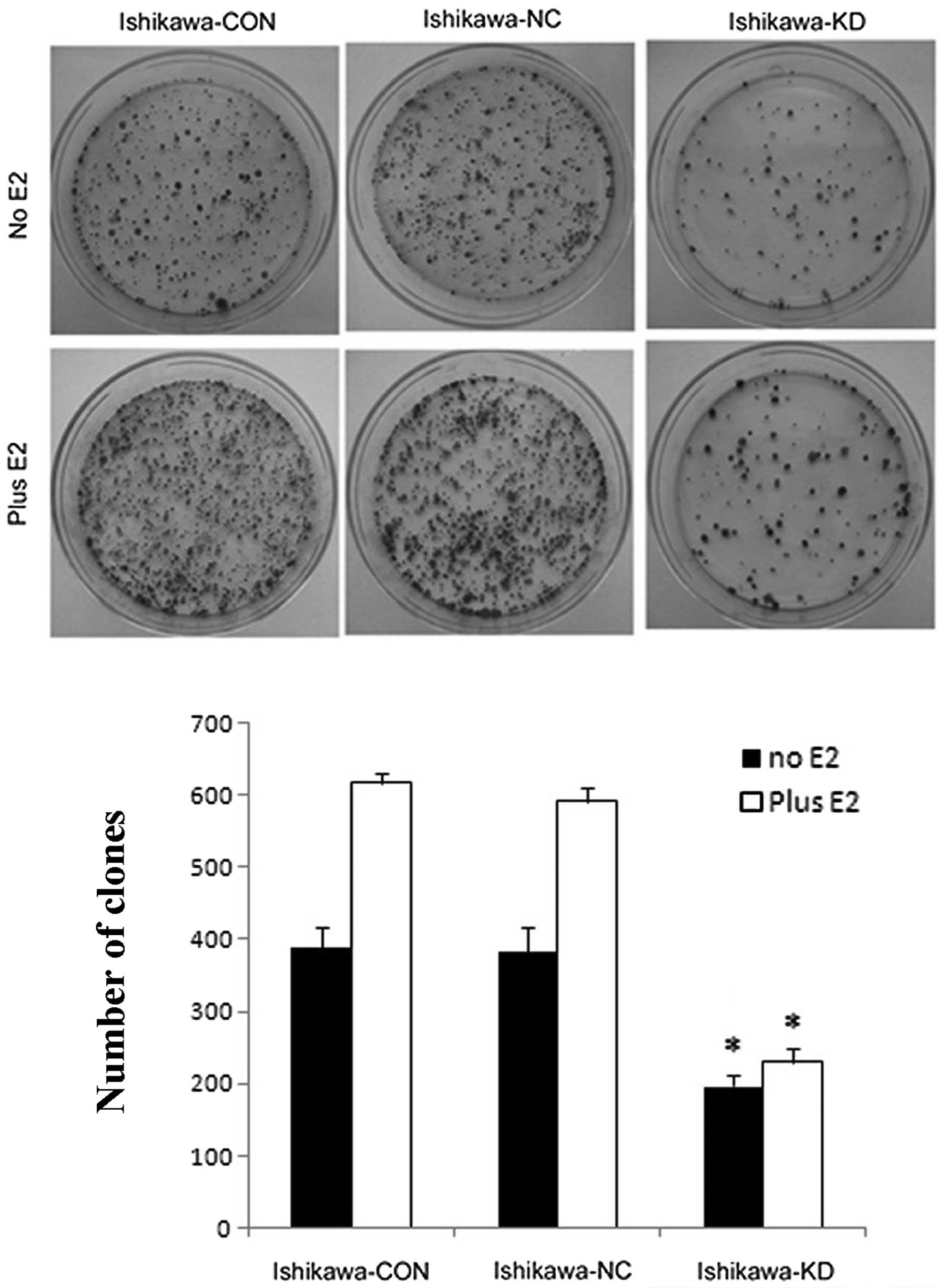
5). We then analyzed the anchorage-dependent growth using a
colony-forming assay. Similarly, Ishikawa-KD cells showed fewer
colonies than Ishikawa-CON and Ishikawa-NC cells, with or without
E2 (10-9 M) treatment; however, there was no difference between the
Ishikawa-NC and Ishikawa-CON cells (Fig. 6).
PELP1/MNAR downregulation reduces cell
migration and invasion
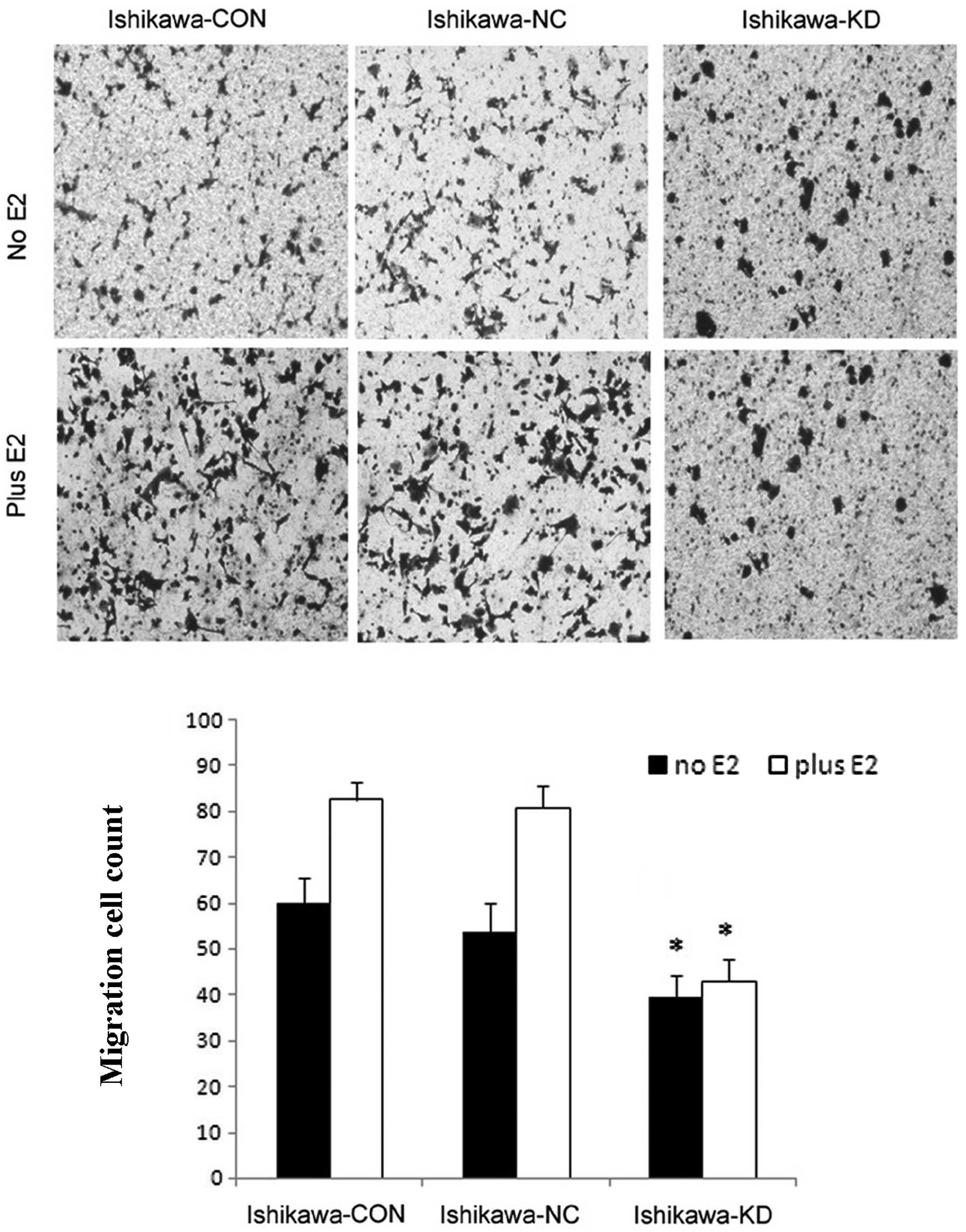
To further examine whether the downregulation of
PELP1/MNAR in Ishikawa cells reduces their migratory potential, we
examined Ishikawa, Ishikawa-NC, Ishikawa-KD cells using Boyden
chamber assay. Compared to the control cells, the knockdown of
PELP1/MNAR in Ishikawa cells resulted in significantly reduced cell
motility (Fig. 6). Moreover, the
accelerated cell migratory effect of E2 was blocked in the
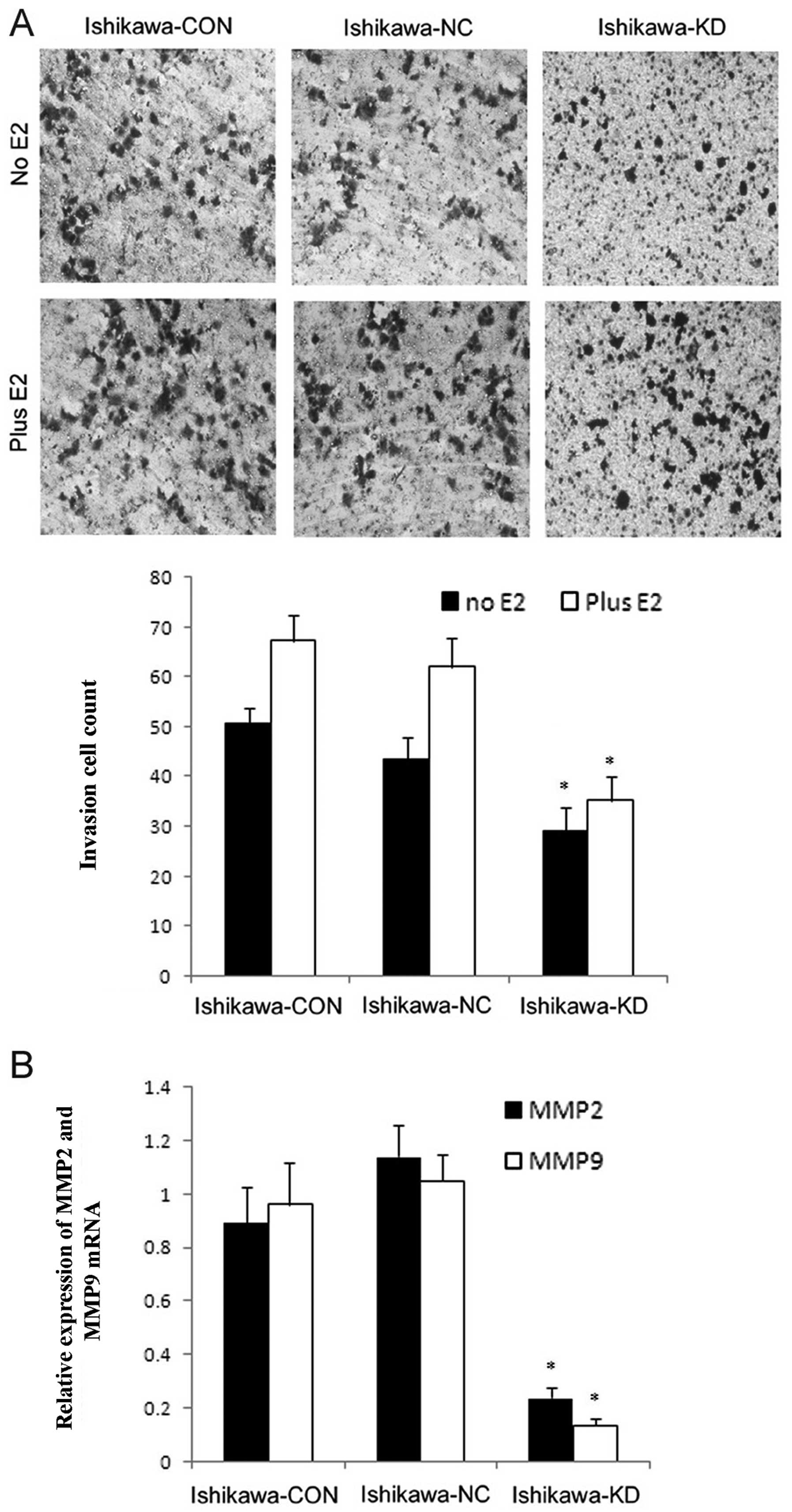
Ishikawa-KD cells. The downregulation of PELP1/MNAR in the Ishikawa
cells also significantly reduced the invasive potential, as shown
by Boyden chamber assay (Fig. 7)
and more importantly, abolished the E2-enhanced invasion
(P<0.05). These results suggest that PELP1/MNAR plays an
important role in cell invasion. To further understand the
mechanism involved, we examined the expression of matrix
metalloproteinase (MMP)-2 and MMP-9 to investigate whether
PELP1/MNAR downregulation altered the expression of MMPs. MMPs
promote cancer progression by enhancing the growth, migration,
invasion and metastasis of endometrial cancer cells. Total RNA
isolated from Ishikawa, Ishikawa-NC and Ishikawa-KD cells was used
for real-time PCR and the results suggested that PELP1/MNAR
downregulation substantially reduced the expression of MMP-2 and
MMP-9 compared to their expression in the control cells (Fig. 8).
Discussion
Although estrogen and ER signaling is considered the
classic etiological factor for endometrial tumorigenesis, the
modulation mechanisms of estrogen that are involved in endometrial
cancer remain unclear. NR co-regulators (co-activators and
co-repressors) are essential elements in regulating NR-mediated
transcription and other cellular events. Disruptions in
co-regulator biology may lead to pathological states, including
cancer. NR-co-regulator proteins have the potential to be
differentially expressed in malignant tumors, and their functions
may be altered, leading to tumor progression. The role of NR
co-regulators as proto-oncogenes is an emerging area in the field
of cancer research and, thus, represents a potential area for
therapeutic targeting (29,30). Several NR co-regulators reported to
be misexpressed in endometrial cancer include amplified in breast
cancer 1 (AIB1), metastasis-associated protein 1 (MTA1) and NR
co-repressor (NcoR). The deregulation of PELP1/MNAR in
endometrial carcinoma was also observed, which led us to
hypothesize that PELP1/MNAR may play a role in endometrial
carcinoma progression and that the downregulation of PELP1/MNAR may
serve as a potential target for therapy.
To examine this hypothesis, we chose 5 endometrial
cancer cell lines to investigate the expression of PELP1/MNAR, and
found that PELP1/MNAR was widely expressed in endometrial cells,
even in ER-negative cells, and the protein was mostly located in
the nucleus. A recent study also showed that PELP1 expression was
retained in ER-negative breast tumors. PELP1 knockdown reduced the
motility and metastatic potential of ER-negative breast cancer
cells in vivo and significantly reduced lung metastatic
nodules in a xenograft assay. Their results suggested that PELP1
has potential to participate in hormone-driven pathologies and
plays a role in the initiation and progression of ER-negative
breast cancer (31). Whether or not
PELP1 contributes to ER-negative endometrial carcinoma progression
is yet to be determined; thus, further research is required. We
then employed the newly developed lentivirus vector encoding shRNAs
against PELP1/MNAR, which has high transfection efficiency and long
duration resulting in stable ablation. After transfection, the
endogenous expression of PELP1/MNAR was significantly knocked down
in the commonly used endometrial carcinoma cell line, Ishikawa, in
order to explore its role on the proliferation, invasion and
metastasis of endometrial carcinoma cells.
Earlier studies have demonstrated that PELP1/MNAR is
essential for the E2-mediated cell proliferation in several
hormone-related cancers, including endometrial cancer. Mechanistic
studies showed that PELP1/MNAR plays a permissive role in
E2-mediated cell cycle progression by enhancing E2 mediated G1-S
progression in breast cancer cells (32). A more recent study revealed that
PELP1/MNAR is a novel substrate of interphase cyclin-dependent
kinases (CDKs) and that its phosphorylation is important for the
proper function of PELP1/MNAR in modulating hormone-driven cell
cycle progression and also for optimal E2F transactivation function
in breast cancer (33). In our
study, the downregulation of PELP1/MNAR was accompanied by a
significant suppression in the ability of estrogen to stimulate the
growth of Ishikawa cells, as well as the basal proliferation
without the addition of E2.
Evidence has shown that PELP1/MNAR also plays a role
in cancer metastasis. PELP1/MNAR expression is deregulated in
metastatic tumors (26,31,34).
PELP1/MNAR protein expression is an independent prognostic
predictor of shorter breast cancer-specific survival, and its
elevated expression is positively associated with markers of poor
outcome (26). PELP1/MNAR plays a
critical role in ovarian cancer cell migration and modulates the
expression of several genes involved in metastasis (34), while its function in endometrial
cancer remains unclear. In the present study, we found that the
knockdown of PELP1/MNAR substantially affected the ability of
endometrial cancer cells to migrate and invade, as shown by Boyden
chamber assay. MMPs are a family of structurally related zinc- and
calcium-dependent endopeptidases that degrade extracellular matrix
components. MMP-mediated proteolysis is involved in various
physiological and pathological processes, such as cancer invasion
and metastasis. MMP-2 and MMP-9 contribute to tumor invasion. Tumor
invasion and angiogenesis are impaired by the combined deficiency
in both metalloproteinases (35).
Our results provide evidence that the production of MMP-2 and MMP-9
was also decreased with PELP1/MNAR knockdown, which contributed to
the inhibition of Ishikawa cell invasion.
Taken together, our study provides evidence that the
downregulation of PELP1/MNAR inhibits the proliferation and
metastasis of endometrial cancer cells in both an
estrogen-dependent and estrogen-independent manner. The findings
suggest that PELP1 may be used as a potential therapeutic target in
endometrial cancer. Future study of the in vivo mechanisms
of PELP1/MNAR activity and profile of the expression of PELP1/MNAR
in a large number of tumor samples would confirm the use of this
novel ER-co-regulatory protein as a diagnostic marker and as a
target for novel therapies.
Acknowledgements
This study was supported by the Science and
Technology Plan Project of Guang Dong Province (2007B030502014,
00429391120223052) and the National Nature Science Foundation of
China (30772332).
References
|
1
|
Whitcomb BP: Gynecologic malignancies.
Surg Clin North Am. 88:301–317. vi2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang H: Endocrine-related cancer.
Encyclopedia of Cancer. Schwab M: 2nd edition. Springer-Verlag;
Heidelberg: pp. 435–439. 2008
|
|
3
|
Shang Y: Molecular mechanisms of oestrogen
and SERMs in endometrial carcinogenesis. Nat Rev Cancer. 6:360–368.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pietras RJ, Levin ER and Szego CM:
Estrogen receptors and cell signaling. Science. 310:51–53. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shao W and Brown M: Advances in estrogen
receptor biology: prospects for improvements in targeted breast
cancer therapy. Breast Cancer Res. 6:39–52. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
O'Lone R, Frith MC, Karlsson EK and Hansen
U: Genomic targets of nuclear estrogen receptors. Mol Endocrinol.
18:1859–1875. 2004. View Article : Google Scholar
|
|
7
|
Bjornstrom L and Sjoberg M: Mechanisms of
estrogen receptor signaling: convergence of genomic and non-genomic
actions on target genes. Mol Endocrinol. 19:833–842. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song RX, Zhang Z and Santen RJ: Estrogen
rapid action via protein complex formation involving ERalpha and
Src. Trends Endocrinol Metab. 16:347–353. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Losel R and Wehling M: Non-genomic actions
of steroid hormones. Nat Rev Mol Cell Biol. 4:46–56. 2004.
View Article : Google Scholar
|
|
10
|
Mckenna NJ, Lanz RB and O'Malley BW:
Nuclear receptor coregulators: cellular and molecular biology.
Endocr Rev. 20:321–344. 1999.PubMed/NCBI
|
|
11
|
Hall JM and Mcdonnell DP: Coregulators in
nuclear estrogen receptor action: from concept to therapeutic
targeting. Mol Interv. 5:343–357. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barnes CJ, Vadlamudi RK and Kumar R: Novel
estrogen receptor coregulators and signaling molecules in human
diseases. Cell Mol Life Sci. 61:281–291. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han SJ, Demayo FJ, Xu J, Tsai SY, Tsai MJ
and O'Malley BW: Steroid receptor coactivator (SRC)-1 and SRC-3
differentially modulate tissue-specific activation functions of the
progesterone receptor. Mol Endocrinol. 20:45–55. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O'Malley BW: Coregulators: from whence
came these ‘master genes’. Mol Endocrinol. 21:1009–1013.
2007.PubMed/NCBI
|
|
15
|
Sakaguchi H, Fujimto J and Sun WS:
Clinical implications of steroid receptor coactivator (SRC)-3 in
uterine endometrial cancers. J Steroid Biochem Mol Biol.
104:237–240. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lanard DM, Lanz RB and O'Malhy BW: Nuclear
receptor coregulators and human disease. Endocr Rev. 28:575–587.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gururaj AE, Singh RR and Rayala SK: MTA1,
a transcriptional activator of breast cancer amplified sequence 3.
Proc Natl Acad Sci USA. 103:6670–6675. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Webb P, Valentine C and Nguyen P: ER beta
binds N-CoR in the presence of estrogens via an LXXLL-like motif in
the N-CoR C-terminus. Nucl Recept. 1:42003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mishra SK, Balasenthil S and Ngnyen D:
Cloning and functional characterization of PELP1/MNAR promoter.
Gene. 330:115–122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nair SS, Nair BC, Cortez V, Chakravarty D,
Metzger E, Schüle R, Brann DW, Tekmal RR and Vadlamudi RK: PELP1 is
a reader of histone H3 methylation that facilitates oestrogen
receptor-alpha target gene activation by regulating lysine
demethylase 1 specificity. EMBO Rep. 11:438–444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheskis BJ, Greger J, Cooch N, et al: MNAR
plays an important role in ERα activation of Src/MAPK and PI3K/Akt
signaling pathways. Steroids. 73:901–905. 2008.
|
|
22
|
Rajhans R and Vadlamudi RK: Comprehensive
analysis of recent biochemical and biologic findings regarding a
newly discovered protein-PELP1/MNAR. Clin Exp Metastasis. 23:1–7.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vadlamudi RK and Kumar R: Functional and
biological properties of the nuclear receptor coregulator
PELP1/MNAR. Nucl Recept Signal. 5:e0042007.PubMed/NCBI
|
|
24
|
Nair SS, Guo Z, Mueller JM, et al:
PELP1/MNAR enhances androgen receptor functions through LIM-only
coactivator FHL2. Mol Endocrinol. 21:613–624. 2007.PubMed/NCBI
|
|
25
|
Rajhans R, Nair S, Holden AH, Kumar R,
Tekmal RR and Vadlamudi RK: Oncogenic potential of the nuclear
receptor coregulator proline-, glutamic acid-, leucine-rich protein
1/modulator of the nongenomic actions of the estrogen receptor.
Cancer Res. 67:5505–5512. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Habashy HO, Powe DG, Rakha EA, et al: The
prognostic significance of PELP1 expression in invasive breast
cancer with emphasis on the ER-positive luminal-like subtype.
Breast Cancer Res Treat. 120:603–612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vadlamudi RK, Balasenthil S, Broaddus RR,
Gustafsson JA and Kumar R: Deregulation of estrogen receptor
coactivator proline-, glutamic acid-, and leucine-rich
protein-1/modulator of nongenomic activity of estrogen receptor in
human endometrial tumors. Clin Endocrinol Metab. 89:6130–6138.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dimple C, Nair SS, Rajhans R, et al: Role
of PELP1/MNAR signaling in ovarian tumorigenesis. Cancer Res.
68:4902–4909. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
O'Malley BW and Kumar R: Nuclear receptor
coregulators in cancer biology. Cancer Res. 69:8217–8222. 2009.
View Article : Google Scholar
|
|
30
|
Chakravarty D, Tekmal RR and Vadlamudi RK:
PELP1: a novel therapeutic target for hormonal cancers. IUBMB Life.
62:163–169. 2010.
|
|
31
|
Roy S, Chakravarty D, Cortez V, De
Mukhopadhyay K, Bandyopadhyay A, Ahn JM, Raj GV, Tekmal RR, Sun L
and Vadlamudi RK: Significance of PELP1 in ER-negative breast
cancer metastasis. Mol Cancer Res. 10:25–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Balasenthil S and Vadlamudi RK: Functional
interactions between the estrogen receptor coactivator PELP1/MNAR
and retinoblastoma protein. J Biol Chem. 278:22119–22127. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nair BC, Nair SS, Chakravarty D, et al:
Cyclin-dependent kinase-mediated phosphorylation plays a critical
role in the oncogenic functions of PELP1. Cancer Res. 70:7166–7175.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chakravarty D, Roy SS, Babu CR, Dandamudi
R, Curiel TJ, Vivas-Mejia P, Lopez-Berestein G, Sood AK and
Vadlamudi RK: Therapeutic targeting of PELP1 prevents ovarian
cancer growth and metastasis. Clin Cancer Res. 17:2250–2259. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|