Introduction
Endometrial cancer (EC) is one of the most common
gynecologic malignancies in developed countries. In the US alone,
46,470 new cases and 8,120 deaths from EC were estimated in 2011
(1). In China, the incidence of
this malignancy has also been observed to increase rapidly,
threatening women’s health (2).
However, the etiology of this malignancy is not clearly understood.
To date, the prevailing hypothesis is ‘unopposed estrogen’. EC is
commonly classified into two major types: type I estrogen-dependent
and type II non-estrogen-dependent. The majority of EC cases are
type I (approximately 80–90%), which generally includes low-grade
endometrioid histologies, often arising from a background of
endometrial hyperplasia and may have more favorable prognoses
(3). Althrough early-stage EC is
often curable with surgery alone, with a 5-year survival rate of
75–93%, the prognosis for late-stage disease is poor and the median
survival for women with advanced or recurrent disease is
approximately 1 year (4). Little is
known concerning the molecular characteristics of EC that predict
who will have recurrence and who should receive a particular type
of treatment. Therefore, early diagnosis is vital, and
identification of novel molecular biomarkers and therapeutic
targets is imperative.
Wnt genes were first discovered by Nusse in 1982
(5). The Wnt family consists of at
least 19 secreted-type glycoproteins with conserved 22–24 cysteine
residues that play key roles in carcinogenesis and embryogenesis.
Among these glycoproteins is the Wnt10a gene, which is located at
human chromosome 2q35. The Wnt10b gene is located at human
chromosome 12q13 (6). The Wnt10a
protein binds to seven transmembrane-type Wnt receptors
(FZD1-FZD10), while the Wnt10b protein has been shown to
functionally interact with FZD5. Wnt10a has been shown to be most
homologous to Wnt10b (59.2% amino acid identity) (7); the two proteins are nearly identical,
suggesting that duplication might have occurred during evolution,
thus conserving these primordial clusters of genes during
evolution. The Wnt signaling pathway regulates diverse
developmental processes, such as cell migration, adhesion,
proliferation and apoptosis. Previous studies have demonstrated
that numerous malignant carcinomas, including osteosarcoma
(8), gastrointestinal (9), prostate (10), breast (11) and ovarian cancer (12), are associated with an abnormal Wnt
signaling pathway. The pathway is best known, however, for its role
in colorectal cancer (CRC), in which greater than 90% of CRC cases
carry an activated mutation in the Wnt signaling pathway, most
frequently in the form of a mutational inactivation of adenomatous
polyposis coli (APC) (13). Studies
have reported that Wnt10a expression is upregulated in CRC cell
lines (14) and Wnt10b expression
is upregulated via the Wnt/β-catenin pathway in breast cancer cell
lines (15). Although previous
reports have shown that 10–45% of all EC cases carry β-catenin
mutations, with a slightly higher propensity in endometrioid EC
(16), the role of Wnt signaling in
EC has not been fully elucidated. The functional relationship and
associated prognostic values between the Wnts and EC have not been
determined. There is little information currently available
concerning the relationship between clinicopathological
characteristics and Wnts. Wang et al(17) reported the characterization of
genomic alterations in five commonly used EC cell lines (HEC1A,
HEC1B, AN3CA, ECC-1 and Ishikawa) and provided valuable genomic
information for research focused on Wnt pathways in EC. The authors
found that the Wnt10a gene was deleted in the HEC1B and AN3CA cell
lines and was normal in the other three EC cell lines; the Wnt10b
gene was amplified in the ECC-1 and Ishikawa cell lines and was
normal in the other three EC cell lines.
In the present study, we investigated the expression
of Wnt10a and Wnt10b in EC samples, and the relationship between
expression levels and the clinicopathologic features of EC was also
evaluated. Furthermore, the effect of the Wnt/β-catenin pathway on
the development of EC was investigated to further understand the
underlying mechanism.
Materials and methods
Reagents and antibodies
Anti-Wnt10a rabbit anti-human and anti-Wnt10b mouse
anti-human monoclonal antibodies were purchased from Abcam (St.
Louis, MO, USA) (ab62051 and ab91201). Anti-β-catenin antibody was
also purchased from Abcam. Anti-APC and anti-c-myc antibodies were
purchased from Fisher Sigma. We used DMEM supplemented with 10%
(vol/vol) fetal bovine serum (FBS) for the cell cultures. The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was obtained from Sigma (St. Louis, MO, USA). An Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit was
obtained from BD Biosciences (San Diego, CA, USA). Unless stated
otherwise, all chemical reagents were purchased from Sigma.
Tissue specimens
All endometrium tissue specimens were obtained from
patients who had undergone curettage or hysterectomy at the Tianjin
Medical University, General Hospital between January 2001 and
December 2010. None of the patients had accepted any radiation,
chemotherapy or hormonal therapy prior to surgery. These specimens
included 84 normal endometrium (48 in the proliferative phase and
36 in the secretory phase), 54 endometrial hyperplasias (18 simple,
6 complex and 30 atypical hyperplasias) and 102 endometrial
carcinomas (83 endometrioid, 12 adenosquamous, 4 uterine mucus, 2
uterine papillary serous and 1 clear cell carcinoma). The median
age of the patients with normal endometrium, endometrial
hyperplasia and carcinoma were 51, 49 and 52 years, respectively.
Of the 102 EC patients, 75 were diagnosed as stage I, 5 as stage II
and 22 as stage III, according to FIGO 2009 staging. Our study was
approved by the local ethics committees of Tianjin Medical
University, General Hospital. All subjects provided written consent
to participate in our investigation. Two gynecological pathologists
reviewed the tumor slides to confirm the original diagnoses. These
tissue specimens were constructed into tissue chips.
Immunohistochemistry staining and
scoring
The specimens were fixed in a 10% formalin solution
and embedded by routine methods in paraffin for sectioning at a
thickness of 3 μm. Immunohistochemical analysis was performed using
the streptavidin-biotin amplification method with a Histofine kit.
Sections were deparaffinized and incubated for 30 min with 3%
H2O2 in methanol to block endogenous
peroxidase activity. After being rinsed in Tris-buffered saline,
the sections were incubated for 6 h at room temperature with a
monoclonal antibody directed against anti-Wnt10a rabbit anti-human
(1:100 dilution) and anti-Wnt10b mouse anti-human monoclonal
antibody (1:200 dilution). The antibody complex was visualized with
3,3′-diaminobenzidine tetrahydrochloride (DAB) solutions.
Pancreatic cancer and breast cancer tissues were used as positive
controls. For the negative control, phosphate-buffered saline was
substituted for the primary antibody.
Two observers blindly and independently assessed the
immunohistochemical expression of Wnt10a and Wnt10b. Positive
immunostaining for Wnt10a and Wnt10b was detected both on the
membrane of the cells and in the cytoplasm. To evaluate Wnt10a and
Wnt10b, we defined a score that corresponded with the sum of the
percentage of positive cells (0, 0–24% immunopositive cells; 1,
25–50% positive cells; 2, 51–74% positive cells; 3, ≥75% positive
cells) and staining intensity (0, negative; 1, weak; 2, moderate;
3, strong). We classified tumors with scores of 0 as having
negative expression, 1–2 as having weak positive expression, 3–4 as
having moderate expression and 5–6 as having high-level
expression.
Cell lines and cell cultures
The EC cell lines Ishikawa3-H-12
(well-differentiated adenocarcinoma) and AN3CA (metastatic
undifferentiated EC) were used in this study. The cell lines were
kindly provided by Tianjin Medical University, General Hospital.
The two cell lines were cultured in DMEM/F12 with 2 mM glutamine
and supplemented with 10% FBS and 1% penicillin-streptomycin. Cells
were incubated at 37°C in a humidified atmosphere of 5%
CO2.
Plasmid transfection and cell
transfection
The pcDNA3.1-Wnt10b expression vector was purchased
from Invitrogen Life Technologies. Full-length WNT10B cDNA was
amplified from human placenta RNA by using a primer set (TGGAAGAAT
GCGGCTCTGAC and AGAGTGACCTTGGAAGGAAATC). Cell transfections were
performed using Lipofectamine 2000 (Invitrogen Life Technologies)
according to the manufacturer’s instructions. Cells
(20×104 in 60-mm dish) were transfected with either the
Wnt10b expression plasmid (pcWnt10b) or the empty control vector
(pcDNA3.1). The cells were harvested for analysis after 48 h.
Western blotting was used to examine the transfection results.
siRNA interference
The target sequence used for WNT10B silencing was
AAGGGUGGGAAGGGAUAAU (small interfering siRNA). Cell transfections
were performed using Lipofectamine 2000 according to the
manufacturer’s instructions. Cells (20×104 in 60-mm
dish) were transfected with 100 nM Wnt10b-specific siRNA or
scrambled siRNA and cells were harvested 48 h after the
transfection. Western blotting was used to examine the transfection
results.
Protein extraction and western blot
analysis
Whole cell extracts were prepared from cultured
cells by homogenizing cells in a lysis buffer (10 mM Tris-HCl (pH
7.5), 150 mM NaCl, 1% NP-40) containing a cocktail of protease
inhibitors. After centrifugation at 15,000 rcf for 30 min at 4°C,
the supernatants were recovered and used for immunoblot analysis.
The proteins were separated by SDS-PAGE and then transferred to
polyvinylidene difluoride (PVDF) membranes (Millipore). Blots were
blocked and then probed with antibodies against Wnt10b (1:500
dilution), β-catenin (1:500 dilution), APC (1:500 dilution) and
c-myc (1:500 dilution). After washing, the blots were incubated
with horseradish peroxidase-conjugated secondary antibodies
(1:1,000 dilution) and visualized using Super ECL detection
reagent.
MTT cell proliferation assay
A total of 200 μl of cells was seeded into a 6-well
microtiter plate at 5×105 cells/ml. We added 10 μl of
MTT reagent to each well 4 h before the end of the incubation.
After the incubation, the supernatant was removed and 200 μl DMSO
was added to dissolve the formazan crystals. The optical density
value (OD) of each sample was measured at a wavelength of 570 nm
(630 nm as a reference) on a microplate reader (Multiskan MK3;
Thermo Lab Systems). The results of the cell viability measurement
were expressed as OD570-OD630. All
experiments were performed in triplicate and the average results
were calculated.
Detection of cell apoptosis
Cells were harvested 48 h post-transfection for
apoptosis detection using the Annexin V-FITC apoptosis detection
kit and subsequently analyzed by flow cytometry.
Statistical analysis
Experimental data were analyzed using the SPSS
software package (version 17.0) (SPSS, Inc., Chicago, IL, USA).
Chi-square and Fisher’s exact tests were used to evaluate the
significance of differences in categorical variables. Spearman’s
correlation coefficients were calculated to evaluate the
correlation between Wnt10a and Wnt10b expression. Survival rate
analyses were compared using Kaplan-Meier survival curves. All
P-values were two-sided and a value <0.05 was considered to
indicate a statistically significant result.
Results
Expression of Wnt10a and Wnt10b proteins
in various endometrial tissues
We used IHC to assess Wnt10a and Wnt10b protein
levels in human endometrial tissues. We tested the expression of
Wnt10a and Wnt10b in EC, simple hyperplasia, complex hyperplasia,
atypical hyperplasia endometrium and control normal endometrium
groups. The distribution of positive expression of Wnt10a in the
proliferative phase, secretory phase, simple hyperplasia, complex
hyperplasia, atypical hyperplasia endometrium and endometrial
carcinoma cases was 39.58% (19/48), 44.44% (16/36), 38.89% (7/18),
33.33% (2/6), 30.00% (9/30) and 53.92% (55/102), respectively
(χ2=2.81, P=0.25). There were no significant differences
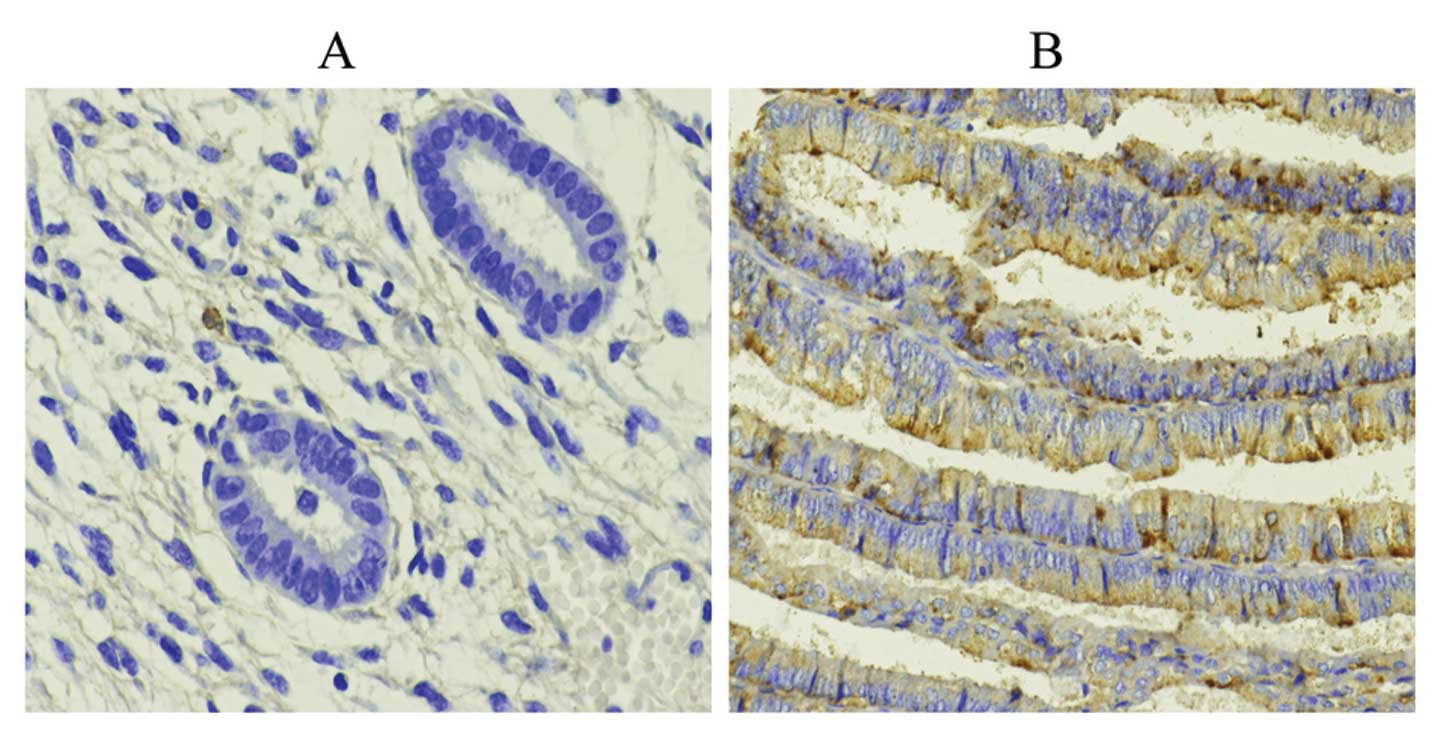
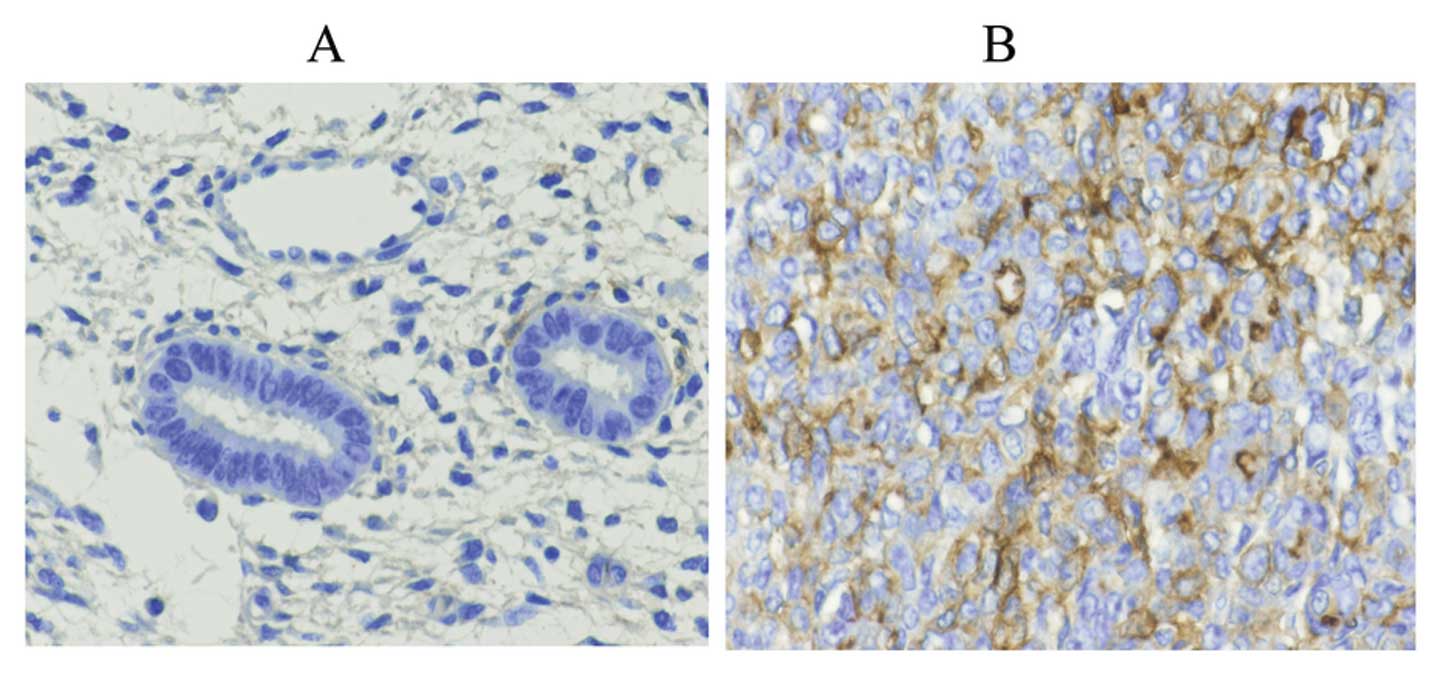
between the positive expression of Wnt10a (Fig. 1). The distribution of positive
expression of Wnt10b in the proliferative phase, secretory phase,
simple hyperplasia, complex hyperplasia, atypical hyperplasia and
endometrial carcinoma cases was 54.17% (26/48), 27.78% (10/36),
55.56% (10/18), 33.33% (2/6), 60.00% (18/30) and 63.73% (65/102),
respectively, and the difference between these groups was
significant (Fig. 2)
(χ2=8.12, P=0.02). The positive expression of Wnt10b in
EC tissues was significantly higher than that in other tissues
(Table I and Figs. 1 and 2).
 | Table IExpression of Wnt10a and Wnt10b in
various endometrial tissues. |
Table I
Expression of Wnt10a and Wnt10b in
various endometrial tissues.
| | Wnt10a | Wnt10b |
|---|
| |
|
|
|---|
| Variables | N | (−) | (+) | (++) | (+++) | (−) | (+) | (++) | (+++) |
|---|
| Normal |
| Proliferative | 48 | 29 | 10 | 6 | 3 | 22 | 11 | 13 | 2 |
| Secretory | 36 | 20 | 10 | 6 | 0 | 26 | 6 | 4 | 0 |
| Hyperplasia |
| Simple | 18 | 11 | 2 | 4 | 1 | 8 | 6 | 2 | 2 |
| Complex | 6 | 4 | 2 | 0 | 0 | 4 | 2 | 0 | 0 |
| Atypical | 30 | 21 | 4 | 1 | 4 | 12 | 10 | 5 | 3 |
| Endometrial
cancer | 102 | 47 | 32 | 17 | 6 | 37 | 42 | 14 | 9 |
Relationship between Wnt10a and Wnt10b
expression and various EC clinicopathological variables
To gain further insight into the clinical
significance of Wnt10a and Wnt10b expression in EC, we performed a
detailed clinical correlation study. The distribution of Wnt10a and
Wnt10b expression among the clinicopathological variables was
investigated. The difference in positive Wnt10a expression in the
different histological types was significant. The positive
expression of Wnt10a was significantly higher in patients with
endometrioid carcinoma (P<0.05); however, the differences in
positive expression between the subgroups for clinicopathological
stage, histologic grade, lymph node metastasis and myometrial
invasion were not significant (P>0.05). Positive expression of
Wnt10b was evaluated among the histological type, grade and FIGO
stage subgroups. The distribution of positive expression of Wnt10b
was significantly higher in patients with endometrioid type, high
grade, no lymph node metastasis, or advanced stage disease
(P<0.05). There were no significant differences, however,
between the myometrial invasion subgroups (P>0.05) (Table II).
 | Table IICorrelation between the expression of
Wnt10a and Wnt10b and clinicopathological characteristics in EC
cases. |
Table II
Correlation between the expression of
Wnt10a and Wnt10b and clinicopathological characteristics in EC
cases.
| | Wnt10a | | | Wnt10b | | |
|---|
| |
| | |
| | |
|---|
| | Negative | Positive | | | Negative | Positive | | |
|---|
| |
| | |
| | |
|---|
| Variables | N | n (%) | n (%) | χ2 | P-value | n (%) | n (%) | χ2 | P-value |
|---|
| Histological
type | | | | 4.75 | 0.03 | | | 4.02 | 0.04 |
| Endometrioid | 95 | 41 (43.2) | 54 (56.8) | | | 32 (33.7) | 63 (66.3) | | |
|
Nonendometrioid | 7 | 6 (87.5) | 1 (12.5) | | | 5 (71.4) | 2 (28.6) | | |
| Grade | | | | 2.06 | 0.36 | | | 6.87 | 0.03 |
| G1 | 42 | 17 (40.5) | 25 (59.5) | | | 11 (26.2) | 31 (73.8) | | |
| G2 | 38 | 17 (44.7) | 21 (55.3) | | | 13 (34.2) | 25 (65.8) | | |
| G3 | 22 | 13 (59.1) | 9 (40.9) | | | 13 (59.1) | 9 (40.9) | | |
| Myometrial
invasion | | | | 0.10 | 0.75 | | | 2.27 | 0.13 |
| None or
<1/2 | 70 | 33 (47.1) | 37 (52.9) | | | 22 (28.6) | 48 (68.5) | | |
| ≥1/2 | 32 | 14 (43.7) | 18 (56.3) | | | 15 (51.6) | 17 (53.1) | | |
| FIGO stage | | | | - | 0.54 | | | - | 0.04 |
| I | 75 | 37 (49.3) | 38 (50.7) | | | 32 (42.7) | 43 (57.3) | | |
| II | 5 | 2 (2/5a) | 3 (3/5a) | | | 2 (2/5a) | 3 (3/5a) | | |
| III | 22 | 8 (36.4) | 14 (63.6) | | | 3 (13.6) | 19 (86.4) | | |
| Lymph-node
metastasis | | | | - | 0.37 | | | - | 0.02 |
| No | 90 | 40 (65.6) | 50 (55.6) | | | 29 (32.2) | 61 (67.8) | | |
| Yes | 12 | 7 (58.3) | 5 (41.7) | | | 8 (66.7) | 4 (33.3) | | |
Correlation of Wnt10a and Wnt10b
expression in EC tissues
There was no obvious correlation between Wnt10a and
Wnt10b expression in EC (P>0.05) (Table III).
 | Table IIICorrelation between Wnt10a and Wnt10b
expression in endometrial cancer tissues. |
Table III
Correlation between Wnt10a and Wnt10b
expression in endometrial cancer tissues.
| | Wnt10b | | |
|---|
| |
| | |
|---|
| Wnt10a | N | − | + | rs | P-value |
|---|
| + | 55 | 18 | 37 | 0.08 | 0.43 |
| − | 47 | 19 | 28 | | |
Analysis of the prognosis for EC and the
expression of Wnt10a and Wnt10b
Twelve of the 102 endometrial carcinoma patients
were lost to follow-up. Of the 17 (18.9%) patients who died, 12
(70.6%) died of cancer and 5 (29.4%) died of other causes,
including heart failure, renal failure and cerebral hemorrhage.
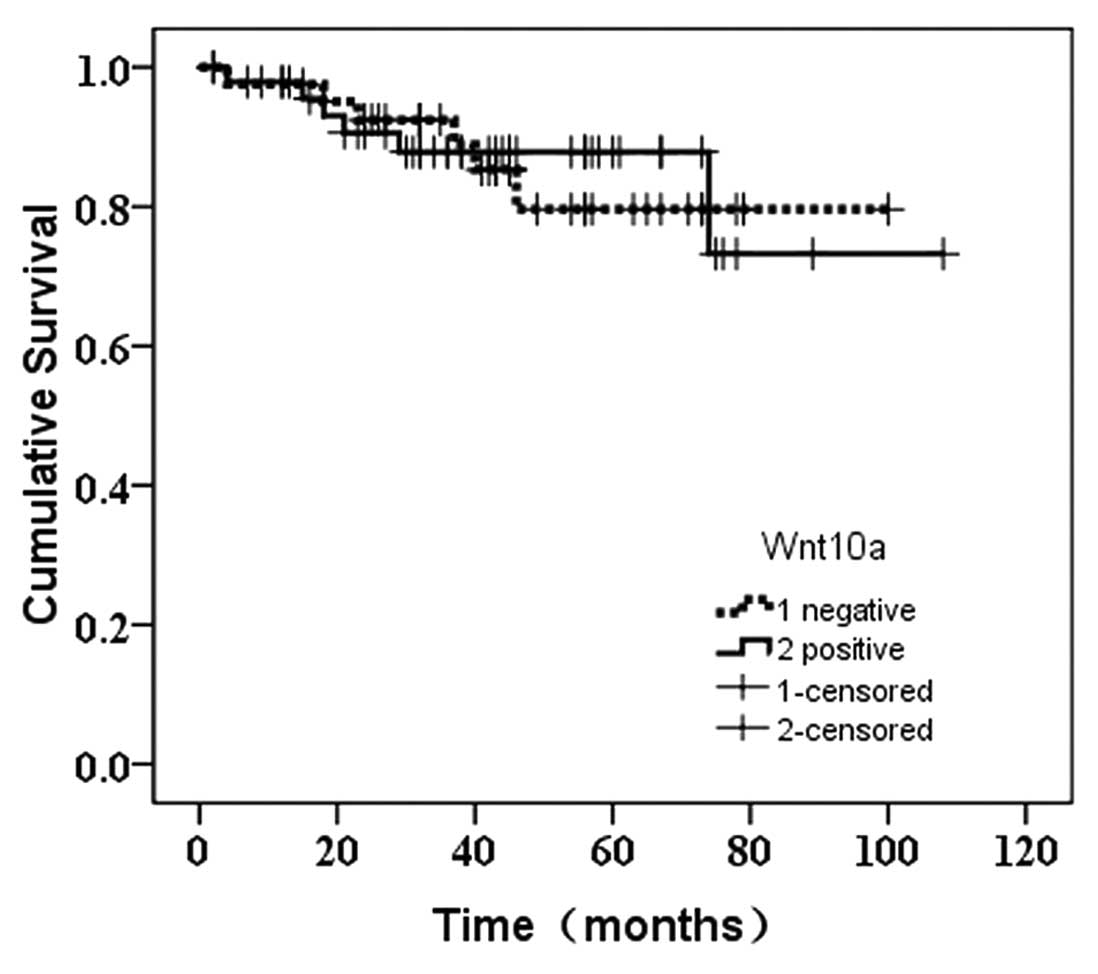
There was no significant difference between the prognosis for
patients with and without positive Wnt10a expression
(χ2=0.027, P=0.868) (Fig.
3). However, the prognosis for patients with positive
expression of Wnt10b was more favorable than that of the negative
carriers (χ2=3.952, P=0.047) (Fig. 4).
Impact of Wnt10b expression on EC cell
proliferation
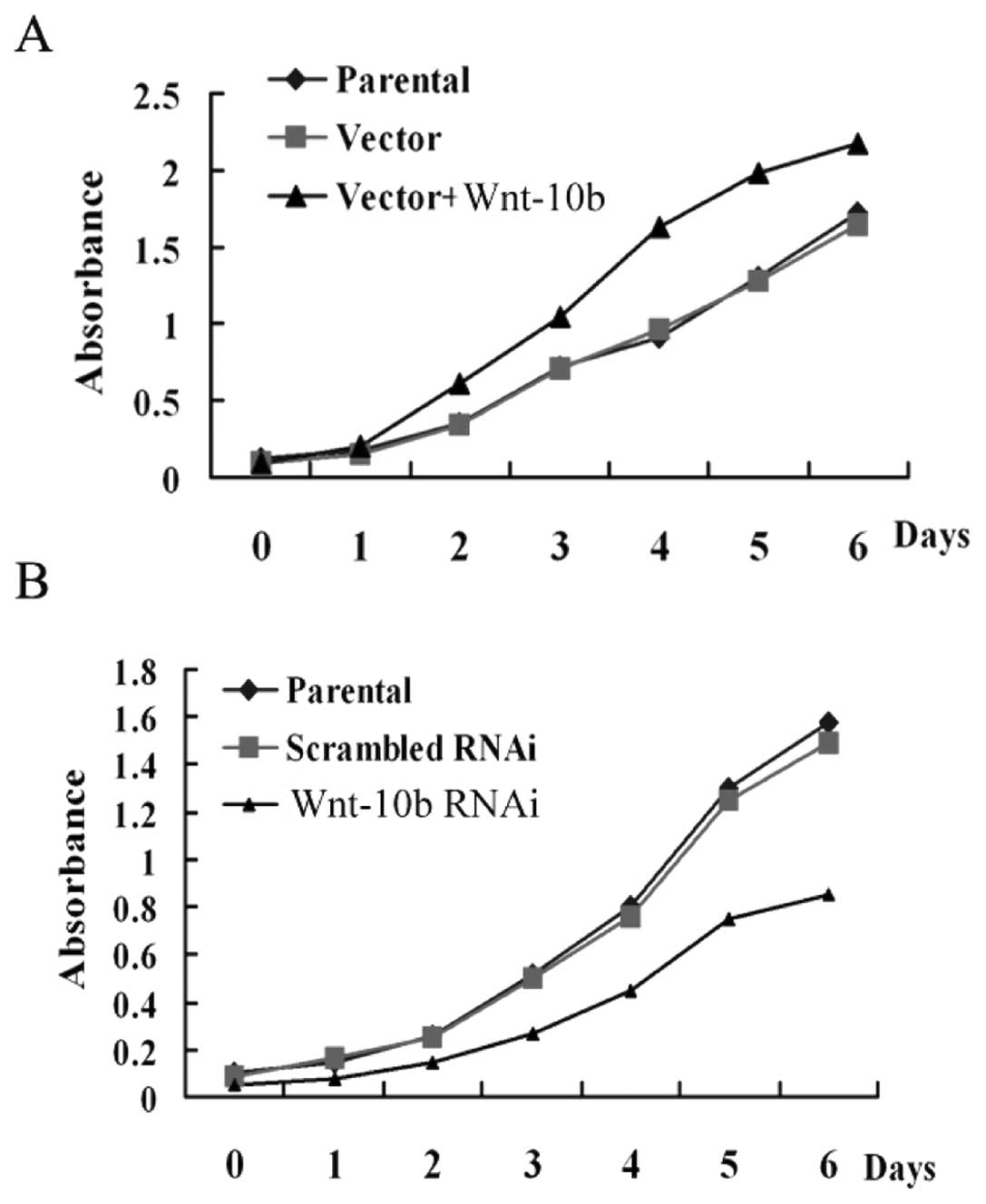
To examine the functional role of Wnt10b
upregulation in EC, we used the EC Ishikawa3-H-12 and AN3CA cell
lines as the in vitro model. The impact of Wnt10b
overexpression on cell proliferation was examined via MTT cell
proliferation assay. AN3CA cells, in which Wnt10b expression is
low, were transfected with the Wnt10b expression vector (pcWnt10b)
or empty control vector (pcDNA3.1). The proliferation of cells with
forced Wnt10b expression was higher than that of the control groups
(Fig. 5A).
To further confirm this result, Ishikawa3-H-12
cells, in which Wnt10b expression is high, were transfected with
Wnt10b-specific siRNA to knockdown Wnt10b expression. The results
showed that the proliferation of cells in the Wnt10b-knockdown
group was lower than that in the control groups (Fig. 5B), showing that Wnt10b expression
promotes cell proliferation.
Impact of Wnt10b expression on EC cell
apoptosis
The impact of Wnt10b overexpression on AN3CA cell
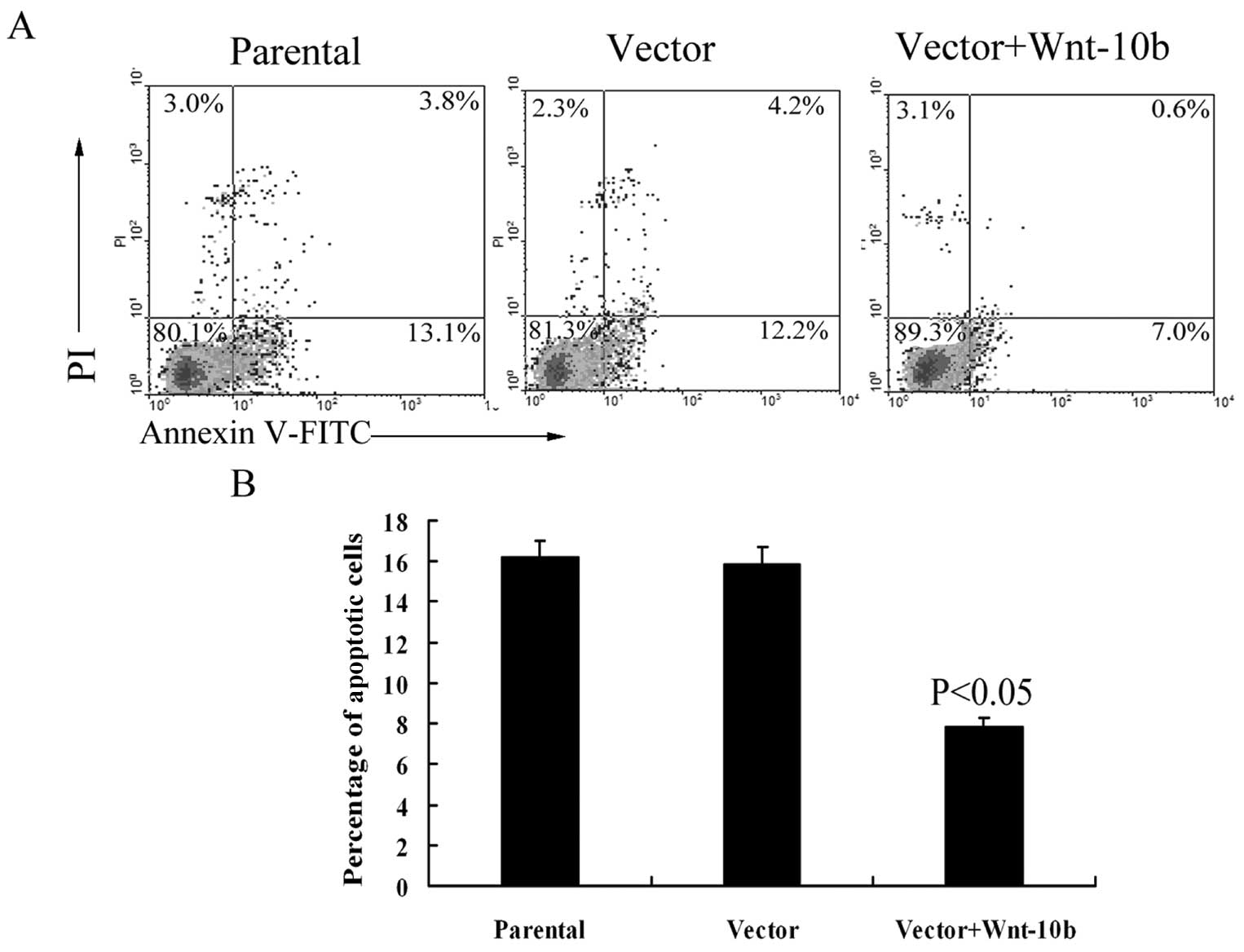
apoptosis was examined via flow cytometry. The percentage of
apoptotic cells with forced Wnt10b expression was significantly
lower than that of the control groups (P<0.05) (Fig. 6A and B). Accordingly, the percentage
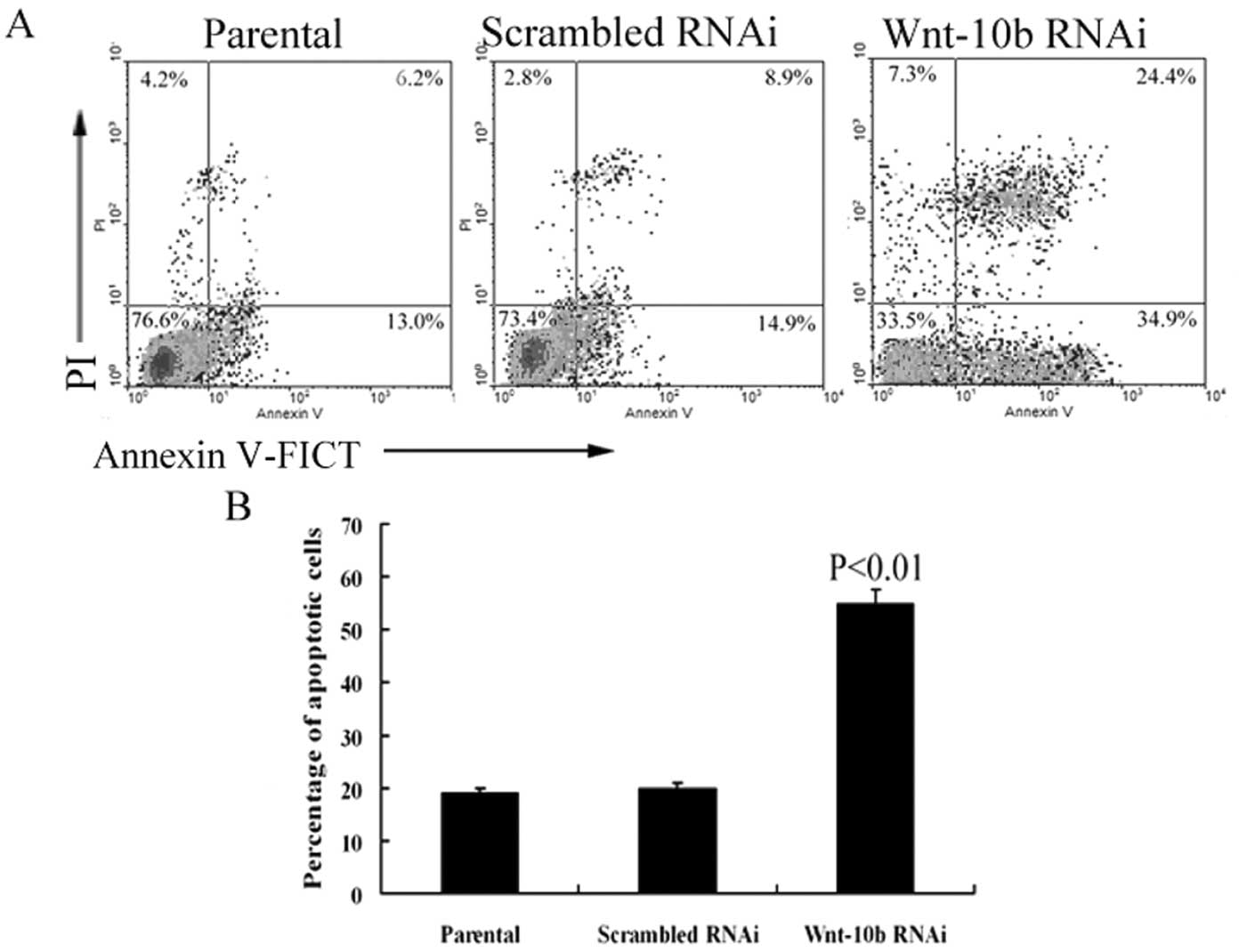
of apoptotic cells in the Wnt10b-knockdown group was significantly
higher than that in the control group (P<0.01) (Fig. 7A and B).
Effects of Wnt10b expression on
β-catenin, APC and c-myc
To determine whether and how the Wnt10b gene plays a
role in human EC, we performed a comprehensive analysis of major
components of the Wnt signaling pathways in the EC cell lines. We
then explored the effects of Wnt10b expression on β-catenin, APC
and c-myc, which are important downstream molecules in the Wnt
signal pathway. Both β-catenin and c-myc expression decreased after
Wnt10b knockdown in Ishikawa3-H-12 cells, while APC expression
increased (Fig. 8A). Conversely,
both β-catenin and c-myc expression increased after Wnt10b-forced
expression in the AN3CA cells, while APC expression decreased
(Fig. 8B). These results showed
that Wnt10b expression had effects on its downstream molecules
β-catenin, APC and c-myc. In our present study, Wnt10b played a
critical role in EC; we expected that Wnt10b would activate the
Wnt/β-catenin pathway in Ishikawa cells. We also observed a
correlation between the upregulation of Wnt10b and major components
of the Wnt/β-catenin pathway. Thus, the role of Wnt10b in the
development of EC may occur through the activation of the
Wnt/β-catenin pathway.
Discussion
A large number of reports have implicated β-catenin
mutations in 10–45% of ECs, however, the precise role of Wnt
signaling in this disease has not been established (18). Wnt ligands play an important role in
regulating the Wnt/β-catenin pathway and have therefore been at the
forefront of research efforts to investigate the mechanism of Wnt
signaling in various solid tumors. Several studies have
demonstrated that Wnt protein overexpression is associated with a
number of malignant diseases. The upregulation of Wnt10a may play
key roles in certain cases of esophageal, gastric and colorectal
cancer (CRC) (19), while the
overexpression of Wnt10b is associated with breast cancer (20). Recent interest in the interactions
between EC and Wnt signaling has focused on mouse studies involving
Wnt10b, a canonical Wnt signaling molecule (21).
Wnt10a overexpression has been reported in
esophageal, gastric and CRCs (19,22).
Similarly, our present results showed that the positive expression
of Wnt10a was higher in EC than hyperplasia or normal endometrium,
but the difference was not significant. The positive expression of
Wnt10b in cancerous endometrial tissues, however, was significantly
higher than hyperplastic or normal endometrium. We also found that
the positive expression of Wnt10a in type I endometrial carcinoma
was slightly higher than that in type II, which suggests that
Wnt10a is likely involved in the estrogen-related carcinogenesis of
EC. However, the exact mechanism promoting the development of EC
has not yet been elucidated. Our study findings of the upregulation
of Wnt10b in both human primary EC tissues and EC cell lines
confirm similar reports in breast (23) and prostate carcinomas (24). Wnt10b overexpression has been
reported in cancers of the breast and prostate. Importantly, our
investigation found that the Wnt10b overexpression was
significantly higher in EC than hyperplastic or normal
endometria.
A number of clinical pathologies predict the
clinical outcome in EC, including stage, grade and histology.
Additionally, the depth of myometrial invasion, lymphovascular
space invasion (LVSI) and pelvic lymph node status predict the
clinical behavior and direct adjuvant treatment options. In our
study, we demonstrated that Wnt10b expression is stage-dependent,
as Wnt10b expression in advanced stage EC tissues was reduced.
Unfortunately, information about the relationship between the
clinicopathological characteristics of EC, Wnt10a and Wnt10b is
still not available. Our present findings showed that Wnt10b
expression is significantly related to histological type, FIGO
stage and lymph node metastasis. The results suggest that higher
levels of Wnt10b may contribute to endometrioid, high-grade,
advanced-stage and no lymph node metastasis, which suggest
favorable prognosis. Regarding the survival curve for Wnt10b, there
was a tendency in the current Chinese study towards a better
prognosis for EC patients with positive Wnt10b expression than
patients who were negative carriers.
We hypothesized that the mechanism of tumor
promotion by Wnt10b in the Ishikawa cell line is regulated through
the Wnt10b-induced upregulation of the Wnt/β-catenin pathway. We
are the first to show that the EC Ishikawa cell line is responsive
to extracellular Wnt signaling. Previous study by our group
demonstrated that Wnt10b DNA was amplified in Ishikawa cell lines
and not amplified in AN3CA cells (17). In the present study, we showed that
the effects of Wnt10b on EC cell proliferation and apoptosis were
dependent on the Wnt/β-catenin pathway. This result established the
Ishikawa cell line as a useful model for the study of Wnt signaling
in EC (25). The Ishikawa cell line
is a well-differentiated, steroid-responsive EC cell line, with
both estrogen and progesterone receptors. The AN3CA cell line is a
metastatic, undifferentiated EC line. Benhaj et al(26) found that overexpression of Wnt10b in
most breast cancer cell lines determined the molecular mechanisms
of Wnt10b-driven mammary tumors via the Wnt/β-catenin signaling
pathway. Fernandez-Cobo et al(27) also reported that Wnt10b was strongly
upregulated in all breast cancer cell lines tested. Their results
suggest that tumors express high levels of nuclear β-catenin. In
addition, the expression of typical Wnt target genes, such as
c-myc, was strongly activated.
The Wnt/β-catenin pathway plays an important role in
EC tumorigenesis and development; it activates target genes through
the stabilization of β-catenin in the nucleus. The Wnt genes
activate Dishevelled, which recruits β-catenin and APC to the
β-catenin-TCF (T cell factor) pathway, and TCF stimulates target
genes, such as c-myc and cyclin D1, that influence cell
proliferation and apoptosis (28).
Aberrant activation of Wnt signaling in tumorigenesis has
frequently been reported. The prime example is CRC, in which
approximately 85% of cases display a loss-of-function mutation in
the tumor-suppressor APC gene (29). In our study, we found that Wnt10b
overexpression enhanced the expression of β-catenin and c-myc and
decreased the expression of APC. Wang et al(30) demonstrated that estrogen promotes
the proliferation of the endometrium by activating the
Wnt/β-catenin signaling pathway, which is also a target for
progesterone which inhibits the proliferation-promoting effects of
estrogen. A number of researchers have suggested that estrogen
increases the proliferation of MCF-7 cells by elevating Wnt10b mRNA
levels (31). It has been
demonstrated that the Wnt family is located upstream of the
mammalian target of rapamycin (mTOR) signaling pathway and that
increased levels of Wnt10b may promote the activation of the mTOR
signaling pathway, resulting in the proliferation of cells.
Furthermore, overactivation of mTOR is common in EC tissues and EC
cell lines (AN3CA, HEC1A, HEC1B, Ishikawa and PL95-2) (32). The development of type I EC has been
attributed to the stimulation of unopposed estrogen and this
relationship between estrogen and the Wnt signal pathway is likely
involved in the carcinogenesis of type I EC. Estrogen enhances the
activation of Wnt signaling by promoting the expression of the Wnt
protein, resulting in increased levels of c-myc that promote the
development of EC. The expression of the Wnt protein promotes
endometrial carcinoma by increasing the activation of the mTOR
signaling pathway. Moreover, the Wnt/β-catenin pathway may also be
activated through other mechanisms. The Wnt signaling pathway is a
complex signaling network, which is characterized by crosstalk with
other altered signaling pathways, such as the Hedgehog and mTOR
pathways.
Although our current study provided significant
results, there are still some limitations. First, the number of our
samples was limited. In the future, large scale research may
provide more reliable information. Second, the Wnt/β-catenin
signaling pathway was involved in the proliferation of EC cells,
however, this may not be the only way Wnt is involved in the
development of EC. Further research may show us more constructive
results.
In summary, the expression level of Wnt10a is higher
in endometrioid carcinoma than in non-endometrioid subtypes;
however, the underlying mechanism remains unclear. Wnt10b plays an
important role in the carcinogenesis of EC, particularly in
endometrioid carcinomas. We propose that the expression of Wnt10b
is involved in the activation of the Wnt/β-catenin pathway, nuclear
accumulation of β-catenin and induction of c-myc overexpression in
human EC cells. It has been suggested that Wnt10b is involved in
the estrogen-promoting development of EC. As the development of EC
is complicated, Wnt10a, Wnt10b and their signaling pathways alone
‘cannot tell the entire story’. However, preventive and therapeutic
strategies targeting Wnt10a and Wnt10b are likely to be of benefit
for EC patients, and further study focused on the relationship
among Wnt10a, Wnt10b and EC is warranted.
Acknowledgements
This study was supported by the Natural Science Fund
of China (no. 30772316), the science and technology development
fund of Tianjin Municipal Education Commission (no. 20100124) and
the foundation of the Fifth Central Hospital of Tianjin (no.
2011208). We also thank Dr Yuanxi Zhu, Wenyan Tian, Huiying Zhang
and Dandan Sun for their help in our study.
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang D and Han L: Year 1969–2003 study on
evolution of endometrial cancer. FuDan Univ J Med Sci. 32:479–483.
2005.
|
|
3
|
Bokhman JV: Two pathogenetic types of
endometrial carcinoma. Gynecol Oncol. 15:10–17. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fleming GF: Systemic chemotherapy for
uterine carcinoma: metastatic and adjuvant. J Clin Oncol.
25:2983–2990. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nusse R: Wnt signaling in disease and in
development. Cell Res. 15:28–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Katoh M: WNT and FGF gene clusters
(Review). Int J Oncol. 21:1269–1273. 2002.PubMed/NCBI
|
|
7
|
Wend P, Wend K, Krum SA and
Miranda-Carboni GA: The role of WNT10B in physiology and disease.
Acta Physiol (Oxf). 204:34–51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Modder UI, Oursler MJ, Khosla S and Monroe
DG: Wnt10b activates the Wnt, notch and NFκB pathways in U2OS
osteosarcoma cells. J Cell Biochem. 112:1392–1402. 2011.PubMed/NCBI
|
|
9
|
White BD, Chien AJ and Dawson DW:
Dysregulation of Wnt/β-catenin signaling in gastrointestinal
cancers. Gastroenterology. 142:219–232. 2012.
|
|
10
|
Robinson DR, Zylstra CR and Williams BO:
Wnt signaling and prostate cancer. Curr Drug Targets. 9:571–580.
2008. View Article : Google Scholar
|
|
11
|
Mukheriee N, Bhattacharya N, Alam N, et
al: Subtype-specific alteration of the Wnt signaling pathway in
breast cancer: clinical and prognostic significance. Cancer Sci.
103:210–220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gatcliffe TA, Monk BJ, Planutis K and
Holcombe RF: Wnt signaling in ovarian tumorigenesis. Int J Gynecol
Cancer. 18:954–962. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carmon KS and Loose DS: Development of
bioassay for detection of Wnt-binding affinities for individual
frizzled receptors. Anal Biochem. 401:288–294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kirikoshi H, Sekihara H and Katoh M:
WNT10A and WNT6, clustered in human chromosome 2q35 region with
head-to-tail manner, are strongly co-expressed in SW480 cells.
Biochem Biophy Res Commun. 283:798–805. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Benhaj K, Akcali KC and Oztuk M: Redundant
expression of canonical Wnt ligands in human breast cancer cell
lines. Oncol Rep. 15:701–707. 2006.PubMed/NCBI
|
|
16
|
Saegusa M, Hashimura M, Yoshida T and
Okayasu I: Beta-catenin mutations and aberrant nuclear expression
during endometrial tumorigenesis. Br J Cancer. 84:209–217. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Yang D, Cogdell D, Hu L, Xue F,
Broaddus R and Zhang W: Genomic characterization of gene
copy-number aberrations in endometrial carcinoma cell lines derived
from endometrioid-type endometrial adenocarcinoma. Technol Cancer
Res Treat. 9:179–189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dellinger TH, Planutis K, Tewari KS and
Holcombe RF: Role of canonical Wnt signaling in endometrial
carcinogenesis. Expert Rev Anticancer Ther. 12:51–62. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kirikoshi H, Inoue S, Sekihara H and Katoh
M: Expression of WNT10A in human cancer. Int J Oncol. 19:997–1001.
2001.PubMed/NCBI
|
|
20
|
Brennan KR and Brown AM: Wnt proteins in
mammary development and cancer. J Mammary Gland Biol Neoplasia.
9:119–131. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barbolina MV, Burkhalter RJ and Stack MS:
Diverse mechanisms for activation of Wnt signaling in the ovarian
tumour microenviroment. Biochem J. 437:1–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kirikoshi H, Sekihara H and Katoh M:
Up-regulation of WNT10A by tumor necrosis factor alpha and
Helicobacter pylori in gastric cancer. Int J Oncol.
19:533–536. 2001.PubMed/NCBI
|
|
23
|
Milovanovic T, Planutis K, Nquyen A, Marsh
JL, Lin F, Hope C and Holcombe RF: Expression of Wnt genes and
frizzled 1 and 2 receptors in normal breast epithelium and
infiltrating breast carcinoma. Int J Oncol. 25:1337–1342.
2004.PubMed/NCBI
|
|
24
|
Kharaishvili G, Simkova D, Makharoblidze
E, et al: Wnt signaling in prostate development and carcinogenesis.
Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 155:11–18.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishida M: The Ishikawa cells from birth
to the present. Hum Cell. 15:104–117. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lustig B and Behrens J: The Wnt signaling
pathway and its role in tumor development. J Cancer Res Clin Oncol.
129:199–221. 2003.PubMed/NCBI
|
|
27
|
Fernandez-Cobo M, Zammarchi F, Mandeli J,
Holland JF and Pogo BG: Expression of Wnt5A and Wnt10b in
non-immortalized breast cancer cells. Oncol Rep. 17:903–907.
2007.PubMed/NCBI
|
|
28
|
Scholten AN, Creutzberg CL, van den Broek
LJ, Noordijk EM and Smit VT: Nuclear beta-catenin is a molecular
feature of type 1 endometrial carcinoma. J Pathol. 201:460–465.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.
|
|
30
|
Wang Y, van der Zee, Fodde R and Blok LJ:
Wnt/B-catenin and sex hormone signaling in endometrial homeostasis
and cancer. Oncotarget. 1:674–684. 2010.PubMed/NCBI
|
|
31
|
Kirikoshi H and Katoh M: Expression and
regulation of WNT10B in human cancer: up-regulation of WNT10B in
MCF-7 cells by beta-estradiol and down-regulation of WNT10B in NT2
cells by retinoic acid. Int J Mol Med. 10:507–511. 2002.PubMed/NCBI
|
|
32
|
Lu Lu KH, Wu W, Dave B, Slomovitz BM,
Burke TW, Munsell MF, Broaddus RR and Walker CL: Loss of tuberous
sclerosis complex-2 function and activation of mammalian target of
rapamycin signaling in endometrial carcinoma. Clin Cancer Res.
14:2543–2550. 2008.PubMed/NCBI
|