Introduction
Hepatoblastoma (HB), the most common primary
malignant liver tumor in children, is associated with an excellent
outcome [3-year survival 96% in standard-risk (SR)-HB] due to the
effectiveness of combined treatment using chemotherapy and surgery.
SR-HB tumors show a high sensitivity to chemotherapy, particularly
to the alkylating-like chemotherapeutic agent cisplatin (CDDP)
(1,2). Neoadjuvant chemotherapy facilitates
tumor resection, and systemic therapy has substantially decreased
the incidence of recurrent metastatic disease (3). CDDP as monotherapy is equally
effective as combined CDDP/DOXO (doxorubicin) in patients with
accurately staged SR-HB regarding complete resection rates. This
approach is, as predicted, less toxic than combination treatment
regimens (3,4). In contrast, children suffering from
high-risk (HR-HB) tumors still present with a poor survival (3-year
survival 69%). A major reason for this fact is multi-drug
resistance, which develops in 80% of initially CDDP- and
DOXO-sensitive tumors after 4–5 courses of chemotherapy (2,5–7).
Exposure to alternating cycles of CDDP is also associated with
clinically significant adverse effects such as irreversible
nephrotoxicity, ototoxicity, neurotoxicity and myelosuppression
(8,9). Other platin-derived drugs are less
toxic but also less effective; therefore, cisplatin remains the
agent of choice for HB and many other childhood solid tumors.
Small-molecular sensitizers, which enhance the
effects of cytotoxic drugs by sensitizing tumor cells to apoptosis,
constitute a promising treatment option for overcoming resistance
and improving outcome (10). One of
these is ABT-737, an antitumor agent that induces apoptosis by
selectively inhibiting the anti-apoptotic proteins Bcl-2, Bcl-XL
and Bcl-W at the mitochondrial cell level (11). HB cells show overexpression of
anti-apoptotic molecules encoded by genes of the Bcl family which
play a central role in drug resistance of several types of
malignancies including HB (11,12).
ABT-737 binds to the Bcl-2 homology domain 3 (BH3) binding groove
of Bcl-2 and facilitates the activation of pro-apoptotic Bcl
proteins tBid, Bad, Bax and Bim leading to an increase in
cytochrome c release and an increase in apoptosis. ABT-737
as a single agent has shown activity against several hematopoietic
cell lines (leukemia, multiple myeloma and cultured lymphoma) and
various solid tumors, including HB in vitro(11,13–18).
Highly synergistic in vivo effects have been described when
combining ABT-737 with established chemotherapeutic drugs commonly
used in treatment protocols of HB, including CDDP (11,19,20).
In this study, we investigated the effects of a
combination therapy consisting of ABT-737 and CDDP in a xenograft
model of HB.
Materials and methods
Gene expression analysis
Gene expression analysis was performed using the
database E-MEXP-1851 (www.ebi.ac.uk) including 25 samples
of HB and 4 samples of normal liver tissue (21). Differential expression was
visualized within the apoptosis pathway using PathVision 2.0.11
(www.pathvision.org).
Drugs
ABT-737 (Abbott GmbH & Co. KG, Wiesbaden,
Germany) was dissolved for in vitro and animal studies as
previously described (14). The
final concentration in the cell culture was 0.01, 0.1, 0.3 and 1
μM. CDDP was provided by Neocorp AG (Weilheim, Germany).
Tumor cells and culture conditions
The HB cell lines HUH6 (22) and HepT1 (23) were used for all experiments. The
cells were transduced with a plasmid encoding Gaussia luciferase
(GLuc, pCMV-GLuc; NEB, Frankfurt, Germany). Stable clones were
isolated and maintained in DMEM (Gibco BRL, Carlsbad, CA)
supplemented with 10% FCS, G418, 1% glutamine, and 1%
penicillin/streptomycin (Gibco, Eggenstein, Germany).
Clonogenic assay
Briefly, an equal number of HUH6 and HepT1 cells at
a concentration of 10,000 cells/ml were seeded on
poly-D-lysine-coated Petri dishes (Becton Dickinson GmbH,
Heidelberg, Germany) with 5 ml medium allowing development of cell
adhesion for 2 h. Cells were incubated with 0.3 μM ABT-737, 1 μg/ml
CDDP or a combination of both drugs for 96 h. The medium was then
replaced, and colonies were washed, fixed with glutaraldehyde (6.0%
v/v), and stained with crystal violet (0.5% w/v). The cell colonies
growing on a surface of 125 mm2 in different regions of
the culture plate were counted using a stereomicroscope (Axioscope
40; Carl Zeiss, Oberkochen, Germany). A colony was defined to
consist of at least 50 cells. All studies were performed in
triplicates.
Animals and xenotransplantation
Xenotransplantation was performed as previously
described (24). All animal studies
were conducted according to criteria outlined in the ‘Guide for the
Care and Use of Laboratory Animals’ [Animal Care and Use: Policy
Issues in the 1990’s, National Institutes of Health/Office for the
Protection from Research Risks (NIH/OPRR), 1989. Proceedings of
NIH/OPRR Conference, Bethesda, MD], and were approved by the local
government’s ethics authority for animal experiments
(Regierungspräsidium Tübingen, Referat 35, number CK1/09). HUH6
cells were injected into the flank of 6- to 8-week-old
NOD/LtSz-scid IL2Rγnull mice (NSG). For each tumor 0.2 ml of tumor
cell suspension (2×106 cells) was injected
subcutaneously into the paravertebral areas. The observation time
was 4 weeks. Each group consisted of 5–6 animals. Treatment was
initiated when tumors had reached a length of 5 mm. CDDP in a 200
μl saline solution was administered i.p. once per day on days 1–4
with a dosage of 1.25 mg/kg (low dose) or 3.0 mg/kg body weight
(high dose), respectively. ABT-737 was injected i.p. with a dosage
of 100 mg/kg body weight alone or in combination with CDDP using
the same schedule. Control animals were left untreated until day 10
unless the tumor volume exceeded 1 cm3. Tumor volumes (V
= 4/3π × a/2 × b/2 × c/2) and body weight of all animals were
determined daily. Relative tumor growth was calculated as the
percentage of tumor volume at each time point compared to day 0.
Blood samples were obtained from the retrobulbar plexus on days 0
and 10. Serum GLuc activity was quantified in fresh serum.
Therefore, 5 μl of serum was added to 50 μl Gaussia GlowJuice
(J.P.K. Instruments AG, Berlin, Germany), and GLuc activity was
measured using a luminometer (Magic® Lite Analysator,
Ciba Corning) after adding 1 μl coelenterazine (100 μM) to acquire
photon counts for 10 sec. Activity was expressed as relative light
units per second (RLU/sec). Tumors were explanted on day 10 and
prepared for histological analysis.
Histology and immunohistochemistry
Paraffin-embedded sections obtained from xenografted
tumors were used for immunohistochemical staining against Ki-67
(mouse anti-human Ki-67, 1:400; Dako GmbH, Hamburg, Germany) as
previously described (14). The
proliferation index was expressed as the mean ± SD of positively
stained cells per microscopic field of two regions in each tumor of
the treatment groups.
Statistical analysis
Statistical analysis of clonogenicity of HB cells
was carried out using two-way ANOVA followed by Bonferroni
post-test using GraphPad Prism 4.00 (GraphPad Software, San Diego,
CA, USA, www.graphpad.com). Relative tumor growth and body
weights for each group of animals were compared to controls using
the Student’s t-test. Data plotted on graphs represent the means ±
SD. Significance was assumed for all p-values <0.05.
Results
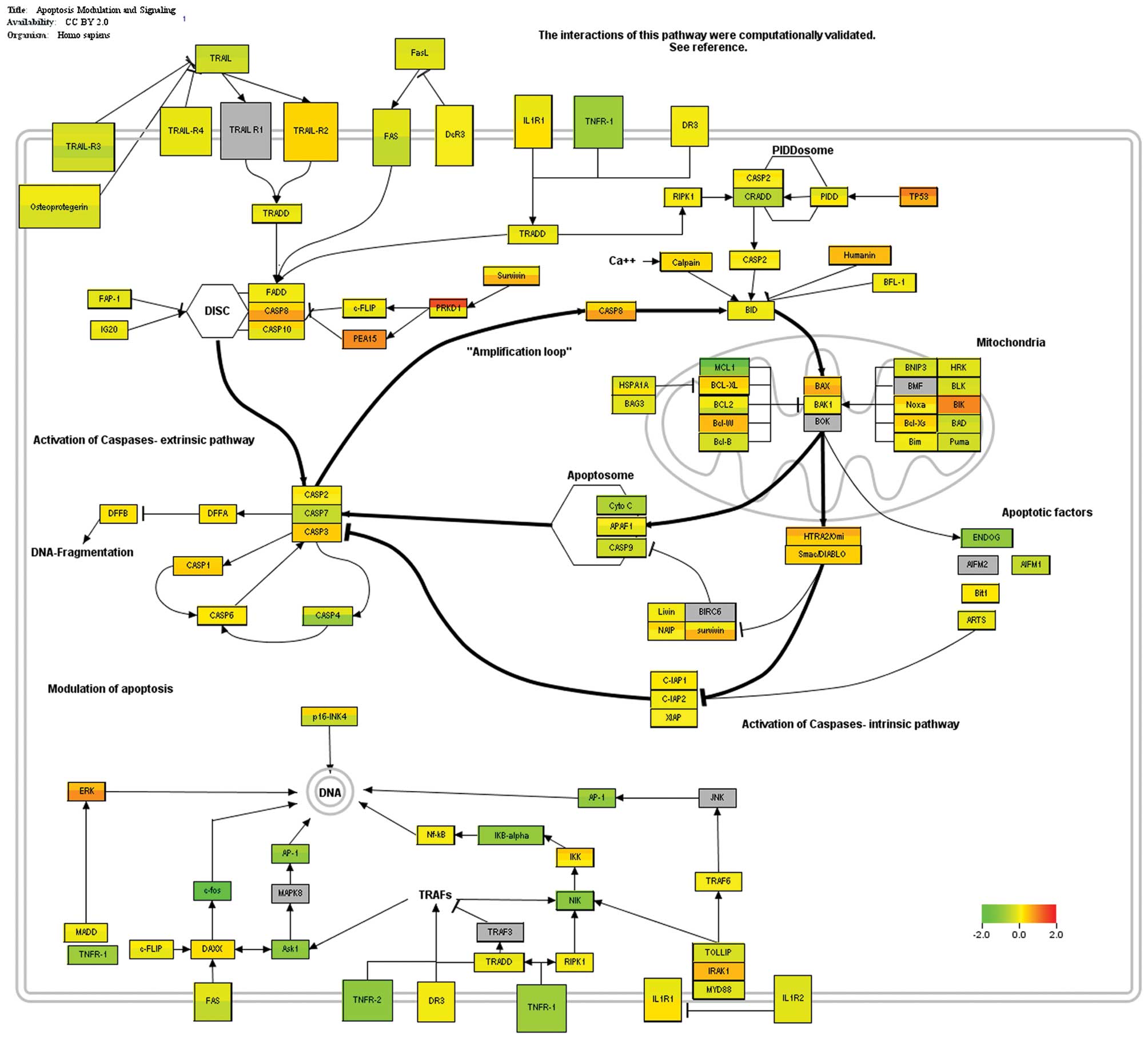
Expression of pro- and anti-apoptotic
proteins in HB and normal liver tissue
Proteins involved in apoptosis modulation and
signalling were visualized using differential gene expression
(Fig. 1). Death receptors of the
TNF-family generally were downregulated in tumors or showed equal
expression levels when compared to normal liver tissue. Caspases,
such as CASP8 and CASP3, were highly expressed in tumors.
Expression levels of the anti-apoptotic Mcl-1 were high in normal
liver tissue and only slightly reduced in tumors; however, still
high expression levels were found. Other anti-apoptotic proteins,
such as Bcl-XL, Bcl-W and survivin were highly expressed in tumors,
whereas pro-apoptotic proteins, such as BAD and PUMA showed low
expression levels. However, Bax, as a promoter of apoptosis, was
present. BAK-1 was equally expressed in tumors and normal liver
tissue. Taken together, an anti-apoptotic state predominated in the
HB tumors compared to normal liver tissue. Consequently, the use of
apoptosis modulators or sensitizers constitutes a promising option
in order to enhance the effects of drugs acting on induction of the
apoptosis cascade.
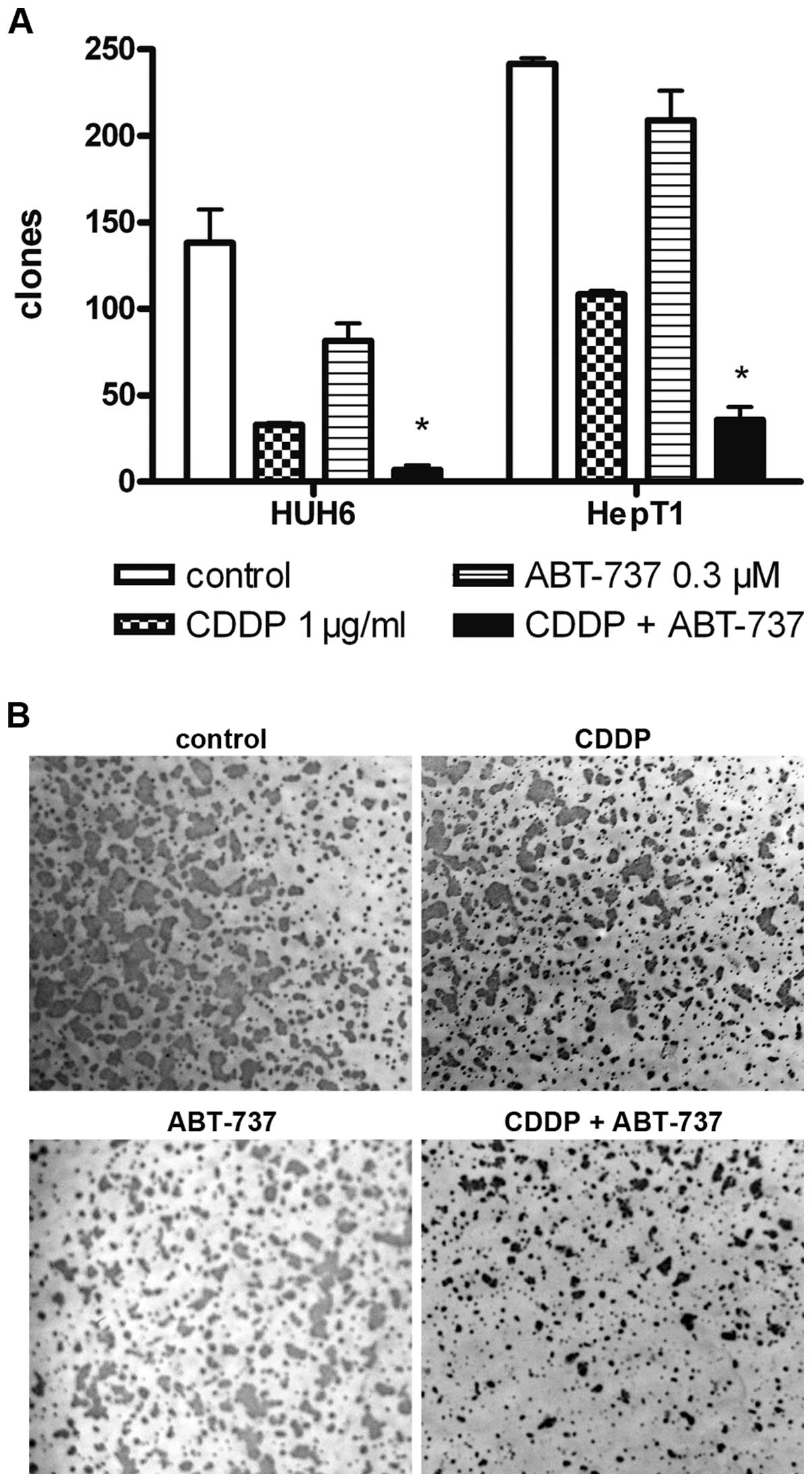
Additive effects of CDDP and ABT-737 in
HB cells
ABT-737 enhances the effect of various cytotoxic
drugs in the combination treatment of tumor cell lines. To
determine the effects of the treatment on HB cells, a clonogenity
assay was carried out. CDDP alone led to a decreased number of
clones in HepT1 and HUH6 cells (Fig.
2). The reduction in clone number following ABT-737 treatment
was inferior to the effect of CDDP and was not significantly
different from the control experiments. CDDP in combination with
ABT-737 significantly decreased the number of clones in HB cell
cultures (p<0.05). The number of clones was more than 5-fold
reduced following the combination treatment compared to treatment
with CDDP alone. In general, HUH6 cells showed higher sensitivity
to CDDP as well as the combined treatment.
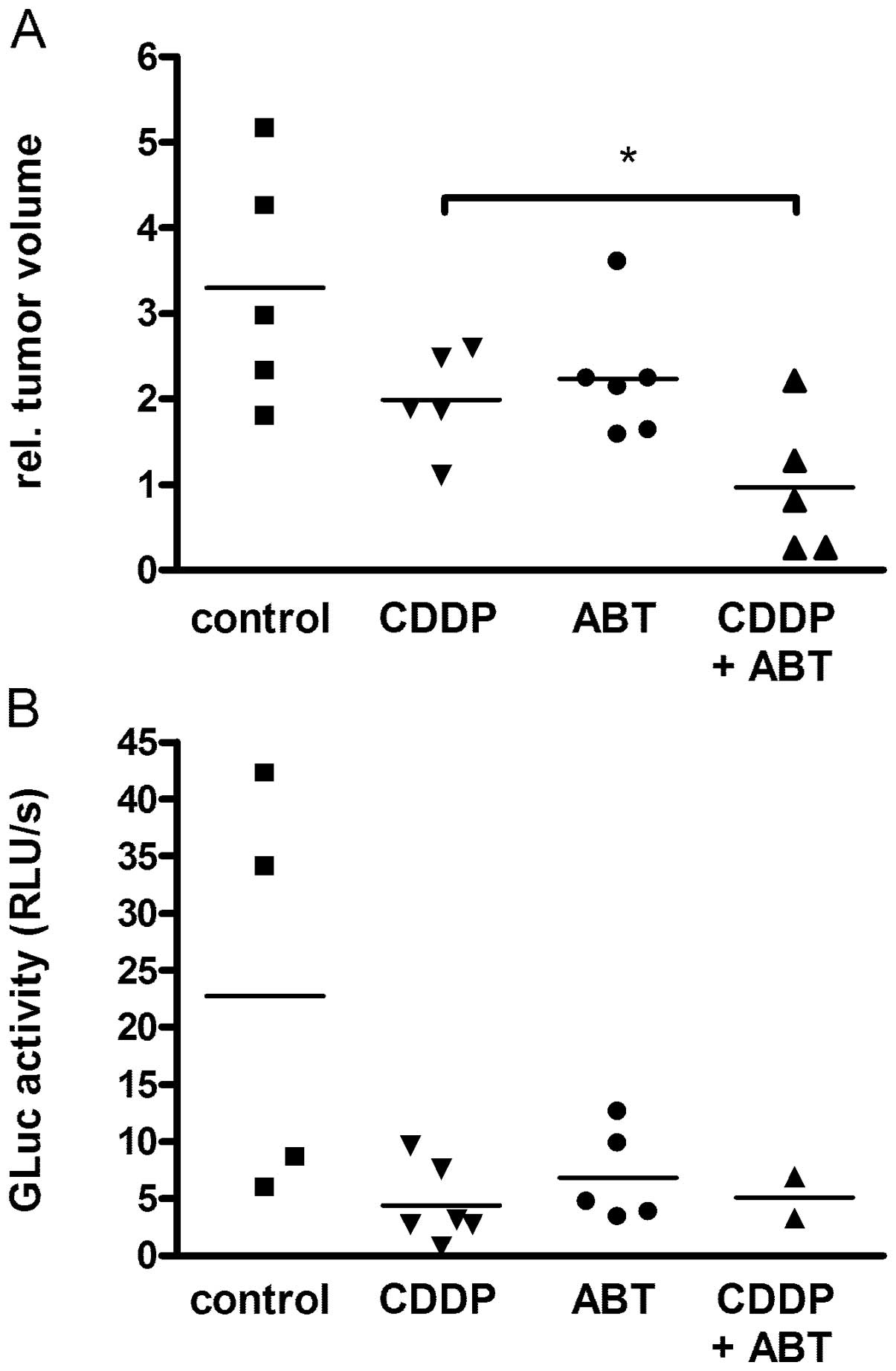
Treatment of HB xenografts with a
combination of CDDP and ABT-737
HUH6 xenografts were used to describe the effects of
ABT-737 in combination with CDDP in vivo. All of the
xenotransplanted animals developed measurable tumors after 4 weeks.
Relative tumor volumes showed maximal growth in the control group
(n=5) (Fig. 3A). Tumor volumes
increased three times within 4 days. In the group treated with low
dose CDDP (1.25 mg/kg for 4 consecutive days; n=6) the
relative tumor volume was duplicated. Significantly reduced tumor
growth was observed in mice after combined treatment (n=5) compared
to treatment with CDDP alone (p<0.02). In the group administered
the combined treatment, two mice did not show tumor growth at all
and two tumors shrank during treatment. As an additional control
parameter, expression of the transgene GLuc was detected in the
blood of all mice at levels >200 RLU/sec, revealing tumor growth
(Fig. 3B). Relative GLuc activity
increased during the experiment in the controls and in mice under
treatment.
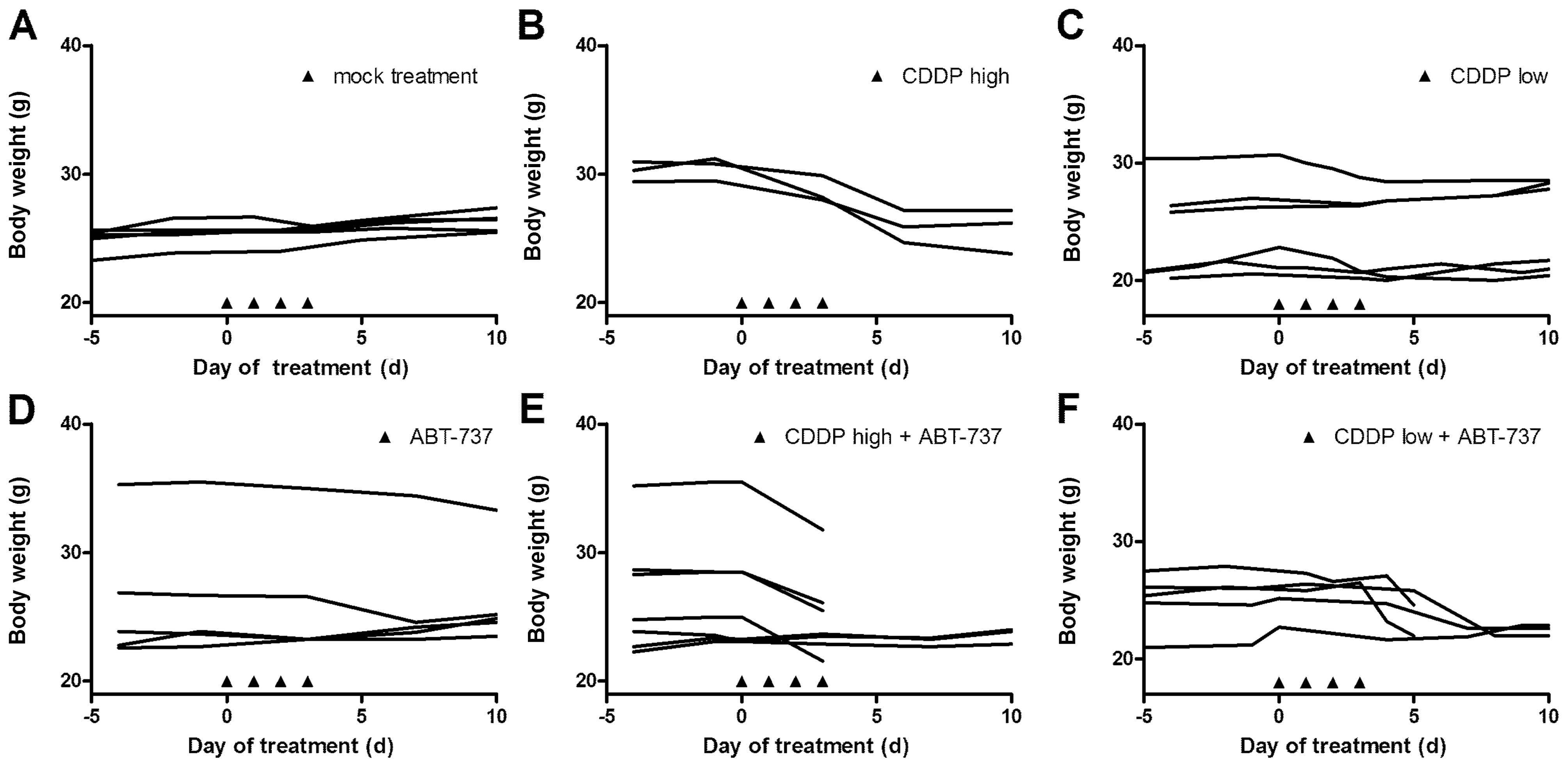
Toxicity was monitored by changes in body weight
during treatment. Tumor-bearing mice in the control group gained
weight or remained constant during the experiment (Fig. 4). Loss of >10% body weight was
observed in the group treated with high dose CDDP (3 mg/kg body
weight) compared to control animals. However, body weights remained
stable after treatment with low dose CDDP (1.25 mg/kg). Treatment
with a combination of ABT-737 and high dose CDDP led to death in 4
of 6 animals, whereas 3 of 5 animals survived the experiment when
treated with combined low dose CDDP. No toxcitiy was observed after
treatment with ABT-737 alone.
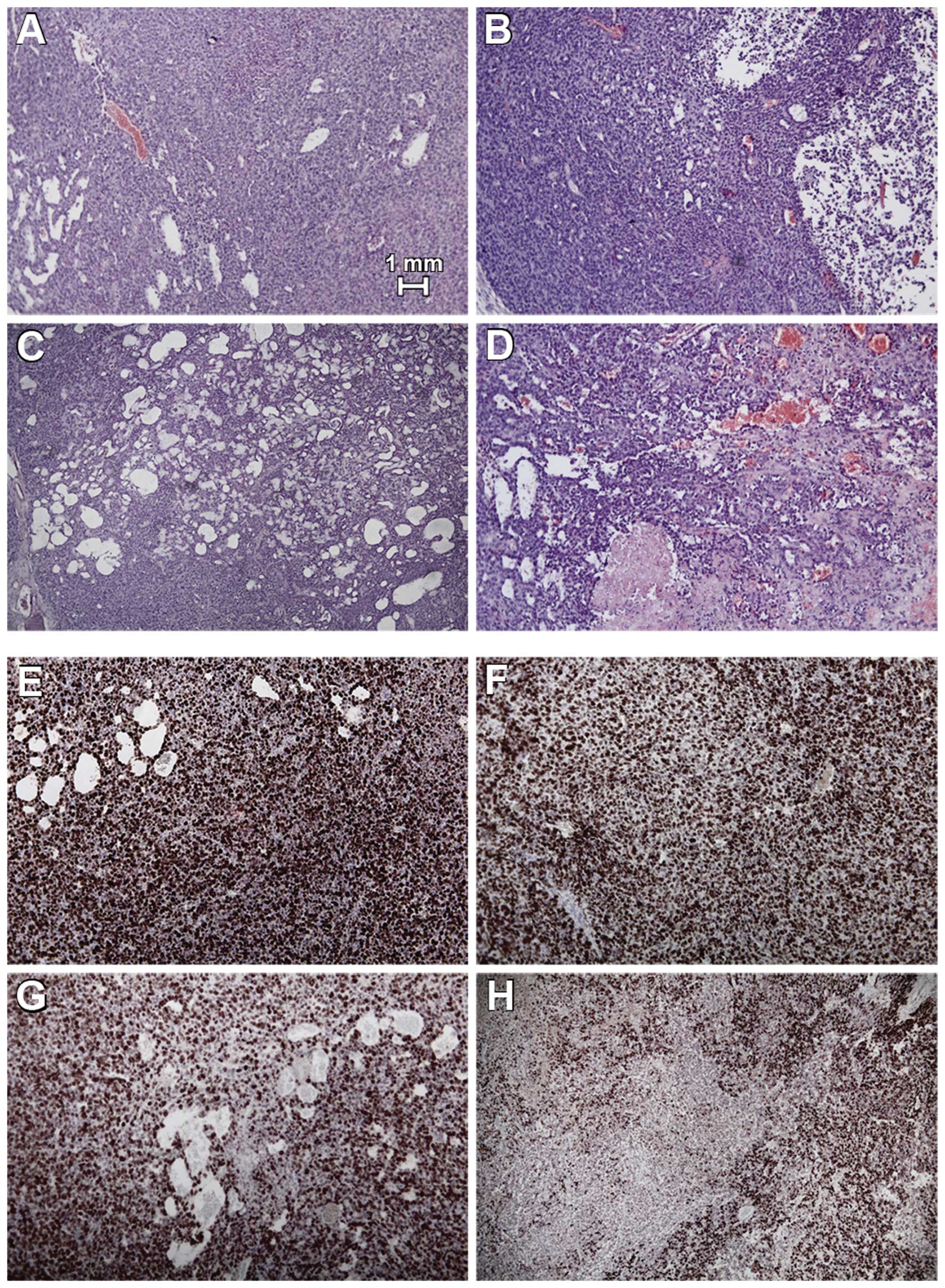
Histological analysis of HB xenografts of the
control group and ABT-737 group showed high density of tumor cells
in H&E staining (Fig. 5). Fewer
vital tumor cells and small areas of necrosis were noted in
xenografts of the CDDP group. After combined treatment, multiple
picnotic cells, hemorrhagic infarction, and large areas of necrosis
were noted in the tumors. In this group, large areas of
disintegrated tumors were detected. Proliferation rates were
evaluated by anti-Ki-67 staining and by mean proliferation index ±
SD. Comparable proliferation was detected in the control tumors
(699±108), tumors treated with low dose CDDP (745±170), and ABT-737
alone (715±98). Cell proliferation was significantly reduced after
combined treatment using ABT-737 and low dose CDDP (475±231) when
compared to all other groups (p<0.5, ANOVA with Bonferroni’s
multiple comparison test).
Taken together, additive effects following CDDP and
ABT-737 treatment were demonstrated in HB xenografts by assessing
tumor growth and histological appearance.
Discussion
Complete tumor resection is mandatory for the
survival of patients suffering from HB. However, 50% of tumors are
primarily unresectable or metastases are presented at diagnosis
(6,25). Therefore, the European Study Groups
for Liver Tumors in Children recommend surgery after neoadjuvant
chemotherapy. HB exhibits a good response to neoadjuvant
chemotherapy resulting in reduced tumor size and better
resectability. CDDP is the most important cytotoxic agent,
particularly in SR-HB, and CDDP treatment leads to an excellent
3-year survival rate of 96% (2).
Various regimens include the use of carboplatin in addition to CDDP
in order to improve treatment efficacy. However, results were not
improved, and therapy was associated with significantly more
toxicity, even though amifostine had been used as a cytoprotective
adjuvant (5). CDDP/DOXO (PLADO)
treatment was also not superior compared to CDDP monotherapy in
SR-HB (2). For HR-HB,
intensification of neoadjuvant chemotherapy with carboplatin/DOXO
alternating with CDDP was recommended in the SIOPEL 3 study, and
this treatment showed tolerable toxicity together with effective
tumor reduction. Shortening the time intervals between the courses
is planned in the SIOPEL 4 for the group of high-risk patients to
further intensify chemotherapy (7).
This may increase the risk of toxicity. Collectively, treatment
results of extended and metastatic HR-HB are still not satisfactory
irrespective of the principle treatment strategy and applied drug
combination. This was also observed and described by the first
Japanese Hepatoblastoma Trial (JPLT-1) and the current German
GPOH-HB99 study (6,7,26). In
addition, multidrug resistance complicates the response to
chemotherapy and develops in 80% of patients initially sensitive to
CDDP and DOXO after 4–5 courses of chemotherapy (27). In order to improve the efficacy of
chemotherapeutic agents several mechanisms are currently under
investigation. Modulation of apoptosis using small BH3 mimetic
molecules, such as ABT-737, is one such mechanism (10,15).
CDDP acts as an alkylating agent and finally induces
apoptosis following two pathways. It activates reactive oxygen
species (ROS), which represent a specific vulnerability of
malignant cells (28). However,
this pathway is inhibited by anti-apoptotic Bcl-2 protein, an
important member of the Bcl family. Various malignancies, such as
HB, demonstrate Bcl-2 overexpression, which plays a central role in
resistance to chemotherapy (29).
In contrast, CDDP increases p53 levels, which activate
pro-apoptotic NOXA, PUMA, Bax and p38. CDDP activates the apoptotic
pathway and MAP kinase signaling (30). In rapidly proliferating tumor cells,
chemotherapeutics may be more effective at physiological apoptosis
homeostasis than in this observed anti-apoptotic state. Therefore,
we used lower dosages of CDDP in combination with the BH3 mimetic
ABT-737 to facilitate induction of apoptosis through ROS.
ABT-737 induces apoptosis as a single drug when
treating various cell lines and has also previously shown additive
effects in HB cell lines when combined with various cytotoxic drugs
despite the expression of Mcl-1 (11,16,19,20,31).
In a previous study, we obtained similar results with a combination
therapy consisting of ABT-737 and paclitaxel in HB xenografts
(14). In the present study, CDDP
also led to a tumor response in xenografts; however, we observed
only reduced growth rates, but not shrinkage of xenografts compared
to initial tumor volume. Additive effects were observed after a
combined therapy using ABT-737 and CDDP, resulting in inhibition of
tumor growth and in certain cases a reduction in tumor volume.
Histologically, we found noticeably less vital tumor cells in HB
xenografts after combination therapy and large areas of
disintegrated tumors were detected.
In this study, high doses of CDDP (3 mg/kg) led to
significant toxicity in NOD mice, whereas low doses of CDDP (1.25
mg/kg) showed transient toxic effects. Clinically significant
adverse effects associated with CDDP exposure in human patients are
well described including irreversible nephrotoxicity, ototoxicity,
neurotoxicity and myelosuppression (8). Thus, lowering the dosage of CDDP along
with treatment in combination with ABT-737, which demonstrates
significantly reduced tumor growth compared to CDDP monotherapy
in vivo, may also maintain or even enhance antitumor
activity in patients. In contrast to CDDP, ABT-737 alone (100 mg/kg
body weight) was not associated with toxic effects in this and
other studies (15,19,20).
In this context, we assume the toxicity to be mouse strain
independent, as has been previously described in nude mice NMRI
(nu/nu) treated with CDDP alone (32). When combining CDDP and ABT-737, the
toxicity was even higher than that following treatment with CDDP
alone. To reduce toxicity due to combination treatment, second
generation orally bioavailable BH3 mimetics, such as ABT-263, may
be used (33). In addition, other
BH3 mimetic drugs have been considered to be attractive candidates
for combination treatments. Currently, obatoclax is under
investigation in several clinical trials including those targeting
solid tumor malignancies and has been described to be well
tolerated without dose-limiting toxicity (34–36).
Obatoclax has already shown additive effects in the treatment of HB
cells when combined with CDDP and therefore will be assessed in
further optimization studies (15).
Finally, the most important advantage of combination treatment is
dose reduction of both the cytostatic and the BH3 mimetic drug,
reducing side effects while maintaining antitumor activity. Based
on this assumption, problematic thrombocytopenia after combination
treatment may be weakened, as it is only described with high
dosages of BH3 mimetic drugs (33,37,38).
However, apoptosis sensitizers may be active only in
those patients with a high anti-apoptotic state, as particularly
high deviations of means were observed in the gene expression
analyses. These findings may recommend the evaluation of the
apoptosis status of patients during the initial treatment phase,
e.g. using an array for apoptosis (11). Based on our results, a combination
therapy of CDDP and BH3 mimetic drugs may serve as a promising
addition to the treatment of advanced HB in clinical settings.
In conclusion, the primary goal of current
chemotherapy in HB is reduction of tumor volume and vitality in
order to enable complete surgical resection. Our results have
confirmed the optimization of chemotherapy by using modulators of
apoptosis. CDDP, which is the most commonly used cytotoxic agent in
most trials of HB, reduces tumor growth when combined with ABT-737.
Sensitizing HB cells to apoptosis may also restore the sensitivity
of resistant HB to established therapeutic regimens.
Acknowledgements
The authors wish to acknowledge Abbott Laboratories
for providing ABT-737.
Abbreviations:
|
HB
|
hepatoblastoma
|
|
SR
|
standard risk
|
|
HR
|
high risk
|
|
MDR
|
multidrug resistance
|
|
CDDP
|
cisplatin
|
|
DOXO
|
doxorubicin
|
|
BH3
|
Bcl-2 homology domain
|
References
|
1
|
Loehrer PJ and Einhorn LH: Drugs five
years later. Cisplatin Ann Intern Med. 100:704–713. 1984.PubMed/NCBI
|
|
2
|
Perilongo G, Maibach R, Shafford E, et al:
Cisplatin versus cisplatin plus doxorubicin for standard-risk
hepatoblastoma. N Engl J Med. 361:1662–1670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pritchard J, Brown J, Shafford E, et al:
Cisplatin, doxorubicin, and delayed surgery for childhood
hepatoblastoma: a successful approach - results of the first
prospective study of the International Society of Pediatric
Oncology. J Clin Oncol. 18:3819–3828. 2000.
|
|
4
|
Sullivan MJ: Hepatoblastoma, cisplatin,
and ototoxicity: good news on deaf ears. Cancer. 115:5623–5626.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ortega JA, Douglass EC, Feusner JH, et al:
Randomized comparison of cisplatin/vincristine/fluorouracil and
cisplatin/continuous infusion doxorubicin for treatment of
pediatric hepatoblastoma: A report from the Children’s Cancer Group
and the Pediatric Oncology Group. J Clin Oncol. 18:2665–2675.
2000.PubMed/NCBI
|
|
6
|
Perilongo G, Shafford E, Maibach R, et al:
Risk-adapted treatment for childhood hepatoblastoma. Final report
of the second study of the International Society of Paediatric
Oncology - SIOPEL 2. Eur J Cancer. 40:411–421. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zsiros J, Maibach R, Shafford E, et al:
Successful treatment of childhood high-risk hepatoblastoma with
dose-intensive multiagent chemotherapy and surgery: final results
of the SIOPEL-3HR study. J Clin Oncol. 28:2584–2590. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cvitkovic E: Cumulative toxicities from
cisplatin therapy and current cytoprotective measures. Cancer Treat
Rev. 24:265–281. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Knight KR, Kraemer DF and Neuwelt EA:
Ototoxicity in children receiving platinum chemotherapy:
underestimating a commonly occurring toxicity that may influence
academic and social development. J Clin Oncol. 23:8588–8596. 2005.
View Article : Google Scholar
|
|
10
|
Chonghaile TN and Letai A: Mimicking the
BH3 domain to kill cancer cells. Oncogene. 27(Suppl 1): 149–157.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lieber J, Kirchner B, Eicher C, et al:
Inhibition of Bcl-2 and Bcl-X enhances chemotherapy sensitivity in
hepatoblastoma cells. Pediatr Blood Cancer. 55:1089–1095. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Adesina AM, Lopez-Terrada D, Wong KK, et
al: Gene expression profiling reveals signatures characterizing
histologic subtypes of hepatoblastoma and global deregulation in
cell growth and survival pathways. Hum Pathol. 40:843–853. 2009.
View Article : Google Scholar
|
|
13
|
Konopleva M, Contractor R, Tsao T, et al:
Mechanisms of apoptosis sensitivity and resistance to the BH3
mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 10:375–388.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lieber J, Eicher C, Wenz J, et al: The BH3
mimetic ABT-737 increases treatment efficiency of paclitaxel
against hepatoblastoma. BMC Cancer. 11:3622011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lieber J, Ellerkamp V, Wenz J, et al:
Apoptosis sensitizers enhance cytotoxicity in hepatoblastoma cells.
Pediatr Surg Int. 28:149–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oltersdorf T, Elmore SW, Shoemaker AR, et
al: An inhibitor of Bcl-2 family proteins induces regression of
solid tumours. Nature. 435:677–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trudel S, Stewart AK, Li Z, et al: The
Bcl-2 family protein inhibitor, ABT-737, has substantial
antimyeloma activity and shows synergistic effect with
dexamethasone and melphalan. Clin Cancer Res. 13:621–629. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
van Delft MF, Wei AH, Mason KD, et al: The
BH3 mimetic ABT-737 targets selective Bcl-2 proteins and
efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized.
Cancer Cell. 10:389–399. 2006.PubMed/NCBI
|
|
19
|
High LM, Szymanska B, Wilczynska-Kalak U,
et al: The Bcl-2 homology domain 3 mimetic ABT-737 targets the
apoptotic machinery in acute lymphoblastic leukemia resulting in
synergistic in vitro and in vivo interactions with established
drugs. Mol Pharmacol. 77:483–494. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mason KD, Khaw SL, Rayeroux KC, et al: The
BH3 mimetic compound, ABT-737, synergizes with a range of cytotoxic
chemotherapy agents in chronic lymphocytic leukemia. Leukemia.
23:2034–2041. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cairo S, Armengol C, De Reynies A, et al:
Hepatic stem-like phenotype and interplay of Wnt/beta-catenin and
Myc signaling in aggressive childhood liver cancer. Cancer Cell.
14:471–484. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Doi I: Establishment of a cell line and
its clonal sublines from a patient with hepatoblastoma. Gann.
67:1–10. 1976.PubMed/NCBI
|
|
23
|
Pietsch T, Fonatsch C, Albrecht S, Maschek
H, Wolf HK and von Schweinitz D: Characterization of the continuous
cell line HepT1 derived from a human hepatoblastoma. Lab Invest.
74:809–818. 1996.PubMed/NCBI
|
|
24
|
Warmann SW, Heitmann H, Teichmann B, et
al: Effects of P-glycoprotein modulation on the chemotherapy of
xenotransplanted human hepatoblastoma. Pediatr Hematol Oncol.
22:373–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Reynolds M: Conversion of unresectable to
resectable hepatoblastoma and long-term follow-up study. World J
Surg. 19:814–816. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haberle B, Bode U and von Schweinitz D:
Differentiated treatment protocols for high- and standard-risk
hepatoblastoma - an interim report of the German Liver Tumor Study
HB99. Klin Padiatr. 215:159–165. 2003.(In German).
|
|
27
|
von Schweinitz D, Byrd DJ, Hecker H, et
al: Efficiency and toxicity of ifosfamide, cisplatin and
doxorubicin in the treatment of childhood hepatoblastoma. Study
Committee of the Cooperative Paediatric Liver Tumour Study HB89 of
the German Society for Paediatric Oncology and Haematology. Eur J
Cancer. 33:1243–1249. 1997.
|
|
28
|
Roux PP and Blenis J: ERK and p38
MAPK-activated protein kinases: a family of protein kinases with
diverse biological functions. Microbiol Mol Biol Rev. 68:320–344.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Warmann SW, Frank H, Heitmann H, et al:
Bcl-2 gene silencing in pediatric epithelial liver tumors. J Surg
Res. 144:43–48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ono K and Han J: The p38 signal
transduction pathway: activation and function. Cell Signal.
12:1–13. 2000. View Article : Google Scholar
|
|
31
|
Chauhan D, Velankar M, Brahmandam M, et
al: A novel Bcl-2/Bcl-X(L)/Bcl-w inhibitor ABT-737 as therapy in
multiple myeloma. Oncogene. 26:2374–2380. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Warmann S, Hunger M, Teichmann B, Flemming
P, Gratz KF and Fuchs J: The role of the MDR1 gene in the
development of multidrug resistance in human hepatoblastoma:
clinical course and in vivo model. Cancer. 95:1795–1801. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tse C, Shoemaker AR, Adickes J, et al:
ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor.
Cancer Res. 68:3421–3428. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hwang JJ, Kuruvilla J, Mendelson D, et al:
Phase I dose finding studies of obatoclax (GX15-070), a small
molecule pan-BCL-2 family antagonist, in patients with advanced
solid tumors or lymphoma. Clin Cancer Res. 16:4038–4045. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Paik PK, Rudin CM, Brown A, et al: A phase
I study of obatoclax mesylate, a Bcl-2 antagonist, plus topotecan
in solid tumor malignancies. Cancer Chemother Pharmacol.
66:1079–1085. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schimmer AD, O’Brien S, Kantarjian H, et
al: A phase I study of the pan bcl-2 family inhibitor obatoclax
mesylate in patients with advanced hematologic malignancies. Clin
Cancer Res. 14:8295–8301. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mason KD, Carpinelli MR, Fletcher JI, et
al: Programmed anuclear cell death delimits platelet life span.
Cell. 128:1173–1186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang H, Nimmer PM, Tahir SK, et al: Bcl-2
family proteins are essential for platelet survival. Cell Death
Differ. 14:943–951. 2007.PubMed/NCBI
|