Introduction
Breast cancer is a serious concern in many
countries. Despite public education, cancer prevention and
screening, increase in early diagnosis and advances in cancer
management, 200,000 women develop breast cancer and more than
40,000 women die of breast cancer in the United States annually
(1,2). Since breast cancer is typically a
hormone-dependent cancer, targeted endocrine therapy has led to a
significant improvement in the outcomes for women with estrogen
receptor (ER)-positive breast cancer. However, not all patients
respond to first-line endocrine treatment, and other patients will
eventually relapse despite an initial response to the treatment
(3). Therapeutic options for these
patients are limited. Additionally, patients with hormone
receptor-negative breast tumors that are not human epidermal growth
factor receptor 2 (HER2)-positive [(i.e., triple-negative breast
cancer (TNBC)] account for ~15% of breast cancer patients and these
TNBC patients have not benefitted from generally well-tolerated,
anti-HER2 drugs or targeted endocrine treatment strategies
(4–6). Although considerable progress has been
achieved through the development of new drugs and treatment
strategies, current therapy is unable to elicit a clinical response
in these patients (7).
In the last two decades, there have been major
efforts to identify the signaling mechanisms responsible for these
patients, and new therapeutic targets are being explored against
cancer signaling pathways. Cyclooxygenase-2 (COX-2) is
overexpressed in approximately 70% of in situ cases and 60%
of invasive breast cancer, and elevated COX-2 expression in
invasive breast cancer leads to increased tumor recurrence and
decreased patient survival (8,9).
COX-2-specific inhibitors have been shown to promote growth arrest
and induce apoptosis in various cancers, including breast cancer,
by down-regulating the prosurvival signaling pathway, protein
kinase B (PKB)/Akt (10,11). The Western New York Exposures and
Breast Cancer Study results showed a significant reduction in
breast cancer risk associated with recent and adult lifetime
non-steroidal anti-inflammatory drug use (odd ratio 0.73, 95%
confidence interval: 0.51–1.03) (12). Luteolin, 3′, 4′, 5,
7-tetrahydroxyflavone, is a common flavonoid that exists in many
types of plants including fruits, vegetables, and medicinal herbs
and acts as antioxidants, estrogenic regulators, and antimicrobial
agents (13). Studies have been
demonstrated that the anticancer property of luteolin is associated
with inducing apoptosis, which involves redox regulation, DNA
damage, protein kinase inhibition to cancel cell proliferation, and
suppression of metastasis and angiogenesis (13,14).
The objective of this study was to examine the
effect of combination treatment of celecoxib and luteolin in human
breast cancer cells and to subsequently determine the mechanism of
action.
Materials and methods
Cell lines
Estrogen receptor-positive MCF-7 human breast cancer
cells and estrogen receptor-negative MDA-MB-231 human breast cancer
cells were obtained from the American Type Culture Collection
(Manassas, VA, USA). The cells were cultured in RPMI-1640 medium
(Sigma Chemical Co., St. Louis, MO, USA) supplemented with heat
inactivated 10% fetal bovine serum with 300 mg/l L-glutamine, 25 mM
HEPES and 25 mM NaHCO3, 100 μg/ml streptomycin, and 100
U/ml penicillin. The cells were grown in a humidified incubator at
37°C and 5% CO2 atmosphere.
Cell survival assays
The effect of celecoxib (Celebrex®,
Pfizer Inc., St. Louis, MO, USA) and luteolin (Sigma Chemical Co.)
on human breast cancer cell growth was determined by cell counting
following XTT (Roche, Mannheim, Germany) assays. The XTT assay was
performed as recommended by the manufacturer. One thousand cells
were seeded in triplicates in 6-well plates with or without
celecoxib and/or luteolin and assayed 72 h later. After incubation,
cells treated with celecoxib were fixed in dimethyl sulfoxide
(DMSO: Sigma Chemical Co.) and cells treated with luteolin were
fixed in isopropyl Alcohol (Duksan pure chemicals, Kyungkido,
Korea). Following fixation, the cells were treated with the XTT
cell proliferation kit (Roche). The 570-nm absorbance was read
using an automated spectrophotometric miniplate reader EL808
(Ultramicroplate reader; Bio-Tek Instruments, Inc., Winooski, VT,
USA). The values were normalized and plotted as the percentage
change compared to control cells (mean ± standard error of the
mean).
Apoptosis assays
Apoptotic MCF-7 and MDA-MB-231 cells were identified
using the 5-bromo-2-deoxyuridine/terminal deoxynucleotidyl
transferase (TdT)-mediated 2′-deoxyuridine 5′-triphosphate
(dUTP)-biotin nick end labeling assay (TUNEL) and Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining.
TUNEL assay (Apoptosis detection kit s7100; Chemicon international
Inc, Temecula, USA) was performed according to the manufacturer’s
protocol. Briefly, total of 1×105 cells/ml were
incubated with celecoxib and/or luteolin for 72 h. Then, the cells
were washed and incubated with staining solution (10 μl of TdT
reaction buffer, 0.75 μl of TdT enzyme, and 8 μl of FITC-dUTP)
overnight. The following day, cells were rinsed and resuspended in
1 ml PI/RNase solution. After incubation in the dark for 30 min at
room temperature, a manual cell count was performed to obtain the
percentage of apoptotic cells.
Annexin V-FITC and PI staining was performed using
the detection kit according to the manufacturer’s protocol (BD
Pharmingen, CA, USA). Total of 1×105 cells/ml were
incubated with celecoxib and/or luteolin for 72 h. The cells were
washed with cold phosphate-buffered saline and suspended in 100 ml
buffer (10 mM HEPES, 10 mM NaOH (pH 7.4), 140 mM NaCl, 2.5 mM
CaCl2). After 5 ml of Annexin V-FITC and 5 ml of PI were
added, the cells were incubated for 15 min at room temperature in
the dark. After this incubation, 400 ml of binding buffer solution
was added, and the cells were analyzed via flow cytometery. The
quadrant containing Annexin V-FITC-positive/PI-negative cells
represents early apoptotic cells, and the quadrant containing
Annexin V-FITC-positive and PI-positive cells represents cells
undergoing the end stage of apoptosis, necrotic cells or dead
cells.
Western blotting
The cells were lysed, and the protein concentration
was determined with the Bio-Rad colorimetric Assay (Bio-Rad
Laboratories, Hercules, CA, USA). Lysates were resolved by western
blot analysis on 10% sodium dodecylsulfate gels. The lanes were
loaded with 50 μg of protein and electrophoresed for 2 h at 90 V.
Proteins were transferred to nitrocellulose membranes. Membranes
were blocked with 5% nonfat dry milk and incubated with antibodies
director against β-catenin (Santa Cruz Biotechnology, Santa Cruz,
CA, USA), Akt (Cell Signaling, Beverly, MA, USA), STAT 3 (Cell
Signaling) and pAkt (Cell Signaling) overnight at 4°C. Membranes
were washed and incubated with secondary antibody for 1 h at room
temperature. Membranes were then developed, and protein signals
were detected using enhanced chemiluminescence western blotting
detection reagents (Amersham Biosciences, Buckinghamshire, UK).
Membranes were incubated with antibody against β-actin (Santa Cruz
Biotechnology) to assess equal protein loading, and results were
subjected to densitometry analysis.
Statistical analysis
Statistical analyses were performed between control
and treatment groups and among the different experimental groups.
Continuous data are presented as the means and standard error
deviations with median values. Comparisons of means were carried
out using One way ANOVA. Differences with a value of P<0.05 were
considered to be statistically significant.
Results
Effect of celecoxib and luteolin on
breast cancer cells in vitro
Breast cancer cells were treated with DMSO,
isopropyl alcohol, celecoxib, luteolin, or a celecoxib + luteolin
combination treatment. DMSO was used as a control for celecoxib and
isopropyl alcohol was used as a control for luteolin. Cell
viability was assessed using XTT assays. MCF-7 cells were treated
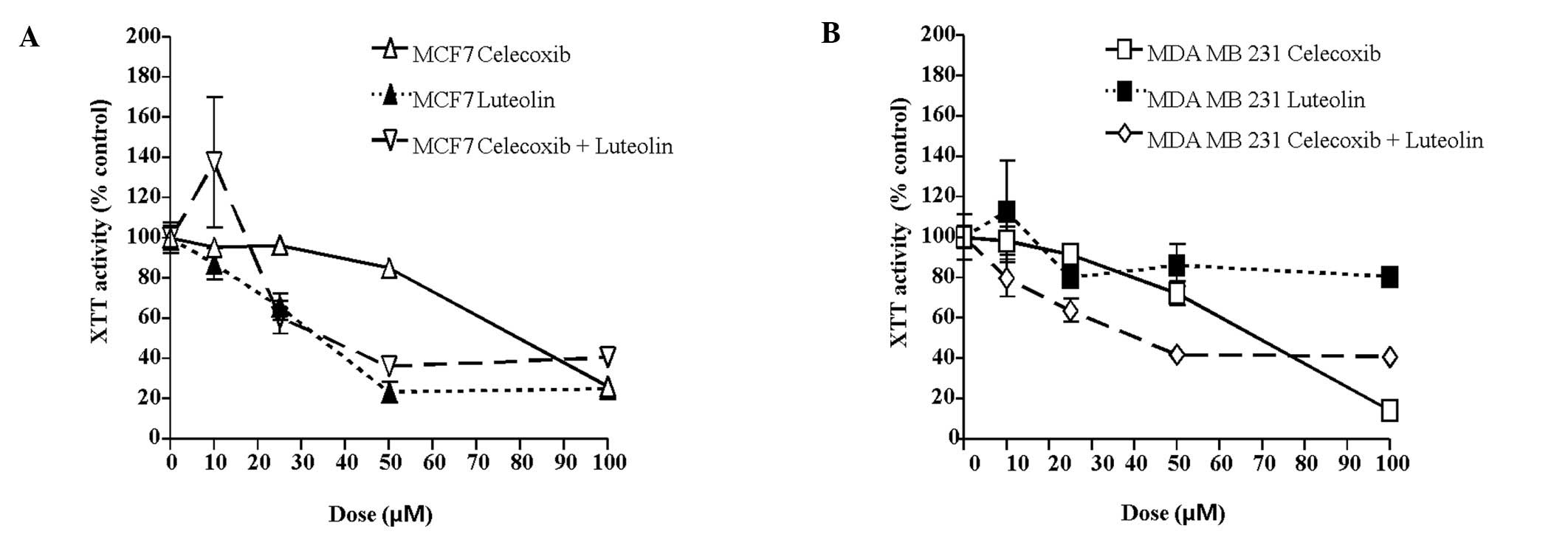
with celecoxib and/or luteolin at various concentrations (0, 10,
25, 50, 75, 100 μM). As shown in Fig.
1A based on the XTT assay results, the viability of the MCF-7
cells was inhibited by treatment with either celecoxib or luteolin
after 72 h of treatment in a concentration-dependent manner
(celecoxib: P=0.03, luteolin: P=0.04). At a concentration of 50 μM,
celecoxib decreased viability to 25.9% compared to controls, and
100 μM celecoxib reduced viability to 73.8% compared to controls.
At a concentration of 25 μM, luteolin decreased viability to 34.2%
compared to controls, and at 50 μM luteolin reduced viability to
76.3% compared to controls. MDA-MB-231 cells were treated with
celecoxib and/or luteolin at various concentration (0, 10, 25, 50,
75, 100 μM). MDA-MB-231 cell viability was inhibited by 72 h of
celecoxib treatment in a concentration-dependent manner (Fig. 1B, P=0.01). However, luteolin alone
did not inhibit MDA-MB-231 cell viability (P=0.78). At a
concentration of 50 μM, celecoxib decreased cell viability to 27.7%
compared to controls, and at 100 μM, it reduced cell viability
81.6% compared to controls. At a concentration of 25 μM, luteolin
decreased cell viability to 20.0% compared to controls, and at 50
μM, it reduced cell viability to 20.8% compared to controls.
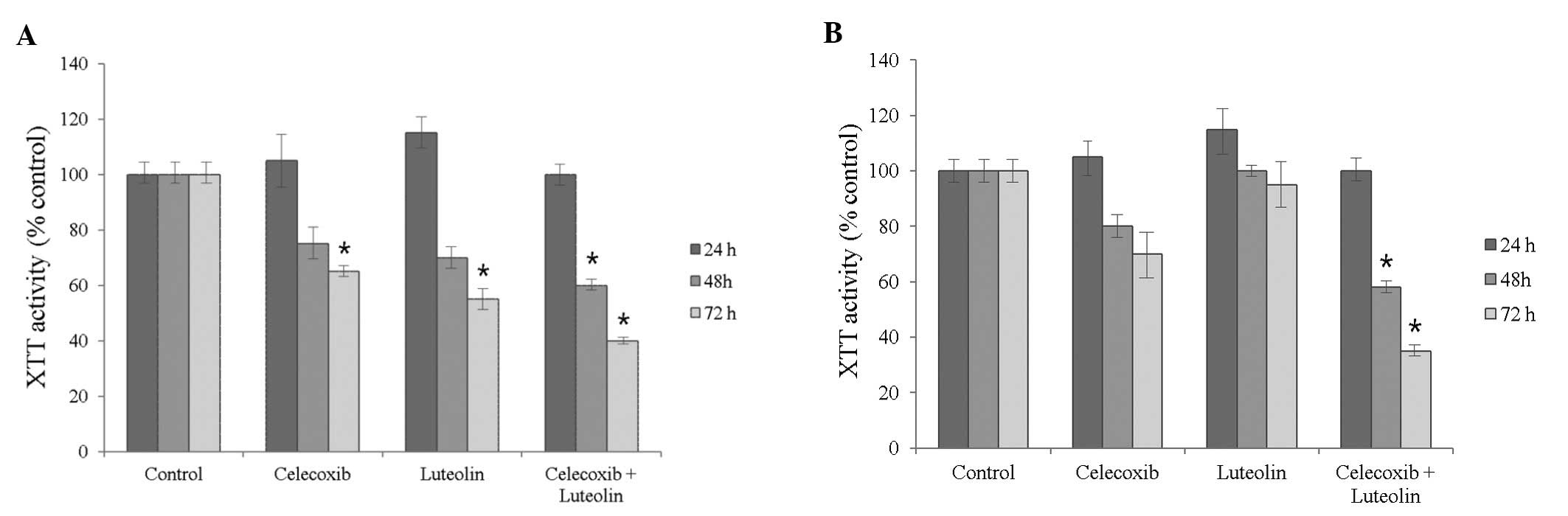
The XTT assays detected no differences in MCF-7 cell
viability following treatment with either celecoxib (75 μM) or
luteolin (50 μM) after 24 h, but did show decreased viability after
48 h and 72 h (Fig. 2, P=0.04 and
0.02, respectively) MDA-MB-231 cell viability showed no differences
following treatment for 24 h, but celecoxib treatment resulted in a
decrease in viability after 72 h of treatment, compared to 48 h
(P=0.04). Luteolin treatment did not inhibit the viability of
MDA-MB-231 cells time-dependently (P=0.13).
As shown in Figs. 1
and 2, the celecoxib and luteolin
combination treatment significantly decreased cell viability and
demonstrated greater efficiency in inducing tumor cell death after
72 h of treatment, compared to treatment with either alone or the
controls in both cell lines (P=0.01). This was observed in a
concentration- and time-dependent manner.
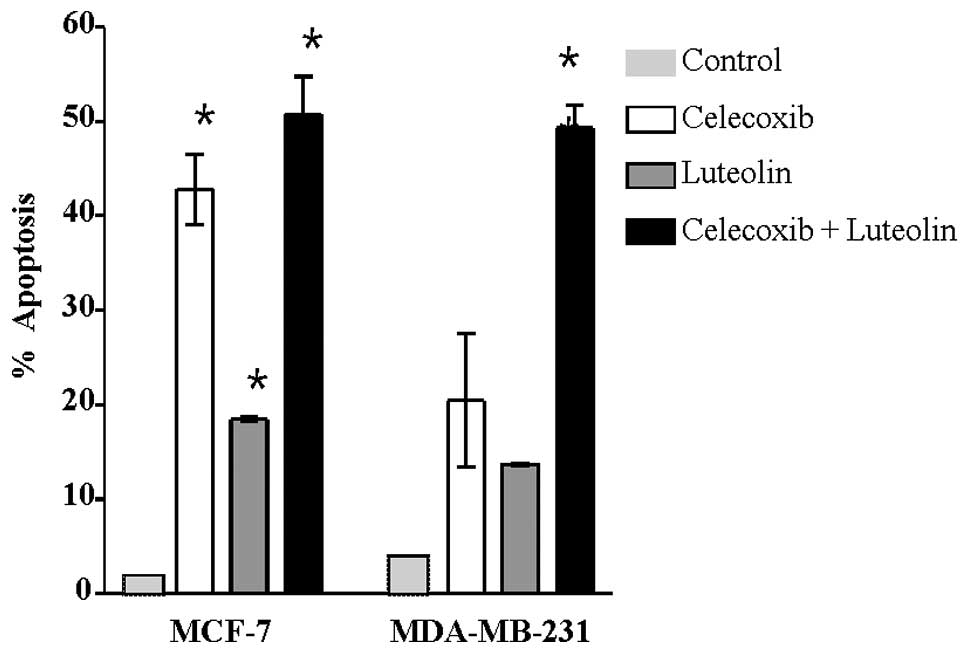
Analysis of apoptosis
The induction of apoptosis was evaluated using TUNEL
assays and Annexin V- FITC/PI staining. The TUNEL assays
demonstrated that the combination treatment resulted in significant
increase in apoptosis induction compared with treatment with a
single drug or controls (Fig. 3).
In the MCF-7 cells, apoptosis was significantly different from
controls after combination treatment (P=0.03). Similarly, in the
MDA-MB-231 cells, the induction of apoptosis was significantly
higher after combination treatment compared to treatment with a
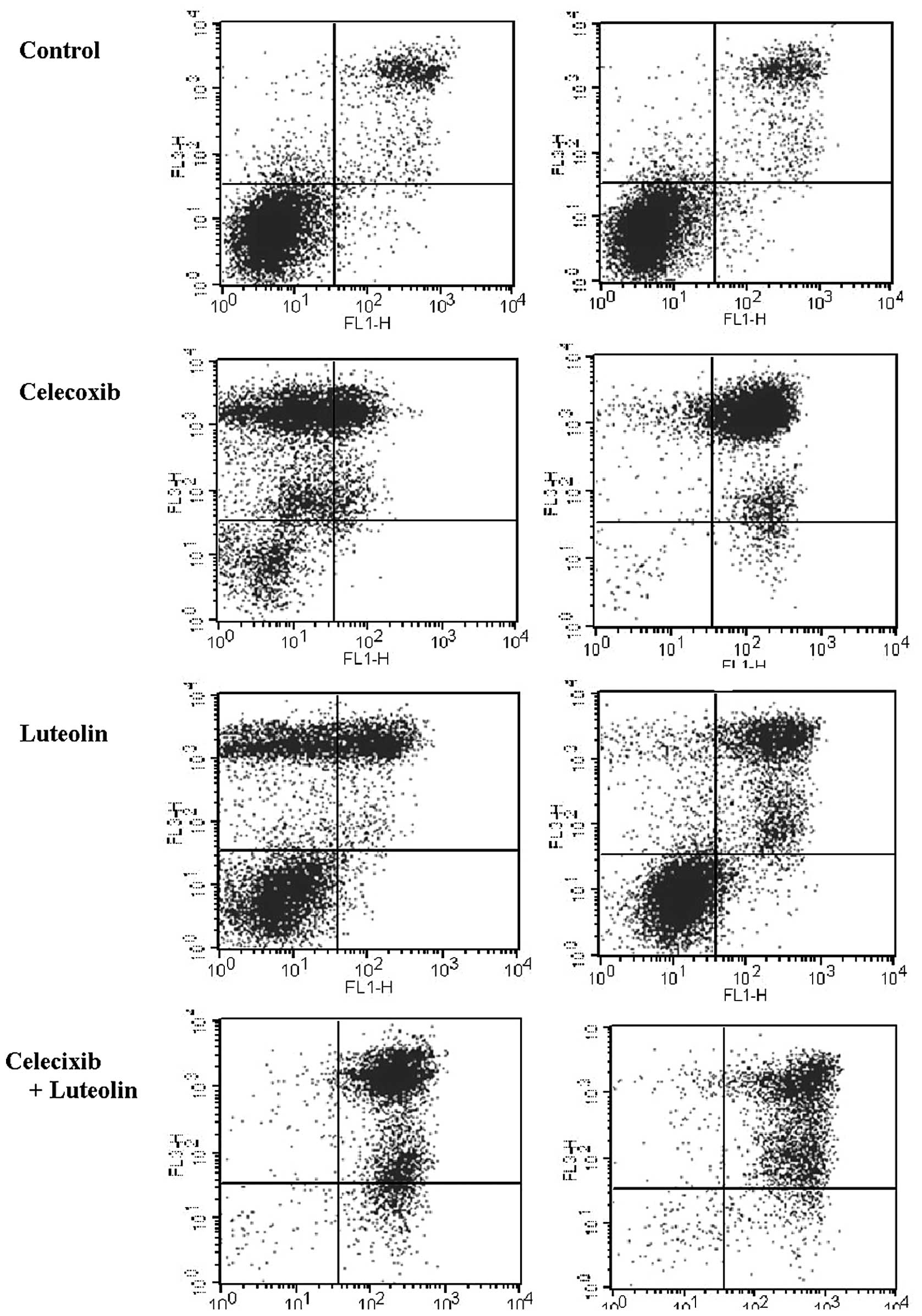
single drug or controls (P<0.01). As shown in the Annexin
V-FITC/PI staining (Fig. 4), the
increase in the proportion of cells undergoing early and late
apoptosis/necrosis was significantly higher following celecoxib and
luteolin combination treatment in both cell lines (P=0.01). The
quantification of the Annexin V-FITC/PI staining is presented in
Table I.
 | Table IQuantification of the analysis of
Annexin V-FITC apoptosis. |
Table I
Quantification of the analysis of
Annexin V-FITC apoptosis.
| Lt. bottom Quadrant
viable cells | Rt. bottom quadrant
Early apoptotic cells | Rt. top quadrant Late
apoptotic cells |
|---|
| MCF-7 |
| Control | 64.27 | 1.09 | 20.51 |
| Celecoxib | 13.75 | 0.69 | 22.53 |
| Luteolin | 50.23 | 1.15 | 21.52 |
| Celecoxib +
Luteolin | 1.14 | 9.50 | 87.56 |
| MDA-MB-231 |
| Control | 83.56 | 1.33 | 11.52 |
| Celecoxib | 4.67 | 1.96 | 88.04 |
| Luteolin | 59.91 | 3.67 | 31.11 |
| Celecoxib +
Luteolin | 2.45 | 6.23 | 88.49 |
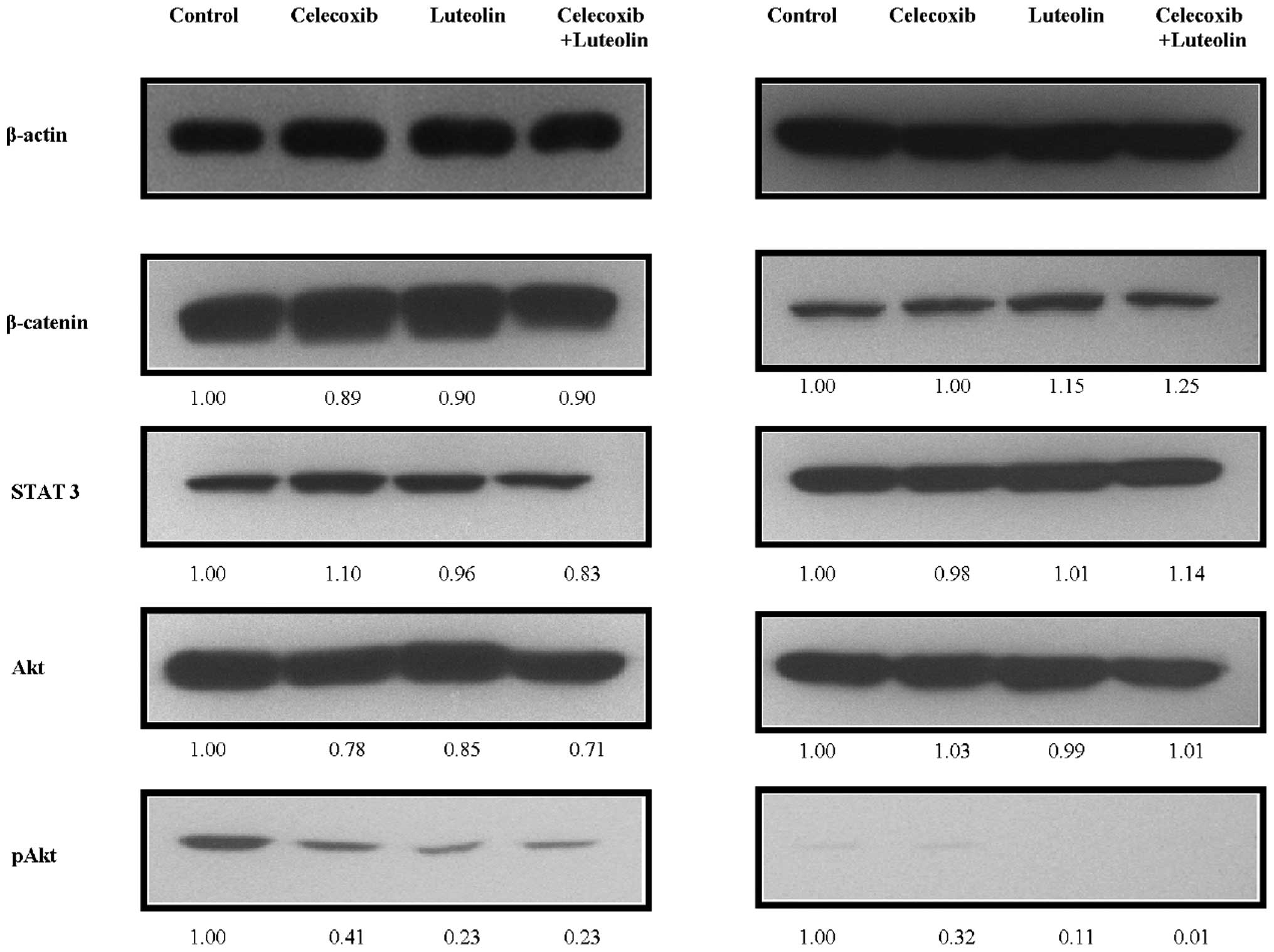
Celecoxib and luteolin combination
decreases expression of pAkt
To determine the effect of celecoxib and/or luteolin
treatment on the expression of representative pro-survival markers,
western blots were performed to analyze the steady-state levels of
β-catenin, STAT3, Akt and pAkt (Fig.
5). Decreased levels of Akt phosphorylation (pAkt) were noted
after combination treatment compared with β-catenin, STAT3, and Akt
(P<0.05). pAkt was significantly decreased in the MCF-7 and
MDA-MB-231 cells following combination treatment (P=0.02).
Discussion
The effect of celecoxib and luteolin combination
treatment for breast cancer has not been reported. However, in the
present study, celecoxib and luteolin combination treatment of
breast cancer cells resulted in a synergistic effect on the cell
death in a concentration- and time-dependent manner when compared
to treatment with either drug alone. Celecoxib was developed as a
selective COX-2 inhibitor for the treatment of chronic pain in
arthritis. However, considering the regulatory role of various
antitumor signaling pathways, inhibition of COX-2 by celecoxib can
induce proapoptotic effects. Suh et al reported that
celecoxib significantly decreased MCF-7/HER2-18 and MDA-MB-436
breast tumor growth in vitro(15). Basu et al reported that
celecoxib treatment altered the expression of genes associated with
angiogenesis, proliferation, apoptosis, and the cell cycle
(16). In vitro results were
corroborated in vivo in tumor-bearing mice treated with
celecoxib. In the present study, we have shown that celecoxib
treatment decreases cell viability and increases cell death after
72 h of treatment in both ER-positive and -negative human breast
cancer cells (P<0.05). Celecoxib inhibited tumor growth by
increasing apoptosis. In the MCF-7 and MDA-MB-231 cell lines, the
percentage of cells undergoing apoptosis increased by 45% and 26%
from the control, respectively.
Luteolin induces several beneficial effects,
including acting as an anti-inflammatory and anticancer agent.
Chiang et al reported that luteolin significantly suppresses
the growth of MCF-7 and MDA-MB-435 breast tumor in
vitro(17). Shinh et al
reported that luteolin significantly decreased MCF-7 and MDA-MB-231
breast tumor growth in vitro(18). We have shown that the viability of
MCF-7 cells was inhibited by luteolin after 72 h of treatment in a
time-dependent (P=0.02) and concentration-dependent manner
(P=0.04). In the MCF-7 cell line, the percentage of cells
undergoing apoptosis increased by 20% from the control to the
treated cells. However, in the MDA-MB-231 cell line, luteolin
treatment did not inhibit cell viability concentration-dependently
(P=0.78) or time-dependently (P=0.13).
In the MCF-7 and MDA-MB-231 cells, the celecoxib and
luteolin combination treatment significantly decreased cell
viability and was more efficient in killing tumor cells after 72 h
of treatment, compared to treatment with only one drug or the
control (P=0.01). The percentage of cells undergoing apoptosis
increased by 52 and 50% from the control in the MCF-7 and
MDA-MB-231 cells, respectively. The antitumor effect of the
celecoxib and luteolin combination treatment was more significant
in the MDA-MB-231 cells compared to treatment with either drug
alone (celecoxib: 20% and luteolin: no effect, and this effect was
more significant than in the MCF-7 cells (P<0.05 vs.
P<0.01).
The pro-oncogenic mechanism of the COX-2 is not
fully understood. Ghosh et al showed that prostaglandin
E2 (PGE2) was the major downstream effector
of COX-2 (19). Activation of the
epidermal growth factor receptor (EGFR) via PGE2 could
result in activation of Ras and the mitogen-activated protein
kinase (MAPK) pathway (20,21). Protein kinase B (Akt/PKB) activity
is implicated in K-Ras-induced expression of COX-2, and the
stabilization of COX-2 mRNA partly depends upon the activation of
Akt/PKB (22). For example,
PGE2 inhibits apoptosis by stimulating the PI3kinase/Akt
pathway. Furthermore, celecoxib was able to bind to and inhibit
3-phosphoinositide-dependent protein kinase-1 (PDK1). PDK1 is an
essential component of cell growth and survival signaling pathways
involving PI3K, upstream of PDK1, and Akt/PKB, downstream of PDK1
(11,23). Since Akt/PKB has a regulatory role
of in pro-oncogenic pathways, the discovery of its inhibition by
celecoxib provides a suitable explanation for the drug’s COX-2
anticancer effects.
As an anticancer agent, luteolin inhibits
proliferation of SCC-9 human oral squamous carcinoma cells, blocks
DMBA-induced DNA adduct formation and cytotoxicity in MCF-7 cells,
and inhibits CYP1A1 and CYP1B1 EROD activity (24–26).
Furthermore, luteolin inhibits the proliferation of several cancer
cell line via the inhibition of the protein tyrosine kinase
activity and autophosphorylation of EGFR, transphosphorylation of
the EGFR downstream effecter protein enolase and activation of
MAPK/ERK (27). Luteolin is able to
inhibit IGF-1-induced activation of IGF-1R and Akt, and
phosphorylation of Akt (28).
To determine whether celecoxib and luteolin alter
prosurvial signaling in breast cancer cells, we used western
blotting to examine Akt phosphorylation. Decreased levels of pAkt
were noted after treatment with either drug (P<0.05). Akt
phosphorylation was further reduced by combination treatment in the
MCF-7 and MDA-MB-231 cells (P=0.02). We demonstrated that enhanced
antitumor activity by celecoxib and luteolin combination treatment
could result in decreased levels of Akt phosphorylation.
Therapeutic selectivity is important in anticancer
treatments. The use of celecoxib has drawn much attention since the
review of cardiac safety compared to another COX-2 inhibitor
(29). Horinaka et al and
Chen et al showed that luteolin induces marginal
cytotoxicity in normal cells (30,31).
These results imply that luteolin is relatively safe when used as
an anticancer agent. The anticancer properties of luteolin have
been tested in conjunction with other anticancer drugs. Luteolin
was observed to increase drug-induced cytotoxicity in a variety of
cancer cells (14). These results
support the idea that a celecoxib and luteolin combination
treatment will provide a better therapeutic strategy to treat
breast cancer than treatment with celecoxib alone.
Our study has several limitations. This study
examines breast cancer cell lines in vitro. Additional
studies are required to demonstrate the efficacy of celecoxib and
luteolin combination treatment in vivo. Even though, the
effect of celecoxib and luteolin combination treatment was
demonstrated in both ER-positive and -negative human breast cancer
cells, breast cancer is a heterogeneous disease, therefore HER2
receptor expression is important for determining definite breast
cancer treatment. This can be answered quite soon with our
collateral studies. In spite of these limitations, our study
demonstrated that a celecoxib and luteolin combination treatment is
effective in killing breast cancer cells. To our knowledge, this is
first study of a celecoxib and luteolin combination treatment in
breast cancer.
In conclusion, we demonstrated that a combination of
celecoxib and luteolin could provide superior inhibition of breast
cancer cell growth than either celecoxib or luteolin treatment
alone. Additional studies, in human breast cancer cells that
overexpress HER2 and in vivo xenograft models, are needed to
demonstrate the effect of celecoxib and luteolin combination in
human breast cancer. These results suggest that celecoxib and
luteolin combination treatment might be a new treatment modality
that could improve prevention of breast cancer and decrease
recurrence after early breast cancer treatment. Celecoxib and
luteolin in combination with other anticancer drugs may improve the
therapeutic value of the combined agents by allowing the use of
lower, sub-toxic doses to achieve a more effective cancer
treatment.
Acknowledgements
The study was funded by Catholic University of Korea
St. Vincent’s Hospital.
References
|
1
|
Glass AG, Carreon JD, Lacey JV Jr and
Hoover RN: Breast cancer incidence, 1980–2006: combined roles of
menopausal hormone therapy, screening mammography, and estrogen
receptor status. J Natl Cancer Inst. 99:1152–1161. 2007.
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
3
|
Ring A and Dowsett M: Mechanisms of
tamoxifen resistance. Endocr Relat Cancer. 11:643–658. 2004.
View Article : Google Scholar
|
|
4
|
Konecny G, Pauletti G, Pegram M, et al:
Quantitative association between HER-2/neu and steroid hormone
receptors in hormone receptor-positive primary breast cancer. J
Natl Cancer Inst. 95:142–153. 2003. View Article : Google Scholar
|
|
5
|
Slamon DJ, Godolphin W, Jones LA, et al:
Studies of the HER-2/neu proto-oncogene in human breast and ovarian
cancer. Science. 244:707–712. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nielsen TO, Hsu FD, Jensen K, et al:
Immunohistochemical and clinical characterization of the basal-like
subtype of invasive breast carcinoma. Clin Cancer Res.
10:5367–5374. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peto R, Boreham J, Clarke M, Davies C and
Beral V: UK and USA breast cancer deaths down 25% in year 2000 at
ages 20–69 years. Lancet. 355:18222000.PubMed/NCBI
|
|
8
|
Boland GP, Butt IS, Prasad R, Knox WF and
Bundred NJ: COX-2 expression is associated with an aggressive
phenotype in ductal carcinoma in situ. Br J Cancer. 90:423–429.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Crawford YG, Gauthier ML, Joubel A, Mantei
K, Kozakiewicz K, Afshari CA and Tlsty TD: Histologically normal
human mammary epithelia with silenced p16(INK4a) overexpress COX-2,
promoting a premalignant program. Cancer Cell. 5:263–273. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Basu GD, Pathangey LB, Tinder TL, Lagioia
M, Gendler SJ and Mukherjee P: Cyclooxygenase-2 inhibitor induces
apoptosis in breast cancer cells in an in vivo model of spontaneous
metastatic breast cancer. Mol Cancer Res. 2:632–642.
2004.PubMed/NCBI
|
|
11
|
Kulp SK, Yang YT, Hung CC, et al:
3-phosphoinositide-dependent protein kinase-1/Akt signaling
represents a major cyclooxygenase-2-independent target for
celecoxib in prostate cancer cells. Cancer Res. 64:1444–1451. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brasky TM, Bonner MR, Moysich KB, et al:
Non-steroidal anti-inflammatory drug (NSAID) use and breast cancer
risk in the Western New York Exposures and Breast Cancer (WEB)
Study. Cancer Causes Control. 21:1503–1512. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Birt DF, Hendrich S and Wang W: Dietary
agents in cancer prevention: flavonoids and isoflavonoids.
Pharmacol Ther. 90:157–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin Y, Shen HM, Shi R and Wang X:
Luteolin, a flavonoid with potential for cancer prevention and
therapy. Curr Cancer Drug Targets. 8:634–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suh YJ, Chada S, McKenzie T, Liu Y,
Swisher SG, Lucci A and Hunt KK: Synergistic tumoricidal effect
between celecoxib and adenoviral-mediated delivery of mda-7 in
human breast cancer cells. Surgery. 138:422–430. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Basu GD, Liang WS, Stephan DA, Wegener LT,
Conley CR, Pockaj BA and Mukherjee P: A novel role for
cyclooxygenase-2 in regulating vascular channel formation by human
breast cancer cells. Breast Cancer Res. 8:R692006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chiang CT, Way TD and Lin JK: Sensitizing
HER2-overexpressing cancer cells to luteolin-induced apoptosis
through suppressing p21(WAF1/CIP1) expression with rapamycin. Mol
Cancer Ther. 6:2127–2138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shih YL, Liu HC, Chen CS, et al:
Combination treatment with luteolin and quercetin enhances
antiproliferative effects in nicotine-treated MDA-MB-231 cells by
down-regulating nicotinic acetylcholine receptors. J Agric Food
Chem. 58:235–241. 2010. View Article : Google Scholar
|
|
19
|
Ghosh N, Chaki R, Mandal V and Mandal SC:
COX-2 as a target for cancer chemotherapy. Pharmacol Rep.
62:233–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sheng H, Shao J, Dixon DA, Williams CS,
Prescott SM, DuBois RN and Beauchamp RD: Transforming growth factor
beta1 enhances Ha-ras-induced expression of cyclooxygenase-2 in
intestinal epithelial cells via stabilization of mRNA. J Biol Chem.
275:6628–6635. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sheng H, Williams CS, Shao J, Liang P,
DuBois RN and Beauchamp RD: Induction of cyclooxygenase-2 by
activated Ha-ras oncogene in Rat-1 fibroblasts and the role of
mitogen-activated protein kinase pathway. J Biol Chem.
273:22120–22127. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sheng H, Shao J and Dubois RN:
K-Ras-mediated increase in cyclooxygenase 2 mRNA stability involves
activation of the protein kinase B1. Cancer Res. 61:2670–2675.
2001.PubMed/NCBI
|
|
23
|
Arico S, Pattingre S, Bauvy C, Gane P,
Barbat A, Codogno P and Ogier-Denis E: Celecoxib induces apoptosis
by inhibiting 3-phosphoinositide-dependent protein kinase-1
activity in the human colon cancer HT-29 cell line. J Biol Chem.
277:27613–27621. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Browning AM, Walle UK and Walle T:
Flavonoid glycosides inhibit oral cancer cell proliferation - role
of cellular uptake and hydrolysis to the aglycones. J Pharm
Pharmacol. 57:1037–1042. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ciolino HP, Wang TT and Yeh GC: Diosmin
and diosmetin are agonists of the aryl hydrocarbon receptor that
differentially affect cytochrome P450 1A1 activity. Cancer Res.
58:2754–2760. 1998.PubMed/NCBI
|
|
26
|
Doostdar H, Burke MD and Mayer RT:
Bioflavonoids: selective substrates and inhibitors for cytochrome
P450 CYP1A and CYP1B1. Toxicology. 144:31–38. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee LT, Huang YT, Hwang JJ, et al:
Blockade of the epidermal growth factor receptor tyrosine kinase
activity by quercetin and luteolin leads to growth inhibition and
apoptosis of pancreatic tumor cells. Anticancer Res. 22:1615–1627.
2002.PubMed/NCBI
|
|
28
|
Fang J, Zhou Q, Shi XL and Jiang BH:
Luteolin inhibits insulin-like growth factor 1 receptor signaling
in prostate cancer cells. Carcinogenesis. 28:713–723. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Solomon SD, McMurray JJ, Pfeffer MA, et
al: Cardiovascular risk associated with Celecoxib in a clinical
trial for Colorectal Adenoma Prevention. N Eng J Med.
352:1071–1080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Horinaka M, Yoshida T, Shiraishi T, et al:
Luteolin induces apoptosis via death receptor 5 upregulation in
human malignant tumor cells. Oncogene. 24:7180–7189. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen D, Chen MS, Cui QC, Yang H and Dou
QP: Structure-proteasome-inhibitory activity relationships of
dietary flavonoids in human cancer cells. Front Biosci.
12:1935–1945. 2007. View
Article : Google Scholar : PubMed/NCBI
|