Introduction
Chondrosarcoma is a malignant cartilage-forming
tumor which accounts for ~20% of bone malignancies (1). The conventional chondrosarcoma is
associated with a significant rate of morbidity and the 10-year
survival rate is low (29–83%) (1,2). In
addition to surgical resection which is the primary treatment
option for patients with chondrosarcoma, conventional chemotherapy
and radiotherapy are still under investigation for treatment
options (2–4). It is known that most chemotherapy
drugs for the treatment of chondrosarcoma are associated with
strong toxicities for normal cells, yet, tumor cells remain
drug-resistant (3). Currently,
chondrosarcomas are relatively chemotherapy- and
radiotherapy-resistant. A recent study reported that the growth of
chondrosarcoma cells can be inhibited by mTOR inhibitor in an in
vivo syngeneic rat model (5),
suggesting a putative chemotherapeutic approach for clinical
applications.
Doxorubicin (Dox) is an antitumor drug that is used
frequently in chemotherapy for a variety of solid tumors (6). Similar to other chemotherapeutic
agents, the efficacy of doxorubicin treatment is limited by drug
resistance (7). Despite
considerable clinical responses initially, the majority of patients
develop resistance to doxorubicin. Although the underlying
mechanism of doxorubicin resistance is not fully understood,
researchers have determined several factors that influence cellular
doxorubicin toxicity. It has been reported that doxorubicin can
induce ROS generation in various tumor cells (8,9) and
inhibition of P-glycoprotein by 20(S)-Rh2 attenuates adriamycin
resistance (10).
Cancer cells, unlike their normal counterparts, use
aerobic glycolysis with reduced mitochondrial oxidative
phosphorylation for glucose metabolism (11). Therefore, the metabolic switch of
cancer cells suggests that targeting metabolic pathway could be a
selective approach not only to treat cancer patients, but also to
override the chemoresistance. It has been reported that the
combination of WZB117 which is a Glut1 inhibitor and cisplatin or
paclitaxel displayed synergistic anticancer effects (12). Another GLUT1 inhibitor phloretin
significantly enhanced daunorubicin’s anticancer effects under
hypoxia (13). Moreover, 3-BrPA
which is a glycolysis inhibitor partially reversed the resistant
phenotype and re-sensitized cells to oxaliplatin and 5-fluorouracil
(14). Lactate dehydrogenase-A
(LDHA) is one of the main isoforms of LDH expressed in breast
tissue, controlling the conversion of pyruvate to lactate of the
cellular glycolytic process. Studies have shown that the LDHA
expression in cancer cells is associated with radiotherapy
sensitivity (15). In addition, it
has been reported LDHA contributed to paclitaxel resistance in
breast cancer, inhibition of LDHA by oxamate overrides the
chemoresistance (16).
In the present study, we reported a novel
therapeutic approach in the treatment of chondrosarcoma cells. LDHA
is highly active in chondrosarcoma cell lines compared with normal
chondrocytes. Doxorubicin treatment induced the LDHA expression
which contributes to the doxorubicin resistance. Meanwhile, the
activity and expression of LDHA were upregulated in
doxorubicin-resistant cells. Moreover, our data showed a strong
correlation between glucose metabolism and doxorubicin resistance
in chondrosarcoma cells. Doxorubicin-resistant cells displayed
highly activated glucose metabolism and depended more on glucose
supply. Finally, we reported a synergistic effect produced by
incorporating doxorubicin with glycolysis inhibitors-oxamate in the
combined treatment of chondrosarcoma cells in vitro and
in vivo.
Materials and methods
Cell lines and cell culture
Hs 819.T and SW1353 human chondrosarcoma cells were
purchased from ATCC. CHON-001 and CHON-002 human normal chondrocyte
cell lines were purchased from ATCC. Primary human chondrocyte was
purchased from Promocell.com. All cells were cultured in RPMI-1640
supplemented with 10% FBS and 1X penicillin-streptomycin-glutamine
(10378-016; Invitrogen) at 37°C in a humidified incubator with 95%
air and 5% CO2. All primary human chondrosarcoma patient
specimens were obtained from patients undergoing surgery for tumor
from 2009 to 2012 at the Cancer Research Center, The 101st Hospital
of PLA, Wuxi, Jiangsu, China and stored in liquid nitrogen until
analysis. All patients provided written informed consent. The study
was approved by the Ethics Committee of the Cancer Research Center,
The 101st Hospital of PLA, Wuxi.
Antibodies and reagents
Antibodies used in this study were purchased from:
LDHA (Cell Signaling Technology #2012); β-actin (Cell Signaling
Technology #4967); doxorubicin and oxamate were purchased from
Sigma-Aldrich (St. Louis, MO, USA).
siRNA transfections
Transfection was performed using the Oligofectamine
Transfection reagent (Invitrogen) according to the manufacturer’s
protocol. siRNA oligonucleotides for LDHA were purchased from
Sigma, with a scrambled siRNA (Sigma) used as a control.
Forty-eight hours after transfection, whole-cell lysates were
prepared for further analysis.
Western blot analysis
Whole cells were lysed in 1X SDS sample buffer and
resolved by electrophoresis using SDS-PAGE and transferred to
nitrocellulose membranes. The membranes were probed with primary
antibodies overnight, and then incubated with appropriate
horseradish peroxidase conjugated secondary antibodies for 3 h
followed by detection with a SuperSignal enhanced chemiluminescence
kit (Pierce, Rockford, IL, USA). For sequential blotting, the
membranes were stripped with Stripping Buffer (Pierce) and
re-probed with proper antibodies.
Cell viability
Cell viabilities were determined using trypan blue
dye exclusion assays. A total of 5×104–1×105
cells/well were seeded in 12-well plates. Twenty-four hours later,
the medium was replaced with different concentrations of
doxorubicin or oxamate. After treatments with multiple
concentrations of drugs, cells were trypsinized and resuspended in
PBS. Viable cell numbers were determined by trypan blue
staining.
Generation of doxorubicin-resistant cell
line
SW1353 cells were treated with gradually increasing
concentrations of doxorubicin in regular cell culture conditions
for the selection of resistant cells. After successive treatments
for up to 3 months, resistant cell clones were pooled and used for
all subsequent experiments in the present study. The resistant
cells were treated by doxorubicin each month for repeating
selection.
Real-time PCR
RNA was extracted from cancer cells using the TRIzol
reagent (Invitrogen, Carlsbad, CA, USA). The cDNA synthesis was
performed using a SuperScript First-Standard Synthesis System for
RT-PCR (Invitrogen) according to the manufacturer’s protocol.
Quantitative PCR analyses were performed using Assay-on-Demand
primers and the TaqMan Universal PCR Master Mix reagent (Applied
Biosystems, Foster City, CA, USA). The samples were analyzed using
an ABI Prism 7700 Sequence Detection System (Applied Biosystems).
The primers for q-PCR were: LDHA: forward,
5′-TGGAGTGGAATGAATGTTGC-3′ and reverse, 5′-ATAGCCCAGGATGTGTAGCC-3′.
The expression levels of β-actin were used to normalize the
relative expression levels. Experiments were triplicated.
Glucose uptake assay
Cells were seeded in 12-well plates at
1×105–3×105 cells/well. Culture media was
collected at 48 h and stored at −20°C until assayed. Glucose uptake
was measured using an Amplex Red Glucose/Glucose Oxidase assay kit
(Molecular Probes). Absorbance was measured at 563 nm using a
SpectraMax M5 plate reader (Molecular Devices) and the results were
normalized to the amount of total protein compared with the control
cells.
Lactate production assay
Lactate production in the medium was detected by
using a Lactate assay kit (BioVision). Results were normalized to
the amount of total protein compared with the control cells.
LDHA activity assay
The total LDHA activity in cell lysates was examined
according to the manufacturer’s instructions of the LDH
cytotoxicity assay kit (BioVision). Briefly, 2×105 cells
were seeded in a 24-well plate one day before assaying and all
samples were analyzed in triplicate. Then, cells were collected,
washed and extracted for protein to measure LDHA activity. Results
were normalized to the amount of total protein compared with the
control cells.
Colony formation assay
For chondrosarcoma cell colony formation assay, 500
cells were seeded on a 10-cm dish with drugs in regular cell
culture medium. The medium was refreshed every two days with drugs.
Cells were grown for three weeks and the surviving colonies were
stained with gentian violet after methanol fixation, and visible
colonies (>50 cells) were counted. Colonies from
randomly-selected image areas of three replicate wells were
enumerated.
Animal experiments
Athymic BALB/c nude mice (5–8 weeks old) were housed
in the Biological Resource Centre of the Department of
Orthopaedics, The 101st Hospital of the People’s Liberation Army.
Mice were implanted subcutaneously in both sides of the flank with
3×106 chondrosarcoma cells without or with doxorubicin
resistance, respectively. When the tumor reached a size of >150
mm3, the mice were randomly divided into groups (8 mice
per group) with the indicated treatments. Mice were weighed weekly
and tumor diameters were measured with calipers twice per week for
>4 weeks. Tumor progress was monitored by tumor size
measurements every other day. All the experiments involving mouse
models complied with both Chinese laws and the guidelines of the
Ethics Committee of Jiangsu Institutes for Biological Sciences.
Statistical analysis
The unpaired Student’s t-test was used for the data
analysis. All data are shown as mean ± standard error (SE).
P<0.05 was considered to indicate a statistically significant
difference.
Results
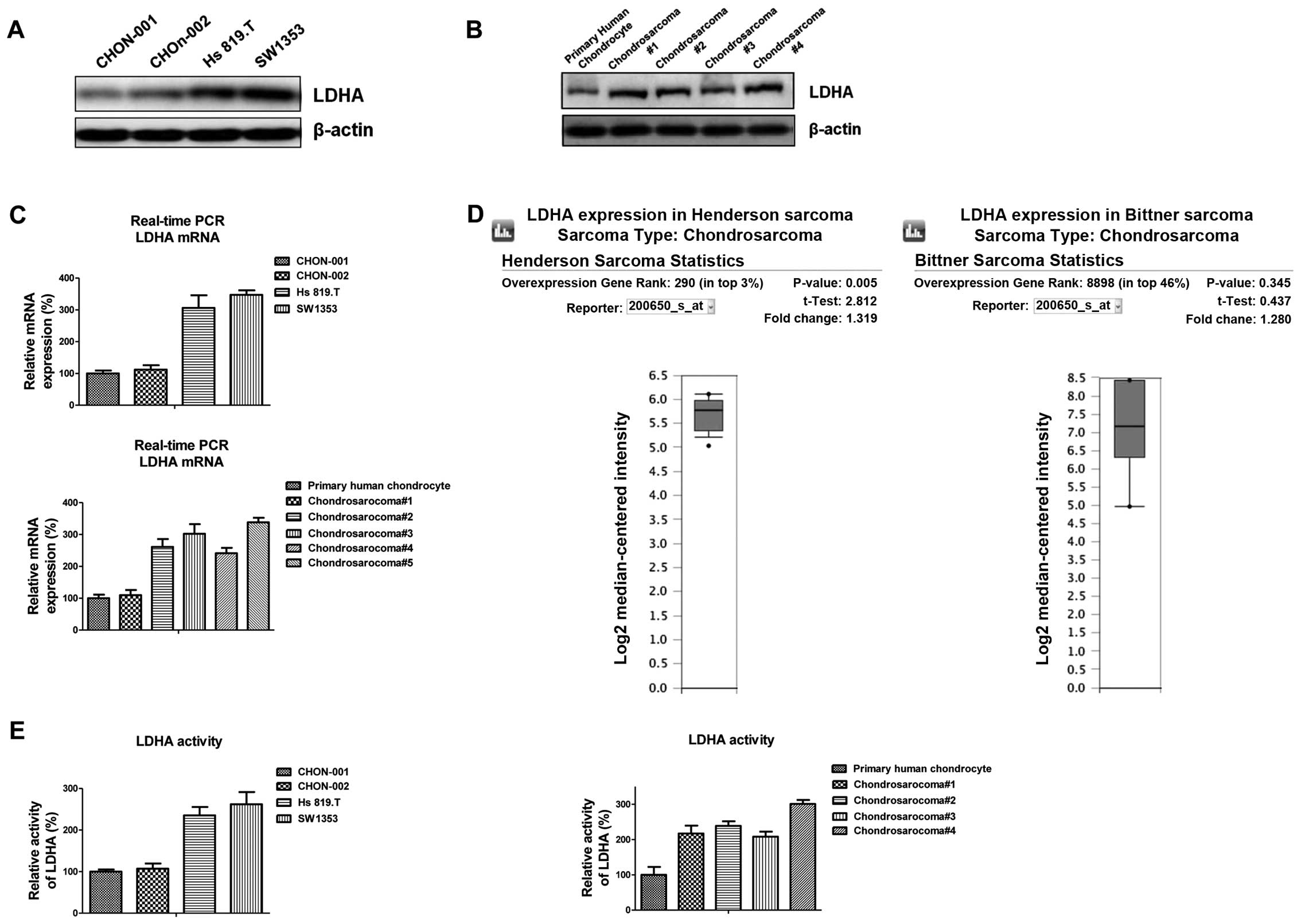
LDHA is highly activated in
chondrosarcoma cells
Cancer cells, unlike their normal counterparts, use
aerobic glycolysis with reduced mitochondrial oxidative
phosphorylation for glucose metabolism (11). Since LDHA is an important key enzyme
of glycolysis, we first measured the LDHA expression in two
malignant chondrosarcoma cell lines compared with benign human
chondrocyte cell lines, CHON-001 and CHON-002. Notably, LDHA was
significantly upregulated in chondrosarcoma cells (Fig. 1A). Consistently, the expression of
LDHA in chondrosarcoma patient tumor samples was also upregulated
compared with primary human chondrocyte (Fig. 1B), indicating the elevated LDHA
expression might be the target for chondrosarcoma chemotherapy.
Real-time PCR results showed the mRNA of LDH-A was also increased
in chondrosarcoma cells (Fig. 1C).
Bioinformatics research through Oncomine.com revealed the
expression of LDHA was upregulated in previously published
chondrosarcoma microarray database (Fig. 1D). We further compared the
activities of LDHA in chondrosarcoma cells and normal chondrocytes.
As we expected, the activities of LDHA were higher in malignant
chondrosarcoma cells than in normal chondrocytes (Fig. 1E). Taken together, our results
suggested the increased activity of LDHA might be an important
biomarker for the clinical treatment of chondrosarcoma
patients.
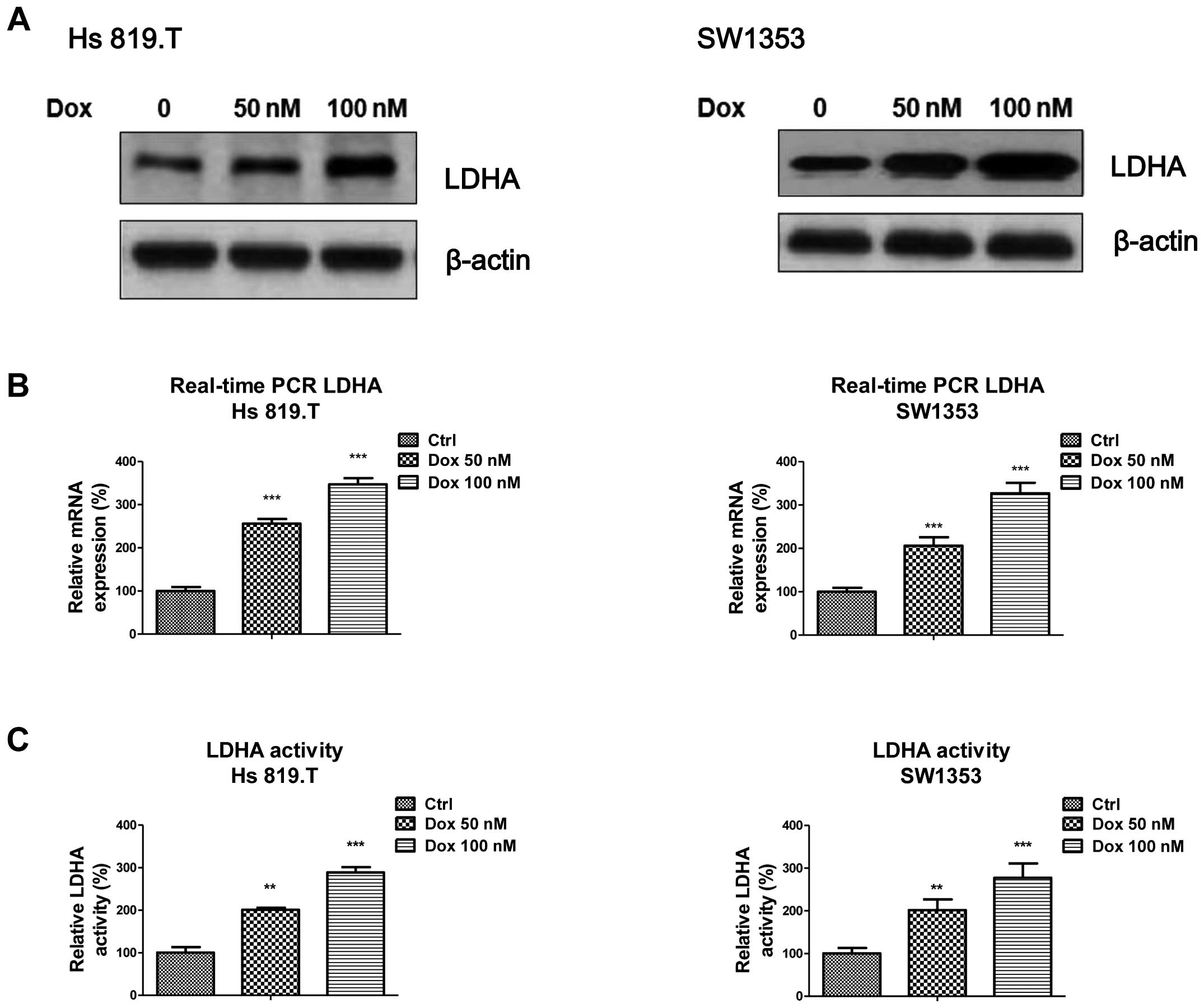
Doxorubicin treatment at low-toxic dosage
induces LDHA expression and activity
It has been reported that doxorubicin acts as a
chemotherapeutic agent against chondrosarcoma cells (17). However, resistance to doxorubicin
represents a major obstacle to successful treatment. To explore the
biological significance of elevated LDHA in chondrosarcoma cells,
we treated chondrosarcoma cells with doxorubicin at a low
concentration which does not induce apoptosis sharply for 72 h
followed by the measurement of LDHA expression. Fig. 2A shows the protein expressions of
LDHA were significantly upregulated by doxorubicin treatments at
low-toxic concentrations in two chondrosarcoma cell lines. The mRNA
levels as well as the kinase activities of LDHA in response to
multiple doxorubicin treatments were also upregulated (Fig. 2B and C) suggesting LDHA and glucose
metabolism are involved in chemotherapy in chondrosarcoma
cells.
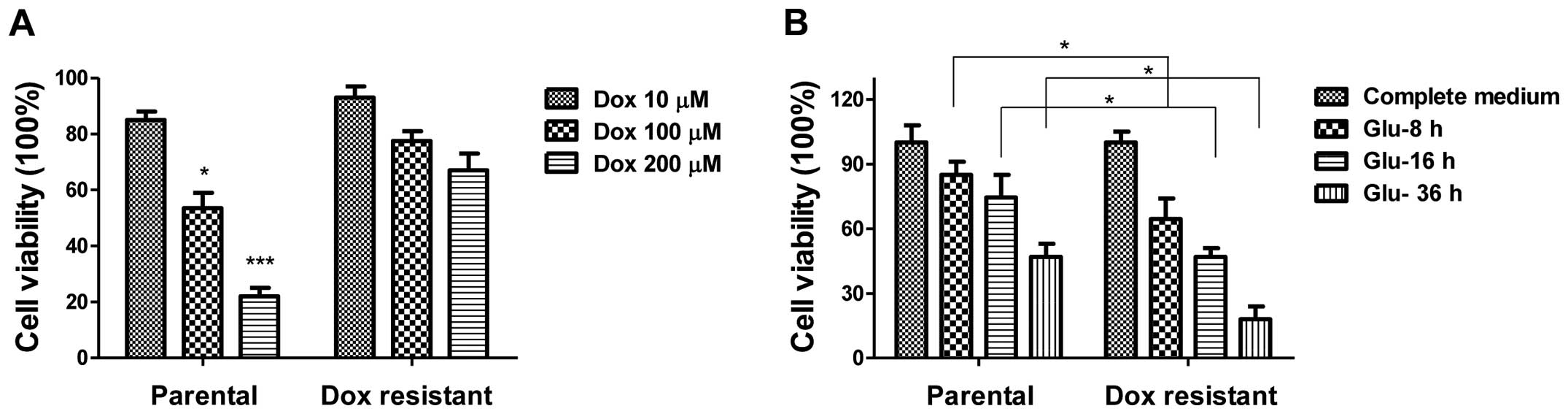
Doxorubicin-resistant chondrosarcoma
cells are more dependent on glucose
To further explore the biological significance of
elevated LDHA in chondrosarcoma cells, we generated
doxorubicin-resistant cell line using SW1353 parental cells by
gradually increasing concentrations of doxorubicin in cell culture
medium for a selection of resistant cells. After successive
treatments for a duration of three months, several
doxorubicin-resistant cell clones were developed and pooled for the
following experiments. To verify the resistance, parental cells and
resistant pool cells were treated with doxorubicin at multiple
concentrations for 72 h. As expected, cell viability assays showed
that SW1353 doxorubicin-resistant cells could tolerate much higher
concentrations of doxorubicin compared with sensitive cells which
exhibited significant inhibition of viability at 100 and 200 μM
(Fig. 3A). As our above results
showed glucose metabolism was highly correlated with doxorubicin
treatments (Fig. 3), we
investigated the susceptibility of doxorubicin-resistant cells
under glucose starvation. SW1353 doxorubicin-resistant cells
exhibited more sensitivity to glucose depletion compared with
SW1353 parental cells (Fig. 3B).
Under low glucose conditions, cell viability of
doxorubicin-resistant cells was decreased more than parental cells
(~25%), suggesting the glucose metabolism in resistant cells was
higher than in sensitive cells and might be targets for clinical
therapeutic agents.
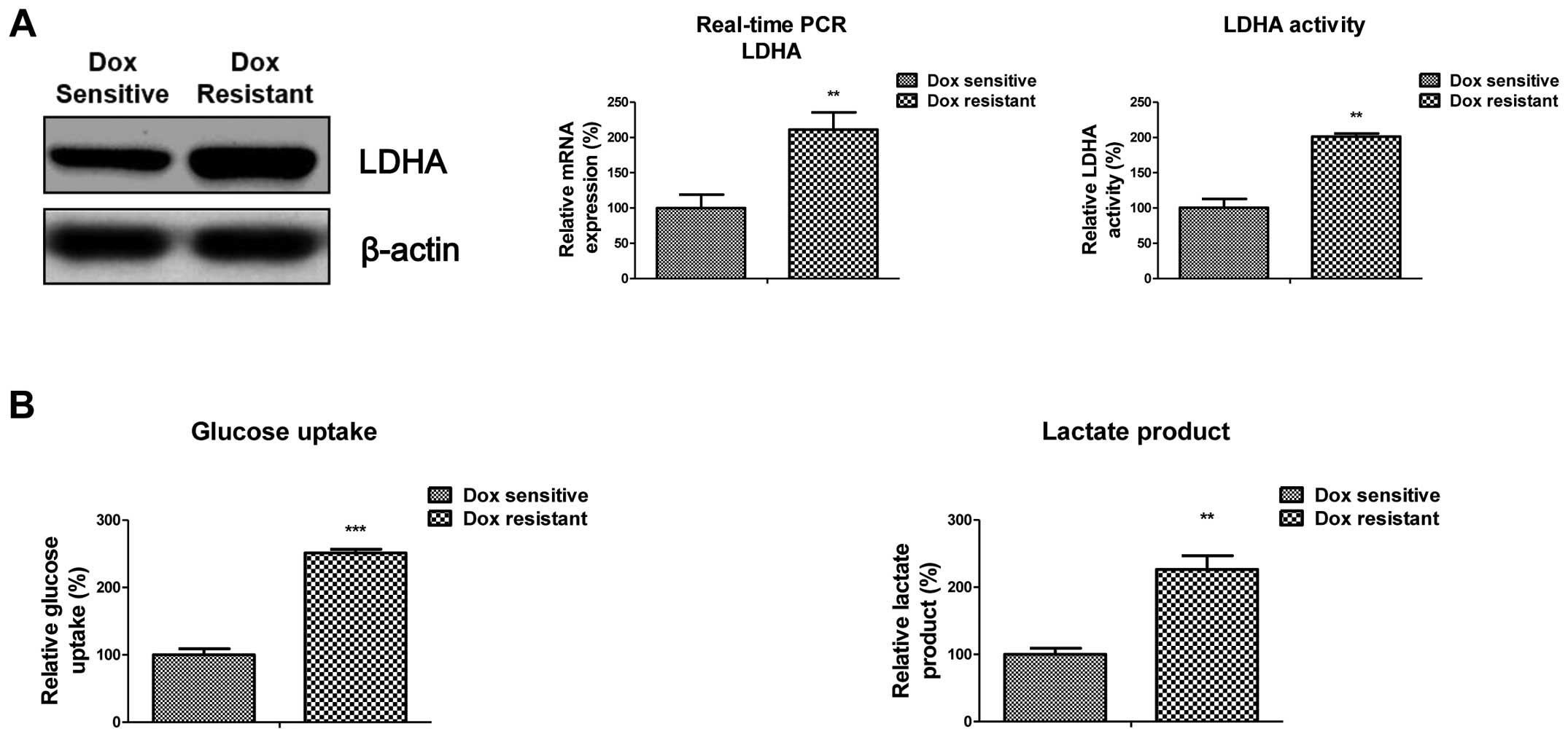
Increased expression and activity of LDHA
in doxorubicin-resistant cells
To examine the role of LDHA in mediating doxorubicin
resistance in human chondrosarcoma cells, the expression of LDHA
was examined in SW1353 parental and doxorubicin-resistant cells
(DoxR). We found that LDHA levels were markedly increased in SW1353
DoxR cells, compared to their parental cells (Fig. 4A, left panel). The mRNA and activity
of LDHA were also increased ~2-fold in resistant cells, compared to
parental cells (Fig. 4A, middle and
right panel). These results indicated that doxorubicin resistance
was correlated with the increased LDHA expression and activity.
Notably, SW1353 DoxR cells showed upregulated glucose metabolism.
The glucoses uptake and lactate product were significantly
increased in resistant cells (Fig.
4B) indicating the metabolic changes contributed to doxorubicin
resistance in chondrosarcoma cells.
Inhibition of LDHA re-sensitizes
doxorubicin-resistant cells
The increased LDHA expression and LDHA activity
detected in doxorubicin-resistant cells suggested that LDHA might
play a critical role in doxorubicin resistance. We hypothesized
that the downregulation of LDH-A by siRNA might re-sensitize
resistant cells to doxorubicin. Therefore, to further verify the
effects of LDHA downregulation in the doxorubicin-induced
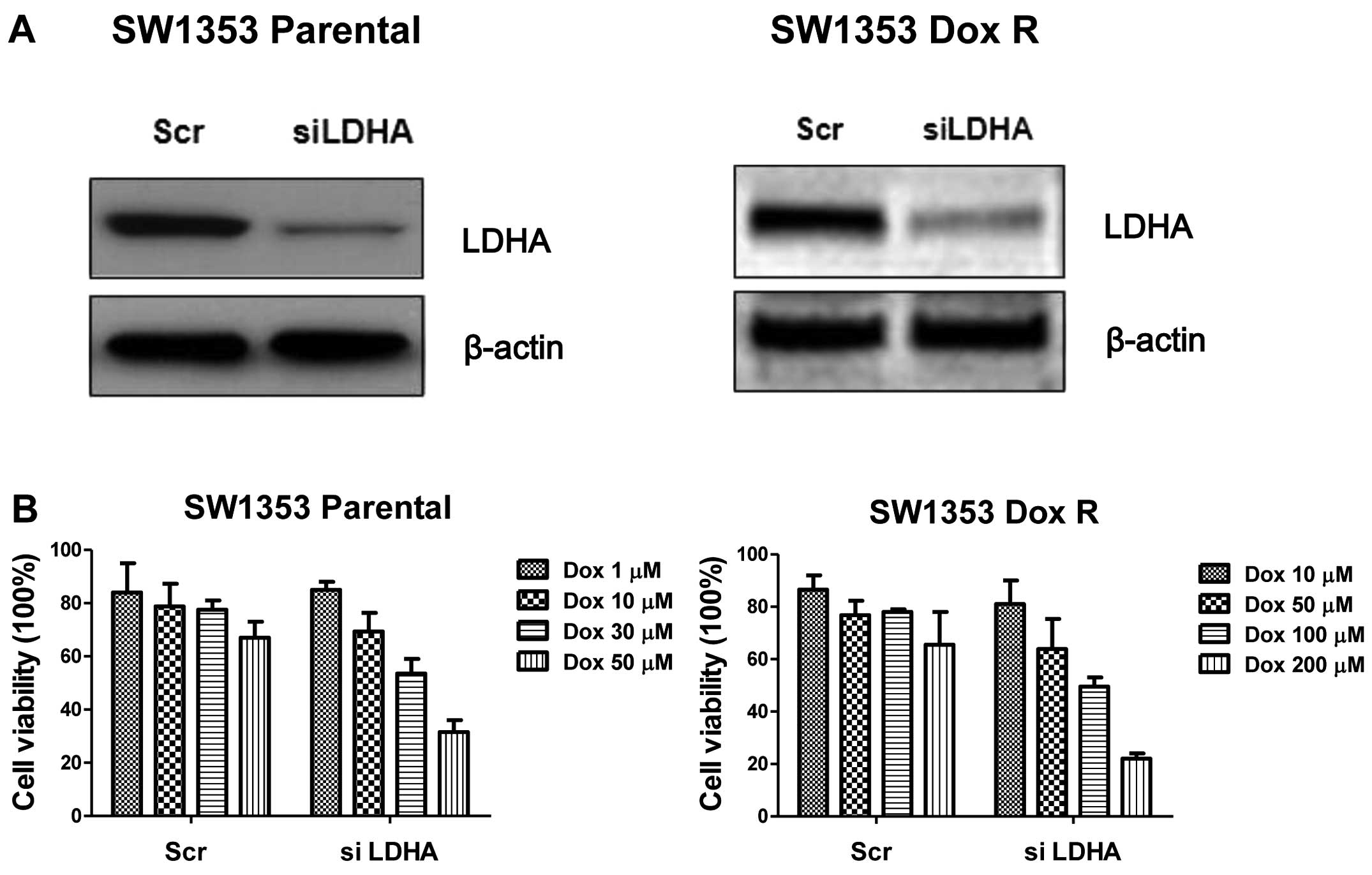
chemotherapy, we knocked down LDHA by specific siRNA in SW1353
parental cells and DoxR cells (Fig.
5A). Consistently, knockdown of LDHA significantly enhanced the
sensitivity of chondrosarcoma cells to doxorubicin treatments in
both parental and resistant cells (Fig.
5B). Knockdown of LDHA decreased the cell viability in parental
cells by 30% at 30 μM and the suppression of cell viability in
doxorubicin-resistant cells was 40% at 200 μM. Since LDHA is a
critical enzyme in the glycolytic pathway, our results demonstrated
LDHA plays an important role in doxorubicin resistance of
chondrosarcoma cells, serving as a promising therapeutic target for
overcoming doxorubicin resistance.
The combination of glycolysis inhibitor
with doxorubicin shows synergistic inhibitory effects on
chondrosarcoma cells in vitro and in vivo
Oxamate is a pyruvate analog that directly inhibits
the converting process of pyruvate to lactate by LDHA to inhibit
cell glycolysis (16). Since
downregulation of LDHA by siRNA significantly inhibited the
viability of the doxorubicin-resistant chondrosarcoma cells, we
further investigated the effects of combining doxorubicin with
glycolysis inhibitor oxamate on the treatment of
doxorubicin-resistant chondrosarcoma cells. In both SW1353 parental
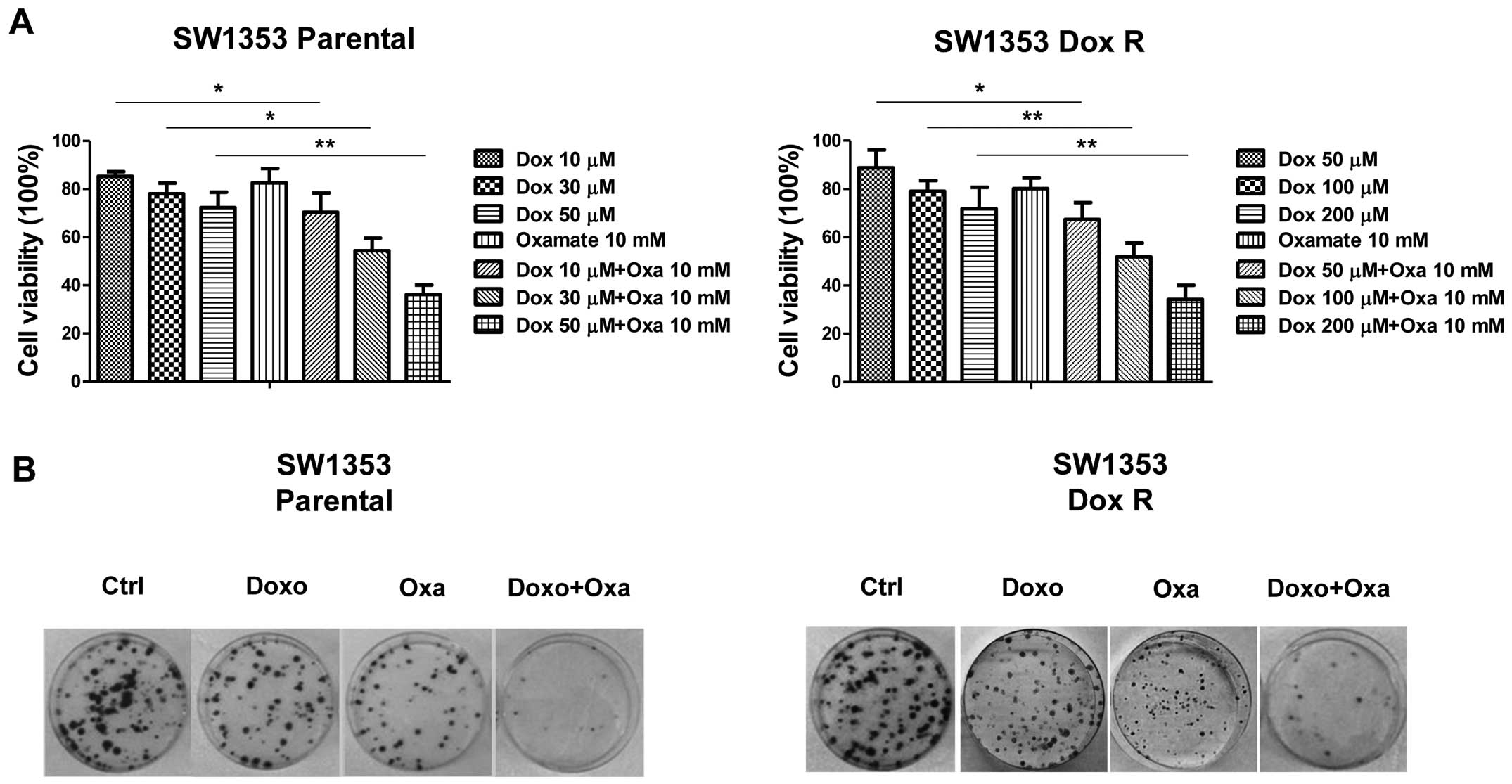
cells and doxorubicin-resistant cells, doxorubicin combined with
oxamate was considerably more effective in inhibiting cell
viability compared with either agent administered alone (Fig. 6A). To further strengthen our results
from the cell viability assay, we performed colony formation assay
for the detection of clonogenicity of chondrosarcoma cells under
treatments of doxorubicin alone, oxamate alone and the combination
of doxorubicin and oxamate. Consistent results from Fig. 6B show significantly increased colony
formation inhibition in fourteen days under the treatments of the
combination of oxamate and doxorubicin compared with control and
treatment with doxorubicin or oxamate alone.
To verify our in vitro results that
inhibition of LDHA by siRNA and inhibitor resulted in the
re-sensitization of doxorubicin-resistant cells to doxorubicin, we
studied the effects of the treatments by the combination of
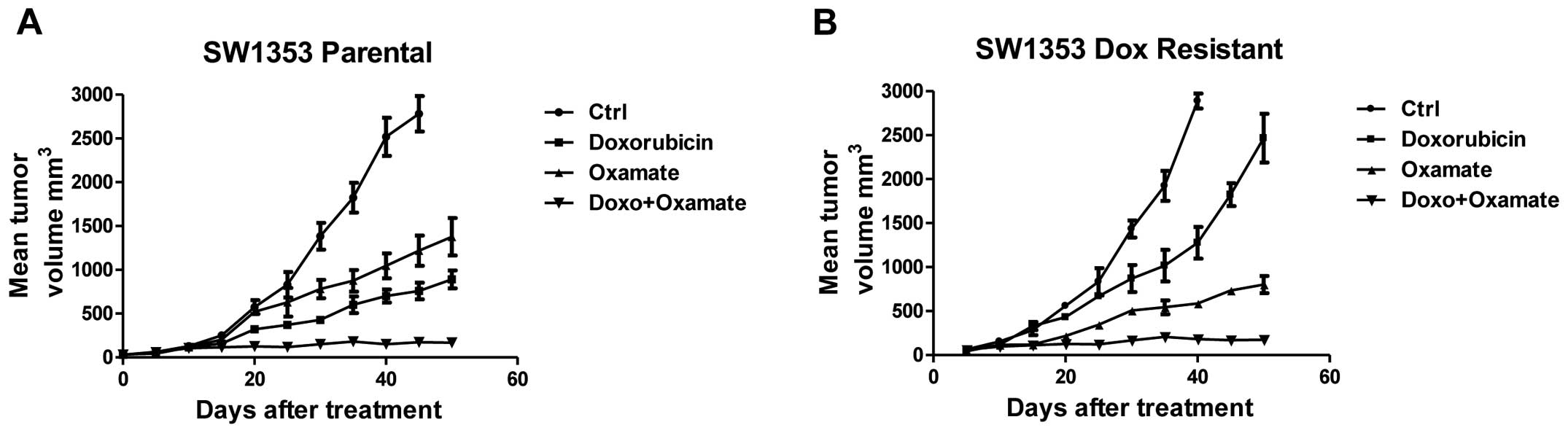
doxorubicin with oxamate on the xenograft tumor growth in nude
mice. We injected SW1353 parental cells or DoxR cells into nude
mice and treated mice with doxorubicin alone, oxamate alone and
doxorubicin with oxamate. After five weeks, mice with the
combination treatment had significantly attenuated tumor sizes
compared with the treatment alone (Fig.
7), indicating the combination of doxorubicin and oxamate
result in strong tumor growth inhibition. Collectively, these
results obtained from both in vitro and in vivo
experiments established that the combination of doxorubicin with
LDHA inhibitor has a greater capacity to inhibit
doxorubicin-resistant chondrosarcoma cells compared to either agent
alone and might lead to a therapeutic aspect for human
chondrosarcoma patients.
Discussion
Approximately 20% of skeletal system cancers are
chondrosarcomas derived from transformed cells that produce
cartilage (1). However,
conventional chemotherapy has very limited efficacy in patients
with advanced chondrosarcoma and it is not considered active
treatment in clinical trial in addition to surgery which is the
primary treatment for this chondrosarcoma (2,3). To
date, the highest benefit from chemotherapy observed is in
mesenchymal and dedifferentiated chondrosarcoma. Therefore,
developments of effective and low-toxic therapeutic approaches are
required to improve chondrosarcoma clinical management. Evasion of
programmed cell death or apoptosis has been recognized as one of
the main alterations that dictate malignant growth and is a
hallmark of most types of cancer. In the present study, we first
reported glucose metabolism is highly active in chondrosarcoma
cells compared with normal chondrocytes which may lead us to induce
cancer cell apoptosis by inhibiting the key enzymes in the glucose
metabolism pathway.
Doxorubicin is frequently used in chemotherapy for a
variety of solid tumors, but the efficacy of doxorubicin treatment
is limited by drug resistance. Multiple mechanisms responsible for
the drug-resistant phenotype in cancer cells have been recognized.
The most common is characterized by the enhanced expression of the
P-glycoprotein, ABCB1 which is a transmembrane pump responsible for
drug efflux from cells (18).
Recently, another study described that loss of HuR, which is an RNA
binding protein involved in the post-transcriptional regulation of
a wide spectrum of mRNAs, is responsible for the doxorubicin
resistance in breast cancer (19).
Restoration of HuR expression in breast cancer cells re-sensitized
resistant cells to doxorubicin (19). Therefore, the purpose of the present
study was to find new, targeted treatment strategies to overcome
chemoresistance for clinical chondrosarcoma patients.
Cancer cells depend mostly on glycolysis, the
anaerobic breakdown of glucose into the energy-storing molecule
ATP, even in the presence of available oxygen while normal cells
rely primarily on the process of mitochondrial oxidative
phosphorylation (11). Therefore,
we sought to use these unique bioenergetic properties to improve
the therapeutic efficacy to inhibit cancer cells. The combination
of Taxol and oxamate was previously reported to enhance the
therapeutic effects on human breast cancer cells (16). In this study, we investigated the
role of LDHA in the acquired doxorubicin resistance in human
chondrosarcoma cells. We identified that compared to parental
cells, doxorubicin-resistant cells possess an increased expression
and activity of LDHA. Downregulation of LDHA resulted in an
increased sensitivity of doxorubicin-resistant cells. In addition,
compared to parental cells, doxorubicin-resistant cells showed a
higher sensitivity to the LDHA inhibitor oxamate. Notably, we found
inhibition of LDHA significantly inhibited chondrosarcoma cell
viability when combined with doxorubicin in vitro and in
vivo, demonstrating the important roles of LDHA in overcoming
chemoresistance in chondrosarcoma cells. In our next study, we will
focus on the mechanisms by which highly active glucose metabolism
makes chondrosarcoma cells evade apoptosis in response to
doxorubicin treatment. Proteomic approaches have been applied to
identify the putative LDHA interaction proteins under the treatment
of doxorubicin, and, more importantly, we will explore more novel
glycolysis inhibitors which contribute to the therapeutic effects
on the treatment of chondrosarcoma patients. In general, the
results of the present study demonstrated that LDHA plays an
important role in doxorubicin resistance and that it may
potentially serve as a therapeutic target for overcoming
chemoresistance in chondrosarcoma patients.
Acknowledgements
The authors thank the staff and faculty working in
the Department of Orthopaedics, The 101st Hospital of the People’s
Liberation Army. We thank Dr Xiangyong Li from the Department of
Hematology and Oncology, The 101st Hospital of the People’s
Liberation Army for the editorial assistance.
References
|
1
|
Gelderblom H, Hogendoorn PC, Dijkstra SD,
van Rijswijk CS, Krol AD, Taminiau AH and Bovée JV: The clinical
approach towards chondrosarcoma. Oncologist. 13:320–329. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fiorenza F, Abudu A, Grimer RJ, Carter SR,
Tillman RM, Ayoub K, Mangham DC and Davies AM: Risk factors for
survival and local control in chondrosarcoma of bone. J Bone Joint
Surg Br. 84:93–99. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Italiano A, Mir O, Cioffi A, Palmerini E,
Piperno-Neumann S, Perrin C, Chaigneau L, Penel N, Duffaud F, Kurtz
JE, Collard O, Bertucci F, Bompas E, Le Cesne A, Maki RG, Ray
Coquard I and Blay JY: Advanced chondrosarcomas: role of
chemotherapy and survival. Ann Oncol. 24:2916–2922. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Onishi AC, Hincker AM and Lee FY:
Surmounting chemotherapy and radioresistance in chondrosarcoma:
molecular mechanisms and therapeutic targets. Sarcoma.
2011:3815642011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Perez J, Decouvelaere AV, Pointecouteau T,
Pissaloux D, Michot JP, Besse A, Blay JY and Dutour A: Inhibition
of chondrosarcoma growth by mTOR inhibitor in an in vivo syngeneic
rat model. PLoS One. 7:e324582012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang S, Konorev EA, Kotamraju S, Joseph J,
Kalivendi S and Kalyanaraman B: Doxorubicin induces apoptosis in
normal and tumor cells via distinctly different mechanisms:
intermediacy of H2O2- and p53-dependent
pathways. J Biol Chem. 279:25535–25543. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Finn NA, Findley HW and Kemp ML: A
switching mechanism in doxorubicin bioactivation can be exploited
to control doxorubicin toxicity. PLoS Comput Biol. 7:e10021512011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gilliam LA, Moylan JS, Patterson EW, Smith
JD, Wilson AS, Rabbani Z and Reid MB: Doxorubicin acts via
mitochondrial ROS to stimulate catabolism in C2C12 myotubes. Am J
Physiol Cell Physiol. 302:C195–C202. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Efferth T, Giaisi M, Merling A, Krammer PH
and Li-Weber M: Artesunate induces ROS-mediated apoptosis in
doxorubicin-resistant T leukemia cells. PLoS One. 2:e6932007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Zhou F, Wu X, Zhang X, Chen Y,
Zha BS, Niu F, Lu M, Hao G, Sun Y, Sun J, Peng Y and Wang G:
Cellular pharmacokinetic mechanisms of adriamycin resistance and
its modulation by 20(S)-ginsenoside Rh2 in MCF-7/Adr cells. Br J
Pharmacol. 165:120–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the Warburg effect: the metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009.PubMed/NCBI
|
|
12
|
Liu Y, Cao Y, Zhang W, Bergmeier S, Qian
Y, Akbar H, Colvin R, Ding J, Tong L, Wu S, Hines J and Chen X: A
small-molecule inhibitor of glucose transporter 1 downregulates
glycolysis, induces cell-cycle arrest, and inhibits cancer cell
growth in vitro and in vivo. Mol Cancer Ther.
11:1672–1682. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao X, Fang L, Gibbs S, Huang Y, Dai Z,
Wen P, Zheng X, Sadee W and Sun D: Glucose uptake inhibitor
sensitizes cancer cells to daunorubicin and overcomes drug
resistance in hypoxia. Cancer Chemotherapy Pharmacol. 59:495–505.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakano A, Tsuji D, Miki H, Cui Q, El Sayed
SM, Ikegame A, Oda A, Amou H, Nakamura S, Harada T, Fujii S, Kagawa
K, Takeuchi K, Sakai A, Ozaki S, Okano K, Nakamura T, Itoh K,
Matsumoto T and Abe M: Glycolysis inhibition inactivates ABC
transporters to restore drug sensitivity in malignant cells. PloS
One. 6:e272222011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou GQ, Tang LL, Mao YP, Chen L, Li WF,
Sun Y, Liu LZ, Li L, Lin AH and Ma J: Baseline serum lactate
dehydrogenase levels for patients treated with intensity-modulated
radiotherapy for nasopharyngeal carcinoma: a predictor of poor
prognosis and subsequent liver metastasis. Int J Radiat Oncol Biol
Phys. 82:e359–e365. 2012. View Article : Google Scholar
|
|
16
|
Zhou M, Zhao Y, Ding Y, Liu H, Liu Z,
Fodstad O, Riker AI, Kamarajugadda S, Lu J, Owen LB, Ledoux SP and
Tan M: Warburg effect in chemosensitivity: targeting lactate
dehydrogenase-A re-sensitizes taxol-resistant cancer cells to
taxol. Mol Cancer. 9:332010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Van Oosterwijk JG, Herpers B, Meijer D,
Briaire-de Bruijn IH, Cleton-Jansen AM, Gelderblom H, van de Water
B and Bovée JV: Restoration of chemosensitivity for doxorubicin and
cisplatin in chondrosarcoma in vitro: BCL-2 family members
cause chemoresistance. Ann Oncol. 23:1617–1626. 2012.PubMed/NCBI
|
|
18
|
Zaja R, Caminada D, Loncar J, Fent K and
Smital T: Development and characterization of P-glycoprotein 1
(Pgp1, ABCB1)-mediated doxorubicin-resistant PLHC-1 hepatoma fish
cell line. Toxicol Appl Pharmacol. 227:207–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Latorre E, Tebaldi T, Viero G, Spartà AM,
Quattrone A and Provenzani A: Downregulation of HuR as a new
mechanism of doxorubicin resistance in breast cancer cells. Mol
Cancer. 11:132012. View Article : Google Scholar : PubMed/NCBI
|