Introduction
Cisplatin is widely used for the treatment of many
types of malignancies, but it results in a number of toxic
side-effects, the most serious of which is nephrotoxicity (1). Cisplatin has dose-dependent toxic and
pro-apoptotic effects on kidney cells (2), and it is also associated with the
local release of inflammatory cytokines/chemokines and consequent
inflammatory cell infiltration (1,3–6). It
has been hypothesized that this inflammatory infiltration into the
damaged kidney tissues further aggravates the cisplatin-induced
nephrotoxic effects. Importantly, however, it was found that
neutrophil depletion had no impact on cisplatin-induced renal
function or tubular necrosis in a mouse model, suggesting that
neutrophil infiltration could be a downstream effect of
cisplatin-induced injury rather than a central driver of this
pathology (7).
The seven ELR-CXC chemokines together play important
roles in the recruitment of neutrophils in inflammatory responses,
and act through their specific receptors, CXCR1 and CXCR2 (8). CXCL8(3–72)K11R/G31P (G31P),
which has been generated as both bovine (9,10) and
human (11,12) CXCL8 analogues, is a full-spectrum
ELR-CXC chemokine antagonist that has high affinity for both CXCR1
and CXCR2 (9,12). It blocks ELR-CXC chemokine signal
transduction through both receptors, such that it has
broad-spectrum anti-inflammatory activity (13–15).
It has also been shown to desensitize a number of heterologous G
protein-coupled receptors (e.g., the receptors for C5a or fMLP) on
CXCR1- or CXCR2-expressing cells, suggesting that its
anti-inflammatory effects could be multi-modal (12). Moreover, given the roles of the
ELR-CXC chemokines as tumor growth and angiogenic factors (16), it makes sense that G31P also
possessed potent inhibitory effects on the growth, metastasis and
angiogenesis of malignant tumors in a mouse model of human prostate
cancer; indeed, it had anti-neoplastic effects comparable to those
of Taxol (17). The ELR-CXC
chemokines also foster the development of resistance to the
cytotoxic effects of chemotherapeutic agents (18,19),
such that antagonizing their activities might be predicted to
augment therapeutic outcomes with these agents. In the present
study, we assessed the impact of G31P and cisplatin co-treatment on
tumor progression and cisplatin-related renal failure in an H22
hepatoma cell model of cancer.
Materials and methods
Reagents
The following reagents were purchased: cisplatin
(Jiangsu Hansoh Pharmaceutical Co., Ltd., Jiangsu, China); RNAiso
Plus, RT reagent kit with gDNA Eraser, and SYBR Premix Ex Taq II
(Takara Bio Inc., Dalian, China); anti-VEGF-D (Bioworld Technology,
Inc); immunohistochemistry (IHC) kit (ZSGB-Bio., Beijing, China);
and blood urea nitrogen (BUN) and serum creatinine (SCr) kits
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Animal model and drug administration
All protocols followed the guidelines of ethics
regarding the treatment of animals used in experiments. This
project was approved by the Dalian Medical University Ethics
Committee in China. Female C57BL/6J mice (6 weeks old; n=5–6
mice/group) were obtained from the Dalian Medical University
Laboratory Animal Center and were randomly divided into 6 groups.
Tumor-bearing mice were treated with saline, 2 mg/kg of cisplatin,
12.5 mg/kg cisplatin with or without 500 μg/kg G31P or 500 μg/kg
G31P alone. A sixth group of healthy mice served as the normal
control animals. We had previously optimized the dose of human G31P
required for use in rodents (Gordon JR, unpublished data), as we
had in multiple other species.
Tumor-bearing mice received 5×106 H22
mouse hepatoma cells (0.2-ml volumes, s.c.). G31P treatment mice
were administered G31P s.c. on alternative days, commencing 7 days
after tumor implantation, while the remaining groups received
normal saline in the same manner. Cisplatin treatment mice were
administered either 12.5 mg/kg (high dose) or 2 mg/kg (low dose)
cisplatin i.p., while the remaining mice received an equal volume
of saline i.p. The clinical status and the body weight of each
mouse were evaluated daily, and on day 21 all mice were sacrificed
for assessment of tumor progression. The primary tumors were
resected from each animal and weighed.
Monitoring of renal function
Blood samples were collected from the angular vein
of the mice under pentobarbital sodium (50 μg/kg) at 4, 8, 12, 24,
48 and 72 h and at day 21 post-tumor cell implantation. Serum
samples were stored at −20°C for blood urea nitrogen (BUN) and
serum creatinine (SCr) detection. BUN and SCr values were
determined according to the supplier’s instructions, as standard
measures of renal function (2). In
addition, the renal coefficient (RC) was determined for each animal
as a surrogate measure of general kidney health (20). To do so, both kidneys were excised
and weighed and the RC was calculated using the following formula:
Kidney weight/body weight. The kidneys were then snap-frozen in
liquid nitrogen, and stored at −80°C prior to real-time PCR
analysis.
Real-time PCR analysis
RNA of renal tissues was isolated using RNAiso Plus
kits according to the supplier’s protocol. Total RNA was reverse
transcribed into cDNA using Takara RT reagent kit with gDNA Eraser.
Briefly, first the genomic DNA was eliminated from each sample by
incubation at 42°C for 5 min and then the reverse-transcription
reactions were performed. PCR parameters were set as 37°C for 15
min and 85°C for 5 sec. The transcriptional levels of IL-1β, KC and
MIP-2 were determined by quantitative real-time PCR (qRT-PCR)
analysis with Takara SYBR Premix Ex Taq II. Melting curve analyses
were used to verify the accuracy of the PCR products following the
amplification reactions. The PCR-primer sequences are indicated in
Table I. The PCR cycling parameters
(40 cycles) were set as: pre-denaturation (95°C for 30 sec);
denaturation (95°C for 5 sec); annealing (59°C for 30 sec); and
extension (72°C for 1 min). The relative expression values were
normalized to that of β-actin. The 2−ΔΔCT method was
used for analysis of the data.
 | Table IPrimer sequences and the length of
amplification products. |
Table I
Primer sequences and the length of
amplification products.
| Name | Primer sequences | Length of product
(bp) |
|---|
| IL-1β | Forward:
5′-tgccaccttttgacagtgatgag-3′ | |
| Reverse:
5′-tgatgtgctgctgcgagattt-3′ | 137 |
| KC | Forward:
5′-gattcacctcaagaacatccaga-3′ | |
| Reverse:
5′-ggacaccttttagcatcttttgg-3′ | 160 |
| MIP-2 | Forward:
5′-aacatccagagcttgagtgtgac-3′ | |
| Reverse:
5′-gccttgcctttgttcagtatctt-3′ | 152 |
| β-actin | Forward:
5′-agagggaaatcgtgcgtgac-3′ | |
| Reverse:
5′-caatagtgatgacctggccgt-3′ | 163 |
Immunohistochemical detection of
VEGF-D
Mice were sacrificed after 21 days of treatment and
each tumor was resected and weighed. Solid tumor samples were fixed
in 4% paraformaldehyde and embedded in paraffin using standard
procedures in preparation for immunohistochemical (IHC) detection
of VEGF-D. Briefly, endogenous peroxidase was blocked with 3%
H2O2 for 10 min at room temperature.
Afterward, the samples were rinsed three times with PBS (5 min
each), incubated for 20 min at room temperature with normal goat
serum as a blocking agent, washed as above with PBS and then
incubated overnight at 4°C with the primary antibody (1:100
dilution). The tissues were again washed with PBS (3 times for 2
min each), incubated for 1 h at 37°C with the secondary antibody,
washed again with PBS as above, incubated in diaminobenzidine (DAB)
solution for 10 min and counterstained with hematoxylin. The
staining results were assessed by image analysis using the
Microsoft Image-Pro 6.0.
Statistical analysis
Statistical analysis of the data and inter-group
comparisons were carried out using the Student’s t-test
(two-tailed). Probability values of <0.05 were considered to
indicate statistically significant results. The results are
expressed as means ± SEM.
Results
Combined treatment with an ELR-CXC
chemokine antagonist and cisplatin augments the tumor
growth-suppressive effects of chemotherapy
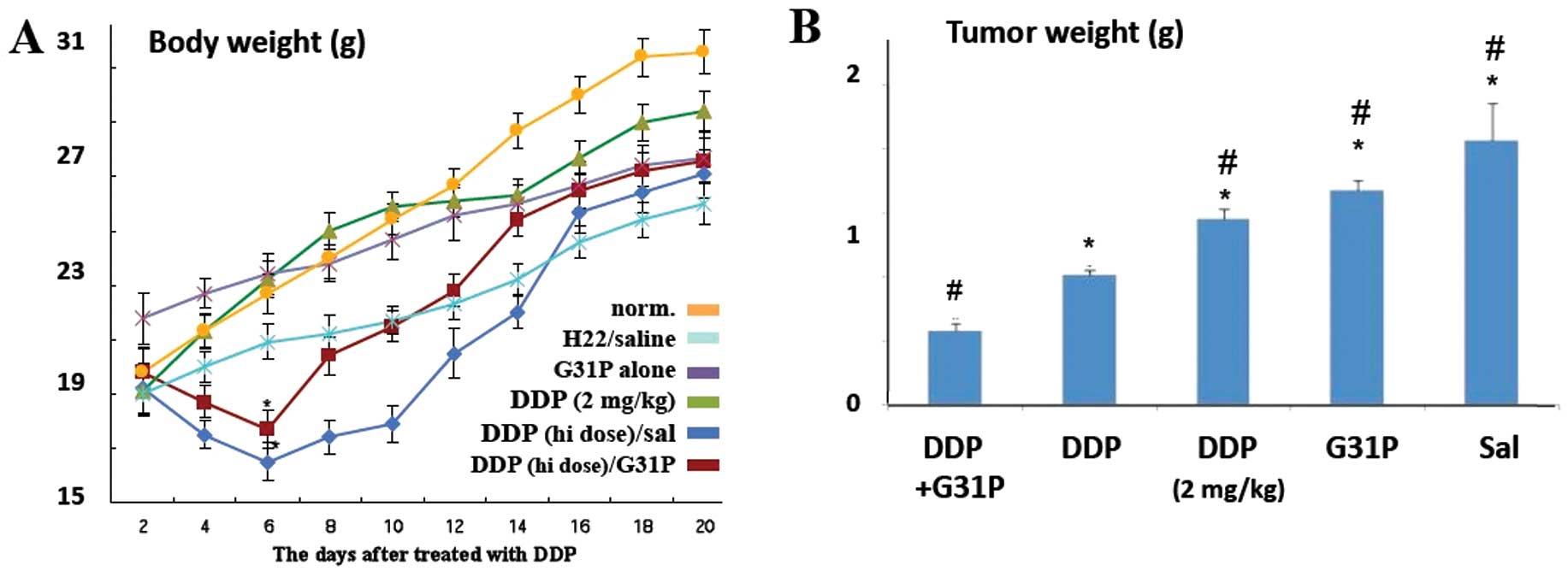
Upon examination, we found that, overall, the
tumor-bearing mice gained less weight than the healthy normal
control mice (Fig. 1A). All
cisplatin-treated animals displayed reduced activity,
hypotrichosis, lackluster attitude and anorexia during the first 72
h following drug treatment. The 12.5 mg/kg dose cisplatin treatment
led to substantial loss of body mass during the first 6 days
post-treatment, whether or not the animals were co-treated with
G31P, while the mice treated with G31P only or a 2 mg/kg dose of
cisplatin lost no weight during this period. Weight gain in the
high-dose cisplatin-treated tumor-bearing animals remained
suppressed for the first 2 weeks, while G31P co-treatment
ameliorated this effect somewhat. On day 21, all tumor-bearing mice
weighed significantly less than the healthy control mice (P≤0.05;
Fig. 1A).
On day 21 we also assessed the weight of the primary
tumor in each animal and found that G31P treatment alone reduced
the sizes of the primary tumors by 19%, while treatment with 2
mg/kg cisplatin reduced the tumor mass by 29%. High-dose cisplatin
treatment alone reduced the size of the tumors in our mice by 52%
(Fig. 1B; P≤0.05), and the addition
of G31P co-therapy to this cisplatin treatment further enhanced the
growth-suppressive effect observed, such that these tumors were 71%
smaller than those in the saline-treated tumor-bearing mice
(P≤0.05; Fig. 1B). The effect of
high-dose cisplatin and G31P co-treatment on the progression of the
primary tumors was additive.
Combined treatment of G31P and cisplatin
reduces lymph node tumor metastasis
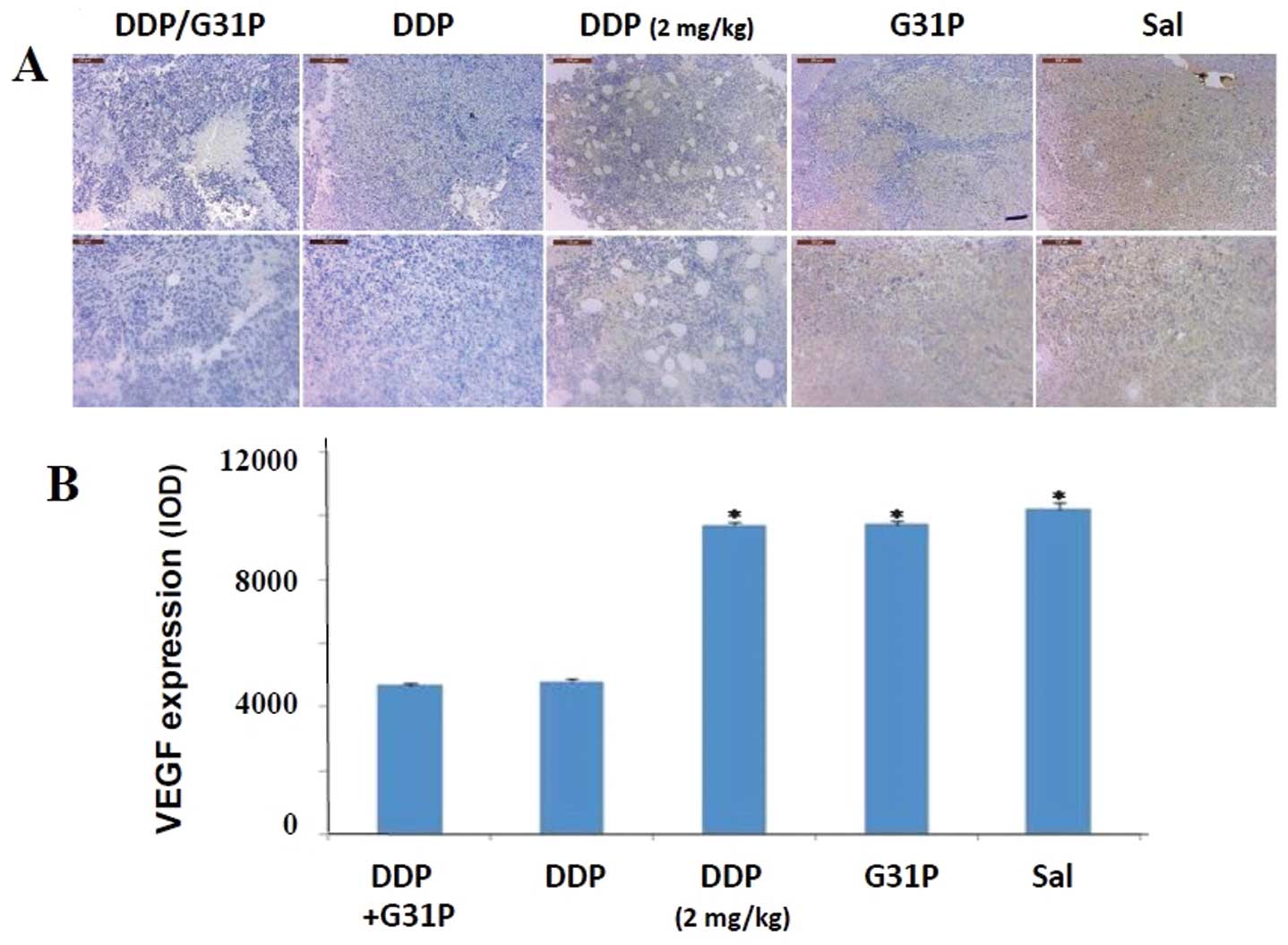
As VEGF-D expression is strongly linked to tumor
dissemination via metastasis (21,22),
we assessed expression of VEGF-D in our H22 hepatoma cell tumors
using immunohistochemical methods and image analysis (Fig. 2). We observed strong expression of
VEGF-D in the tumors of the saline-treated mice, and neither
treatment with the ELR-CXC chemokine antagonist G31P alone nor
low-dose cisplatin therapy had discernible effects on this
expression. In contrast, high-dose cisplatin therapy, either alone
or in combination with G31P co-therapy, reduced expression of
VEGF-D in the tumors by ~50% (Fig
2B). Given the associations between VEGF-D production and
metastatic disease, and the prognostic implications of extrahepatic
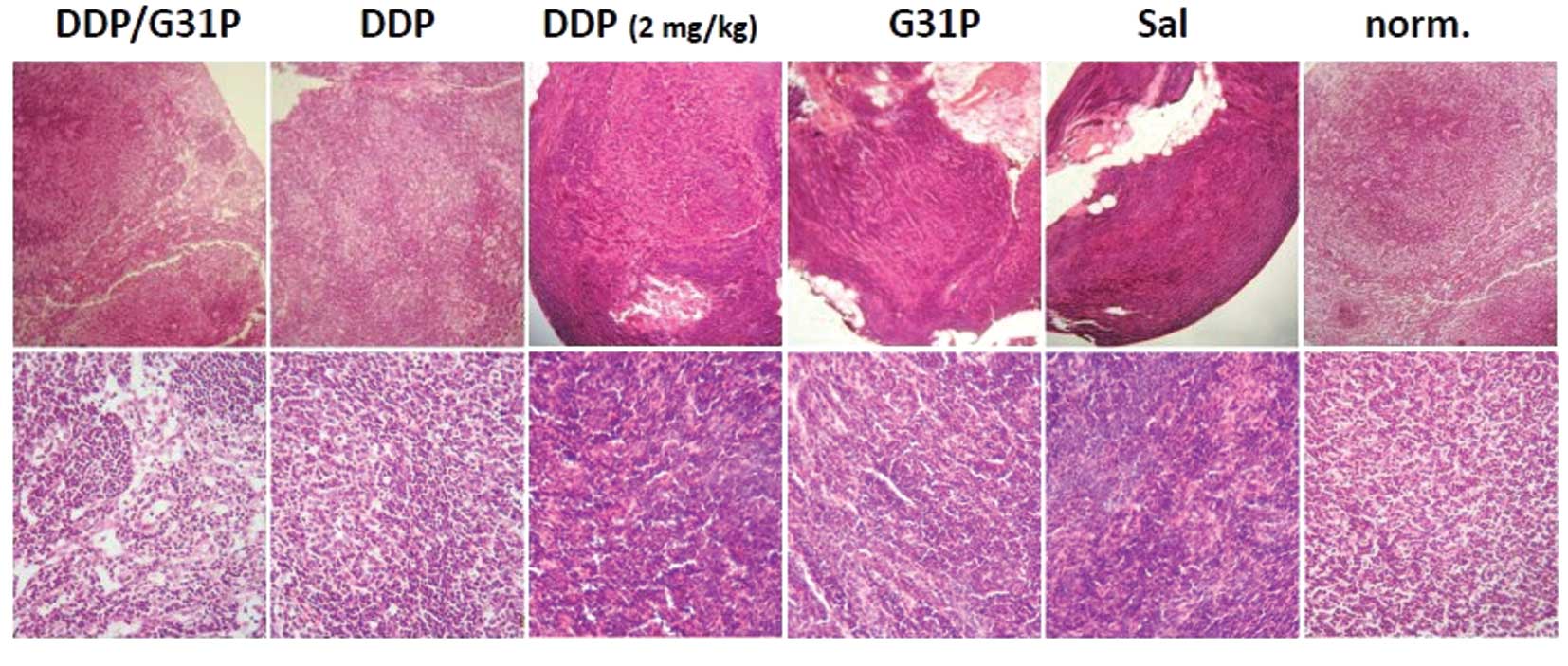
metastasis in hepatocellular carcinoma (23), we also assessed the impact of
ELR-CXC chemokine antagonism and cisplatin therapy on lymph node
metastasis in our model. We resected the inguinal lymph nodes of
our mice at the time of sacrifice and examined them directly for
evidence of metastases using H&E-stained tissue sections
(Fig. 3). We found abundant
metastases in the lymph nodes of the saline-treated H22 hepatoma
cell tumor-bearing mice, and evidence of metastases in the G31P
only and low-dose cisplatin-treated animals. Few tumors were noted
in the mice treated with high-dose cisplatin and particularly with
the combination of G31P/high-dose cisplatin (Fig. 3).
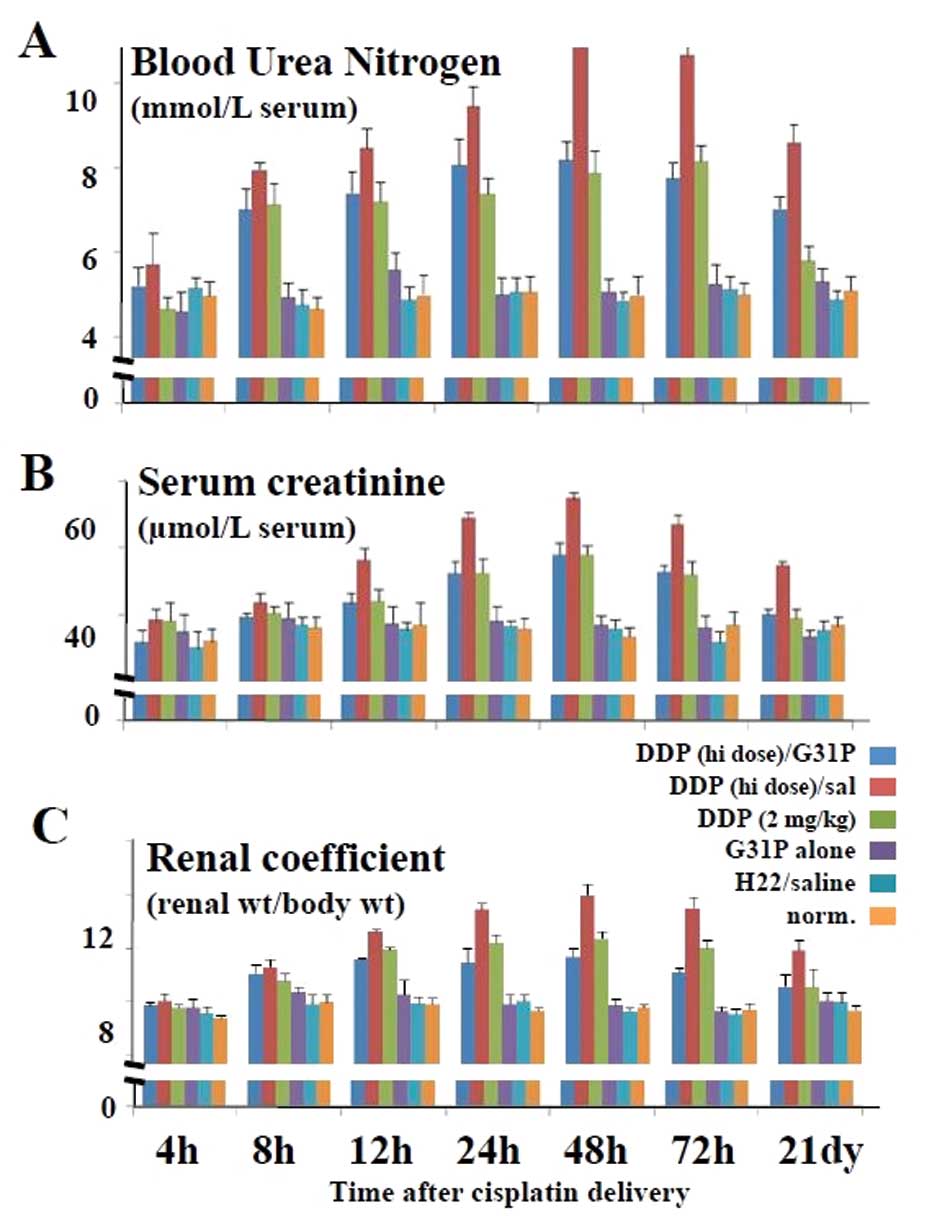
Impact of CXCR1/CXCR2 antagonism on renal
function in the cisplatin-treated hepatoma cell tumor-bearing
mice
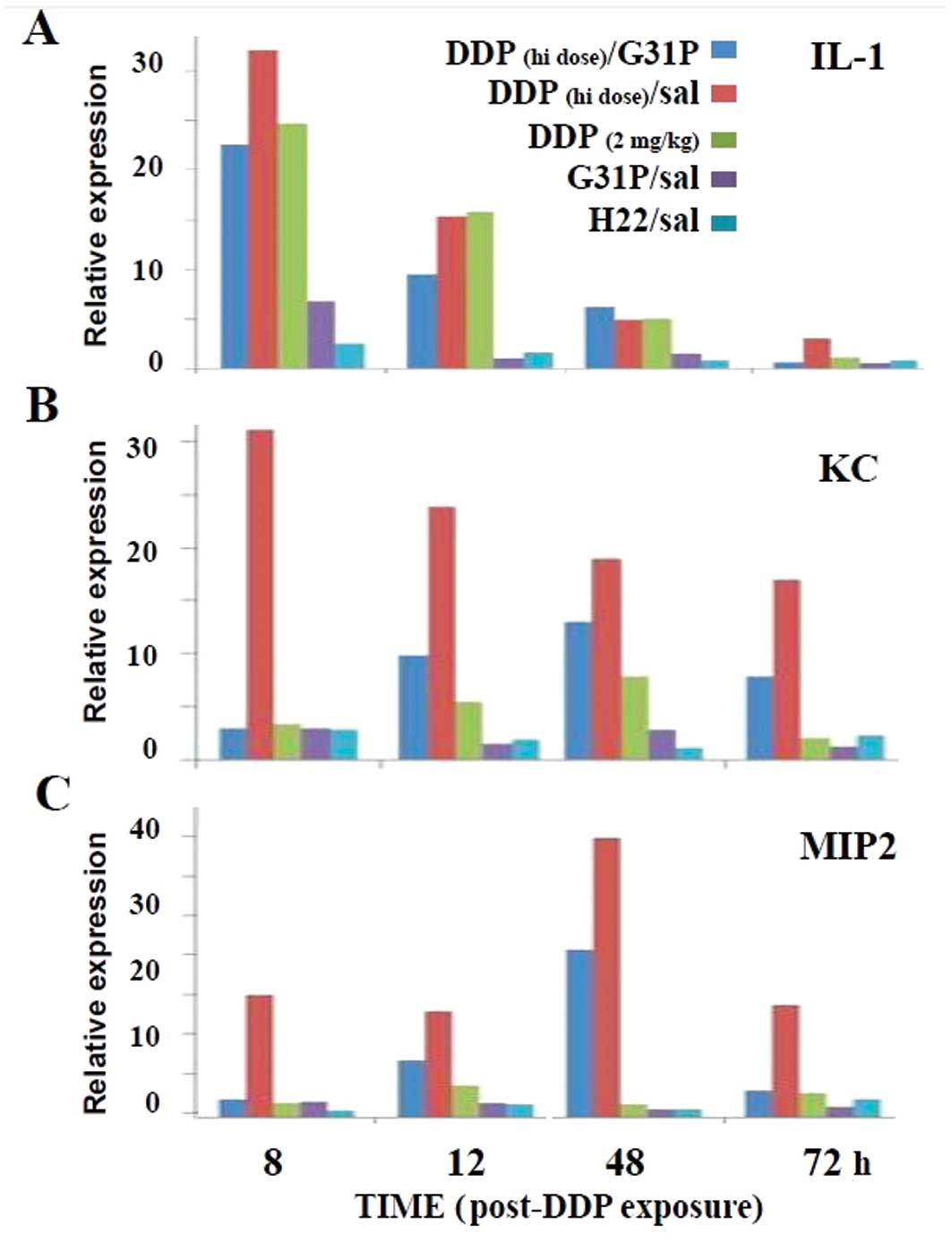
High-dose cisplatin therapy has been reported to
induce renal inflammation and toxicity (2). Therefore, we assessed the expression
of inflammatory mediators in our cisplatin and G31P-treated mice
(Fig. 4), as well as the renal
function, as determined by serum levels of blood urea nitrogen
(BUN) and serum creatinine (SCr) and the renal coefficients
(Fig. 5). We found that the saline-
and G31P-treated mice displayed slight renal IL-1, KC and MIP2
expression thoughout the 3 days of observation (Fig. 4). Notably, the high- and low-dose
cisplatin-treated mice expressed substantial levels of IL-1 during
the 48 h following drug infusion, and the G31P co-treatment only
modestly reduced this response. By day 3 the IL-1 response in the
mice treated with low-dose cisplatin and G31P/high-dose cisplatin
was again at background, while there remained a small IL-1 response
at this time in the high-dose cisplatin-treated animals (Fig. 4A). The low-dose cisplatin treatment
had a slight effect on inducing an ELR-CXC chemokine (i.e., KC or
MIP2) response, while the high-dose cisplatin therapy markedly
elevated the expression of both chemokines, albeit with distinct
kinetics (Fig. 4B and C). The G31P
co-treatment appeared to fully off-set the 8-h KC and MIP2 response
to cisplatin, but by 48 h these animals also expressed substantial
levels of both of these potent neutrophil agonists.
We also ascertained whether the mice were suffering
from cisplatin-induced nephrotoxicity and whether CXCR1/CXCR2
antagonism had an impact. Neither the saline- nor the G31P
only-treated hepatoma tumor-bearing mice displayed discernible
evidence of kidney damage, while administration of cisplatin led to
dose-dependent nephrotoxicity, as determined by increases in BUN,
SCr and RC values (Fig. 5), with
the high-dose therapy being overtly toxic. While G31P co-treatment
significantly augmented the therapeutic outcome of high-dose
cisplatin in terms of reducing tumor progression in our model, this
combined therapy had a substantial beneficial impact on
nephrotoxicity, as measured by serum levels of BUN and SCr and the
RC values (P≤0.05, for G31P/high-dose cisplatin vs. high-dose
cisplatin therapy alone). Indeed, the BUN and SCr levels in the
mice treated with G31P/12.5 mg/kg cisplatin were approximately
equivalent to those in the low-dose cisplatin-treated mice.
Moreover, the RC values in the mice treated with G31P/high-dose
cisplatin were lower overall than these values in the mice treated
with low-dose cisplatin, indicating that co-delivery of G31P
allowed the use of higher, more tumor-growth suppressive doses of
cisplatin while at the same time protecting the animals against
these cisplatin-associated toxic side-effects.
Discussion
We demonstrated that high-dose cisplatin
chemotherapy was highly beneficial in terms of blocking H22
hepatoma tumor progression and metastasis, although this dose also
caused substantial nephrotoxicity. More importantly, we also found
that co-treatment with the CXCR1/CXCR2 antagonist G31P increased
the antitumor activity of cisplatin, which was not unexpected given
its reported antitumor activity in a mouse model of prostate cancer
(17).
Cisplatin is a chemotherapeutic drug used clinically
for the treatment of many types of cancers. In target cells it
causes the formation of DNA adducts that activate multiple signal
transduction pathways (e.g., ATR, p53, p73) that lead to tumor cell
apoptosis (24). Yet, as it is
excreted mainly by the kidneys, cisplatin causes substantial
dose-dependent toxic renal side-effects even within its therapeutic
dose (2). However, this toxicity is
a compromise due to the therapeutic antitumor effects of the agent,
yet this limits its use as a chemotherapeutic agent. Numerous
chemotherapeutic agents induce the expression by tumor cells of
ELR-CXC chemokines (e.g., CXCL8), which can reduce tumor cell death
through regulation of the pro-apoptotic protein
caspase-8-inhibitory protein c-FLIP (reviewed in ref. 18). Indeed, we previously reported that
G31P reverses the anti-apoptotic effects of the ELR-CXC chemokines
on neutrophils (12). Thus, ELR-CXC
chemokine antagonism might be expected to halt primary tumor
progression, as noted above, and to reduce the toxicity of
chemotherapeutic agents, thereby allowing the use of higher doses
of these drugs with, consequently, better therapeutic outcomes. Our
data confirmed that high-dose cisplatin therapy strongly induced
intratumoral expression of the ELR-CXC chemokines KC and MIP2,
while antagonizing these chemokines via G31P co-treatment reduced
the nephotoxicity associated with high-dose cisplatin therapy to
levels equivalent to those observed with low-dose cisplatin. While
G31P should reduce some of the inflammatory responses observed in
damaged kidneys, our data confirmed that it does not ablate this
response, as assessed by expression of IL-1, KC or MIP2. This is
consistent with the above-mentioned assumptions that neutrophil
inflammation is not the primary cause of nephrotoxicity under
cisplatin chemotherapy, but is rather a consequence of the toxicity
(7). We hypothesized that G31P
treatment activated anti-apoptotic processes in the kidneys of our
cisplatin-treated mice and this was the mechanism behind its
ability to reduce nephrotoxicity. Taken together, our results
showed that ELR-CXC chemokine antagonism with an agent such as G31P
can alleviate the renal injury caused by cisplatin without
compromising its therapeutic efficacy. This approach may be
clinically beneficial for the treatment of patients at risk for
serious drug-induced renal injury.
Acknowledgements
The present study was supported by grants from the
National Science Foundation of China (NSFC30772023) and the Natural
Sciences and Engineering Research Council (NSERC) of Canada
(J.R.G.).
Abbreviations:
|
G31P
|
CXCL8(3–72)K11R/G31P
|
|
BUN
|
blood urea nitrogen
|
|
SCr
|
serum creatinine
|
|
RC
|
renal coefficient
|
|
qRT-PCR
|
quantitative real-time PCR
|
|
IHC
|
immunohistochemical
|
|
DAB
|
diaminobenzidine
|
|
PBS
|
phosphate-buffered saline
|
|
VEGF
|
vascular endothelial growth factor
|
|
H&E staining
|
hematoxylin and eosin staining
|
|
KC
|
keratinocyte-derived chemokine
|
|
MIP2
|
macrophage inflammatory protein-2
|
|
DNA
|
deoxyribonucleic acid
|
|
ATR
|
ATM/Rad3-related
|
|
cFLIP
|
cellular FLIP
|
|
FLIP
|
FLICE (caspase-8) inhibitory
protein
|
References
|
1
|
Zernecke A, Weber KSC, Erwig LP, Kluth DC,
Schroppel B, Rees AJ and Weber C: Combinatorial model of chemokine
involvement in glomerular monocyte recruitment: role of CXC
chemokine receptor 2 in infiltration during nephrotoxic nephritis.
J Immunol. 166:5755–5562. 2001. View Article : Google Scholar
|
|
2
|
Miller RP, Tadagavadi RK, Ramesh G and
Reeves WB: Mechanisms of cisplatin nephrotoxicity. Toxins.
2:2490–2518. 2010. View Article : Google Scholar
|
|
3
|
Eliopoulos N, Zhao J, Bouchentouf M,
Forner K, Birman E, Yuan S, Boivin MN and Martineau D: Human
marrow-derived mesenchymal stromal cells decrease cisplatin
renotoxicity in vitro and in vivo and enhance survival of mice
post-intraperitoneal injection. Am J Physiol Renal Physiol.
299:F1288–F1298. 2010. View Article : Google Scholar
|
|
4
|
Fram RJ: Cisplatin and platinum analogues:
recent advances. Curr Opin Oncol. 4:1073–1079. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gonçalves GM, Zamboni DS and Câmara NO:
The role of innate immunity in septic acute kidney injuries. Shock.
34(Suppl 1): 22–26. 2010.
|
|
6
|
Matsushima H, Yonemura K, Ohishi K and
Hishida A: The role of oxygen free radicals in cisplatin-induced
acute renal failure in rats. J Lab Clin Med. 131:518–526. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Faubel S, Lewis EC, Reznikov L, Ljubanovic
D, Hoke TS, Somerset H, Oh DJ, Lu L, Klein CL, Dinarello CA and
Edelstein CL: Cisplatin-induced acute renal failure is associated
with an increase in the cytokines interleukin (IL)-1β, IL-18, IL-6,
and neutrophil infiltration in the kidney. J Pharmacol Exp Ther.
322:8–15. 2007.
|
|
8
|
Mukaida N: Pathophysiological roles of
interleukin-8/CXCL8 in pulmonary diseases. Am J Physiol Lung Cell
Mol Physiol. 284:L566–L577. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li F, Zhang X and Gordon JR:
CXCL8(3–73)K11R/G31P antagonizes ligand binding to the
neutrophil CXCR1 and CXCR2 receptors and cellular responses to
CXCL8/IL-8. Biochem Biophys Res Commun. 293:939–944. 2002.
|
|
10
|
Li F, Zhang X, Mizzi C and Gordon JR:
CXCL8(3–73)K11R/G31P antagonizes the neutrophil
chemoattractants present in pasteurellosis and mastitis lesions and
abrogates neutrophil influx into intradermal endotoxin challenge
sites in vivo. Vet Immunol Immunopathol. 90:65–77. 2002.
|
|
11
|
Zhao X, Li F, Town JR, Zhang X, Wang W and
Gordon JR: Humanized forms of the CXCR1/CXCR2 antagonist, bovine
CXCL8(3–74)K11R/G31P, effectively block ELR-CXC
chemokine activity and airway endotoxemia pathology. Int
Immunopharmacol. 7:1723–1731. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao X, Town JR, Li F, Zhang X, Cockcroft
DW and Gordon JR: ELR-CXC chemokine receptor antagonism targets
inflammatory responses at multiple levels. J Immunol.
182:3213–3222. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gordon JR, Li F, Zhang X, Wang W, Zhao X
and Nayyar A: The combined CXCR1/CXCR2 antagonist
CXCL8(3–74)K11R/G31P blocks neutrophil infiltration,
pyrexia, and pulmonary vascular pathology in endotoxemic animals. J
Leukoc Biol. 78:1265–1272. 2005.PubMed/NCBI
|
|
14
|
Zhao X, Town JR, Li F, Li W, Zhang X and
Gordon JR: Blockade of neutrophil responses in aspiration pneumonia
via ELR-CXC chemokine antagonism does not predispose to airway
bacterial outgrowth. Pulm Pharmacol Ther. 23:22–28. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao X, Town JR, Yang A, Zhang X, Paur N,
Sawicki G and Gordon JR: A novel ELR-CXC chemokine antagonist
reduces intestinal ischemia reperfusion-induced mortality, and
local and remote organ injury. J Surg Res. 162:264–273. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Waugh DJ and Wilson C: The interleukin-8
pathway in cancer. Clin Cancer Res. 14:6735–6741. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, Peng J, Sun W, Yang S, Deng G, Li
F, Cheng JW and Gordon JR: G31P, an antagonist against CXC
chemokine receptors 1 and 2, inhibits growth of human prostate
cancer cells in nude mice. Tohoku J Exp Med. 228:147–156. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wilson C, Purcell C, Seaton A, Oladipo O,
Maxwell PJ, O’Sullivan JM, Wilson RH, Johnston PG and Waugh DJ:
Chemotherapy-induced CXC-chemokine/CXC-chemokine receptor signaling
in metastatic prostate cancer cells confers resistance to
oxaliplatin through potentiation of nuclear factor-kappaB
transcription and evasion of apoptosis. J Pharmacol Exp Ther.
327:746–759. 2008. View Article : Google Scholar
|
|
19
|
Wilson C, Wilson T, Johnston PG, Longley
DB and Waugh DJ: Interleukin-8 signaling attenuates TRAIL- and
chemotherapy-induced apoptosis through transcriptional regulation
of c-FLIP in prostate cancer cells. Mol Cancer Ther. 7:2649–2661.
2008. View Article : Google Scholar
|
|
20
|
Kim YS, Moon JI, Kim DK, Kim SI and Park
K: Ratio of donor kidney weight to recipient bodyweight as an index
of graft function. Lancet. 357:1180–1181. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jüttner S, Wissmann C, Jons T, Vieth M,
Hertel J, Gretschel S, Schlag PM, Kemmner W and Hocker M: Vascular
endothelial growth factor-D and its receptor VEGFR-3: two novel
independent prognostic markers in gastric adenocarcinoma. J Clin
Oncol. 24:228–240. 2006.PubMed/NCBI
|
|
22
|
Moldobaeva A, Baek A, Eldridge L and
Wagner EM: Differential activity of pro-angiogenic CXC chemokines.
Microvasc Res. 80:18–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Katyal S, Oliver JH III, Peterson MS,
Ferris JV, Carr BS and Baron RL: Extrahepatic metastases of
hepatocellular carcinoma. Radiology. 216:698–703. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Siddik and Zahid H: Cisplatin: mode of
cytotoxic action and molecular basis of resistance. Oncogene.
22:7265–7279. 2003. View Article : Google Scholar : PubMed/NCBI
|