Introduction
Diffuse large B-cell lymphomas (DLBCL) essentially
encompass a heterogeneous group of B-cell lymphomas which typically
have an aggressive clinical course. For many years CHOP
(cyclophosphamide, adriamycin, vincristine and prednisone) was used
as the standard treatment for DLBCL patients, however by adding
rituximab (CD20 antibody) to this regime, prognosis has improved
significantly (1).
The most commonly used prognostic tool in DLBCL is
the International Prognostic Index (IPI), which takes into account
clinical parameters such as age, clinical stage and performance
status. Nonetheless, many studies have identified certain molecular
markers of prognostic relevance, such as the classification of
DLBCL as germinal center B-like (GC) or activated B cell-like (ABC)
groups using expression profiling techniques (2,3). In
addition, many individual biomarkers have been identified and some
are prognostic even in the rituximab era (4).
Recently, microRNA (miRNA) expression has been shown
to be important in understanding the biology and clinical course of
DLBCL, as well as other tumors. miRNAs are endogenous small
noncoding RNAs, approximately 22 nucleotides in length (5). In mammals, miRNAs mediate
translational suppression by binding to the 3′UTR of messenger-RNAs
(6). miRNAs have been shown to be
involved in many cellular processes such as differentiation and
apoptosis, as well as tumorigenesis (7,8).
Furthermore, miRNAs have been reported to predict outcome in many
different tumor types (9–14).
One miRNA of interest is miR-200c, which is
activated by p53. This activation of miR-200c is accomplished by
p53-binding to the miR-200c promoter (15). When miR-200c is upregulated it has
been found to affect three different pathways.
The first pathway involves the inhibition of ZEB1
and ZEB2, transcriptional repressors of E-cadherin which is an
epithelial marker (16,17). This inhibition of the repressors
leads to higher expression of E-cadherin and probably contributes
to less cancer progression and metastasis by hampering the tumor
and epithelial-to-mesenchymal transition (EMTs), which has been
shown to occur in metastatic cancer cells (16,17).
It has also been shown in DLBCL that GC-subtype patients, who often
have longer survival compared to ABC-subtype patients, express
lower amounts of ZEB1 compared to the ABC-subtype (18). Of note, this low expression of ZEB1
in the GC-subtype was also correlated with high expression of
BCL-6, a zinc finger transcription factor that is highly expressed
in normal germinal centre B-cells (18). It has also been shown that patients
with high expression of BCL-6 have longer survival than patients
with relatively low expression of BCL-6 in DLBCL (19).
miR-200c is also involved in the regulatory pathway
of BMI1; when miR-200c is upregulated it leads to a downregulation
of BMI1, which is a protein that maintains cell stemness properties
(20,21). By negating the cell stemness
properties through induction of high miR-200c expression, it has
been showed that clonal expansion of breast cancer cells is
inhibited, while the growth of embryonal carcinoma cells in
vitro is suppressed by high expression of miR-200c (20).
However, while both of these studies purport that
high expression of miR-200c would be of benefit for cancer patients
in terms of survival, recently, evidence of a third alternative
pathway has been found which shows that breast cancer patients with
high expression of miR-200s actually have a worse outcome compared
to patients with low expression (22). Korpal et al showed that high
levels of miR-200s inhibit Sec23a and the secretory pathway which
leads to a downregulation of metastasis-suppressive proteins such
as insulin-like growth factor binding protein 4 (Igfbp4) and
tubulointerstital nephritis antigen-like 1 (Tinagl 1). These
findings indicate a possible mechanism by which a higher
mesenchymal-to-epithelial transition (MET) rate occurs, a feature
typically found in metastasis (22).
Moreover, it has also been shown that high
expression of miR-200c induces chemoresistance in esophageal cancer
leading to a shorter survival for those patients expressing high
levels of miR-200c (23). This
chemoresistance is probably caused by miR-200c-induced
downregulation of PPP2R1B expression, a subunit of protein
phosphatase 2A, which results in activation of Akt signalling,
known to be involved in chemoresistance in various cancers
(23–26). Noteworthy, it has been shown that
DLBCL patients with high p-AKT expression displayed a poorer
survival compared to those with low p-AKT expression (26).
In the present study, we investigated the expression
levels of miR-200c in 61 de novo DLBCL cases by reverse
transcriptase-PCR. The miR-200c expression levels were compared to
clinical features, including overall survival and GC subtype. We
also compared the expression of miR-200c in tumor tissue, to that
in surrounding tissue and normal controls.
Materials and methods
Diagnostic lymph nodes from 61 patients with de
novo DLBCL were included in the study. The clinical
characteristics for the patients for whom a Pfaffl-value was
obtained are presented in Table I.
We also used 13 normal lymph nodes as controls. All DLBCL biopsies
were evaluated by a pathologist, and the areas with the most
typical lymphoma tissue were marked. After the evaluation, the
areas with typical lymphoma tissue and the ‘surrounding tissue’,
with a more normal morphology, were microdissected with a scalpel
into two parts: ‘tumor tissue’ and ‘surrounding tissue’.
 | Table IClinical features of the patients with
a known miR-200c value. |
Table I
Clinical features of the patients with
a known miR-200c value.
| Low miR-200c (n) | High miR-200c
(n) | P-value |
|---|
| Total patients | 34 | 27 | |
| Age, years |
| Mean | 63 | 69 | 0.1a |
| Median | 64.5 | 69 | |
| Range | 17–90 | 37–88 | |
| Gender | | | 0.59b |
| Females | 10 | 10 | |
| Males | 24 | 17 | |
| Stage | | | 0.43b |
| I–II | 15 | 10 | |
| III–IV | 15 | 16 | |
| NA | 4 | 1 | |
| aaIPI | | | 0.41b |
| 0–1 | 20 | 14 | |
| 2–3 | 10 | 12 | |
| NA | 4 | 1 | |
| CHOP-like
treatment | | | 1b |
| Yes | 24 | 18 | |
| No | 3 | 3 | |
| NA | 7 | 6 | |
| Rituximab | | | 0.06b |
| Yes | 17 | 8 | |
| No | 12 | 17 | |
| NA | 5 | 2 | |
| GC/non-GC | | | 0.43b |
| GC | 12 | 13 | |
| Non-GC | 19 | 13 | |
| NA | 3 | 1 | |
Total miRNA was extracted from the dissected tissue
using Recoverall Total Nucleic Acid Isolation (Ambion, Austin, TX,
USA) according to the standard protocol. For the reverse
transcriptase reaction, 2 μl or ~5 ng of RNA was used, and the
amplification was carried out in a Gene Amp 9700 PCR machine
(Applied Biosystems, CA, USA) according to the manufacturers
protocol.
Primers for specific reverse transcription of
miR-200c (Applied Biosystems) were used to create cDNA. RNU6b, a
housekeeping gene, was used as a reference. In the quantification
of miRNA, all sample reactions were performed in triplicate and all
amplifications were carried out in a TaqMan Real-Time PCR machine
(Applied Biosystems), as described by the manufacturer.
The relative expression of miR-200c compared to
RNU6b was calculated using a modified Pfaffl method due to the fact
that no controls were available from each corresponding DLBCL
(27). Our modified Pfaffl method
was as follows: log2 [(Efficiencies of RNU6bCycle number of
RNU6b)/(Efficiencies of miR-200cCycle number of
miR-200c)].
The patients were assigned to GC or non-GC
categories based on immunohistochemistry according to the algorithm
presented by Hans et al(28). The clinical information was obtained
from patient records. The study was approved by the Ethics
Committee of Uppsala University.
Statistical methods
To calculate whether the relative expression of
miR-200c was of any prognostic importance, the values of relative
expression were divided in to two groups according to the mean and
median values. The overall survival rates of the groups of DLBCL
patients with either low or high relative expression of miR-200c
were compared by Kaplan-Meier survival analysis and log-rank test
to investigate the prognostic importance of miR-200c expression.
Overall survival was calculated from the date of diagnosis until
the last follow-up or death.
To evaluate whether there were any differences in
the relative expression of miR-200c in the tumor tissues, the
surrounding tissue and the normal controls, we compared the three
groups pairwise using a t-test. To determine the differences in
(mean) age at diagnosis between groups with high versus low
expression of miR-200c, an unpaired t-test was performed. A
Fisher’s exact test was used to examine for differences in
proportions between the same groups regarding different clinical
parameters (Table I).
All statistical analyses were performed using
Statistica 10.0 (StatSoft, Tusla, OK, USA) and the R statistical
program (http://www.r-project.org/).
Probabilities of <0.05 were judged as an indication of
statistical significance.
Results
Twenty-seven (44%) patients had high relative
expression (above the mean; mean Pfaffl-value of −6.248818) and 34
(56%) patients had low relative expression of miR-200c (below the
mean).
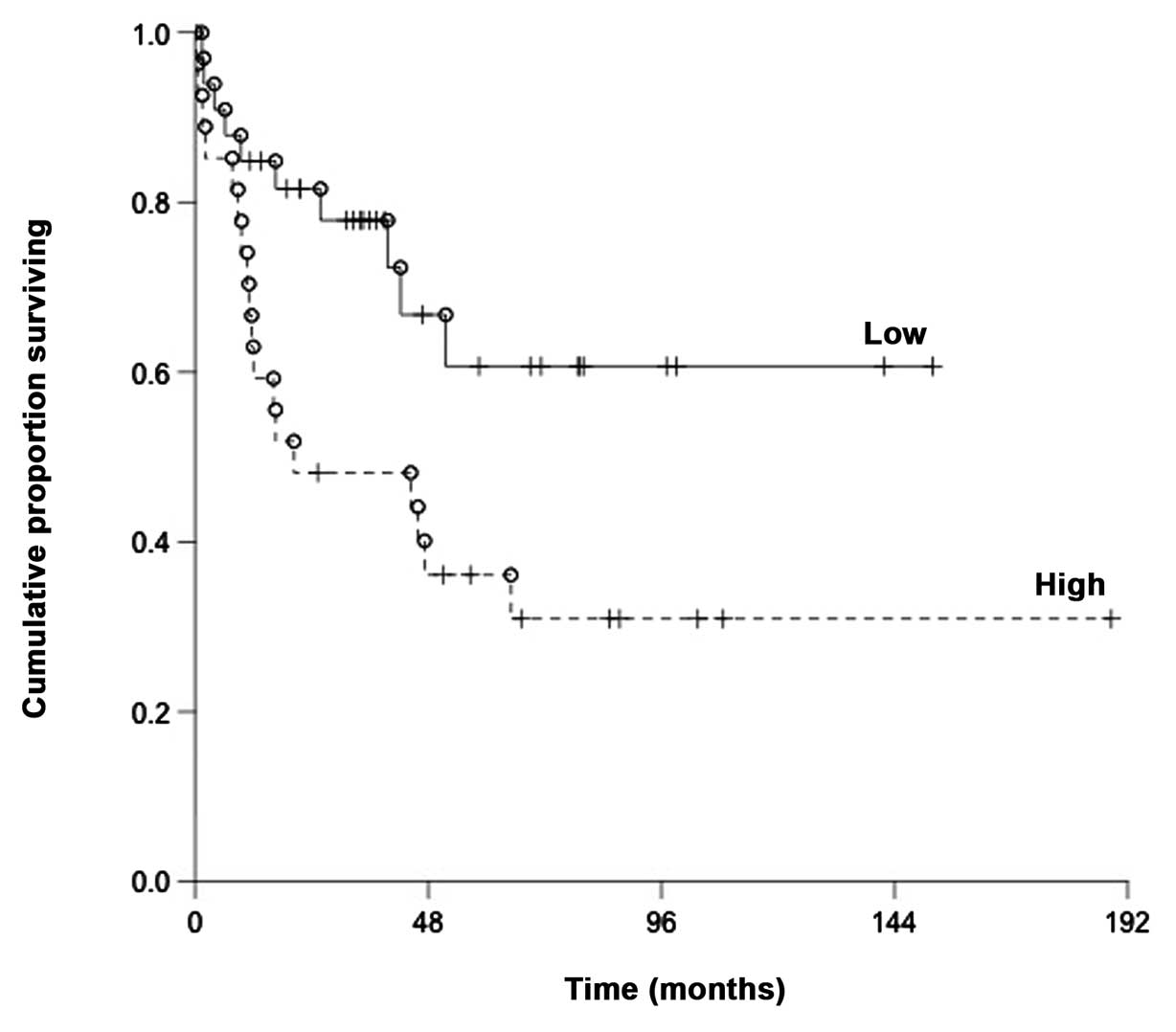
The DLBCL patients with high relative expression had
a significantly shorter overall survival as compared to those with
a low expression; with a median of 20.3 months and 35.8 months,
respectively (P=0.019) (Fig.
1).
The mean age at diagnosis was 69 years for patients
with high expression of miR-200c and 63 years for patients with low
expression of miR-200c. The median age at diagnosis was 68 years
for patients with high expression of miR-200c and 63 years for
patients with low expression. No statistically significant
differences were found between the groups regarding age at
diagnosis.
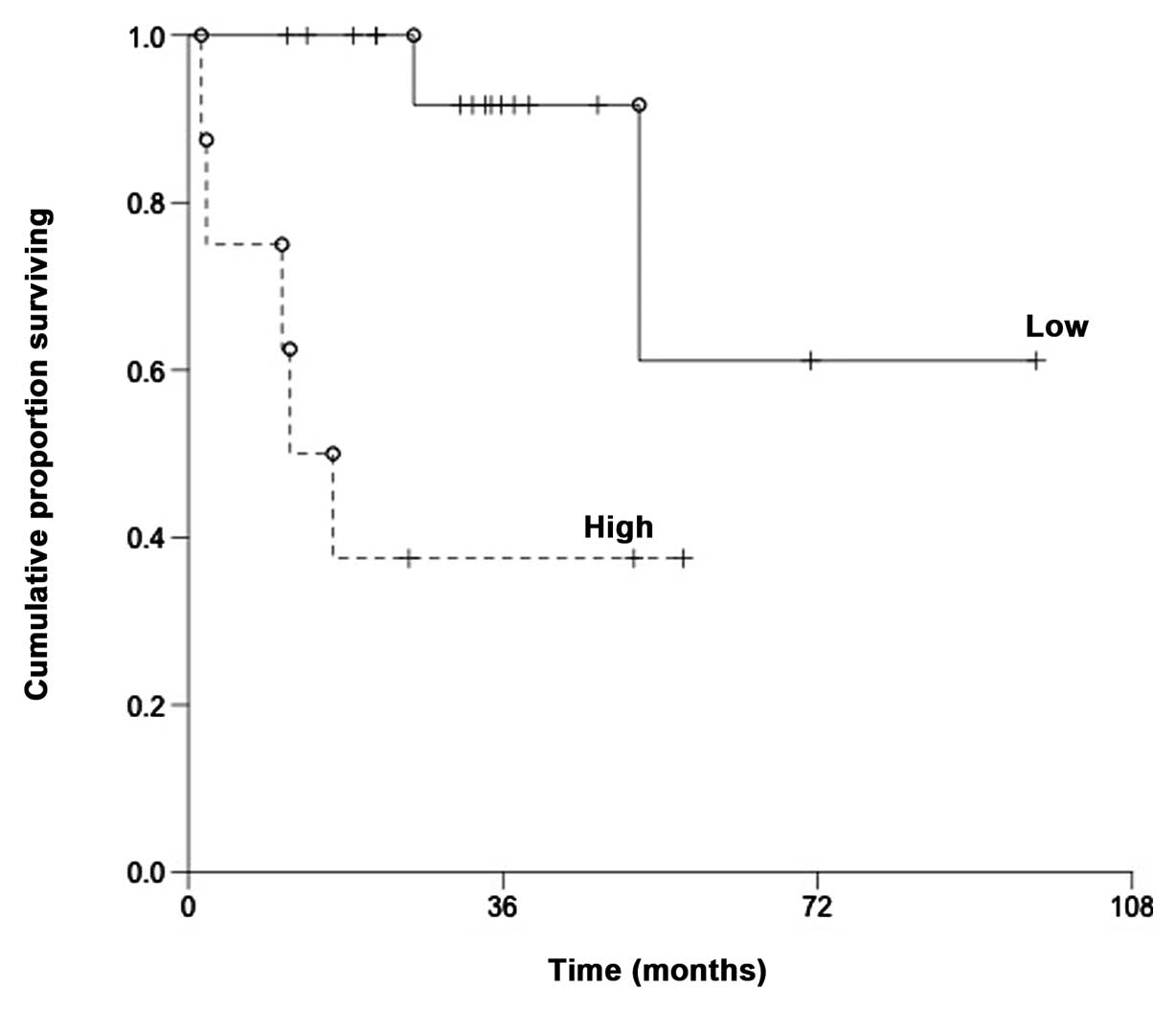
For patients treated with R-CHOP (n=25), there was a
statistically significant difference (P=0.0036) in overall survival
between patients with high relative expression of miR-200c (above
the mean) and low relative expression of miR-200c (below the mean)
(Fig. 2).
Patients with high relative expression of miR-200c
had a 50% survival at 20.3 months whereas 15 patients with low
relative expression of miR-200c were still alive at the last
follow-up date. For patients not treated with R-CHOP (n=29), no
difference in overall survival was found (P=0.35).
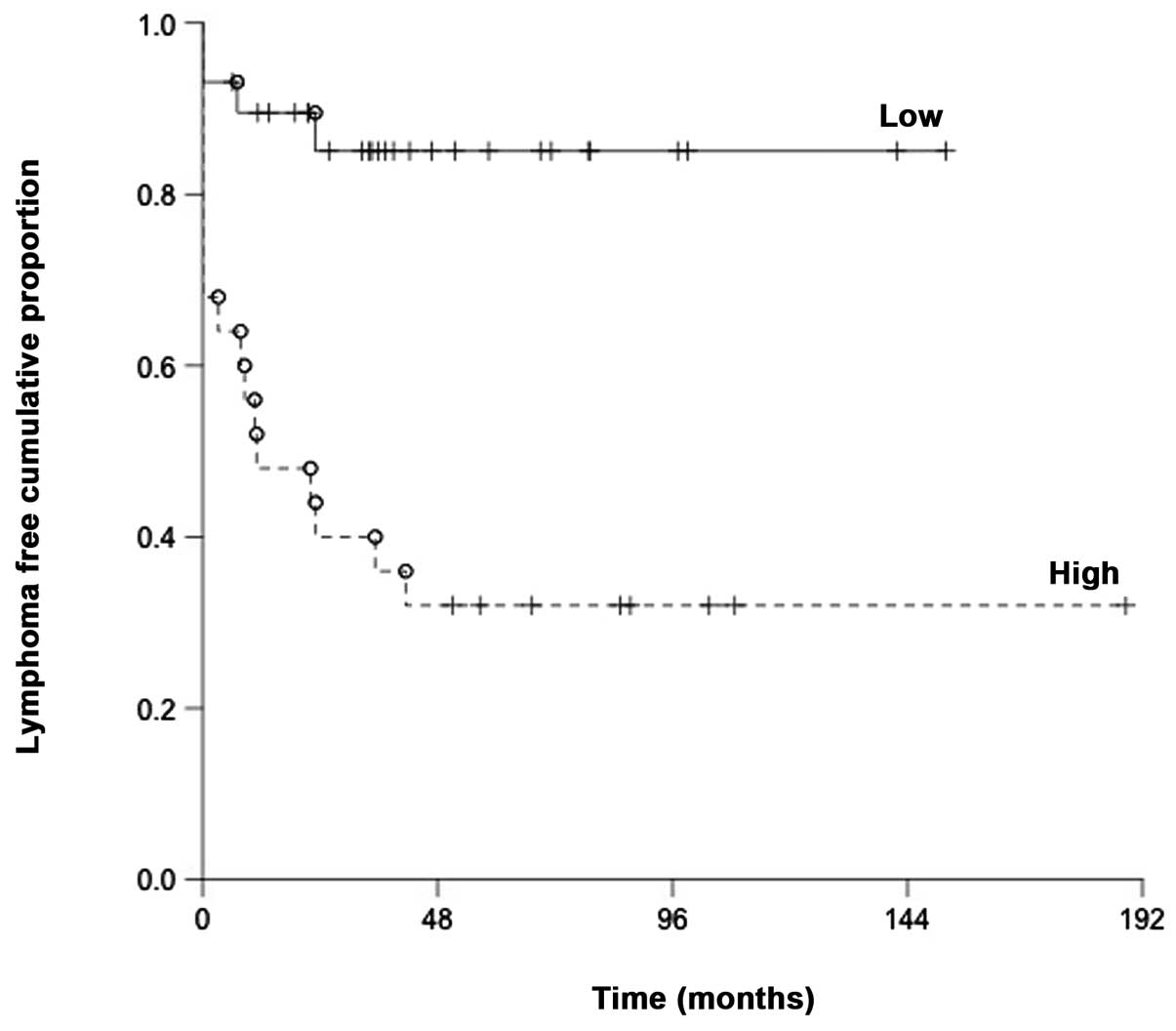
When measuring the time from initial diagnosis to
the first relapse we found a clear difference where early relapsing
patients expressed high miR-200c to a greater extent than patients
with low miR-200c expression (P=0.0001) (Fig. 3).
There were no differences between the high or low
miR-200c expression groups in terms of age, IPI and stage, either
when the groups were determined by mean (Table I) or median value of relative
miR-200c expression. There was no statistically significant
difference in survival between the genders (P=0.59) in either the
GC or the non-GC subtype (P=0.43).
When the tumor tissue, surrounding tissue and normal
lymph nodes were compared in terms of miR-200c expression, no
significant differences were observed between them (normal lymph
nodes: mean Pfaffl-value of −6.244954, surrounding tissue: mean
Pfaffl-value of −6.30193 and tumor tissue: mean Pfaffl-value of
−6.248818).
Discussion
miR-200c has not been well studied in lymphomas;
however, it has been shown that the GC-subtype of DLBCL expresses
low amounts of the protein ZEB1 (18). Of note, a low expression of ZEB1
correlates with a high expression of miR-200c and taking into
account the fact that GC-subtype patients have a proven longer
survival compared to the ABC-subtype, it is tempting to hypothesize
that high expression of miR-200c leads to a significantly better
survival (16–19).
However, our results instead indicated the opposite
trend, where patients with high expression of miR-200c showed a
significantly worse outcome compared to patients with low
expression of miR-200c (P=0.019). This finding was rather
remarkable and somewhat unexpected. This correlation could, of
course, be explained by the relatively low sample size in our
study, but it would be rather unfortunate if our results are in
total disagreement with the true biological impact. We did not find
any connection between the expression of miR-200c and the
GC-subtype or non-GC-subtype (P=0.37) indicating that the
expression of miR-200c is probably independent of DLBCL subtype.
This finding was also rather unexpected considering that high
expression of miR-200c leads to low expression of ZEB1 and low
expression of ZEB1 has been reported for the GC-subtype (16–19).
However, considering that our data indicate that
high miR-200c expression correlates with a significantly poorer
outcome for DLBCL patients, it is tempting to speculate that the
development of DLBCL and the biology of the tumor is to some extent
explained by Korpal et al findings. They reported that
breast cancer patients with high expression of miR-200s actually
had a worse outcome compared to patients with low expression of
miR-200s, which is in line with our results showing a worse outcome
for DLBCL patients with high levels of miR-200c (22). According to Korpal et al,
high levels of miR-200s inhibits Sec23a and the secretory pathway
which lead to a downregulation of certain metastasis-suppressive
proteins such as insulin-like growth factor binding protein 4
(Igfbp4) and tubulointerstitial nephritis antigen-like 1 (Tinagl 1)
(22). This pathway can possibly
lead to a higher mesenchymal-to-epithelial transition (MET), which
has been shown to promote the development of metastasis (22).
Another plausible explanation for the correlation
between high miR-200c expression and poor prognosis in DLBCL is
that high miR-200c expression induces chemoresistance, which has
been noted in esophageal cancer (23). Even though the chemotherapy for
esophageal cancer and DLBCL is not the same, the finding that
miR-200c activates Akt signaling in esophageal cancer fits well
with the finding that DLBCL patients with high p-AKT expression
have a poorer survival compared to patients with low p-AKT
expression (23,26). This explanation for miR-200c-induced
chemoresistance can perhaps also be supported by our findings that
DLBCL patients treated with R-CHOP and high miR-200c expression had
a significantly shorter survival compared to patients treated with
R-CHOP and low miR-200c expression (P=0.0036) and also that the
time to relapse was significantly shorter for patients with high
miR-200c (P=0.0001).
In summary, we showed that DLBCL patients with high
expression of miR-200c have a significantly worse prognosis
compared to DLBCL patients with low expression of miR-200c which
can be seen in terms of overall survival particularly for DLBCL
patients treated with R-CHOP. However, it is necessary to explore
the biology behind this finding and further investigate the impact
of chemoresistance in DLBCL to confirm our results.
References
|
1
|
Habermann TM, Weller EA, Morrison VA, et
al: Rituximab-CHOP versus CHOP alone or with maintenance rituximab
in older patients with diffuse large B-cell lymphoma. J Clin Oncol.
24:3121–3127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alizadeh AA, Eisen MB, Davis RE, et al:
Distinct types of diffuse large B-cell lymphoma identified by gene
expression profiling. Nature. 403:503–511. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rosenwald A, Wright G, Chan WC, et al: The
use of molecular profiling to predict survival after chemotherapy
for diffuse large-B-cell lymphoma. N Engl J Med. 346:1937–1947.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ninan MJ, Wadhwa PD and Gupta P:
Prognostication of diffuse large B-cell lymphoma in the rituximab
era. Leuk Lymphoma. 52:360–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim VN, Han J and Siomi MC: Biogenesis of
small RNAs in animals. Nat Rev Mol Cell Biol. 10:126–139. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Valencia-Sanchez MA, Liu J, Hannon GJ and
Parker R: Control of translation and mRNA degradation by miRNAs and
siRNAs. Genes Dev. 20:515–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davis BN and Hata A: microRNA in cancer:
the involvement of aberrant microRNA biogenesis regulatory
pathways. Genes Cancer. 1:1100–1114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hobert O: Common logic of transcription
factor and microRNA action. Trends Biochem Sci. 29:462–468. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alencar AJ, Malumbres R, Kozloski GA, et
al: MicroRNAs are independent predictors of outcome in diffuse
large B-cell lymphoma patients treated with R-CHOP. Clin Cancer
Res. 17:4125–4135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calin GA, Ferracin M, Cimmino A, et al: A
microRNA signature associated with prognosis and progression in
chronic lymphocytic leukemia. N Engl J Med. 353:1793–1801. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marcucci G, Radmacher MD, Maharry K, et
al: MicroRNA expression in cytogenetically normal acute myeloid
leukemia. N Engl J Med. 358:1919–1928. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Montes-Moreno S, Martinez N,
Sanchez-Espiridion B, et al: miRNA expression in diffuse large
B-cell lymphoma treated with chemoimmunotherapy. Blood.
118:1034–1040. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwind S, Maharry K, Radmacher MD, et al:
Prognostic significance of expression of a single microRNA,
miR-181a, in cytogenetically normal acute myeloid leukemia: a
Cancer and Leukemia Group B study. J Clin Oncol. 28:5257–5264.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yanaihara N, Caplen N, Bowman E, et al:
Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang CJ, Chao CH, Xia W, et al: p53
regulates epithelial-mesenchymal transition and stem cell
properties through modulating miRNAs. Nat Cell Biol. 13:317–323.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Korpal M, Lee ES, Hu G and Kang Y: The
miR-200 family inhibits epithelial-mesenchymal transition and
cancer cell migration by direct targeting of E-cadherin
transcriptional repressors ZEB1 and ZEB2. J Biol Chem.
283:14910–14914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Papadopoulou V, Postigo A, Sanchez-Tillo
E, Porter AC and Wagner SD: ZEB1 and CtBP form a repressive complex
at a distal promoter element of the BCL6 locus. Biochem J.
427:541–550. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lossos IS, Jones CD, Warnke R, et al:
Expression of a single gene, BCL-6, strongly predicts survival in
patients with diffuse large B-cell lymphoma. Blood. 98:945–951.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimono Y, Zabala M, Cho RW, et al:
Downregulation of miRNA-200c links breast cancer stem cells with
normal stem cells. Cell. 138:592–603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wellner U, Schubert J, Burk UC, et al: The
EMT-activator ZEB1 promotes tumorigenicity by repressing
stemness-inhibiting microRNAs. Nat Cell Biol. 11:1487–1495. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Korpal M, Ell BJ, Buffa FM, et al: Direct
targeting of Sec23a by miR-200s influences cancer cell secretome
and promotes metastatic colonization. Nat Med. 17:1101–1108. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hamano R, Miyata H, Yamasaki M, et al:
Overexpression of miR-200c induces chemoresistance in esophageal
cancers mediated through activation of the Akt signaling pathway.
Clin Cancer Res. 17:3029–3038. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu LZ, Zhou XD, Qian G, Shi X, Fang J and
Jiang BH: AKT1 amplification regulates cisplatin resistance in
human lung cancer cells through the mammalian target of
rapamycin/p70S6K1 pathway. Cancer Res. 67:6325–6332. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thompson JE and Thompson CB: Putting the
rap on Akt. J Clin Oncol. 22:4217–4226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Uddin S, Hussain AR, Siraj AK, et al: Role
of phosphatidylinositol 3′-kinase/AKT pathway in diffuse large
B-cell lymphoma survival. Blood. 108:4178–4186. 2006.
|
|
27
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hans CP, Weisenburger DD, Greiner TC, et
al: Confirmation of the molecular classification of diffuse large
B-cell lymphoma by immunohistochemistry using a tissue microarray.
Blood. 103:275–282. 2004. View Article : Google Scholar : PubMed/NCBI
|