Introduction
In women, cervical cancer is the second most common
cancer in developing regions with more than 85% of the global
burden occurring in developing countries (1). Although cervical cancer screening has
reduced its incidence, ~30% of patients are still diagnosed at an
advanced stage and ultimately show recurrence and metastasis
(2). Therefore, investigation of
the mechanisms of tumor invasion and metastasis will provide
further insight into the occurrence and development of cervical
cancer. In recent years, many genes such as secreted protein acidic
and rich in cysteine (3),
metastasis-associated 1 (4), and
twist homolog 2 (5) have been found
to be correlated with the progression of cervical cancer. However,
few studies have explored the relationship between fibulin-4 and
cervical cancer progression and prognosis.
Fibulin-4, also known as endothelial growth factor
(EGF)-containing fibulin-like extracellular matrix protein 2
(EFEMP2), mutant p53 binding protein 1 (MBP1) or UPH1, is a
443-amino acid secreted protein that contains six EGF-like
calcium-binding domains and belongs to the fibulin family (6). Fibulins have been shown to modulate
cell morphology, growth, adhesion and motility, and are closely
associated with the development of a wide variety of carcinomas
(7). As tumor-suppressor genes,
fibulin-2 (8,9) and fibulin-5 (10–12)
were widely considered to be associated with the suppression of
tumor growth, invasion and angiogenesis. Research findings on the
role of fibulin-1 and fibulin-3 in different tumor tissues have
been controversial. Few researchers have reported oncogenic
activities (13–19), whereas others have reported
tumor-suppressive activities (20–27).
This discrepancy may be attributable to the influence of the tumor
microenvironment on tumor-associated genes in promoting
angiogenesis and metastasis (28).
Fibulin-4 is essential for connective tissue
development and elastic fiber formation and may also play an
important role in vascular patterning and collagen biosynthesis
(29). Fibulin-4 plays a role in
many clinical conditions such as cutis laxa (30), aortic aneurysms (31), osteoarthritis (32) and cancer (22,33).
In their study on colon tumors, Gallagher et al found that
the fibulin-4 gene was localized on chromosome 11q13 (33); translocations, amplifications and
other rearrangements in this region are associated with a variety
of human cancers (34,35). Reverse transcriptase (RT)-polymerase
chain reaction (PCR) of RNA from paired human colon tumors and
adjacent normal tissue showed that tumors had a 2–7 fold increase
in the level of fibulin-4 mRNA expression (33). However, in prostate cancer (22), fibulin-4 was found to be
significantly downregulated and weakly expressed in carcinoma cell
lines compared to normal prostate epithelial cells. Against this
background of controversies in the research addressing the role of
fibulin-4, more studies are needed to elucidate the relationship
between fibulin-4 and cancer. To our knowledge, the role of
fibulin-4 in cervical cancer remains unexplored.
The purpose of this study was to assess whether
fibulin-4 expression is associated with the progression of cervical
cancer and to investigate the relationship between fibulin-4 and
angiogenesis.
Materials and methods
Cell lines
Highly invasive subclones (HeLa-1 and SiHa-1) and
low-invasive subclones (HeLa-25 and SiHa-23) were derived from the
HeLa and SiHa human cervical cancer cell lines, using the limited
dilution method. Next, the cell electrophoretic mobility (EPM) of
each clone was measured to study the charge-related properties
using microcapillary electrophoresis chips. Finally, the MTT assay,
soft agar colony formation assay, Matrigel invasion assay and cell
migration assay were performed and tumor xenografts were generated
in nude mice to confirm that highly invasive subclones and
low-invasive subclones had high and low metastatic potential,
respectively (3). Cells were
cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented
with 10% fetal bovine serum (FBS) and antibiotics (Gibco-BRL,
Rockville, MD, USA).
Tissue specimens
A total of 270 human cervical tissue specimens
obtained with written informed consent from patients were used for
this study. Two hundred and thirty cervical cancer patients were
enrolled from the Department of Gynecology and Obstetrics, Shandong
Provincial Hospital between 2006 and 2010. There were 60 cervical
intraepithelial neoplasia (CIN) cases [age range, 28–55 years; mean
(SD), 40 (8)], 140 squamous cell
carcinoma cases [age range, 30–65 years; mean (SD), 45 (10)] and 30 adenocarcinoma cases [age
range, 35–60; mean (SD), 43 (6)].
All cervical cancer patients were clinically staged according to
the revised International Federation of Gynecology and Obstetrics
(FIGO) staging system (FIGO stage I, 76 cases; FIGO stage II, 81
cases; and FIGO stage III and IV, 13 cases). None of the cervical
cancer patients received preoperative radiation or chemotherapy.
All patients were treated consecutively and were followed up
regularly; 9 patients were lost to follow-up and 25 patients died
during the study period. Follow-up duration was between 2 and 7
years by the end of 2012. Forty normal cervical tissue specimens
[age range, 30–60 years; mean (SD), 47 (9)] were obtained from the Department of
Gynecology and Obstetrics, Shandong Provincial Hospital. The study
was approved by the Institutional Medical Ethics Committee of
Shandong University.
Blood samples
Blood samples were obtained with written informed
consent from the same 230 cervical cancer patients (60 CIN cases,
140 squamous cell carcinoma cases and 30 adenocarcinoma cases) at
the Department of Gynecology and Obstetrics, Shandong Provincial
Hospital between 2006 and 2010. None of the cervical cancer
patients received preoperative radiation or chemotherapy. Forty
control blood samples were obtained with written informed consent
from age-matched examinees undergoing health examinations at
Shandong Provincial Hospital. Control subjects had no history of
disease and no abnormalities on laboratory examinations. The study
was approved by the Institutional Medical Ethics Committee of
Shandong University.
Enzyme-linked immunosorbent assay
Levels of fibulin-4 in serum samples were measured
using sandwich enzyme-linked immunosorbent assay (ELISA) with human
fibulin-4 ELISA assay kits (Immuno-Biological Laboratories,
Fujioka, Gunma, Japan). Serum was diluted with enzyme immunoassay
(EIA) buffer (1% bovine serum albumin, 0.05% Tween-20 in phosphate
buffer) and incubated for 2 h at 37°C. After 4 washes with EIA
buffer, horseradish peroxidase-conjugated antibodies were added and
incubated for 30 min at 4°C. After 4 washes, 100 μl of
tetramethylbenzidine solution was added and incubated for 30 min at
room temperature. The reaction was stopped with 100 μl of 1 N
sulfuric acid and measured using the ELISA reader at 450 nm.
Immunohistochemistry
According to standard streptavidinbiotin-peroxidase
complex procedures, immunohistochemistry (IHC) was performed on
formalin-fixed, paraffin-embedded sections (5-μm thick) and cell
slides were fixed in 4% paraformaldehyde. Briefly, after dewaxing,
rehydration, and antigen retrieval, the sections were incubated
with anti-human fibulin-4 antibodies (ab125073; Abcam and MAB2644;
Millipore) with working dilutions of 1:200 for ab125073 and 1:500
for MAB2644 at 4°C overnight, and stained with the enzyme substrate
3′,3-diaminobenzidine tetrahydrochloride (Sigma, St. Louis, MO,
USA). Human breast cancer paraffin-embedded sections
(fibulin-4-positive) were used as positive controls. A negative
control was obtained by replacing the primary antibody with normal
rabbit or mouse immunoglobulin (IgG). Positive expression of
fibulin-4 protein was defined as the presence of brown granules in
the cytoplasm.
Immunohistochemistry analysis
A semi-quantitative scoring system derived from the
method by Soumaoro et al (36) for both the intensity of staining and
the percentage of positive cells was used to evaluate fibulin-4
expression. The intensity of fibulin-4-positive staining was scored
from 0 to 3 (negative, 0; weak, 1; moderate, 2; or strong, 3), and
the percentage of positively stained cells was scored as 0 (0%), 1
(1–25%), 2 (26–50%), 3 (51–75%) and 4 (76–100%). The sum of the
intensity and percentage scores was used as the final staining
score (0–7). The sum-indices (−), (+), (++), and (+++) indicated
final staining scores of 0, 1–3, 4–5 and 6–7, respectively. For
statistical analysis, sum-indices (−) and (+) were defined as low
fibulin-4 expression, while sum-indices (++) and (+++) were defined
as high fibulin-4 expression. Each section was independently scored
by two pathologists. In cases of an inconsistency, a third
pathologist was consulted to arrive at a consensus. To assess
reproducibility, we invited three other pathologists to score all
sections independently. The interobserver reliability and
intraobserver reproducibility of IHC experiments were evaluated
using kappa (κ) statistical evaluation.
Microvessel assessment
Microvessel density (MVD) was assessed according to
CD34 immunohistochemical staining of tumor vessels. Any
immune-positive single endothelial cell or endothelial cell
clusters and microvessels in the tumor were considered to be
individual vessels and were counted, as described by Weidner et
al (37). Peritumoral
vascularity, vascularity in areas of necrosis, and vessels with a
thick muscle wall or having a diameter larger than 8 erythrocytes,
were not counted. The sections were scanned at low power (×100) to
select the most vascularized (hot-spots) areas. The microvessels in
the hot-spots were then counted, and an average count in three hot
spots was calculated as the MVD. All counts were performed
independently by three observers who were blinded to the
corresponding clinicopathological data.
Quantitative real-time-polymerase chain
reaction
Total RNA was extracted using TRIzol reagent
(Invitrogen) and reverse transcribed. Quantitative real-time RT-PCR
analysis was performed using ABI Prism 7500 Real-Time PCR System
(Applied Biosystems). Each well (20-μl reaction volume) contained
10 μl Power SYBR-Green PCR Master Mix (Applied Biosystems), 1 μl of
each primer (5 μmol/l) and 1 μl template. The following primers
were used: fibulin-4, 5′-GCTGCTACT GTTGCTCTTGGG-3′ and
5′-GGGATGGTCAGACACTCGT TG-3′; β-actin 5′-CCACGAAACTACCTTCAACTCCA-3′
and 5′-GTGATCTCCTTCTGCATCCTGTC-3′.
Statistical analysis
IHC data were analyzed using the Chi-square test.
Measurement data were expressed as means ± SE. The interobserver
reliability and intraobserver reproducibility of IHC experiments
were evaluated using the κ statistical evaluation. The strength of
agreement was interpreted as follows: excellent (κ ≥0.80), good
(0.60–0.79), moderate (0.40–0.59), poor (0.20–0.39) and very poor
(<0.20) (38). For comparison of
means between two groups, a two-tailed t-test was used and for
comparison of means among three groups, one-way ANOVA was used.
Survival curves were calculated using the Kaplan-Meier method and
analyzed using the log-rank test. Correlations of fibulin-4
expression with VEGF expression and MVD were analyzed using the
Pearson correlation test. Multivariate Cox proportional hazards
models were used to define the potential prognostic significance of
individual parameters. Statistical analysis was performed using
SPSS software version 13.0. Two-sided P-values of <0.05 were
considered to indicate statistically significant differences.
Results
Fibulin-4 expression in the human
cervical tissues
Fibulin-4 protein expression was extremely low in
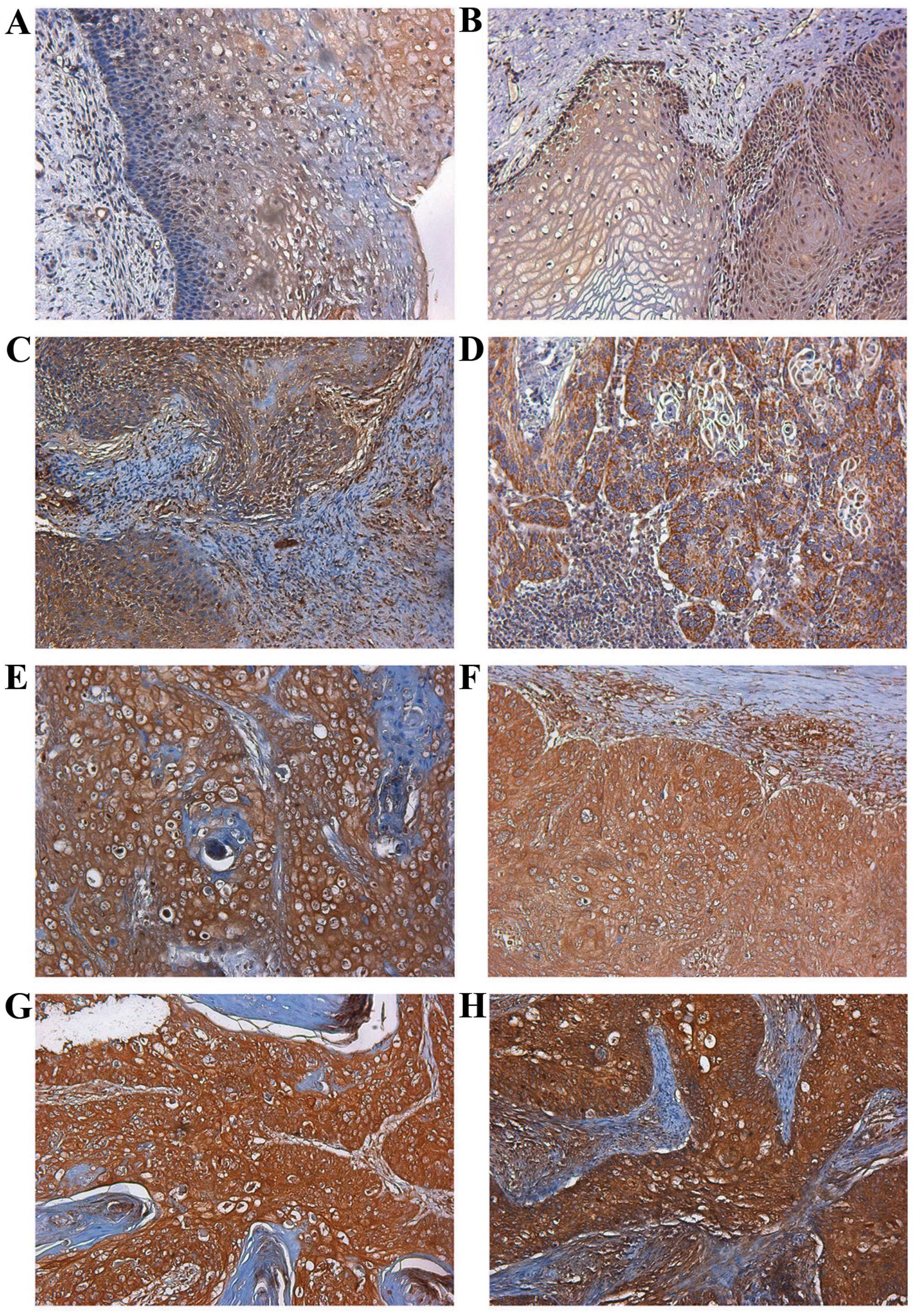
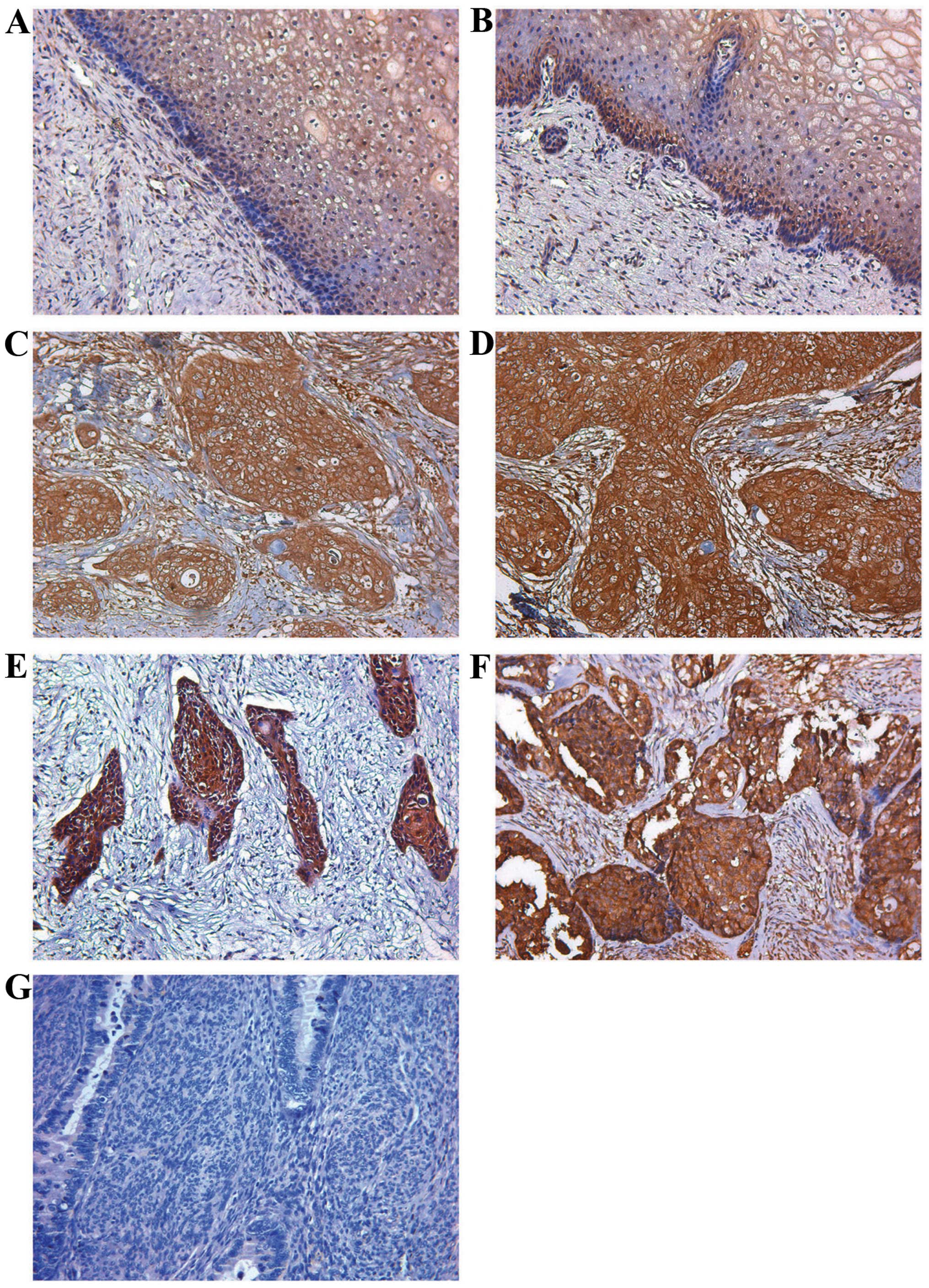
the normal human cervical tissue and CIN (Figs. 1A and B; 2A and B). However, in most cervical
carcinomas, fibulin-4 immunoreactivity was high, and high fibulin-4
protein expression was detected in the cytoplasm of cervical cancer
cells (Figs. 1C–H; 2C–E). Moreover, high fibulin-4 protein
expression was associated with low differentiation, advanced stage
and positive lymph node status of the cervical carcinoma cases
(Tables I and II). The interobserver reliability
coefficients were 0.86 and 0.81 for the first and second
assessments, with an intraobserver reproducibility coefficient of
0.85. The interobserver reliability and intraobserver
reproducibility of the IHC experiments were excellent. Similarly
results were also found for the real-time RT-PCR experiment;
fibulin-4 mRNA expression was also extremely low in the normal
cervical tissues and CIN, and significantly high fibulin-4
expression was noted in the cervical carcinoma cases. Moreover,
high fibulin-4 mRNA expression was also associated with low
differentiation, advanced stage and positive lymph node status of
the cervical carcinomas (Table
III). To evaluate the prognostic value of fibulin-4 in cervical
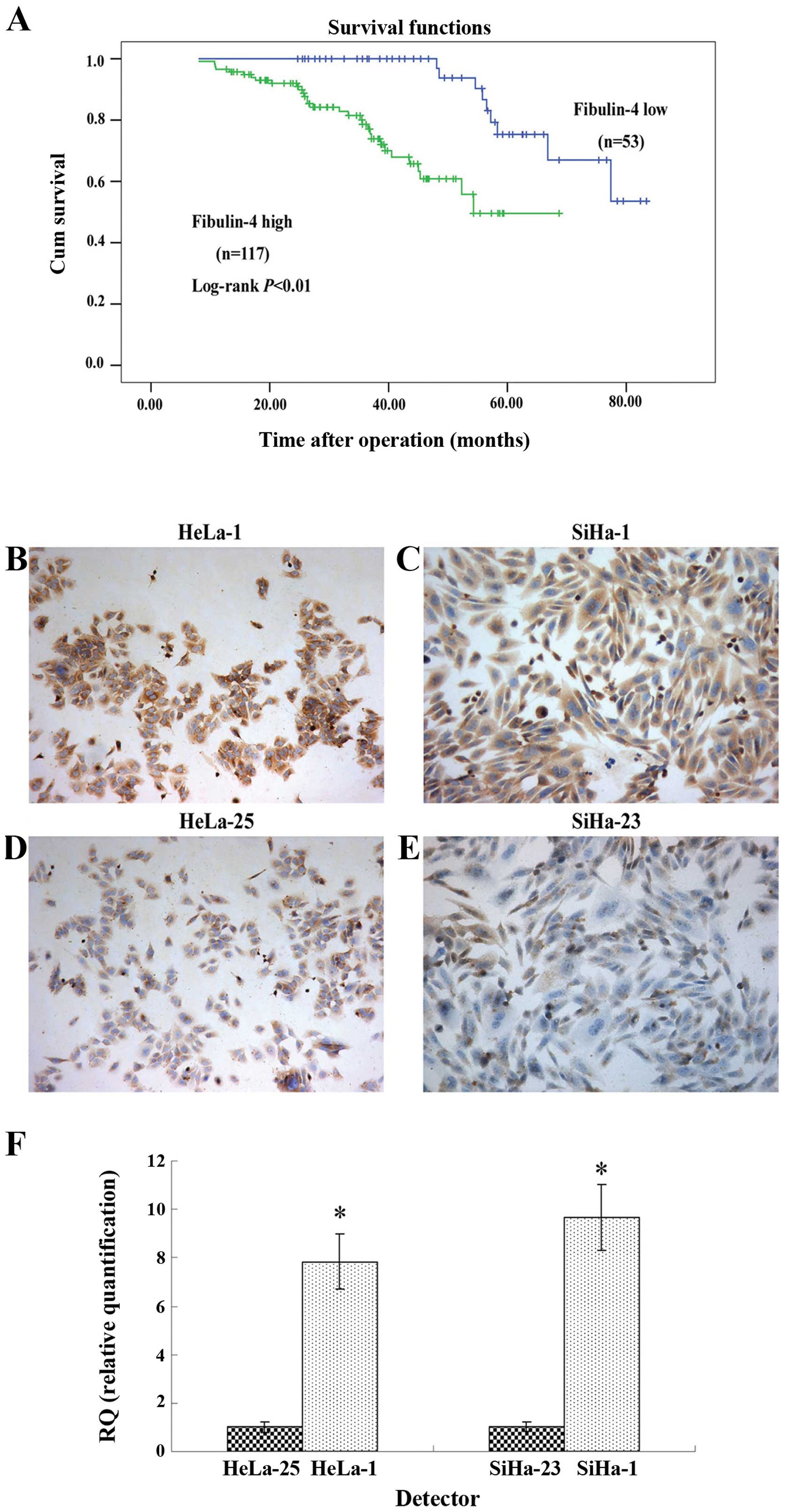
cancer, we performed survival analysis using Kaplan-Meier analysis.
The results showed that patients with high fibulin-4 expression had
a much worse prognosis than those with low fibulin-4 expression
(log-rank, P<0.01) (Fig. 3A). In
the multivariate analysis, considering all histological and
molecular features together, the significant prognostic factors
were lymph node metastasis (P=0.002; hazard ratio 2.129), fibulin-4
expression (P=0.002; hazard ratio 1.019) and tumor stage (P=0.007;
hazard ratio 3.175) (Table
IV).
 | Table IProtein expression of fibulin-4 in
the human cervical tissues with the ab125073 antibody. |
Table I
Protein expression of fibulin-4 in
the human cervical tissues with the ab125073 antibody.
| | Fibulin-4 low
(−/+) | Fibulin-4 high
(++/+++) | | |
|---|
| |
|
| | |
|---|
| N | n | (%) | n | (%) | χ2 | P-value |
|---|
| Normal | 40 | 37 | (92.5) | 3 | (7.5) | 66.05 | <0.01 |
| CIN | 60 | 44 | (73.3) | 16 | (26.7) | | |
| Carcinoma | 170 | 53 | (31.2) | 117 | (68.8) | | |
| Pathological
type | | | | | | 0.02 | >0.05 |
| Squamous cell
carcinoma | 140 | 44 | (31.4) | 96 | (68.6) | | |
|
Adenocarcinoma | 30 | 9 | (30.0) | 21 | (70.0) | | |
| Cell
differentiation | | | | | | 25.57 | <0.01 |
| High and
medium | 89 | 43 | (48.3) | 46 | (51.7) | | |
| Low | 81 | 10 | (12.3) | 71 | (87.7) | | |
| Tumor stage | | | | | | 26.07 | <0.01 |
| I | 60 | 32 | (53.3) | 28 | (46.7) | | |
| II | 59 | 19 | (32.2) | 40 | (67.8) | | |
| III and IV | 51 | 4 | (7.8) | 47 | (92.2) | | |
| Nodal status | | | | | | 18.26 | <0.01 |
| Positive | 66 | 8 | (12.1) | 58 | (87.9) | | |
| Negative | 104 | 45 | (43.3) | 59 | (56.7) | | |
 | Table IIProtein expression of fibulin-4 in
the human cervical tissues with the MAB2644 antibody. |
Table II
Protein expression of fibulin-4 in
the human cervical tissues with the MAB2644 antibody.
| | Fibulin-4 low
(−/+) | Fibulin-4 high
(++/+++) | | |
|---|
| |
|
| | |
|---|
| N | n | (%) | n | (%) | χ2 | P-value |
|---|
| Normal | 40 | 36 | (90) | 4 | (10) | 63.468 | <0.01 |
| CIN | 60 | 42 | (70) | 18 | (30) | | |
| Carcinoma | 170 | 50 | (29.4) | 120 | (70.6) | | |
| Pathological
type | | | | | | 0.270 | >0.05 |
| Squamous cell
carcinoma | 140 | 40 | (28.6) | 100 | (71.4) | | |
|
Adenocarcinoma | 30 | 10 | (33.3) | 20 | (66.7) | | |
| Cell
differentiation | | | | | | 21.705 | <0.01 |
| High and
medium | 89 | 40 | (44.9) | 49 | (55.1) | | |
| Low | 81 | 10 | (12.3) | 71 | (87.7) | | |
| Tumor stage | | | | | | 32.759 | <0.01 |
| I | 76 | 39 | (51.3) | 37 | (48.7) | | |
| II | 81 | 11 | (13.6) | 70 | (86.4) | | |
| III and IV | 13 | 0 | (0) | 13 | (100) | | |
| Nodal status | | | | | | 24.525 | <0.01 |
| Positive | 66 | 6 | (9.1) | 60 | (90.9) | | |
| Negative | 104 | 47 | (45.2) | 57 | (54.8) | | |
 | Table IIImRNA expression of fibulin-4 in the
human cervical tissues. |
Table III
mRNA expression of fibulin-4 in the
human cervical tissues.
| N | Fibulin-4 mRNA | P-value |
|---|
| Control | 40 | 0.0096±0.0064 | |
| CIN | 60 | 0.0091±0.0048 | >0.05a |
| Carcinoma | 170 | 0.0769±0.0089 | <0.05b |
| Pathological
type | | | >0.05 |
| Squamous cell
carcinoma | 140 | 0.0648±0.0115 | |
|
Adenocarcinoma | 30 | 0.0796±0.0127 | |
| Cell
differentiation | | | <0.05 |
| High and
medium | 89 | 0.0284±0.0078 | |
| Low | 81 | 0.0932±0.0105 | |
| Tumor stage | | | <0.05 |
| I | 76 | 0.0267±0.0073 | |
| II | 81 | 0.0541±0.0081 | |
| III and IV | 13 | 0.0946±0.0126 | |
| Nodal status | | | <0.05 |
| Positive | 66 | 0.0979±0.0117 | |
| Negative | 104 | 0.0243±0.0059 | |
 | Table IVPredictive factors of survival by
multivariate analysis (Cox proportional hazards model). |
Table IV
Predictive factors of survival by
multivariate analysis (Cox proportional hazards model).
| Prognostic
factors | HR (95% CI) | P-value |
|---|
| Fibulin-4 | 1.019
(1.007–1.031) | 0.002 |
| Pathological
type | 0.978
(0.429–2.230) | 0.957 |
| Cell
differentiation | 1.012
(0.999–1.026) | 0.065 |
| Tumor stage | 3.175
(1.361–7.403) | 0.007 |
| Lymph node
metastasis | 2.129
(1.319–3.435) | 0.002 |
| Tumor size | 1.095
(0.986–1.216) | 0.089 |
| Age (years) | 0.981
(0.957–1.005) | 0.125 |
Differential expression of fibulin-4 in
the highly invasive subclones and the low-invasive subclones
The highly invasive subclones (HeLa-1 and SiHa-1)
and the low-invasive subclones (HeLa-25 and SiHa-23) were derived
from the HeLa and SiHa human cervical cancer cell lines, using the
limited dilution method. Since the cell lines have similar genetic
backgrounds, they are suitable for comparative analysis. As shown
in Fig. 3B–F, fibulin-4 protein and
mRNA expression levels were extremely high in the highly invasive
subclones (HeLa-1 and SiHa-1), compared to the low-invasive
subclones (HeLa-25 and SiHa-23).
Serum levels of fibulin-4 in human
cervical cancer patients and healthy control
As shown in Table V,
the serum fibulin-4 level in cervical carcinoma patients was much
higher than that in the healthy controls and CIN patients
(P<0.05). No significant difference was found between healthy
controls and CIN (P>0.05). Moreover, high serum levels of
fibulin-4 were associated with low differentiation, advanced stage
and positive lymph node status of the cervical carcinoma cases
(P<0.05). There were no significant differences among the
different pathological types of cervical carcinoma (P>0.05).
 | Table VSerum levels of fibulin-4 in the
patients with cervical tumors. |
Table V
Serum levels of fibulin-4 in the
patients with cervical tumors.
| N | Fibulin-4
(ng/ml) | P-value |
|---|
| Control | 40 | 108.43±11.86 | |
| CIN | 60 | 116.57±13.24 | >0.05a |
| Carcinoma | 170 | 356.49±22.15 | <0.05b |
| Pathological
type | | | >0.05 |
| Squamous cell
carcinoma | 140 | 267.26±16.54 | |
|
Adenocarcinoma | 30 | 271.32±18.61 | |
| Cell
differentiation | | | <0.05 |
| High and
medium | 89 | 106.93±10.22 | |
| Low | 81 | 347.56±23.47 | |
| Tumor stage | | | <0.05 |
| I | 76 | 109.84±13.51 | |
| II | 81 | 213.47±15.69 | |
| III and IV | 13 | 368.51±24.85 | |
| Nodal status | | | <0.05 |
| Positive | 66 | 357.34±20.06 | |
| Negative | 104 | 113.92±13.43 | |
Relationships of fibulin-4 with VEGF
expression and MVD
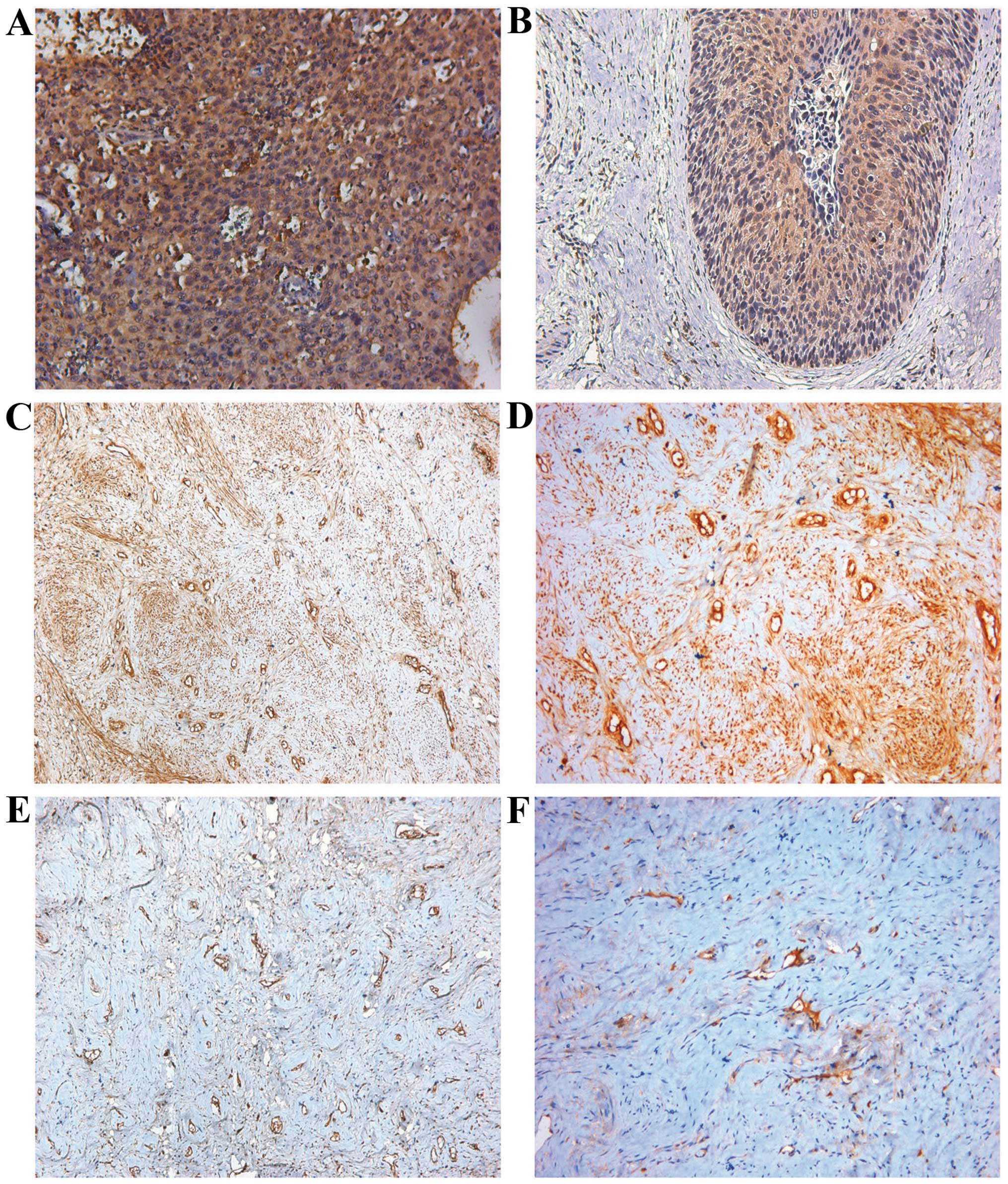
Fig. 4 shows the
representative immunohistochemical staining images for VEGF and
CD34. The immunohistochemical expression of VEGF and fibulin-4 was
evaluated using software Image-Pro Plus 6.0 to detect photodensity.
In brief, five positive fields in a section were selected at random
and then read using Image-Pro Plus 6.0, and the average densities
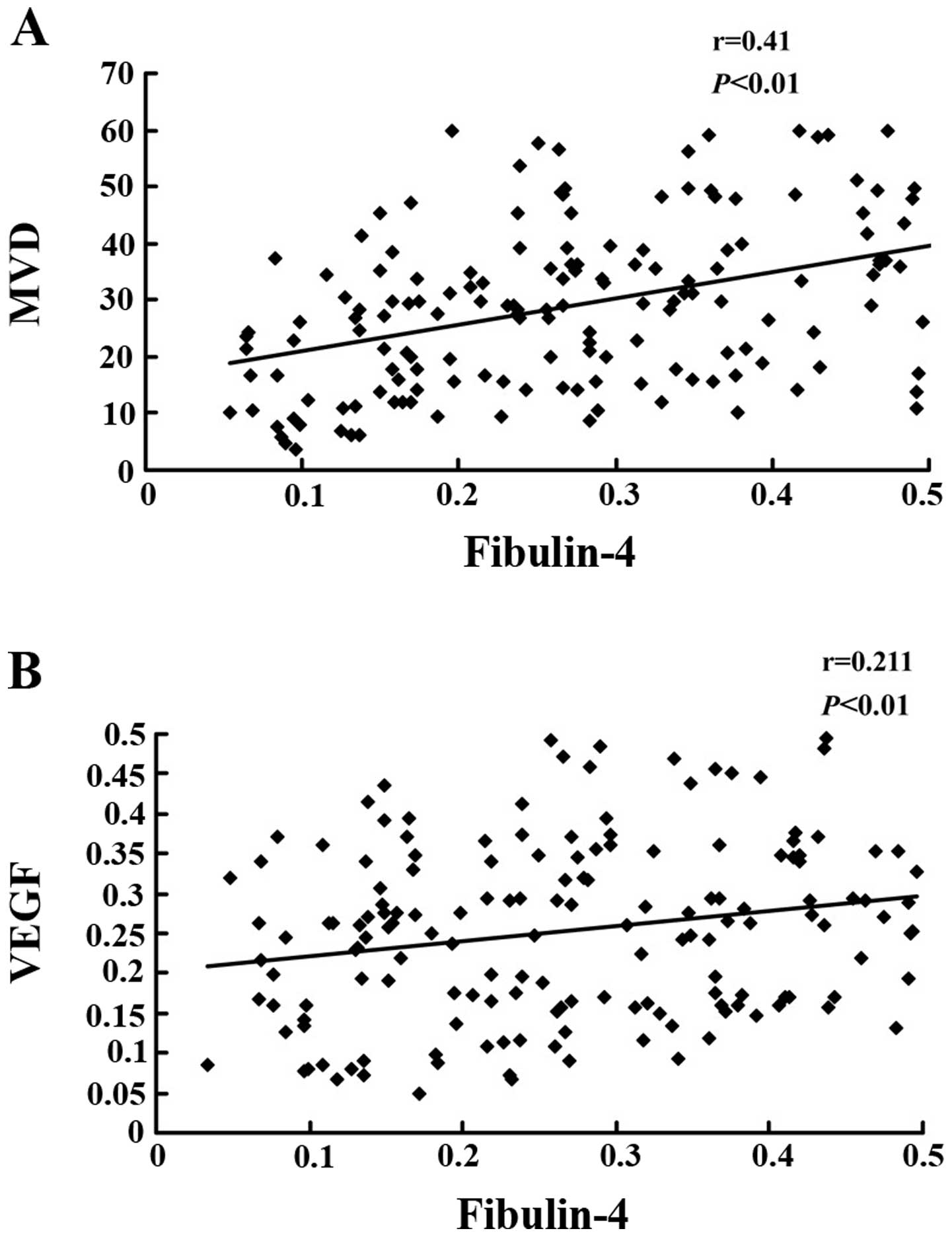
were then calculated. Pearson correlation tests of MVD (Fig. 5A, P<0.01) and VEGF expression
(Fig. 5B, P<0.01) vs. fibulin-4
revealed strong positive correlations.
Discussion
In the present study, we demonstrated for the first
time that the expression of fibulin-4 is associated with poor
prognostic clinicopathologic features, neovascularization and poor
outcome in human cervical carcinoma patients.
Our immunohistochemical studies showed an
upregulation of fibulin-4 expression in cervical carcinoma tissues,
compared with normal cervical tissues and CIN. Fibulin-4 is rich in
elastic fiber tissue, and is mainly involved in the synthesis and
arrangement of elastic fibers (39). In the present study, the expression
of fibulin-4 in the extracellular matrix was found to be much less
than that in the cancer cell cytoplasm, which probably was due to
the presence of fewer elastic fibers in cervical tissue. In the
basement membranes, however, we found stronger expression of
fibulin-4 in CIN when compared with the normal tissue. Real-time
RT-PCR experiments confirmed that mRNA expression of fibulin-4 was
also upregulated in the cervical carcinoma tissues. Moreover, high
fibulin-4 expression was associated with low differentiation,
advanced stage and positive lymph node status in the cervical
carcinomas. Similar results have been reported in earlier studies
on colon cancer; dysregulated expression of the fibulin-4 gene was
shown to be associated with human colon tumorigenesis (33). However, contrasting results have
also been reported for prostate cancer. By microarray analysis, the
fibulin-4 genes were found to be significantly downregulated in
prostate cancer and this result was corroborated by qRT-PCR
(22). In this study, fibulin-4 was
overexpressed in cervical cancer and was shown to play an important
role in tumor development. As is the case for other fibulins, there
are controversies in research on fibulin-4; these discrepancies may
be attributable to the fact that the tumor microenvironment
influences the functions of tumor-associated genes.
Angiogenesis is the process of formation of new
microvessels from preexisting vasculature. Once the tumor volume
exceeds a few millimeters in diameter, hypoxia and nutrient
deprivation trigger tumor cells to exploit their microenvironment
by releasing cytokines and growth factors, which then activate
normal, quiescent cells around them and initiate a cascade of
events resulting in tumor progression. For example, tumor
cell-derived VEGF stimulates the sprouting and proliferation of
endothelial cells. VEGF is considered the most potent candidate for
angiogenesis induction during tumor growth (40). Since angiogenesis is essential for
tumor growth and metastasis, controlling tumor-associated
angiogenesis is a promising strategy for inhibiting cancer
progression. In our study, we sought to determine whether fibulin-4
is associated with angiogenesis. To this end, the Pearson
correlation coefficient was calculated to assess the correlation of
fibulin-4 with MVD and VEGF expression. We found that fibulin-4
expression was positively correlated with MVD and VEGF expression,
which indicated that fibulin-4 may promote angiogenesis. No
previous studies concerning fibulin-4 have reported an association
with tumor angiogenesis, although its highly homologous proteins,
fibulin-3 and fibulin-5 were found to be associated with tumor
angiogenesis. Exogenous and endogenous fibulin-5 were shown to be
anti-angiogenic (41). Fibulin-3
was initially found to exert an anti-angiogenic effect (42), but in recent years, several studies
have reported that fibulin-3 can promote angiogenesis, particularly
in pancreatic adenocarcinoma and cervical cancer. Fibulin-3 gene
transfection elevates VEGF expression and MVD (16,17).
Since fibulin-4 is highly homologous to fibulin-3 and fibulin-5, we
speculate that fibulin-4 may play a significant role in tumor
angiogenesis. Pearson correlation tests of MVD and VEGF expression
versus the corresponding expression of fibulin-4 revealed strong
direct correlations. Hence, we conclude that fibulin-4 may promote
cervical tumor angiogenesis. However, further studies are needed to
confirm our speculation, including cell transfection experiments,
chorioallantoic membrane assays and tumor xenograft models in nude
mice.
High serum levels of fibulin-4 were found in
cervical carcinoma patients when compared with healthy controls and
CIN patients, and high fibulin-4 levels were associated with low
differentiation, advanced stage, and positive lymph node status in
cervical carcinomas. This discovery may aid in determining the
diagnosis and prognosis of cervical carcinoma. In recent years,
fibulins have been recognized as biomarkers for many diseases, such
as osteoarthritis, pleural mesothelioma and breast carcinoma.
Fibulin-3 and fibulin-4 may play pathogenic roles in osteoarthritis
(32,43). The plasma fibulin-3 and fibulin-1
levels were found to be elevated in patients with mesothelioma and
breast carcinoma, respectively (44,45).
Novel specific biomarkers can help detect diseases at an earlier
stage and tailor treatment strategies for individualized
management. Fibulin-4 may be exploited as a tool for the early
detection of cervical carcinoma.
In conclusion, fibulin-4 is a newly identified gene
that is overexpressed in cervical carcinoma, promotes angiogenesis,
and is associated with poor prognosis. Serum levels of fibulin-4
may be helpful in early diagnosis and determining the prognosis in
cases of cervical cancer. Fibulin-4 may also serve as a novel
therapeutic target in patients with cervical carcinoma.
Acknowledgements
This study was supported by the Independent
Innovation Foundation of Shandong University (2012TS088), the
National Nature Science Foundation of China (81202056), and the
Foundation of Shandong Provincial Science and Technology
Development Program (no. 2012G0021820). The funding organizations
had no role in the study design, data collection and analysis,
decision to publish or preparation of the manuscript.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Waggoner SE: Cervical cancer. Lancet.
361:2217–2225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen J, Shi D, Liu X, Fang S, Zhang J and
Zhao Y: Targeting SPARC by lentivirus-mediated RNA interference
inhibits cervical cancer cell growth and metastasis. BMC Cancer.
12:4642012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rao Y, Wang H, Fan L and Chen G: Silencing
MTA1 by RNAi reverses adhesion, migration and invasiveness of
cervical cancer cells (SiHa) via altered expression of p53, and
E-cadherin/β-catenin complex. J Huazhong Univ Sci Technolog Med
Sci. 31:1–9. 2011.PubMed/NCBI
|
|
5
|
Li Y, Wang W, Wang W, et al: Correlation
of TWIST2 up-regulation and epithelial-mesenchymal transition
during tumorigenesis and progression of cervical carcinoma. Gynecol
Oncol. 124:112–118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
de Vega S, Iwamoto T and Yamada Y:
Fibulins: multiple roles in matrix structures and tissue functions.
Cell Mol Life Sci. 66:1890–1902. 2009.PubMed/NCBI
|
|
7
|
Gallagher WM, Currid CA and Whelan LC:
Fibulins and cancer: friend or foe? Trends Mol Med. 11:336–340.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Law EW, Cheung AK, Kashuba VI, et al:
Anti-angiogenic and tumor-suppressive roles of candidate
tumor-suppressor gene, Fibulin-2, in nasopharyngeal carcinoma.
Oncogene. 31:728–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yi CH, Smith DJ, West WW and Hollingsworth
MA: Loss of fibulin-2 expression is associated with breast cancer
progression. Am J Pathol. 170:1535–1545. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schluterman MK, Chapman SL, Korpanty G,
Ozumi K, Fukai T, Yanagisawa H and Brekken RA: Loss of fibulin-5
binding to beta1 integrins inhibits tumor growth by increasing the
level of ROS. Dis Model Mech. 3:333–342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu Z, Ai Q, Xu H, et al: Fibulin-5 is
down-regulated in urothelial carcinoma of bladder and inhibits
growth and invasion of human bladder cancer cell line 5637. Urol
Oncol. 29:430–435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yue W, Sun Q, Landreneau R, Wu C,
Siegfried JM, Yu J and Zhang L: Fibulin-5 suppresses lung cancer
invasion by inhibiting matrix metalloproteinase-7 expression.
Cancer Res. 69:6339–6346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moll F, Katsaros D, Lazennec G, et al:
Estrogen induction and overexpression of fibulin-1C mRNA in ovarian
cancer cells. Oncogene. 21:1097–1107. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Greene LM, Twal WO, Duffy MJ, et al:
Elevated expression and altered processing of fibulin-1 protein in
human breast cancer. Br J Cancer. 88:871–878. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bardin A, Moll F, Margueron R, et al:
Transcriptional and posttranscriptional regulation of fibulin-1 by
estrogens leads to differential induction of messenger ribonucleic
acid variants in ovarian and breast cancer cells. Endocrinology.
146:760–768. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seeliger H, Camaj P, Ischenko I, et al:
EFEMP1 expression promotes in vivo tumor growth in human pancreatic
adenocarcinoma. Mol Cancer Res. 7:189–198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song EL, Hou YP, Yu SP, et al: EFEMP1
expression promotes angiogenesis and accelerates the growth of
cervical cancer in vivo. Gynecol Oncol. 121:174–180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song EL, Chen SG and Wang HQ: The
expression of EFEMP1 in cervical carcinoma and its relationship
with prognosis. Gynecol Oncol. 117:417–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu B, Thirtamara-Rajamani KK, Sim H and
Viapiano MS: Fibulin-3 is uniquely upregulated in malignant gliomas
and promotes tumor cell motility and invasion. Mol Cancer Res.
7:1756–1770. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kanda M, Nomoto S, Okamura Y, Hayashi M,
Hishida M, Fujii T, Nishikawa Y, Sugimoto H, Takeda S and Nakao A:
Promoter hypermethylation of fibulin 1 gene is associated with
tumor progression in hepatocellular carcinoma. Mol Carcinog.
50:571–579. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng YY, Jin H, Liu X, et al: Fibulin 1
is downregulated through promoter hypermethylation in gastric
cancer. Br J Cancer. 99:2083–2087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wlazlinski A, Engers R, Hoffmann MJ, et
al: Downregulation of several fibulin genes in prostate cancer.
Prostate. 67:1770–1780. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie L, Palmsten K, MacDonald B, et al:
Basement membrane derived fibulin-1 and fibulin-5 function as
angiogenesis inhibitors and suppress tumor growth. Exp Biol Med.
233:155–162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hwang CF, Chien CY, Huang SC, et al:
Fibulin-3 is associated with tumour progression and a poor
prognosis in nasopharyngeal carcinomas and inhibits cell migration
and invasion via suppressed AKT activity. J Pathol. 222:367–379.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sadr-Nabavi A, Ramser J, Volkmann J, et
al: Decreased expression of angiogenesis antagonist EFEMP1 in
sporadic breast cancer is caused by aberrant promoter methylation
and points to an impact of EFEMP1 as molecular biomarker. Int J
Cancer. 124:1727–1735. 2009. View Article : Google Scholar
|
|
26
|
Hu Y, Pioli PD, Siegel E, et al: EFEMP1
suppresses malignant glioma growth and exerts its action within the
tumor extracellular compartment. Mol Cancer. 10:1232011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim EJ, Lee SY, Woo MK, et al: Fibulin-3
promoter methylation alters the invasive behavior of non-small cell
lung cancer cell lines via MMP-7 and MMP-2 regulation. Int J Oncol.
40:402–408. 2012.PubMed/NCBI
|
|
28
|
Chen L, Sun B, Zhang S, et al: Influence
of microenvironments on microcirculation patterns and tumor
invasion-related protein expression in melanoma. Oncol Rep.
21:917–923. 2009.PubMed/NCBI
|
|
29
|
Argraves WS, Greene LM, Cooley MA and
Gallagher WM: Fibulins: physiological and disease perspectives.
EMBO Rep. 4:1127–1131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Berk DR, Bentley DD, Bayliss SJ, Lind A
and Urban Z: Cutis laxa: a review. J Am Acad Dermatol.
66:842.e1–17. 2012.PubMed/NCBI
|
|
31
|
Huang J, Yamashiro Y, Papke CL, et al:
Angiotensin-converting enzyme-induced activation of local
angiotensin signaling is required for ascending aortic aneurysms in
fibulin-4-deficient mice. Sci Transl Med. 5:183ra581–11.
2013.PubMed/NCBI
|
|
32
|
Xiang Y, Sekine T, Nakamura H, et al:
Fibulin-4 is a target of autoimmunity predominantly in patients
with osteoarthritis. J Immunol. 176:3196–3204. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gallagher WM, Greene LM, Ryan MP, Sierra
V, Berger A, Laurent-Puig P and Conseiller E: Human fibulin-4:
analysis of its biosynthetic processing and mRNA expression in
normal and tumour tissues. FEBS Lett. 489:59–66. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ying J, Shan L, Li J, et al: Genome-wide
screening for genetic alterations in esophageal cancer by aCGH
identifies 11q13 amplification oncogenes associated with nodal
metastasis. PLoS One. 7:e397972012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ormandy CJ, Musgrove EA, Hui R, Daly RJ
and Sutherland RL: Cyclin D1, EMS1 and 11q13 amplification in
breast cancer. Breast Cancer Res Treat. 78:323–335. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soumaoro LT, Uetake H, Higuchi T, Takagi
Y, Enomoto M and Sugihara K: Cyclooxygenase-2 expression: a
significant prognostic indicator for patients with colorectal
cancer. Clin Cancer Res. 10:8465–8471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weidner N, Folkman J, Pozza F, et al:
Tumor angiogenesis: a new significant and independent prognostic
indicator in early-stage breast carcinoma. J Natl Cancer Inst.
84:1875–1887. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Landis JR and Koch GG: The measurement of
observer agreement for categorical data. Biometrics. 33:159–174.
1977. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
McLaughlin PJ, Chen Q, Horiguchi M, et al:
Targeted disruption of fibulin-4 abolishes elastogenesis and causes
perinatal lethality in mice. Mol Cell Biol. 26:1700–1709. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Weis SM and Cheresh DA: Tumor
angiogenesis: molecular pathways and therapeutic targets. Nat Med.
17:1359–1370. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yanagisawa H, Schluterman MK and Brekken
RA: Fibulin-5, an integrin-binding matricellular protein: its
function in development and disease. J Cell Commun Signal.
3:337–347. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Albig AR, Neil JR and Schiemann WP:
Fibulins 3 and 5 antagonize tumor angiogenesis in vivo. Cancer Res.
66:2621–2629. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Henrotin Y, Gharbi M, Mazzucchelli G,
Dubuc JE, De Pauw E and Deberg M: Fibulin 3 peptides Fib3-1 and
Fib3-2 are potential biomarkers of osteoarthritis. Arthritis Rheum.
64:2260–2267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pass HI, Levin SM, Harbut MR, et al:
Fibulin-3 as a blood and effusion biomarker for pleural
mesothelioma. N Engl J Med. 367:1417–1427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Piura E and Piura B: Autoantibodies to
tumor-associated antigens in breast carcinoma. J Oncol.
2010:2649262010. View Article : Google Scholar : PubMed/NCBI
|