Introduction
NPC is one of the most common malignant tumors in
southern China and southeast Asia with incidence rates of 20–50 per
100,000 (1). The most effective
treatment for NPC is radiotherapy, which achieves an overall 5-year
survival rate of 65% (2).
Nevertheless, NPC local recurrence remains a serious barrier to
successful treatment in many cases. Since overfull radiotherapy
easily leads to undesirable complications, development of novel
therapeutic approaches to NPC is necessary.
PDT is a minimally invasive modality in the
treatment of a variety of malignant tumors. It is a form of
photochemotherapy that uses a photosensitizer, light and oxygen.
PDT involves the targeting of cells or tissues that have been
sensitized to light by administration of a photosensitizing agent.
Photosensitizers used in this process include 5-aminolevulinic acid
(5-ALA) and its lipophilic derivative, methyl-aminolevulinate (MAL)
(3). Because of its high
selectivity and low toxicity, attention has been focused on its
application in PDT (4–8).
Apoptosis is the main way for PDT-induced cell
death. PDT can lead plasma membrane and lysosome to necrosis,
whereas mitochondrial activity can lead to programmed cell death,
including the extrinsic death pathway (death receptor-dependent)
and the intrinsic pathway (mitochondria-dependent). In the
extrinsic pathway, apoptosis is stimulated upon the binding of
specific ligands to death receptors triggering the recruitment of
the adaptor Fas- and TNFR-associated death domain protein (FADD and
TRADD) and initiator caspase-8 into a death-inducing signaling
complex (DISC). In the intrinsic pathway, various stress conditions
cause the release of cytochrome c into the cytosol, a process that
is tightly regulated by pro- and anti-apoptotic proteins of the
Bcl-2 family. Once released, cytochrome c binds to the adapter
protein Apaf-1, which enables the activation of the initiator
caspase-9 in a high-molecular-weight complex, called the apoptosome
(9–11). Generally, mitochondrion plays an
important role in PDT initiating and executing apoptosis in several
types of cells.
Apoptin is a small apoptosis-inducing protein
derived from chicken anemia virus (CAV) (12). It is also commonly called VP3, which
is a 14-kDa non-structural protein (13,14).
Apoptin can induce programmed cell death in a broad range of
transformed and cancer cells but not in non-transformed or primary
cells (15,16). It was reported that apoptin can
induce selective death of a variety of malignant cells and
transformed cells, including melanoma, lymphoma, hepatoma, colon
carcinoma, breast and lung cancer (17,18).
Many human tumor cell lines were shown to be susceptible to
apoptin. However, apoptin does not induce apoptosis in normal cells
such as human endothelial cells and hepatocytes (17). Apoptin induces tumor cell death
mainly through mitochondrial pathway independently of p53 (19). In this pathway, apoptin could
trigger cytochrome c release and the activation of caspase-9
(20).
In this study, we supposed that apoptin would
potentiate PDT therapy in NPC. We evaluated efficacy of apoptin
with PDT treatment in vivo and in vitro experiment
and confirmed apoptin to have synergic efficiency in PDT.
Materials and methods
Cell culture
NPC cell CNE-2 used in this study was obtained from
Cancer Research Institute, Central South University. CNE-2 cells
were cultured in RMPI-1640 medium (HyClone, Thermo Scientific, USA)
supplemented with 10% of fetal bovine serum (FBS, Hyclone) and was
maintained at 37°C in an incubator at a humidified atmosphere with
5% CO2 in air. VP3-transfected CNE-2 cells and mock
vector-transfected as control cells were maintained in a medium
supplemented with 10% FBS and G418 (500 μg/ml for CNE-2
clone selection).
Plasmids and stable transfection
The vector pcDNA™3.1(−) and control plasmid
pcDNA3.1/CAT were purchased from Invitrogen. The full-length cDNA
of VP3 gene was amplified by PCR and cloned into pcDNA3.1(−) to
form expression vector PcDNA3.1(−)VP3-Myc (PVP3). The vector was
transformed into competent E. coli that was cultured in the
selecting medium with 50 μg/ml of spectinomycin. The
selected plasmid was confirmed by DNA sequencing. For stable
transfection, CNE-2 cells seeded in 24-well plates were transfected
with 0.8 μg of either PVP3 or pcDNA3.1(−) using
Lipofectamine™ 2000 (Invitrogen, CA, USA) according to the
manufacturer’s instructions. After no less than 14 days of
selection in RPMI-1640 containing 10% FBS and G418 (500
μg/ml), individual G418-resistant colonies were isolated and
expanded. The expression of VP3 was verified by reverse
transcription-polymerase chain reaction (RT-PCR) and western
blotting.
Reverse transcription-PCR analysis
TRIzol reagent (Invitrogen) was used for total RNA
extraction. After confirmation of the RNA integrity on an agarose
gel, total RNA was dissolved in RNase-free water. The cDNA was
synthesized from 2 μg of total RNA using M-MuLV RT
Transcriptase and oligo(dT) (Fermentas Life Sciences). For PCR, 2
μl of cDNA products was mixed with PCR mix buffer (Tiangen,
China) and 0.1 μmol concentrations of VP3 PCR primers, which
synthesized according to the cDNA sequence in GenBank. The primer
sequences utilized in this study are VP3 forward:
5′-CGGAATTCATGAACGCTCTCC AAGAAG-3′, VP3 reverse:
5′-CGGAATTCTTACAGTCTTATA CG-3′; GAPDH forward:
5′-CAAGGTCATCCATGACAACTTT G-3′, GAPDH reverse:
5′-CAAGGTCATCCATGACAACTT TG-3′. RT product was amplified using the
following conditions: 95°C for 5 min; then 30 cycles at 95°C for 45
sec, 56°C for 45 sec and 72°C for 45 sec; and an extension at 72°C
for 5 min. PCR product was electrophoresed on 1.5% agarose gel,
FluorChem FC2 (San Leandro, CA) was applied for detection of the
intensity of bands. PCR experiments were repeated three times.
Western blotting
Total protein (40 μg) was resolved by
SDS-PAGE and transferred onto a PVDF membrane. The membranes was
incubated with primary antibody: anti-VP3 (Santa Cruz, 1:200), then
goat anti-mouse IgG horseradish peroxidase (Beyotime, Beijing,
China, 1:2000 dilution) was used as secondary antibody. Bands were
visualized by employing the ECL Plus Detection system (Beyotime).
Signal intensity was analyzed using GeneTools software (Syngene,
Frederick, MD, USA). The level of β-actin was used as a loading
control. The results of western blot analysis represented the
average of three individual experiments.
Photodynamic therapy (PDT)
For cells, after incubated with 5-ALA (Sigma
Chemical Co., 1 mmol/l) for 6 h, cells received PDT treatment with
different laser energy density (630 nm, l, 3 and 6.25
J/cm2). The nude mice received 20% 5-ALA at a dose of
100 mg/kg, following by PDT (630 nm, 100 J/cm2, 100
mW/cm2) at 3–3.5 h later (21). The diameter of the irradiating laser
beam entirely covered the tumor.
Cell proliferation
Cell proliferation was analyzed using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
assay (Beyotime). Briefly, the cells (5×103 cells/well)
were planted in the 96-well culture plates overnight at 37°C. After
8-h PDT treatment, cell proliferation was monitored by using MTT.
MTT reagent was added into each well, incubating for 4 h, then MTT
reagent was removed and DMSO was added per well. After shaking for
10 min, optical densities (OD) were determined on a microtiter
plate reader at 570 nm. Three independent experiments were done.
Proliferation was calculated using the following equation:
Proliferation (%) = 1 - (OD control group - OD treatment group)/OD
control group × 100%.
Flow cytometry analysis of cell
apoptosis
Cell apoptosis was detected by Annexin V-FITC
apoptosis detection kit (Beyotime). The cells were seeded into
6-well culture plates at 2×105 per well. After harvested
at 6 h after PDT treatment, cells were stained using Annexin-V-FITC
for 10 min at room temperature, then were stained using propidium
iodure (PI) for 10 min in dark. The cells were analyzed on a FSCAN
flow cytometer (BD Biosciences, USA) immediately. All samples were
assayed in triplicate.
Transmission electron microscopy
(TEM)
To identify cell morphology, TEM was performed on
the Mock vector or PVP3 vector transfected CNE-2 cells at 6 h after
PDT treatment. The cells were incubated with 5-ALA at the
concentration of 1 mmol/l for 6 h before PDT treatment at the
intensity of 3 J/cm2. Fixed cells were postfixed in 2%
glutaraldehyde, dehydrated in graded alcohol, and flat embedded in
Epon 812. After stained with uranyl acetate and lead citrate,
ultrathin sections (100 nm) were examined under an electron
microscopy (H-7500, Lympus, Japan).
Animal experiments and tumor model
Male Balb/c nude mice, 6–8 weeks old and weighing
18–22 g (n=32) were used. The mice were purchased from the
Institute of Laboratory Animal Sciences of the Chinese Academy of
Medical Sciences (CAMS). The study was approved by the Ethics
Committee for Biomedical Research of Xiang Ya School of Medicine,
Changsha, China. All the animals were bred at the Animal Center of
Xiangya Medical School. Mock- or PVP3-vector stably transfected
CNE-2 cells were cultured and implanted subcutaneously
(1×106) in the nude mice. When tumor grew to a size of
5–8 mm in diameter, the animals were divided into four groups (8
animals per group), including Mock group, PVP3 group, Mock + PDT
group and PVP3 + PDT group. At 14 days after irradiation, the mice
in groups were sacrificed. Tumor sizes and weights were recorded on
days 0, 3, 7 and 14.
Hematoxylin and eosin (H&E)
staining
A part of each tumor was excised and fixed in 3.7%
formaldehyde for routine paraffin embedding. A series of sections
were excuted for each specimen, laid on polylysine-coated glass
slides, then dried on a hot plate, and H&E staining was carried
out on at least three sections of each specimen.
Statistical analyses
All statistical analyses were carried out using SPSS
for Windows version 13.0 (SPSS). Student’s t-test and one-way
analysis of variance (ANOVA) were used to analyze the data. All
cell culture experiments were performed in triplicate. Data are
presented as mean ± standard deviation (SD). Differences were
considered statistically significant for P<0.05.
Results
Apoptin is expressed in PVP3 stable
transfection CNE-2 cells
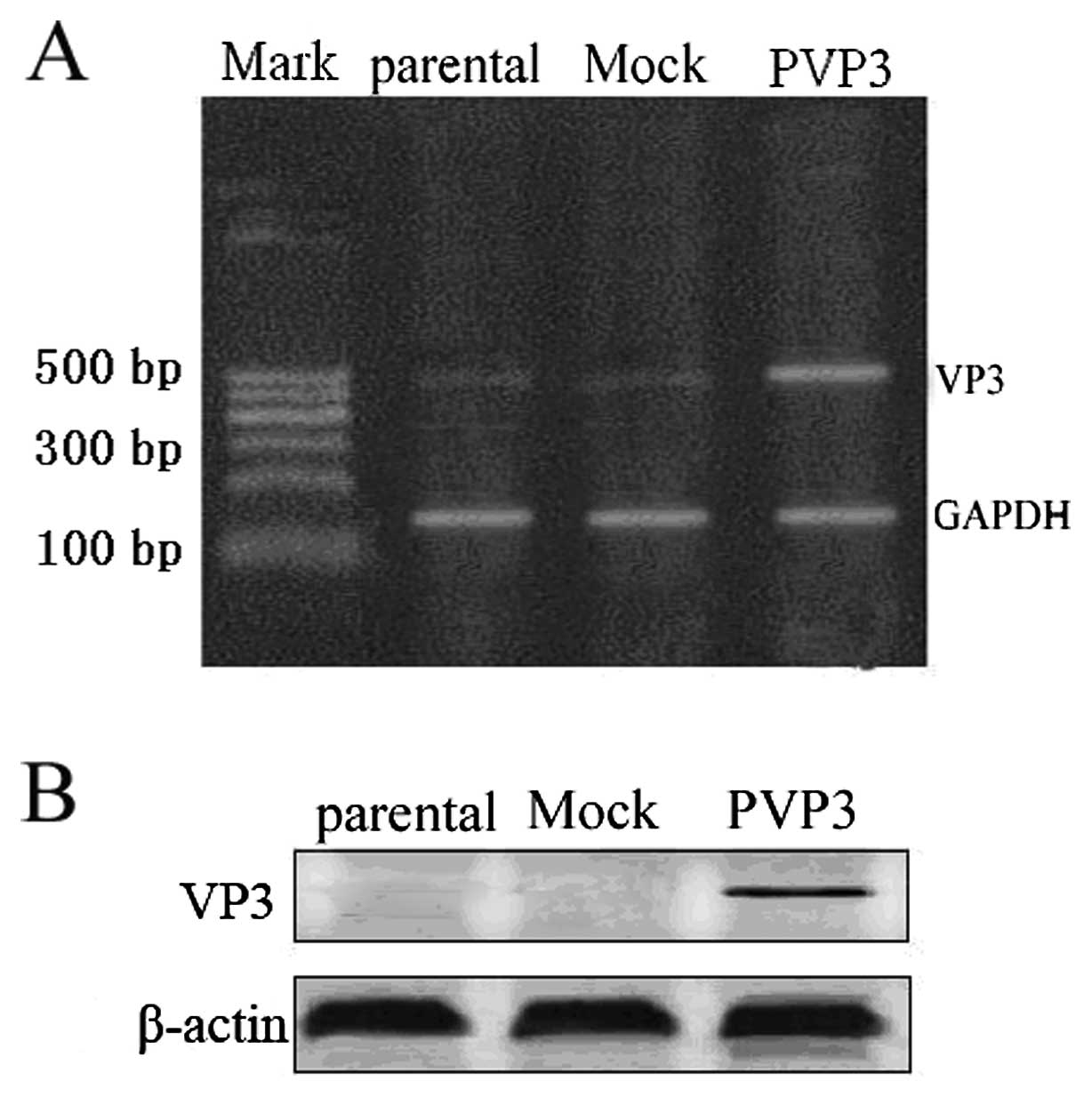
In order to express apoptin in CNE-2 cells, we
applied PVP3 plasmid to transfect CNE-2 cells. RT-PCR and western
blotting were used to detect apoptin expression. RT-PCR analysis
confirmed apoptin expression in PVP3 transfected CNE-2 cells while
no apoptin expression in mock vector transfected CNE-2 cells
(Fig. 1A). As shown in Fig. 1B, western blot analysis also
demonstrated that apoptin was expressed in PVP3-CNE-2 cells while
not in Mock-CNE-2 cells.
Apoptin restrains cell proliferation and
enhances cell apoptosis
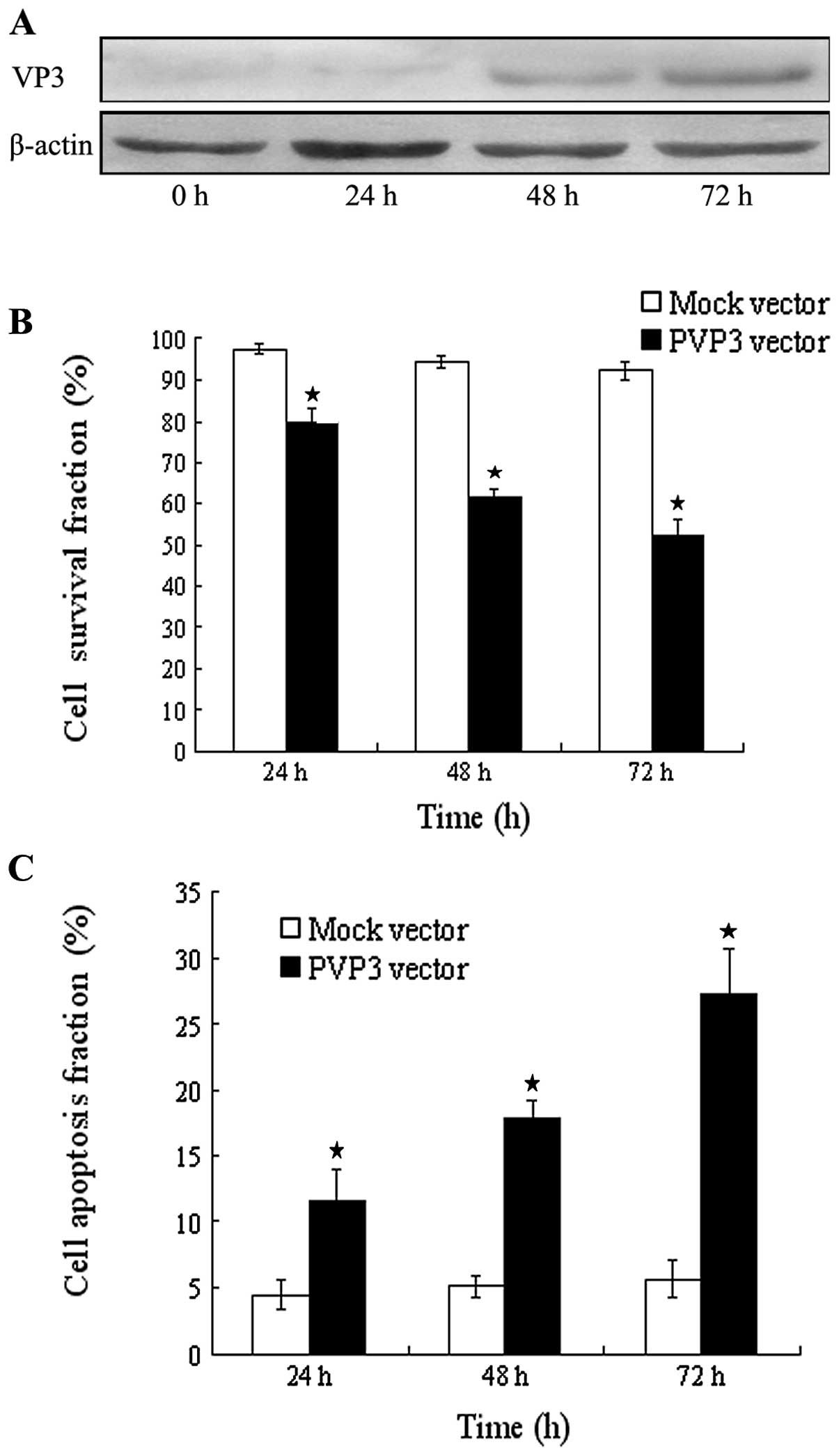
We observed an important change of cell
proliferation and apoptosis induced by apoptin in CNE-2 cells. We
used PVP3 vector and Mock vector treated CNE-2 cells at different
time intervals (24, 48 and 72 h) for transient transfection. The
expression of apoptin was detected by western blotting and reached
the strongest expression at 72 h (Fig.
2A). As shown in Fig. 2B, after
transient transfection, Mock-CNE-2 cells show cell proliferation of
97.6±1.4, 94.2±1.7 and 92.3±2.1% at 24, 48 and 72 h, respectively,
while PVP3-CNE-2 cells show cell proliferation of 79.7±3.6,
61.5±2.3 and 52.4±3.8% at 24, 48 and 72 h respectively, which
indicates that expression of apoptin could restrain cell
proliferation (P<0.001, Fig.
2B). In addition, after transient transfection, 11.6±2.4,
17.8±1.4 and 27.3±3.3% apoptotic cells were observed in PVP3-CNE-2
cells while 4.5±1.2, 5.1±0.8 and 5.7±1.4% apoptotic cells were
observed in Mock-CNE-2 cells at 24, 48 and 72 h, respectively
(P<0.001, Fig. 2C).
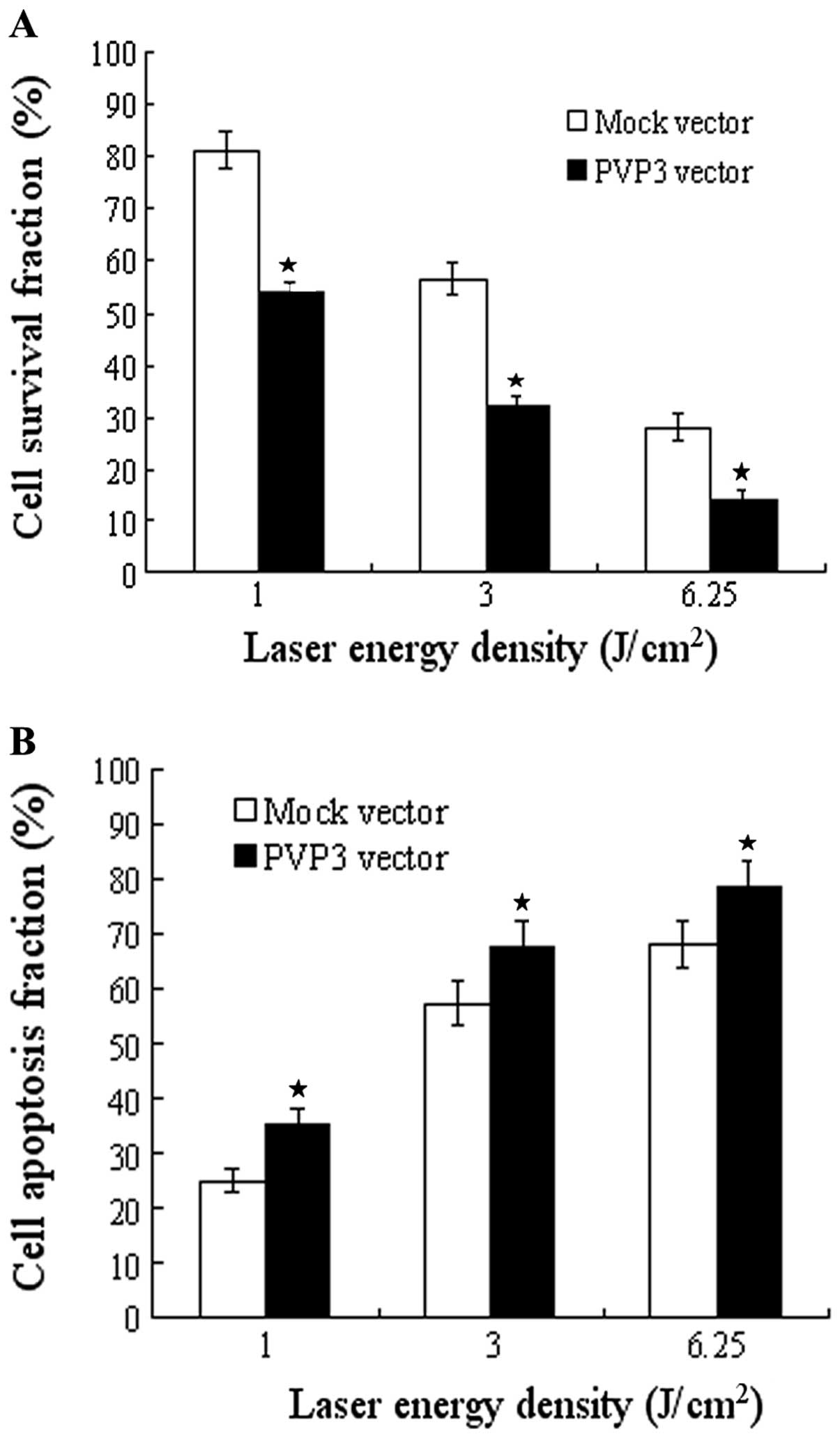
Apoptin reinforces the effect of PDT
To test whether apoptin has a synergistic effect in
PDT, we used PVP3 stable transfection CNE-2 cells and its control
cells to apply PDT treatment. The efficacy was evaluated by cell
proliferation and apoptosis test. As showed in Fig. 3A, PDT significantly inhibited cell
growth of both PVP3-CNE-2 cells and Mock-CNE-2 cells in different
laser energy density (l, 3 and 6.25 J/cm2) with maximal
inhibition rate at 6.25 J/cm2. The inhibition rate of
PVP3-CNE-2 cells is much higher than that of Mock-CNE-2 cells in
different laser energy density (P<0.001). Moreover, PDT
significantly induced cell apoptosis of both PVP3-CNE-2 and
Mock-CNE-2 cells in the different laser energy density and the
apoptotic rate reached the maximum at 6.25 J/cm2
(Fig. 3B). The apoptotic rate of
PVP3-CNE-2 cells is much higher than that of Mock-CNE-2 cells in
the different laser energy density (P<0.001).
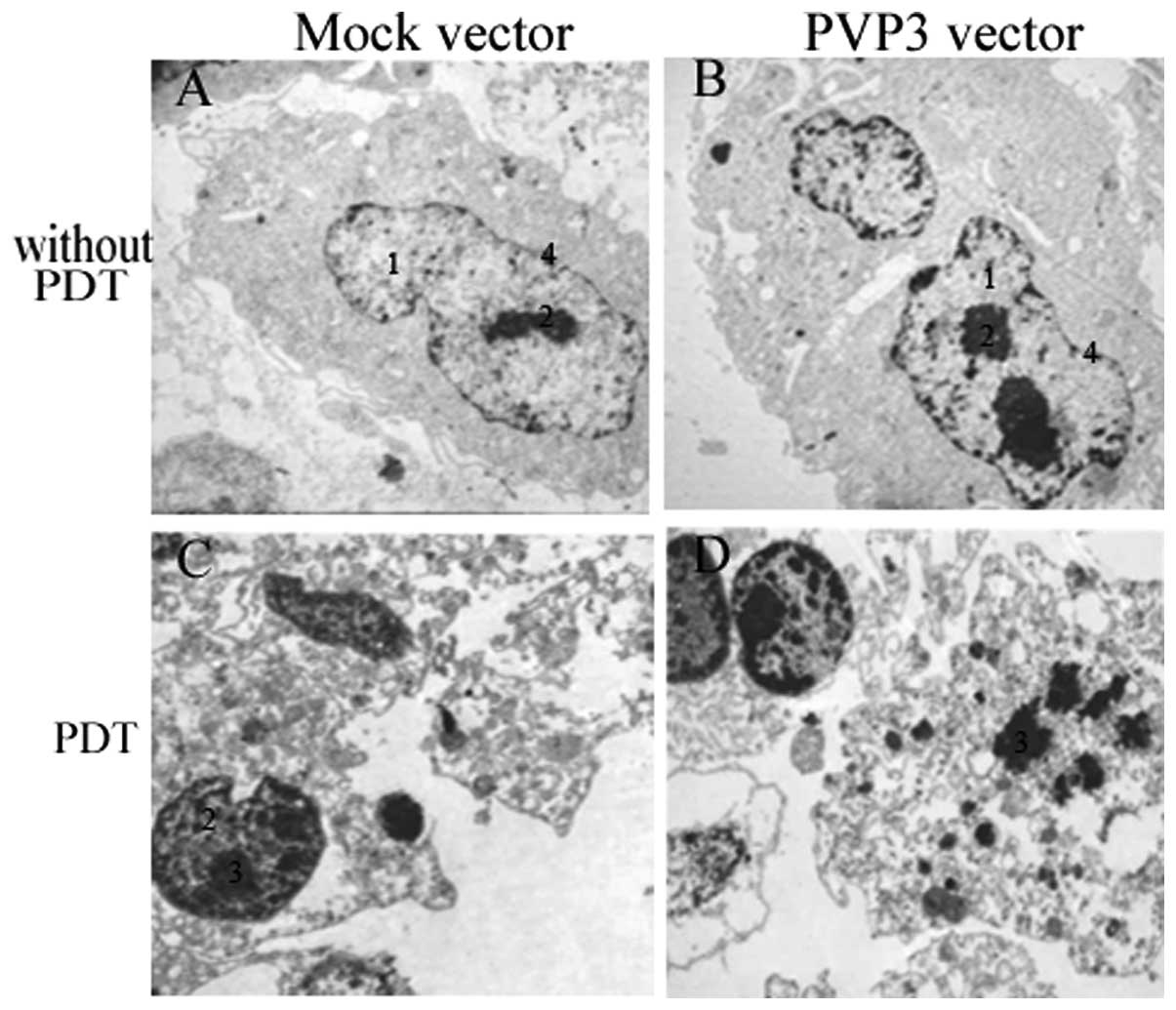
Ultrastructural morphology
The ultrastructural morphology of PVP3-CNE-2 and
Mock-CNE-2 cells after PDT treatment was investigated using TEM. As
shown in Fig. 4A and B, compare
with Mock-CNE-2 cells, PVP3-CNE-2 cells showed grievous
deformation, nuclear condensation, but mitochondria and endoplasmic
reticulum still clear without PDT. However, after PDT treatment,
PVP3-CNE-2 cells showed more severe chromatin margination, swollen
mitochondria and endoplasmic reticulum, nuclear fragmentation and
apoptotic bodies compared with Mock-CNE-2 cells in Fig. 4C and D.
Effects of VP3 and PDT therapy in
vivo
5-ALA dose of 100 mg/kg and laser energy density of
100 J/cm2 were selected for the in vivo studies.
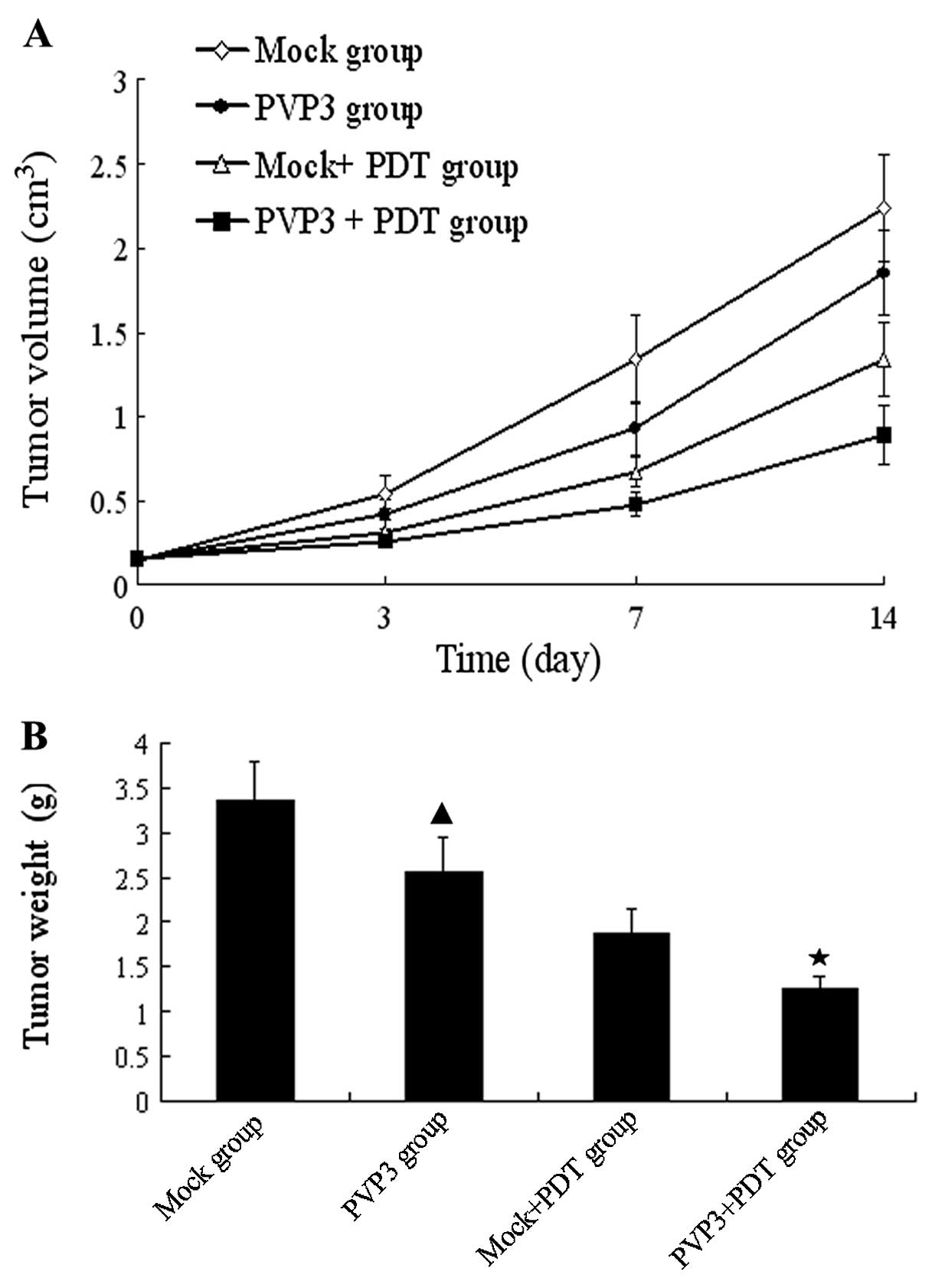
The mean tumor volumes before PDT were 0.150±0.026, 0.148±0.021,
0.155±0.031 and 0.153±0.022 cm3 in Mock group, PVP3
group, Mock + PDT group and PVP3 + PDT group, respectively. After
PDT treatment, we found that the tumor of PVP3 group was delayed
and the nodules were smaller than in the Mock group. In addition,
PVP3 + PDT group produced slow-growing tumors and smaller tumor
sizes than the others. The mean tumor volumes on day 14 were
2.237±0.316, 1.850±0.247, 1.338±0.221 and 0.887±0.174
cm3 in Mock group, PVP3 group, Mock + PDT group and PVP3
+ PDT group, respectively (Fig.
5A). The tumor weight of PVP3 group was less than Mock group,
and the tumor weight of PVP3 + PDT group was less than the others
(Fig. 5B). These results suggest
PVP3 + PDT group has the strongest xenograft inhibition of the four
groups.
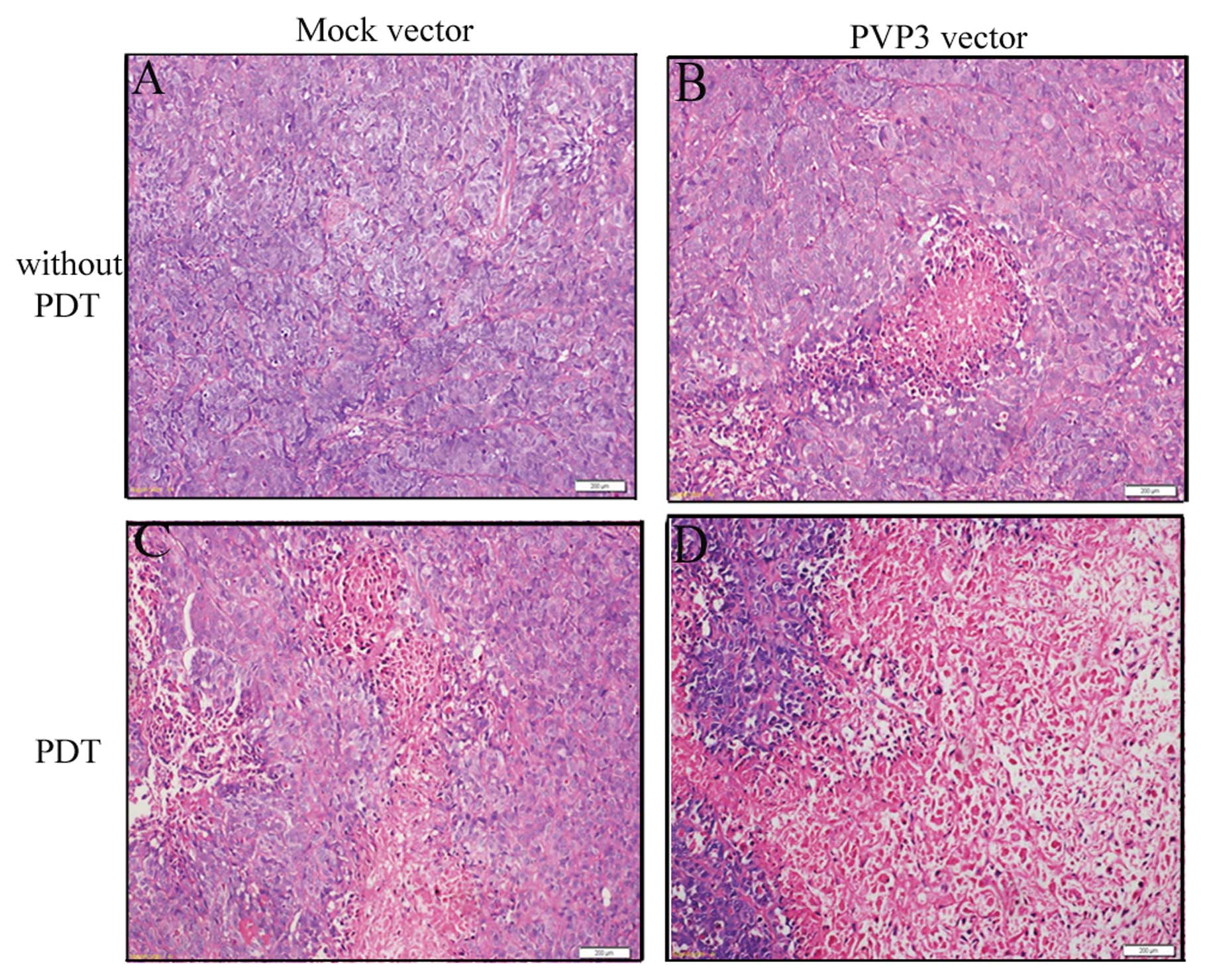
Histological findings
H&E staining of slices was used to observe the
xenograft morphological changes. As shown in the Fig. 6A, there were numerous mitotic tumor
cells around the blood vessels and in the peripheral regions of the
tumors in the Mock group and a few scattered necrotic areas were
observed in PVP3 group (Fig. 6B).
Extensive degenerated and pyknotic cells were observed in Mock +
PDT group and PVP3 + PDT group after PDT (Fig. 6C and D). Additionally, there were
numerous necrotic areas and some residual tumor cell islands on
PVP3 + PDT group (Fig. 6D).
Discussion
PDT for the treatment of malignant tumors has been
proved to be a feasible, safe and reliable method. It has many
advantages. First, PDT on malignant tumors is highly selective,
less damage to normal tissue or killing normal cells, high efficacy
and celerity. Second, it is suitable for applying in the elderly
and patients with severe complications or for those refused surgery
for less impact on the liver, kidney and bone marrow system. Third,
the operation of PDT is easy and simple, and PDT also has the
advantage of direct effect of tumor location with high reliability
(22–25).
However, this method also has some shortcomings.
Since PDT is a kind of oxygen-dependent treatment, tumor hypoxia
can be easily reduced by PDT, which greatly reduces the
tumor-killing effect of PDT in return. The limitation of the depth
also restricts the role of PDT in tumor treatment. How to enhance
the antitumor effect of PDT is a serious challenge for researchers.
In recent years, the combination of PDT and gene therapy is
becoming a new strategy for the treatment of cancer. Currently,
PDT/gene therapy is mainly used with immune genes, tumor suppressor
gene and suicide gene combination, and this therapy has achieved
good effect (26,27).
In this study, we have demonstrated apoptin was able
to significantly inhibit CEN-2 cells proliferation and enhanced
cell apoptosis. To further investigate whether apoptin has a
synergistic effect in PDT, we expressed apoptin in CNE-2 cells and
dealt with PDT treatment. Our study has shown that apoptin with PDT
treatment had stronger cell killing effect than apoptin or PDT
treatment alone. We also found apoptin with PDT treatment induced
severe ultrastructural morphology changes compare with apoptin or
PDT alone. In addition, we demonstrated that apoptin with PDT
treatment had the highest tumor-killing effect in vivo.
These data clearly indicate that the tumor-killing effect of
apoptin with PDT therapy is significantly stronger than single gene
therapy or PDT.
Apoptin is located in the nucleus of tumor cells, it
can associate with different interaction partners including death
effector domain-associated factor (DEDAF), Nmi, anaphase-promoting
complex/cyclosome (APC/C) and promyelocytic leukemia protein (PML)
in the nucleus, which facilitates apoptin cracking the nucleus and
induces tumor cells to apoptosis (28–31).
On the other hand, the reactive oxygen species (ROS) produced by
PDT can directly impact on the cell membrane, mitochondria,
lysosomes, endoplasmic reticulum and golgi apparatus, which can
induce tumor cell apoptosis. Apoptin with PDT therapy might through
the nucleus and cytoplasm to promote apoptosis, therefore, tumor
cell apoptosis is significantly increased in this kind of
therapy.
In conclusion, our data showed that apoptin had
certain cell killing effect in NPC cells, and apoptin with PDT
therapy has significantly stronger tumor-killing effect than
apoptin gene therapy or PDT alone.
Acknowledgements
This study was supported by Scientific Research Fund
of Hunan Provincial Education Department.
References
|
1
|
Yu MC and Yuan JM: Epidemiology of
nasopharyngeal carcinoma. Semin Cancer Biol. 12:421–429. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chow E, Payne D, O’Sullivan B, Pintilie M,
Liu FF, Waldron J, Warde P and Cummings B: Radiotherapy alone in
patients with advanced nasopharyngeal cancer: comparison with an
intergroup study. Is combined modality treatment really necessary?
Radiother Oncol. 63:269–274. 2002. View Article : Google Scholar
|
|
3
|
Fritsch C, Goerz G and Ruzicka T:
Photodynamic therapy in dermatology. Arch Dermatol. 134:207–214.
1998. View Article : Google Scholar
|
|
4
|
Roscetti G, Franzese O, Comandini A and
Bonmassar E: Cytotoxic activity of Hypericum perforatum L on K562
erythroleukemic cells: differential effects between methanolic
extract and hypericin. Phytother Res. 18:66–72. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kiesslich T, Krammer B and Plaetzer K:
Cellular mechanisms and prospective applications of hypericin in
photodynamic therapy. Curr Med Chem. 13:2189–2204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim CH, Chung CW, Choi KH, Yoo JJ, Kim do
H, Jeong YI and Kang DH: Effect of 5-aminolevulinic acid-based
photodynamic therapy via reactive oxygen species in human
cholangiocarcinoma cells. Int J Nanomed. 6:1357–1363.
2011.PubMed/NCBI
|
|
7
|
Kamoshima Y, Terasaka S, Kuroda S and
Iwasaki Y: Morphological and histological changes of glioma cells
immediately after 5-aminolevulinic acid mediated photodynamic
therapy. Neurol Res. 33:739–746. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park JH, Moon YH, Kim DJ, Kim SA, Lee JB,
Ahn SG and Yoon JH: Photodynamic therapy with hexenyl ester of
5-aminolevulinic acid induces necrotic cell death in salivary gland
adenocarcinoma cells. Oncol Rep. 24:177–181. 2010.PubMed/NCBI
|
|
9
|
Los M, Wesselborg S and Schulze-Osthoff K:
The role of caspases in development, immunity, and apoptotic signal
transduction: lessons from knockout mice. Immunity. 10:629–639.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schwerk C and Schulze-Osthoff K:
Regulation of apoptosis by alternative pre-Mrna splicing. Mol Cell.
19:1–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fischer U, Jänicke RU and Schulze-Osthoff
K: Many cuts to ruin: a comprehensive update of caspase substrates.
Cell Death Differ. 10:76–100. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Noteborn MH, Todd D, Verschueren CA, de
Gauw HW, Curran WL, Veldkamp S, Douglas AJ, McNulty MS, van der
EBAJ and Koch G: A single chicken anemia virus protein induces
apoptosis. J Virol. 68:346–351. 1994.PubMed/NCBI
|
|
13
|
Maddika S, Mendoza FJ, Hauff K, Zamzow CR,
Paranjothy T and Los M: Cancer selective therapy of the future:
apoptin and its mechanism of action. Cancer Biol Ther. 5:10–19.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noteborn MH: Chicken anemia virus induced
apoptosis: underlying molecular mechanisms. Vet Microbiol.
98:89–94. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heilman DW, Teodoro JG and Green MR:
Apoptin nucleocytoplasmic shuttling is required for cell
type-specific localization, apoptosis, and recruitment of the
anaphase-promoting complex/cyclosome to PML bodies. J Virol.
80:7535–7545. 2006. View Article : Google Scholar
|
|
16
|
Poon IK, Oro C, Dias MM, Zhang J and Jans
DA: Apoptin nuclear accumulation is modulated by a CRM1-recognized
nuclear export signal that is active in normal but not in tumor
cells. Cancer Res. 65:7059–7064. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Backendorf C, Visser AE, de Boer AG,
Zimmerman R, Visser M, Voskamp P, Zhang YH and Noteborn M: Apoptin:
therapeutic potential of an early sensor of carcinogenic
transformation. Annu Rev Pharmacol Toxicol. 48:143–169. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tavassoli M, Guelen L, Luxon BA and Gäken
J: Apoptin: specific killer of tumor cells? Apoptosis. 10:717–724.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhuang SM, Shvarts A, van Ormondt H,
Jochemsen AG, van der Eb AJ and Noteborn MH: Apoptin, a protein
derived from chicken anemia virus, induces p53-independent
apoptosis in human osteosarcoma cells. Cancer Res. 55:486–489.
1995.PubMed/NCBI
|
|
20
|
Burek M, Maddika S, Burek CJ, Daniel PT,
Schulze-Osthoff K and Los M: Apoptin-induced cell death is
modulated by Bcl-2 family members and is Apaf-1 dependent.
Oncogene. 25:2213–2222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie Y, Wei ZB, Zhang Z, Wen W and Huang
GW: Effect of 5-ALA-PDT on VEGF and PCNA expression in human
NPC-bearing nude mice. Oncol Rep. 22:1365–1371. 2009.PubMed/NCBI
|
|
22
|
Krieger MD, Couldwell WT and Weiss MH:
Assessment of long term remission of acromegaly following surgery.
J Neurosurg. 98:719–724. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barnes LD, Giuliano EA, Ota J, Cohn LA and
Moore CP: The effect of photodynamic therapy on squamous cell
carcinoma in a murine model: evaluation of time between
intralesional injection to laser irradimion. Vet J. 180:60–65.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yung A, Stables GI, Fernandez C, Williams
J, Boiar RA and Goulden V: Microbiological effect of photodynarnic
therapy(PDT) in healthy volunteers: a comparative study using
methyl aminolaevulinate and hexyl aminolaevulinate cream. Clin Exp
Dermatol. 32:716–721. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su ZA, Yao K, Shen J, Jiang JK, Fang XY,
Lin JJ and DU XH: Evaluation of photodynamic therapy in idiopathic
choroidal neovascularization. Zhonghua Yan Ke Za Zhi. 43:509–513.
2007.PubMed/NCBI
|
|
26
|
Luna MC, Chen X, Wong S, Tsui J, Rucker N,
Lee AS and Gomer CJ: Enhanced photodynamic therapy efficacy with
inducible suicide gene therapy controlled by the grp promoter.
Cancer Res. 62:1458–1461. 2002.PubMed/NCBI
|
|
27
|
Laden BP, Tang Y and Porter TD: Cloning,
heterologous expression, andenzymological characterization of human
squalene monooxygenase. Arch Biochem Biophys. 374:381–388. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Teodoro JG, Heilman DW, Parker AE and
Green MR: The viral protein Apoptin associates with the
anaphase-promoting complex to induce G2/M arrest and apoptosis in
the absence of p53. Genes Dev. 18:1952–1957. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Danen-van Oorschot AA, Voskamp P, Seelen
MC, van Miltenburg MH, Bolk MW, Tait SW, Boesen-de Cock JG, Rohn
JL, Borst J and Noteborn MH: Human death effector domain-associated
factor interacts with the viral apoptosis agonist Apoptin and
exerts tumor-preferential cell killing. Cell Death Differ.
11:564–573. 2004.
|
|
30
|
Janssen K, Hofmann TG, Jans DA, Hay RT,
Schulze-Osthoff K and Fischer U: Apoptin is modified by SUMO
conjugation and targeted to promyelocytic leukemia protein nuclear
bodies. Oncogene. 26:1557–1566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Krieghoff-Henning E and Hofmann TG: Role
of nuclear bodies in apoptosis signaling. Biochim Biophys Acta.
1783:2185–2194. 2008. View Article : Google Scholar : PubMed/NCBI
|