Introduction
Colorectal cancer (CRC) is one of the most common
neoplasms worldwide (1). A large
body of epidemiological evidence suggests that obesity increases
CRC risk in men (relative risks of ~1.5–2.0) and women (relatively
risks of ~1.2–1.5) (2,3). Although the molecular mechanisms
underlying this association are unknown, data acquired from
experiments performed in vitro suggest the direct
involvement of fat tissue in CRC development. Adipocytes and
preadipocytes stimulate the growth of CRC cells (4), and the principal hormone synthesized
by adipocytes is leptin. Leptin is encoded by the obese gene and
functions as a neuroendocrine hormone that has attracted attention
since its identification in 1995 (5). Leptin regulates appetite, bone
formation, reproduction and angiogenesis (6). These biological activities suggest
that it plays an important role in proliferation, invasion and
metastasis of cancer cells (7). In
humans, circulating leptin levels correlate with body mass index
and are significantly elevated in obese individuals (8). Recent data clearly indicate that the
mitogenic, anti-apoptotic, proinflammatory and angiogenic
properties of leptin promote the development and progression of
different types of cancers (9).
Several reports have described the mitogenic effects of leptin on
gastric (10), breast (11), ovarian (12), prostate (13) and endometrial cancer cells (14). Two studies demonstrated that
increased leptin levels are associated with greater risk of CRC
development, particularly in males (15,16).
Furthermore, in colon epithelial cells, leptin was found to induce
chemokine production associated with macrophage activation similar
to that observed in an adenomatous polyposis coli genotype
(17,18). The aims of the present study were to
investigate the expression of leptin in 80 patients with CRC and to
determine the effects of leptin on the malignant properties of CRC
cell lines.
Materials and methods
Case selection and immunohistochemical
assessment
For immunohistochemical staining, we obtained
formalin-fixed, paraffin-embedded tissue samples from 80 patients
diagnosed with CRC at Chonnam National University Hospital,
Gwangju, Korea. The histology of the tumors was analyzed, and the
pathological stage was estimated according to the TNM score.
Patients (stage I, n=20; stage I, n=20; stage III, n=20 and stage
IV, n=20) were randomly selected from each of the stage categories.
The specimens were fixed in 10% neutral-buffered formalin, embedded
in paraffin and stained with hematoxylin and eosin. The present
study was approved by the Institutional Review Board of Chonnam
National University Hospital.
For immunohistochemical staining, tissue sections
were deparaffinized, rehydrated and subjected to epitope retrieval.
To block endogenous peroxidase activity, tissues were treated with
Peroxidase-Blocking solution (Dako, Carpinteria, CA, USA) and
incubated with a polyclonal rabbit anti-leptin antibody (A-20;
Santa Cruz Biotechnology, Santa Cruz, CA, USA), which was diluted
1:100 using goat serum and incubated with the sections at room
temperature for 1 h. After three 2-min washes with
phosphate-buffered saline (PBS), the sections were incubated with a
biotinylated goat anti-rabbit secondary antibody for 30 min (Dako).
After three 2-min washes in PBS, horseradish
peroxidase-streptavidin (Dako) was added to the sections for 30
min, followed by another three washes for 2 min in PBS. Reactions
were detected using with 3,3′-diaminobenzidine substrate (Vector
Laboratories, Burlington, ON, Canada) for 1 min, and the cells were
counterstained using Mayer’s hematoxylin. Then slides were
dehydrated following a standard procedure and sealed with
coverslips.
Tissue specimens reacted with the anti-leptin
antibody were examined at low and then at high magnification by two
pathologists blinded to the identities of the samples. In cases of
heterogeneous patterns in some sections, the classification was
determined by the dominant pattern, and the intensity of stained
cells was designated as negative, weak, moderate or strong.
Cell culture and leptin
pretreatments
We selected three human CRC cell lines (LS174T, HCT
116 and CaCo-2) since they express leptin at high levels. They were
obtained from the Korean Cell Line Bank (KCLB) and cultured at 37°C
in a humidified atmosphere containing 5% CO2. Cells were
grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 4,500
mg/l glucose, 100 mg/l streptomycin and 2 mM L-glutamine
supplemented with 10% fetal bovine serum (FBS) (both from Gibco
Invitrogen Inc., USA). After 24 h of serum starvation, the culture
media were replaced with serum-free media containing the indicated
treatments. After a further 24 h incubation, 10 ng/ml human
recombinant leptin (Sigma-Aldrich Corp., St. Louis, MO, USA) was
added for different times.
Spheroid formation assay
Cells were grown to 70% confluence, trypsinized and
plated in 100 cm2 diameter culture dishes at a density
of 1,000 cells/ml in serum-free DMEM containing 10 ng/ml human
recombinant basic fibroblast growth factor, (bFGF) and 10 ng/ml
human epidermal growth factor (hEGF) (both from R&D Systems,
Minneapolis, MN, USA). A density of at least 1,000 cells/ml was
established for forming tumorspheres/colonospheres (serving as an
in vitro model of cancer stem cells). All cultures were
incubated at 37°C in a humidified atmosphere containing 5%
CO2. Cells were grown as suspension cultures for 1–2
weeks for tumorsphere formation. Colonies were counted in 10
randomly selected fields at ×10 magnification using an Olympus IX50
inverted microscope.
Western blotting
To extract proteins, cells were lysed with RIFA
buffer (1 M Tris-HCl, 150 mM NaCl, 1% Triton X-100 and 2 mM EDTA)
containing 1 mM phenylmethanesulfonyl fluoride (PMSF) and Halt™
protease inhibitor cocktail (Thermo, Rockford, IL, USA). The
protein concentrations of the cell lysates were quantitated using
the BCA™ protein assay (Thermo) with bovine serum albumin (BSA) as
a standard. The lysates (25 μg protein) were subjected to
electrophoresis on 10% SDS-polyacrylamide gels and then
electrophoretically transferred to polyvinylidene fluoride
membranes (Millipore, Billerica, MA, USA). The membranes were
incubated for 1 h in blocking solution [5% BSA in TBS-Tween-20
buffer (TBST)] and sequentially blotted with primary antibodies at
4°C overnight. Antibodies against Janus kinase 2 (JAK2),
phospho-JAK2, AKT, phospho-AKT, ERK, phospho-ERK, MAPK,
phospho-MAPK and β-actin were purchased from Cell Signaling
Technology (Beverly, MA, USA). After rinsing in TBST, membranes
were incubated with horseradish peroxidase (HRP)-labeled
anti-rabbit or anti-mouse immunoglobulin secondary antibodies
(1:2,000 dilution) (Cell Signaling Technology) at room temperature
for 1 h. Enhanced chemiluminescence was used to detect the bands,
which were visualized using a Fuji LAS-3000 image analyzer (Fuji
Film, Tokyo, Japan).
Adhesion assay
The 96-well plates were prepared. Three human CRC
cell lines were detached from the surfaces of culture flasks with 5
mM EDTA in PBS, resuspended in culture medium containing 0.02% BSA
to 2.4×105 cells/ml, and 100 μl of cell suspension was
added to each well. All cells were assayed in quadruplicate. After
incubation for 12 h at 37°C in an atmosphere containing 5%
CO2, the supernatant from each well was removed. After
washing out non-adherent cells, adherent cells were incubated for 4
h in medium containing 500 μg/ml
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
solution. The absorbance of the reaction product was measured at
550 nm. Adherent cells were counted in three random area of each
well.
Invasion assay
Cell invasion assays were performed using Transwell
filter chambers (8.0-μm pores) coated with 1% gelatin overnight and
dried at room temperature. Human CRC cell lines were harvested,
washed once in serum-free media, and seeded at 2×105
cells in 120 μl of medium containing 0.2% BSA in the upper chamber.
Then, 400 μl of 0.2% BSA medium containing 20 μg/ml of human plasma
fibronectin (Calbiochem, La Jolla, CA, USA), a chemotactic factor,
was loaded into the lower chamber. The Transwell apparatus was
incubated for 24 h at 37°C. Cells that invaded the bottom surface
of the upper chamber were fixed with 70% ethanol and stained with
Diff-Quik solution (Sysmex, Kobe, Japan) following the
manufacturer’s protocol. The non-invasive cells on the top surface
were wiped off with cotton balls, and the stained cells on the
bottom surface were counted in five selected fields (each 0.5
mm2) of six random squares using a hematocytometer
placed on the stage of a light microscope. Results are expressed as
the means ± standard error of the mean (SEM) of the number of
cells/field of three individual experiments.
Statistical analysis
The statistical significance of differences between
data sets was determined using the paired t-test. The λ2
and Fisher’s exact test, where appropriate, were used to compare
expression of leptin with various tumor stages. All reported
p-values were two-sided and p≤0.05 was considered to indicate a
statistically significant result. The Statistical Package for the
Social Sciences (SPSS)/PC 20.0 (Chicago, IL, USA) was used to
perform the calculations.
Results
Association of leptin expression and
tumor stage
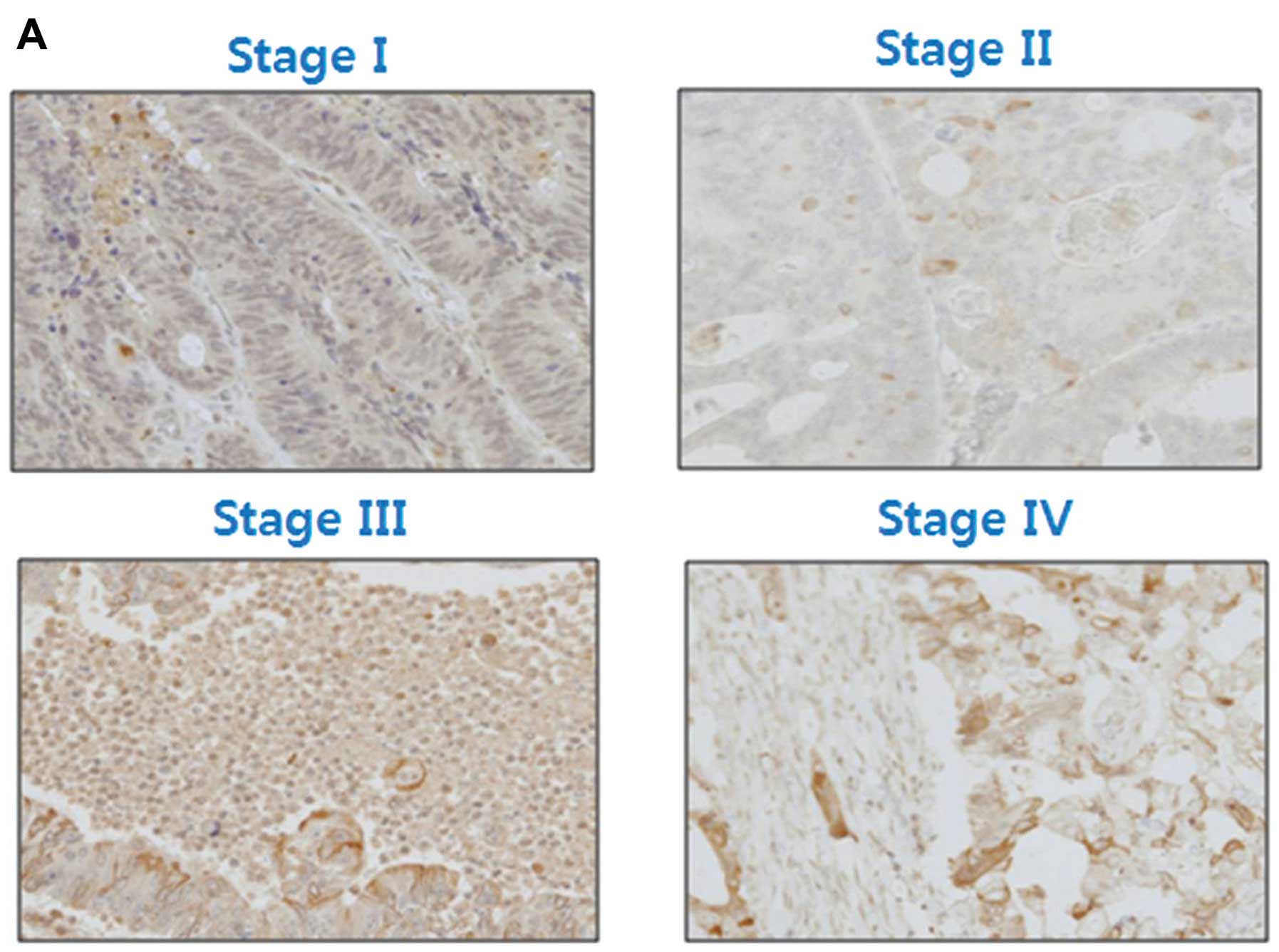
We determined the level of expression of leptin in
80 CRCs of different stages. Leptin was clearly expressed in the
cytoplasm of the CRC cells. Leptin expression was ‘undetectable’ in
19/20 (95%) patients with stage I CRC and 5/20 (25%) patients with
stage IV CRC. In contrast, leptin was ‘moderately to strongly’
expressed in 0/20 patients with stage I CRC and in 10/20 (50%)
patients with stage IV CRC. Expression of leptin was significantly
associated with tumor stage (Fig.
1, p<0.0001).
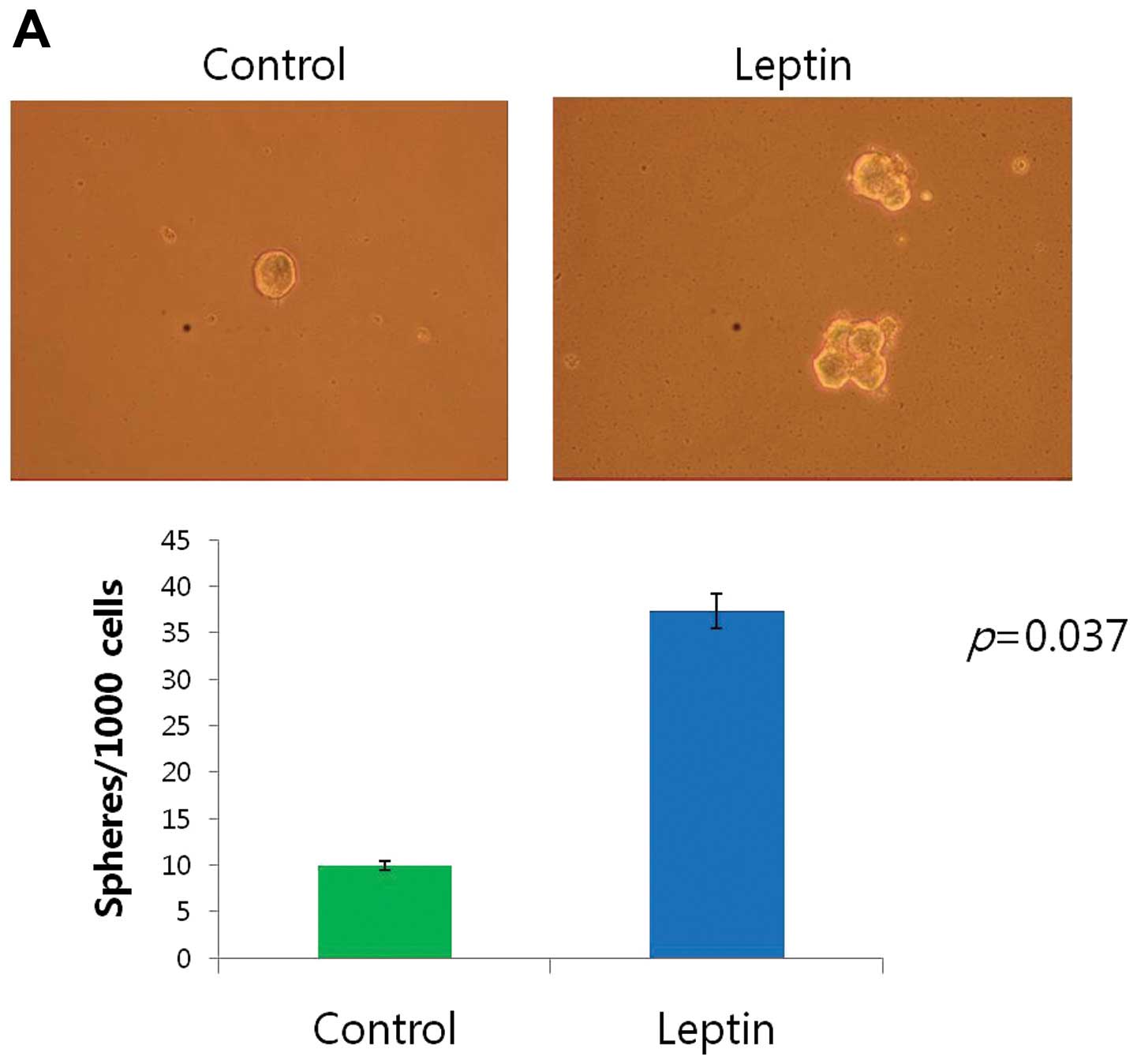
Spheroid formation induced by leptin
Cancer cells can be cultured in suspension to form
spheres in serum-free replacement medium. To test whether human CRC
cell lines could form spheres, CaCo-2, LS174T and HCT 116 cells
were cultured in a suspension-culture system. Tumorsphere formation
by CaCo-2, LS174T and HCT 116 cells was observed on day 5,
accounting for 3.73±0.25, 2.80±0.26 and 3.13±0.32% of the total
cell population by day 11, respectively. A greater number of large
aggregates and colonies formed in the leptin-treated cultures
relative to the controls (p<0.05). Leptin exposure increased the
average colony size formed by each cell line (Fig. 2).
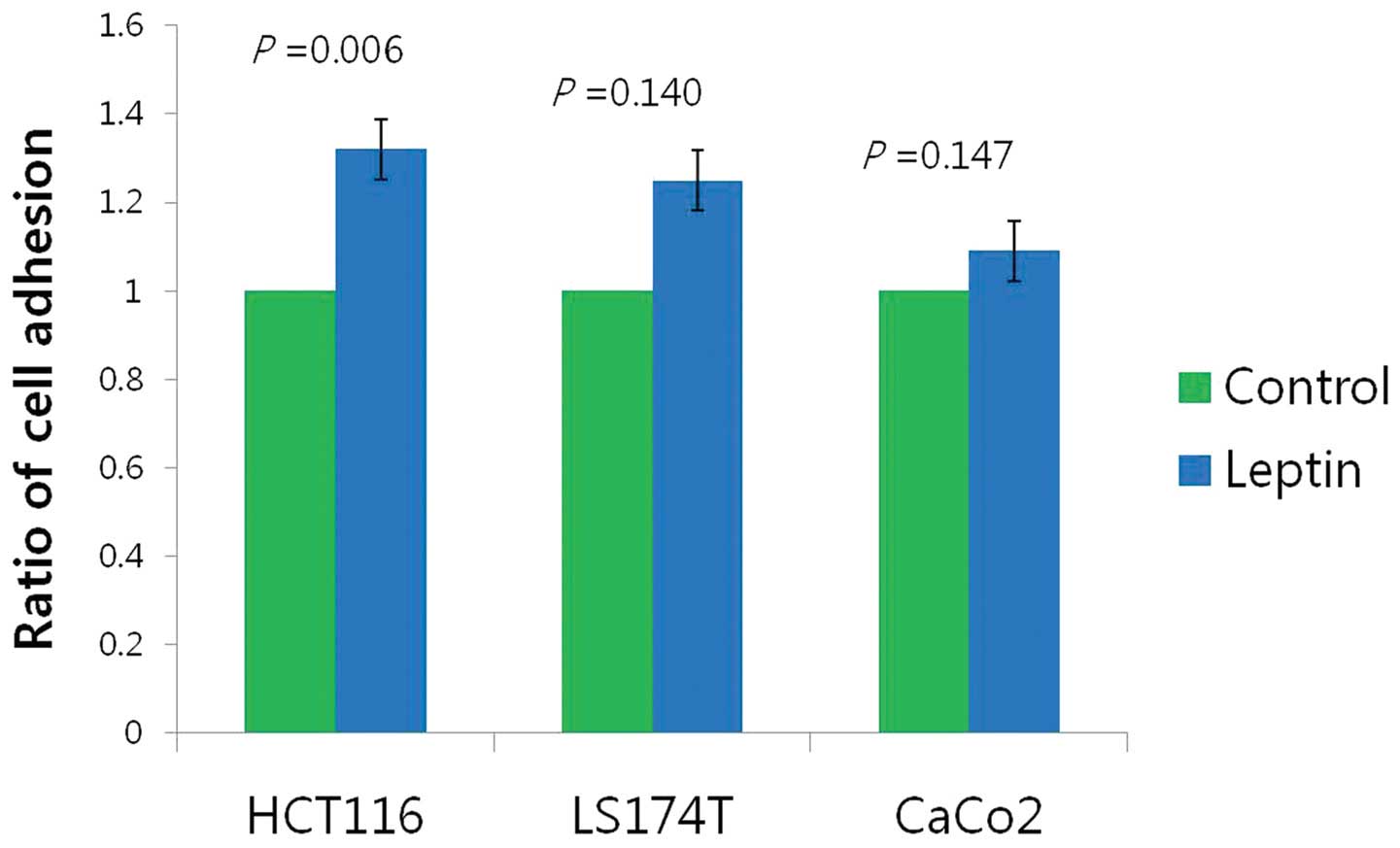
Induction of adhesion and invasion in
leptin-treated cells
In the adhesion assays, leptin treatment for 12 h
enhanced cell-cell adhesion of HCT 116 cells compared with the
untreated HCT 116 cells (p=0.006); however, there was no
statistically significant difference between the treated and
untreated LS174T (p=0.140) and CaCo-2 cells (p=0.147, Fig. 3).
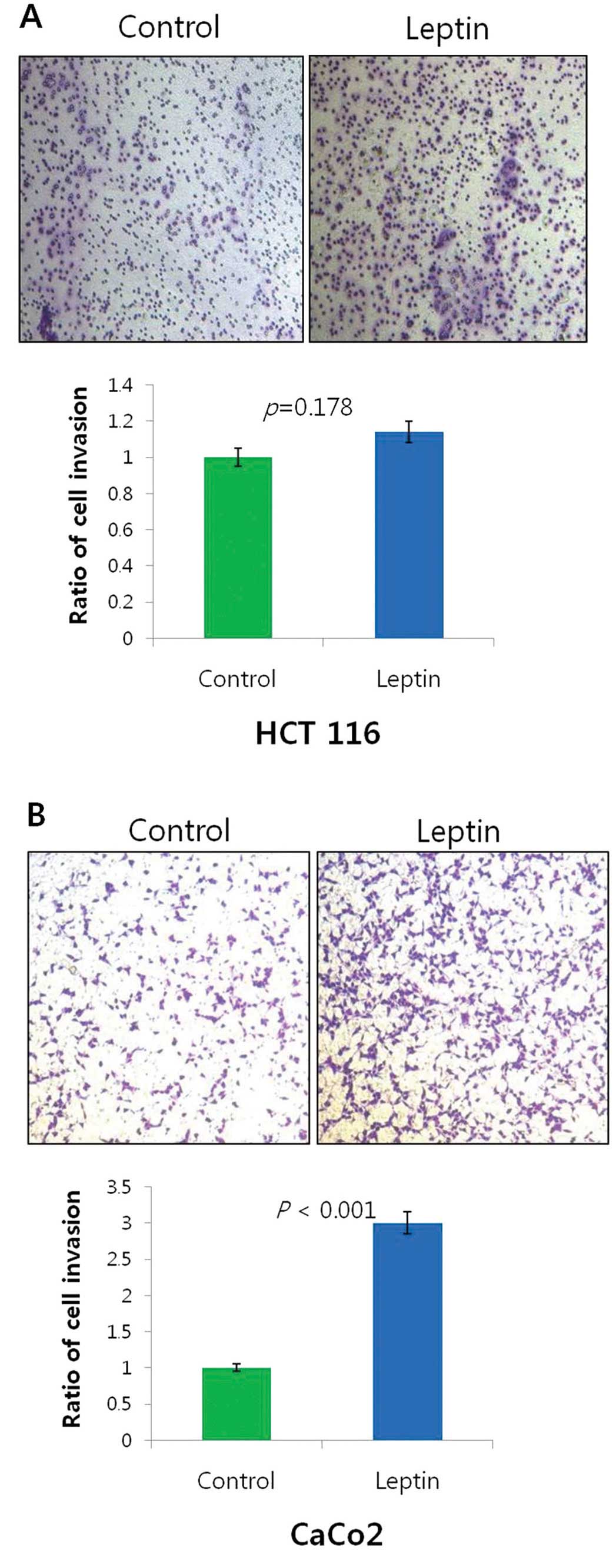
We next determined whether leptin treatment
influences the invasiveness of the colon cancer cell lines The
number of invading HCT 116 cells treated with leptin (10 ng/ml) was
574.3±562.5 when compared with the control value of 502.6±502.2
(Fig. 4A, p=0.178). The number of
invading CaCo-2 cells treated with leptin (10 ng/ml) was
3,395.6±1571.2 when compared with the control value of
1,512.6±850.9 (Fig. 4B,
p<0.001).
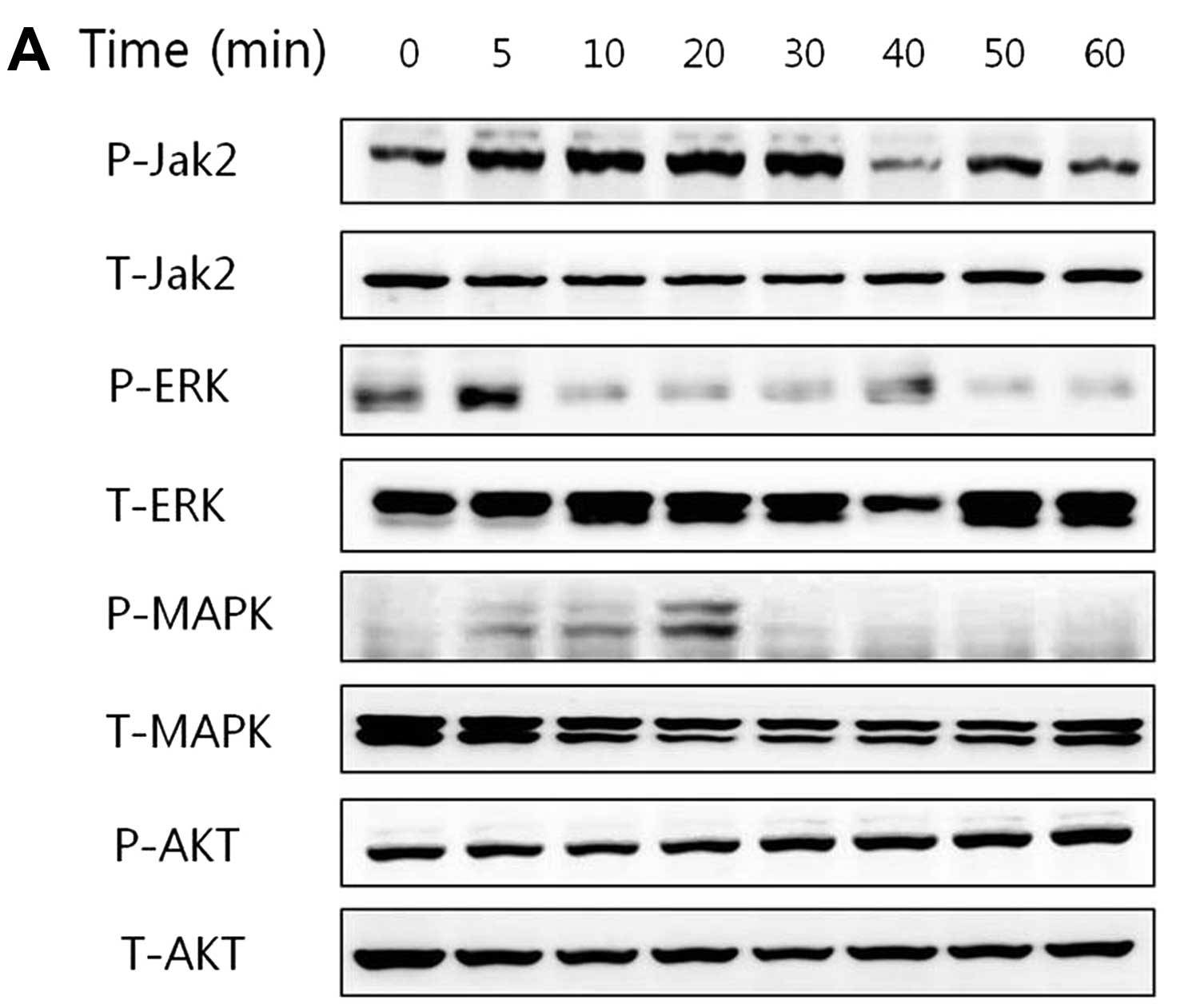
Activation of JAK2 and ERK signaling in
cells treated with leptin
In the CRC CaCo-2 cells, leptin (10 ng/ml)
stimulated the phosphorylation of JAK2 at the different treatment
times. Increased phosphorylation of JAK2 was observed within 30 min
after leptin treatment followed by a decline. In the LS174T and HCT
116 cells, leptin stimulated the phosphorylation of ERK. The levels
of total (T) JAK2, ERK, MAPK and AKT were not altered in the three
CRC cell lines after leptin treatment (Fig. 5).
Discussion
In the present study, we demonstrated that leptin
enhanced the malignant phenotypes of three cell lines derived from
human CRCs.
Epidemiologic studies suggest that obesity is a risk
factor for colon cancer development (16,19).
In contrast, Stattin et al suggested that leptin is simply
an innocent bystander, serving merely as a correlate of other
obesity-induced adverse alterations in metabolism that may be the
true cause of CRC (16). In human
CRC tissue, but not in normal mucosa or adenomas, leptin and its
receptor are overexpressed, which correlates with the expression of
the proneoplastic transcriptional regulator, hypoxia-inducible
factor 1, and a more advanced tumor phenotype (21,22).
Moreover, leptin is gradually expressed in the progression of
normal cells to adenomas and subsequently to carcinomas, suggesting
that leptin may be involved in multistep colorectal carcinogenesis
(3).
In the present study, we determined the expression
of leptin in tissue samples from 80 Korean patients with CRC and
the associated clinicopathologic factors. We showed that patients
with more advanced stage tumors expressed higher levels of leptin.
Expression levels of leptin and its receptor were previously found
to correlate with the grade of tumor differentiation, depth of
bowel wall invasion, Dukes’ stage and distant metastasis in CRC
patients, suggesting that the binding of leptin to its cellular
receptor promotes the proliferation of CRC cells (23). However, data for the leptin
concentration in the sera of patients with CRC are discrepant. For
example, three studies report that decreased serum leptin levels
are associated with tumor aggressiveness (21,24,25).
Cancer stem cells are rare, but play an important
role in the maintenance of cancer homeostasis (26). Stem cells of the gastrointestinal
tract may serve as a principal target of tumorigenic mutations due
to their naturally long life-span and capacity for self-renewal.
The concept of stem cell-driven tumorigenesis in CRC is supported
by the identification and phenotypic characterization of a
subpopulation of colon cancer cells capable of initiation and
sustained reproduction of human colon carcinomas in
immune-compromised mice (tumor-initiating cells or colorectal tumor
stem cells) (27). Cancer stem
cells (CSCs) form spheres when cultured in vitro in
serum-free suspension cultures. This sphere-formation technique was
used to isolate putative CSCs from freshly isolated brain (28), breast (29) and colon tumors (30). Furthermore, spheroid culture (or
colonospheres) generated from a limited number of human CRC-derived
cell lines are enriched for cells that express colonic CSC markers
(31,32). Increasing evidence suggests that
stem cells play a decisive role in the progression and metastasis
of CRC.
CSCs possess the ability to self-renew and
differentiate into different cell types. Moreover, CSCs play an
important role in the maintenance of cancer homeostasis,
metastasis, resistance to therapy and subsequent tumor recurrence
(26,33). In the present study, leptin
increased the number and size of tumorspheres formed by HCT 116,
LS174T and CaCo-2 cells. Bartucci et al reported that leptin
enhances the formation of tumorsphere by increasing their size and
number (27). Therefore, leptin may
affect the growth and survival of CRC stem cells that promote
colorectal carcinogenesis.
Cancer progression is a multistep process that
enables tumor cells to migrate to points far from a given primary
tumor mass (20). Here we showed
that leptin enhanced the invasive potential of CaCo-2 cells and the
adhesive potential of HCT 116 cells. These effects are consistent
with the ability of leptin to enhance the malignant phenotypes of
CRC cells, such as local invasiveness and the formation of distant
metastasis. Moreover, these results are consistent with our
confirmation here of leptin expression in human CRC tissue, which
was associated with advanced tumor stage.
To elucidate the signaling pathways involved in
leptin-mediated induction of malignant properties of CRC cells, we
showed that leptin rapidly induced the phosphorylation of JAK2 and
ERK, thus activating these key signal transduction pathways
associated with cell growth. These results are consistent with
those of others that demonstrated the enhanced activation of the
JAK/STAT signaling pathway and elevated expression of genes that
are targeted by the STAT3 signaling pathway in colorectal adenomas
compared with normal colorectal tissues (34). Although we did not study the
pharmacologic inhibition of this pathway here, our results suggest
that leptin-mediated JAK and ERK signaling may control invasion and
migration. Further investigations are required to clarify the role
of CSC in the invasion and migration of CRC cells.
Our results were inconsistent regarding adhesion and
invasion, signaling pathways and spheroid formation in the three
CRC cell lines. These cell lines mirror the features of the
original, individual, diverse tumors from which they were derived
and reflect different stages of tumors occurring in the same organ
as well as their metastatic cells. Therefore, each of these three
colon cancer cell lines may possess distinct combinations of unique
oncogenes and tumor-suppressor mutations (35).
In conclusion, in the present study, we demonstrated
that leptin affected the spheroid formation of colorectal cancer
cell lines and regulated adhesion and invasion of colorectal
carcinomas through activation of the JAK and ERK signaling
pathways.
References
|
1
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2006. CA Cancer J Clin. 56:106–130. 2006. View Article : Google Scholar
|
|
2
|
Renehan AG, Tyson M, Egger M, Heller RF
and Zwahlen M: Body-mass index and incidence of cancer: a
systematic review and meta-analysis of prospective observational
studies. Lancet. 371:569–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Calle EE and Thun MJ: Obesity and cancer.
Oncogene. 23:6365–6378. 2004. View Article : Google Scholar
|
|
4
|
Amemori S, Ootani A, Aoki S, et al:
Adipocytes and preadipocytes promote the proliferation of colon
cancer cells in vitro. Am J Physiol Gastrointest Liver Physiol.
292:G923–G929. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Halaas JL, Gajiwala KS, Maffei M, et al:
Weight-reducing effects of the plasma protein encoded by the obese
gene. Science. 269:543–546. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang L and Li C: Leptin: a
multifunctional hormone. Cell Res. 10:81–92. 2000. View Article : Google Scholar
|
|
7
|
Somasundar P, McFadden DW, Hileman SM and
Vona-Davis L: Leptin is a growth factor in cancer. J Surg Res.
116:337–349. 2004. View Article : Google Scholar
|
|
8
|
Zhang F, Chen Y, Heiman M and Dimarchi R:
Leptin: structure, function and biology. Vitam Horm. 71:345–372.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garofalo C and Surmacz E: Leptin and
cancer. J Cell Physiol. 207:12–22. 2006. View Article : Google Scholar
|
|
10
|
Pai R, Lin C, Tran T and Tarnawski A:
Leptin activates STAT and ERK2 pathways and induces gastric cancer
cell proliferation. Biochem Biophys Res Commun. 331:984–992. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rose DP, Komninou D and Stephenson GD:
Obesity, adipocytokines, and insulin resistance in breast cancer.
Obes Rev. 5:153–165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi JH, Park SH, Leung PC and Choi KC:
Expression of leptin receptors and potential effects of leptin on
the cell growth and activation of mitogen-activated protein kinases
in ovarian cancer cells. J Clin Endocrinol Metab. 90:207–210. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Somasundar P, Frankenberry KA, Skinner H,
et al: Prostate cancer cell proliferation is influenced by leptin.
J Surg Res. 118:71–82. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharma D, Saxena NK, Vertino PM and Anania
FA: Leptin promotes the proliferative response and invasiveness in
human endometrial cancer cells by activating multiple
signal-transduction pathways. Endocr Relat Cancer. 13:629–640.
2006. View Article : Google Scholar
|
|
15
|
Stattin P, Palmqvist R, Söderberg S, et
al: Plasma leptin and colorectal cancer risk: a prospective study
in Northern Sweden. Oncol Rep. 10:2015–2021. 2003.PubMed/NCBI
|
|
16
|
Stattin P, Lukanova A, Biessy C, et al:
Obesity and colon cancer: Does leptin provide a link? Int J Cancer.
109:149–152. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fenton JI, Hursting SD, Perkins SN and
Hord NG: Leptin induces an Apc genotype-associated colon epithelial
cell chemokine production pattern associated with macrophage
chemotaxis and activation. Carcinogenesis. 28:455–464. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fenton JI, Lavigne JA, Perkins SN, et al:
Microarray analysis reveals that leptin induces autocrine/paracrine
cascades to promote survival and proliferation of colon epithelial
cells in an Apc genotype-dependent fashion. Mol Carcinog.
47:9–21. 2008. View
Article : Google Scholar
|
|
19
|
Yu F, Yao H, Zhu P, et al: let-7
regulates self renewal and tumorigenicity of breast cancer cells.
Cell. 131:1109–1123. 2007. View Article : Google Scholar
|
|
20
|
Yamaguchi H, Wyckoff J and Condeelis J:
Cell migration in tumors. Curr Opin Cell Biol. 17:559–564. 2005.
View Article : Google Scholar
|
|
21
|
Paik SS, Jang SM, Jang KS, Lee KH, Choi D
and Jang SJ: Leptin expression correlates with favorable
clinicopathologic phenotype and better prognosis in colorectal
adenocarcinoma. Ann Surg Oncol. 16:297–303. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koda M, Sulkowska M, Kanczuga-Koda L,
Surmacz E and Sulkowski S: Overexpression of the obesity hormone
leptin in human colorectal cancer. J Clin Pathol. 60:902–906. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu H, Wan D, Pan Z, et al: Expression and
biological significance of leptin, leptin receptor, VEGF, and CD34
in colorectal carcinoma. Cell Biochem Biophys. 60:241–244. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bolukbas FF, Kilic H, Bolukbas C, et al:
Serum leptin concentration and advanced gastrointestinal cancers: a
case controlled study. BMC Cancer. 4:292004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sălăgeanu A, Tucureanu C, Lerescu L, et
al: Serum levels of adipokines resistin and leptin in patients with
colon cancer. J Med Life. 3:416–420. 2010.PubMed/NCBI
|
|
26
|
Scopelliti A, Cammareri P, Catalano V,
Saladino V, Todaro M and Stassi G: Therapeutic implications of
cancer initiating cells. Expert Opin Biol Ther. 9:1005–1016. 2009.
View Article : Google Scholar
|
|
27
|
Bartucci M, Svensson S, Ricci-Vitiani L,
et al: Obesity hormone leptin induces growth and interferes with
the cytotoxic effects of 5-fluorouracil in colorectal tumor stem
cells. Endocr Relat Cancer. 17:823–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dey D, Saxena M, Paranjape AN, et al:
Phenotypic and functional characterization of human mammary
stem/progenitor cells in long term culture. PLoS One. 4:e53292009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Todaro M, Alea MP, Di Stefano AB, et al:
Colon cancer stem cells dictate tumor growth and resist cell death
by production of interleukin-4. Cell Stem Cell. 1:389–402. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan X, Ouyang N, Teng H and Yao H:
Isolation and characterization of spheroid cells from the HT29
colon cancer cell line. Int J Colorectal Dis. 26:1279–1285. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanwar SS, Yu Y, Nautiyal J, Patel BB and
Majumdar AP: The Wnt/β-catenin pathway regulates growth and
maintenance of colonospheres. Mol Cancer. 9:2122010.
|
|
33
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
34
|
Uchiyama T, Takahashi H, Sugiyama M, et
al: Leptin receptor is involved in STAT3 activation in human
colorectal adenoma. Cancer Sci. 102:367–372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vécsey-Semjén B, Becker KF, Sinski A, et
al: Novel colon cancer cell lines leading to better understanding
of the diversity of respective primary cancers. Oncogene.
21:4646–4662. 2002.PubMed/NCBI
|