Introduction
Schizophrenia and cancer are disorders characterized
by a broad spectrum of clinical phenotypes and complex genetics.
The hypothesis that the incidence of cancer in patients with
schizophrenia is reduced has been confirmed in clinical reports,
although this hypothesis has been reported and formulated since
1909 (Commissioners in Lunacy for England and Wales: Annual Report.
London. HMSO, 1909). Since then, even though there are conflicting
results on this hypothesis, in some population-based incidence
studies a decreased cancer risk has been found in patients with
schizophrenia (1–5). Furthermore, in a recent study, cancer
risk was associated with the duration and age of onset of
schizophrenia in a large sample of patients with schizophrenia and
bipolar disease and specifically, duration was inversely correlated
with cancer risk (6).
Schizophrenia and cancer are heterogeneous syndromes
of different disorders that share clinical symptoms and features
(7). Both conditions are mediated
by common etiological factors; genetic and environmental factors
play a distinctive role in the development of both syndromes. The
development of cancer is characterized by increased gene expression
that leads to uncontrolled cell proliferation, whereas the
development of schizophrenia is characterized by the reduced
expression of genes whose products suppress cellular proliferation
(tumor suppressor gene activity) (8,9) and
increase the rate of apoptosis (10).
MicroRNAs (miRNAs) are a class of small, non-coding
RNAs that play an important role in various biological processes
(11). Bioinformatics have
predicted that approximately one-third of human genes are targeted
by miRNAs. There is increasing evidence demonstrating the
involvement of miRNAs in human cancer. Previous studies have
revealed that 98 out of 186 miRNA genes located in
cancer-associated genomic regions may frequently be found in
different types of tumor, whereas the altered expression of let-7
and miR-155 in lung cancer has been shown to correlate with patient
survival (12,13). Additionally, a number of studies
have shown that the pathogenesis of schizophrenia may be related to
the dysregulation of miRNA expression (14,15).
In this study, we investigated the potential role of
miRNAs in the development of cancer in 2 groups of patients with
schizophrenia, in an attempt to provide further evidence for the
low incidence of cancer risk among schizophrenic patients. The 1st
group consisted of patients with schizophrenia, whereas the 2nd
group included patients with schizophrenia and a concomitant
diagnosis of a solid tumor.
Materials and methods
Subjects
Six patients (male/female, 2/4) (study group) were
recruited from the Psychiatric Hospital of Attika and from the
Oncology Outpatient Department of the University General Hospital
‘Attikon’ between February and June 2011. Patients were assessed
according to the Structured Clinical Interview for DSM-IV Axis I
Disorders (SCID-IV) criteria (16)
and by Positive and Negative Syndrome subscales (PANSS) (17). Exclusion criteria included a history
of any neurological disease and current substance misuse or
dependence in the preceding 6 months as defined by the Diagnostic
and Statistical Manual of Mental Disorders, fourth edition (DSM-IV)
[American Psychiatric Association (APA), 1994]. All patients were
in a stabilized psychological state. The diagnosis of cancer was
made according to the medical records of the patient. It should be
noted that it is extremely difficult to find patients with this
type of co-morbidity (schizophrenia and cancer).
The second group of patients (control group)
consisted of 8 schizophrenic patients (male/female, 1/7), which had
no evidence of cancer disease. Inclusion criteria for the control
group were an age of 30–70 years, body weights within 10% of an
appropriate body mass index, no other serious diseases other than
schizophrenia, no clinically significant abnormal laboratory values
and no pathological findings upon thorough clinical examination.
Additionally, individuals in the control group had no history of
hypersensitivity (asthma, urticaria and eczema), autoimmune
disorders, such as systemic lupus erythematosus, uncontrolled
hypertension or serious heart, lung, liver or renal conditions.
All patients were in a stabilized psychological
state and their medication had not been altered over a period of at
least 6 months pior to enrollment in this study. Patients with a
smoking history were included in this study. However, exclusion
criteria included a recent history (>1 year) of alcoholism, use
of recreational drugs or drug addiction. No consumption of alcohol
was allowed within 48 h prior to blood collection. All female
participants were tested for pregnancy and the results were
negative. All patients were on regular medical treatment with
antipsychotics. All participants were able to communicate
effectively, were informed of the nature of the study and provided
written informed consent. The study was approved by the
institutional review board and ethics committee of both
participating hospitals and was conducted in accordance with Good
Clinical Practice principals and applicable local regulations. The
patients in the control group were clinically followed-up for 1
year after blood collection and showed no evidence of cancerous
disease.
Sample collection and preservation for
microarray analysis
miRNA profiling for each patient was performed on
whole blood samples. Preservation of the gene expression status of
the samples was achieved by collecting 500 μl of whole blood
from each patient to an RNAprotect Animal Blood Tube (Qiagen,
Hilden, Germany). Following gentle inversion of the tubes for 8–10
times, the tubes were incubated for 2 h at ambient temperature,
according to the manufacturer’s instructions, to allow for
efficient cell lysis. All tubes were then stored at −70°C prior to
RNA purification.
RNA purfication and miRNA microarray
analysis
Total RNA purification that contained small RNA,
including miRNA, was carried out using the miRNeasy Protect Animal
Blood kit (Qiagen). All further experiments that included sample
RNA quality control and miRNA profiling were conducted by Exiqon
Services (Vedbaek, Denmark). Briefly, RNA quality control and
measurement were carried out using an Agilent 2100 Bioanalyzer and
a nanodrop instrument. All samples and a reference RNA sample,
labeled with Hy3™ and Hy5™ fluorescent labels, respectively, were
mixed pair-wise and hybridized to the miRCURY LNA™ miRNA Array 6th
gen (Exiqon), which contains capture probes targeting all miRNAs
for humans, mice or rats registered in the miRBASE 16.0. The
hybridization was performed using a Tecan HS4800™ hybridization
station (Tecan, Grödig, Austria). The miRCURY LNA™ miRNA Array
slides were scanned using the Agilent G2565BA Microarray Scanner
System (Agilent Technologies, Inc., Santa Clara, CA, USA) and the
image analysis was carried out using the ImaGene® 9
(miRCURY LNA miRNA Array Analysis Software, Exiqon).
Statistical analysis
Principal component analysis was used in order to
explore, based on the expression profile, the naturally arising
sample classes. It was revealed that the samples cluster according
to their biology. A statistical analysis of the miRNA expression
between the control and study group was also carried out by Exiqon;
The Student’s t-test was used for determination of the statistical
significance of the relative expression of miRNAs between the 2
groups and the Bonferroni multiple testing adjustment method was
subsequently applied to the P-values for the control of possible
false positive results.
Results
In total, 345 different miRNAs were analyzed by the
miRCURY LNA miRNA Array. Fig. 1
shows a heat map diagram that depicts the expression of the 50
miRNAs with the highest standard deviation on all samples.
Statistical analysis of the results showed that only miR-183 showed
a significantly differential expression between the 2 groups of
patients (P<0.05) and specifically, a significantly greater
level of miR-183 expression was recorded for the control group of
patients. The specific result suggested the possibility that the
expression level of miR-183 may be directly related to the absence
of a solid tumor in the presence of schizophrenia.
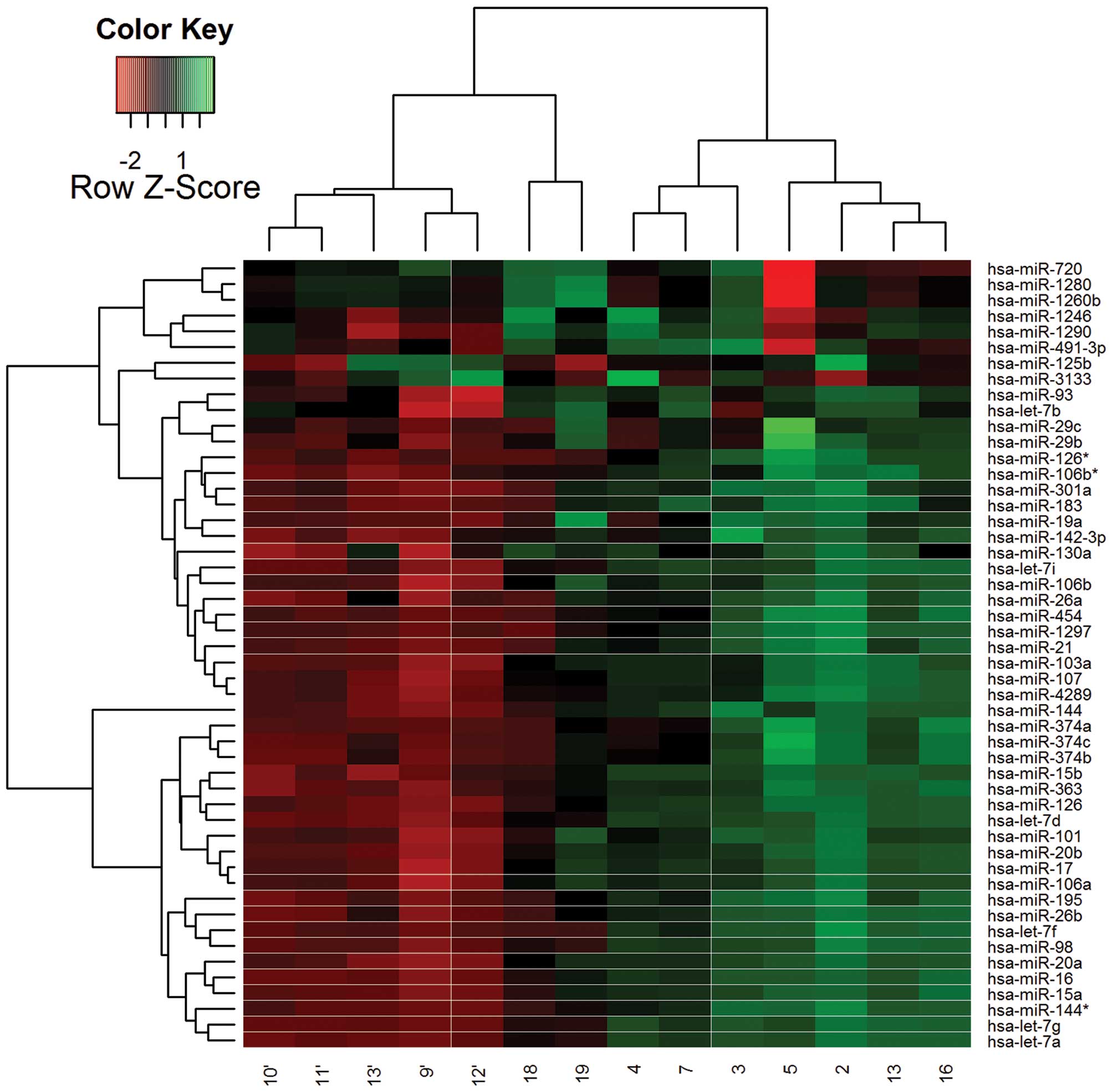 | Figure 1Heat map diagram showing the
expression of the 50 miRNAs with the highest standard deviation in
all samples. The color scale illustrates the relative expression
level of miRNAs and specifically, red color represents an
expression level below the reference channel, whereas green color
represents an expression higher than the reference. Samples 9′,
10′, 11′, 12′, 13′, 16, 18 and 19 correspond to the study group of
patients (cases of schizophrenia with tumor formation), whereas
samples 2, 3, 4, 5, 7 and 13 correspond to the control group of
patients (cases of schizophrenia without tumor formation). The
expression of hsa-miR-183, which was found to be significantly
higher in the samples of the control group of patients, is also
indicated. |
Discussion
The hypothesis that schizophrenia is associated with
a reduced risk of cancer has been addressed in a number of studies;
however, there are conflicting results. The implication of several
biological characteristics such as the wingless-related family of
proteins (Wnt) pathways of inactivation, dopamine effects and
enhanced natural killer cell activities has been reported in
clinical studies (10,18). Furthermore, antipsychotic agents
have been proposed to account for the reduced cancer risk in
patients with schizophrenia in several studies (1,19).
Specifically, a high dose of phenothiazine has been shown to reduce
the risk of prostate cancer, whereas another study reported that
the long-term use of antipsychotics may decrease the risk of
rectal, colon and prostate cancer (20). Thus, the anticarcinogenic effect of
antipsychotic agents should be considered for the decreased cancer
risk among patients with chronic schizophrenia.
miRNAs, as mentioned above, are emerging as
important mechanisms implicated in the modulation of gene
expression (21), and are thus
genetic factors contributing to the etiology of both psychiatric
disorders and cancer. It is estimated that miRNAs regulate <30%
of human gene expression at the post-transciptional and
translational levels (11).
Moreover, miRNAs are involved in the modulation of a wide range of
biological processes, including programmed cell death (apoptosis
and autophagy) (22–24) and those that are expressed in the
brain affect neuronal differentiation, synaptosomal complex
localization and synapse plasticity, all functions thought to be
disrupted in schizophrenia (25).
In the present study, we investigated miRNA expression in plasma
from a sample of patients with schizophrenia and another sample of
patients with schizophrenia and cancer. In this way, we
investigated the possible role (positive or negative) of miRNAs in
the development of cancer in patients with schizophrenia, in an
attempt to obtain evidence regarding their role cancer protection
(onco-suppressor activity). Our results showed an overexpression of
miR-183 in the group of schizophrenic patients without a history of
cancer. On the contrary, the absence of miR-183 expression in the
group of patients with schizophrenia and cancer may be an
indication that this miRNA is a protective factor against
cancer.
miR-183 has recently been implicated in the
modulation of different stages of apoptotis and autophagy through
the regulation of apoptosis and autophagy-related genes (24,26,27).
Specifically, the knockdown of miR-183 expression has been shown to
induce autophagic cell death in medullary thyroid cancer, through
the regulation of certain tumor suppressive signaling pathways,
indicating that miR-183 may be an attractive therapeutic target
(28). A recent study reported that
the overexpression of miR-183 correlated with the metastatic
potential of lung cancer cells (29). Furthermore, the overexpression of
miR-183 has been shown to inhibit the migration and invasion of
lung cancer cells. The oncogenic role of miR-183 has been revealed
in a recent study by targeting the transcription factor, EGR1, and
promoting tumor cell migration in different types of cancer, such
as sarcomas and colon tumors (30).
Thus, miR-183 may play a tumor suppressor role, possibly by
activating the expression of tumor suppressor genes that control
cell differentiation or apoptosis (31). The onco-supressor role of miR-183 is
indicated in the study by Zhu et al (32), who demonstrated that the
downregulation of miR-183 promotes the migration and invasion of
osteosarcoma cells by targeting Ezrin. Moreover, it has recently
been shown that Tiam1, that is downregulated by miR-183, presents
an overexpression pattern in ovarian cancer cells. Particularly,
there seems to be an implication of Tiam1 in the migration,
invasion, viability and, in general, in the aggressive profile of
ovarian cancer cells (33). Another
similar miRNA with an onco-suppressor activity is let-7, which
negatively regulates Ras and leads to apoptosis or cellular
senescence (34). miRNAs
functioning as oncogenes, such as miR-21, target the tumor
suppressors, tropomyosin 1 (35)
and programmed cell death 4, in breast cancer cells (36). The miR-17–92 cluster may be regarded
as a family of oncogenes, directly targeting many genes implicated
in apoptotic pathways. Therefore, miRNAs can act both as oncogenes
and tumor suppressors, depending on the particular miRNA and cell
type.
The implication of miRNAs in the etiology of
schizophrenia and bipolar disorder, has been reported in several
studies. In the study by Perkins et al, 16 miRNAs were
identified as dysregulated in a post-mortem brain sample consisting
of 13 individuals with schizophrenia and 2 individuals with
schizoaffective disorder (37). A
more recent study by Kim et al, confirmed the implication of
miRNAs in the prefrontal cortex of individuals with schizophrenia
and bipolar disorder (25).
Finally, the differential expression of miRNAs has been reported in
other neurodegenerative disorders, such as autism (38), in Parkinson’s disease (39) and Alzheimer’s disease (40).
One of the limitations of our study was the rather
small sample size. It should also be noted that it is extremely
difficult to find patients with this type of co-morbidity
(schizophrenia and cancer). The level of expression of miRNAs was
analyzed in blood and not in the brain, thus representing an
indirect analysis of brain miRNA expression levels. Consequently,
the data obtained in this study present a general genetic
predisposition regarding the implication of miR-183 in the
co-morbidity of schizophrenia and cancer. This is the first
clinical study that analyzes the level of a large number of miRNAs
in patients with both diseases, in order to examine this hypothesis
and provide an explanation as to the low cancer risk in
schizophrenic patients. Although, early clinical studies have
confirmed the decrease cancer risk in schizophrenic sample patients
(3) the relationship between
miR-183 expression levels in the peripheral blood and the brain, in
our study remains unclear.
As a conclusion, our data indicate that the
overexpression of miR-183 may be associated with the presence of
schizophrenia and the absence of a solid tumor, while the low
expression of miR-183 may be linked with schizophrenia and the
presence of a solid tumor. Our data provide a possible molecular
explanation regarding the low cancer risk in patients suffering
from schizophrenia. Further studies are warranted with a larger
sample size, in order to establish the crucial role of miRNAs in
the development of major diseases, such as schizophrenia and cancer
and to elucidate the possible associations between them and the
molecular pathways involved.
References
|
1
|
Mortensen PB: The incidence of cancer in
schizophrenic patients. J Epidemiol Commun Health. 43:43–47. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lawrence D, Holman CD, Jablenski AV,
Threlfall TJ and Fuller SA: Excess cancer mortality in Western
Australian psychiatric patients due to higher case fatality rates.
Acta Psychiatr Scand. 101:382–388. 2000. View Article : Google Scholar
|
|
3
|
Barak Y, Achiron A, Mandel M, Mirecki I
and Aizenberg D: Reduced cancer incidence among patients with
schizophrenia. Cancer. 104:2817–2821. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leucht S, Bunkard T, Henderson J, Maj M
and Sartorius N: Physical illness and schizophrenia: a review of
the literature. Acta Psychiatr Scand. 116:317–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chou FHC, Tsai KY, Su CY and Lee CC: The
incidence and relative risk factors for developing cancer among
patients with schizophrenia: a nine-year follow-up study. Schizophr
Res. 129:97–103. 2011.PubMed/NCBI
|
|
6
|
Lin GM, Chen YJ, Kuo DJ, Jaiteh LES, Wu
YC, Lo TS and Li YH: Cancer incidence in patients with
schizophrenia or bipolar disorder: a nationwide population-based
study in Taiwan, 1997–2009. Schizophr Bull. 1:1–10. 2011.
|
|
7
|
Fanous AH and Kendler KS: Genetic
heterogeneity, modifier genes, and quantitative phenotypses in
psychiatric illness: searching for a framework. Mol Psychiatry.
10:6–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feinberg AP and Tycko B: The history of
cancer epigenetics. Nat Rev Cancer. 4:143–153. 2004. View Article : Google Scholar
|
|
9
|
Rybakowski JK, Skibinska M, Kapelski P,
Kaczmarek L and Hauser J: Functional polymorphism of the matrix
metalloproteinase-9 (MMP-9) gene in schizophrenia. Schizophr Res.
109:90–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Catts VS and Catts SV: Apoptosis and
schizophrenia: is the tumour suppressor gene, p53, a candidate
susceptibility gene? Schizophr Res. 41:405–415. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calin GA, Dumitru CD, Shimizu M, Bichi R,
Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, Rassenti L,
Kipps T, Negrini M, Bullrich F and Croce CM: Frequent deletions and
down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in
chronic lymphocytic leukaemia. Proc Natl Acad Sci USA.
99:15524–15529. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takamizawa J, Konishi H, Yanagisawa K,
Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y,
Mitsudomi T and Takahashi T: Reduced expression of the let-7
microRNAs in human lung cancers in association with shortened
postoperative survival. Cancer Res. 64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hansen T, Olsen L, Lindow M, Jakobsen K,
Ullum H, Jonsson E, Adreassen O, Djurovic S, Melle I, Agartz I,
Hall H, Timm S, Wang AG and Werge T: Brain expressed microRNAs
implicated in schizophrenia etiology. Plos One. 9:1–7. 2007.
|
|
15
|
Beveridge NJ, Tooney PA, Carroll AP,
Gardiner E, Bowden N, Scott RJ, Tran N, Dedova I and Cairns MJ:
Dysregulation of miRNA 181b in the temporal cortex in
schizophrenia. Human Mol Genet. 17:1156–1168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
First MB, Spitzer RL, Gibbon M and William
JBM: Structured Clinical Interview for DSM-IV Axis I Disorders
Patient edition. New York State Psychiatric Institute, Biometrics
Research; New York: 1997
|
|
17
|
Kay SR, Opler LA and Lindenmayer JP: The
Positive and Negative Syndrome Scale (PANSS): rational and
standardization. Br J Psychiatry. 155(Suppl): 59–65. 1987.
|
|
18
|
Cui DH, Jiang KD, Jiang SD, Xu YF and Yao
H: The tumor suppressor adenomatous polyposis coli gene is
associated with susceptibility to schizophrenia. Mol Psychiatry.
10:669–677. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lichtermann D, Ekelund J, Pukkala E,
Tanskanen A and Lonnqvist J: Incidence of cancer among persons with
schizophrenia and their relatives. Arch Gen Psychiatry. 58:573–578.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dalton SO, Johansen C, Poulsen AH,
Norgaard M, Sorensen HT, McLaughlin JK, Mortensen PB and Friis S:
Cancer risk among users of neuroleptic medication: a
population-based cohort study. Br J Cancer. 95:934–939. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cao X, Yeo G, Muotri AR, Kuvabara T and
Gage FH: Noncoding RNAs in the mammalian central nervous system. An
Rev Neurosci. 29:77–103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lima RT, Busacca S, Almeida GM, Gaudino G,
Fennel DA and Vasconcelos MH: MicroRNA regulation of core apoptosis
pathways in cancer. Eur J Cancer. 47:163–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cho WC: MicroRNAs in cancer - from
research to therapy. Biochim Biophys Acta. 1805:209–217.
2010.PubMed/NCBI
|
|
24
|
Fu LI, Wen X, Bao JK and Liu B:
MicroRNA-modulated autophagic signaling networks in cancer. Int J
Biochem Cell Biol. 44:733–736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim A, Reimers M, Maher B, Williamson V,
McMichael O, McClay J, van den Oord E, Riley B, Kendler K and
Vladimirov V: MicroRNA expression profiling in the prefrontal
cortex of individuals affected with schizophrenia and bipolar
disorders. Schizophr Res. 124:183–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li ZY, Yang Y, Ming M and Liu B:
Mitochondrial ROS generation for regulation of autophagic pathways
in cancer. Biochem Biophys Res Commun. 414:5–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jian X, Xiao-yan Z, Bin H, Yu-feng Z, Bo
K, Zhi-nong W and Xin N: Mir-204 regulate cardiomyocyte autophagy
induced by hypoxia-reoxygenetion through LC3-II. Int J Cardiol.
148:111–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abraham D, Jackson N, Gundara JS, Zhao J,
Gill AJ, Delbridge L, Robinson BG and Sidhu SB: MicroRNA profiling
of sporadic and hereditary medullary thyroid cancer identifies
predictors of nodal metastasis, prognosis, and potential
therapeutic targets. Clin Cancer Res. 17:4772–4781. 2011.
View Article : Google Scholar
|
|
29
|
Wang G, Mao W and Zheng S: MicroRNA-183
regulates Ezrin expression in lung cancer cells. FEBS Lett.
582:3663–3668. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sarver A, Li L and Subramanian S: MicroRNA
miR-183 functions as an oncogene by targeting the transcriptional
factor ERG1 and promoting tymor cell migration. Cancer Res.
70:9570–9580. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Babashah S and Soleimani M: The oncogenic
and tumour suppressive roles of microRNAs in cancer and apoptosis.
Eur J Cancer. 47:1127–1137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu J, Feng Y, Ke Z, Yang Z, Zhou J, Huang
X and Wang L: Down-regulation of miR-183 promotes migration and
invasion of osteosarcoma by targeting Ezrin. Am J Pathol.
180:2440–2451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li J, Liang S, Jin H, Xu C, Ma D and Lu X:
Tiam1, negatively regulated by miR-22, miR-183 and miR-31, is
involved in migration, invasion and viability of ovarian cancer
cells. Oncol Rep. 27:1835–1842. 2012.PubMed/NCBI
|
|
34
|
Hermeking H: P53 enters the microRNA
world. Cancer Cell. 12:414–418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu S, Si ML, Wu H and Mo YY: MicroRNA-21
targets the tumor suppressor gene tropomyosin 1 (TPM1). JBC.
282:14328–14336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Frankel LB, Christoffersen NR, Jakobsen A,
Lindow M, Krogh A and Lund AH: Programmed cell death 4 (PDCD4) is
an important functional target of the microRNA miR-21 in breast
cancer cells. J Biol Chem. 283:1026–1033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Perkins DO, Jeffries CD, Jarskog LF,
Thomson JM, Woods K, Newman MA, Parker JS, Jim JP and Hammond SM:
MicroRNA expression in the prefrontal cortex of individuals with
schizophrenia and schizoaffective disorder. Genome Biol. 8:R272007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abu-Elneel K, Liu T, Gazzaniga FS,
Nishimura Y, Wall DP, Geschwind DH, Lao K and Kosik KS:
Heterogenous dysregulation of microRNAs across the autism spectrum.
Neurogenetics. 9:153–161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Junn E, Lee KW, Jeong BS, Chan TW, Imb J
and Mouradian MM: Repression of alpha-synuclein expression and
toxicity by microRNA-7. Proc Natl Acad Sci USA. 106:13052–13057.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cogswell JP, Ward J, Taylor A, Waters M,
Shi Y, Cannon B, Kelnar K, Kemppainen J, Brown D, Chen C, Prinjha
RK, Richardson JC, Saunders AM, Roses AD and Richards CA:
Identification of miRNA changes in Alzheimer’s disease brain and
CSF yields putative biomarkers and insights into disease pathways.
J Alzheimer Dis. 14:27–41. 2008.
|















