Introduction
Cancer chemoprevention involves the use of natural,
synthetic, or biological agents to suppress or delay the initial
phases of carcinogenesis or the progression of premalignant cells
to invasive disease. These agents are administered to individuals
without overt disease who harbor pre-cancerous lesions or who are
genetically predisposed to developing cancer. Because
chemopreventive drugs are administered to generally healthy
individuals over a long period of time, they must exhibit
little-to-no toxicity. This requirement has been a longstanding
barrier to moving chemopreventive agents to the clinic; and, as a
result, the FDA has only approved a very small number of agents for
cancer risk reduction across all subtypes of cancer (1). For breast cancer prevention, the
selective estrogen receptor modulators (SERMs) tamoxifen and
raloxifene have been approved for use in women at high risk for the
disease, and in women with ductal carcinoma in situ (DCIS)
who have undergone breast surgery and radiation. However, despite
their proven efficacy, these drugs have failed to gain acceptance
from patients and health care providers due to their potential to
cause hot flashes, induce thromboembolic events, and increase the
risk of uterine cancer (2,3). Thus, there is a critical need for the
identification and development of new chemopreventive agents for
breast cancer.
The molecular targets for cancer chemoprevention
include factors involved in DNA damage/repair, inflammation,
cellular metabolism, apoptosis, angiogenesis, and signal
transduction. One such factor is signal transducer and activator of
transcription 3 (STAT3). STAT3 is one of the seven members of a
family of transcription factors that regulates cell proliferation,
differentiation, apoptosis, and the immune response. Upon ligand
binding, cytokine and growth factor receptors such as the IL6
receptor (IL6-R), epidermal growth factor receptor (EGFR), vascular
endothelial growth factor receptor (VEGFR), and platelet-derived
growth factor receptor (PDGFR) dimerize, resulting in the
recruitment and subsequent activation of Janus kinases (JAKs).
Activated JAKs in turn phosphorylate tyrosine residues on the
cytoplasmic domain of the receptor, creating a docking site for the
src-homology 2 (SH2) domain of STAT3 and enabling the
phosphorylation and activation of the STAT3 protein (4). Upon activation, STAT3 dimerizes via its
SH2 domain and translocates to the nucleus where it promotes the
expression of numerous target genes involved in cell proliferation
and survival [cyclin D1 (5), c-myc
(6), Bcl-XL (7), survivin (8)], migration and invasion [MMPs (9)], angiogenesis [VEGF (10), HIF-1 (11)], and immune suppression [TGFβ, IL-10
(12)]. STAT3 can also be activated
in a receptor-independent manner by the Src and Abl kinases
(4).
In normal cells, the activation of STAT3 is
transient and is highly regulated by phosphatases, ubiquitinases,
and the suppressor of cytokine signaling (SOCS) and protein
inhibitor of activated STAT (PIAS) proteins (4). However, in many types of cancer,
including breast (13), ovarian
(14), prostate (15), colon (16), renal (17), brain (18), and pancreatic cancer (19), STAT3 is constitutively active. This
correlation, combined with the findings that transgenic mice
expressing constitutively active STAT3 exhibit an increased rate of
tumor formation and a greater tumor burden than their wild-type
counterparts (20,21), and that the reduction or inactivation
of the STAT3 protein prevents transformation and promotes apoptosis
in animal models of cancer (22,23),
supports a role for STAT3 in carcinogenesis and suggests that STAT3
could serve as a target for preventive intervention.
Targeting STAT3 is especially appealing for the
prevention of breast cancer. STAT3 is constitutively active in over
40% of all breast cancers, particularly in triple-negative breast
cancers which lack the expression of the estrogen receptor (ER),
progesterone receptor (PR), and human epidermal growth factor
receptor 2 (HER2/Neu) (24).
Activated STAT3 has also been shown to induce estrogen biosynthesis
and the subsequent proliferation of ER-positive breast epithelial
cells (25), and is thought to play a
role in the maintenance of tumor recurrence-promoting stem
cell-like breast cancer cells and in the conversion of a non-cancer
stem cell population to breast cancer stem cell-like cells
(26). Thus, STAT3 inhibitors offer a
unique advantage over the FDA-approved breast cancer preventive
agents tamoxifen and raloxifene in that they could potentially
prevent multiple breast cancer subtypes. In addition, because STAT3
inhibitors have a distinct mechanism of action from the SERMs
tamoxifen and raloxifene, such inhibitors may also be particularly
useful against ER-positive breast cancers that have developed
resistance to these drugs.
GLG-302 (S3I-201, NSC 74859) is a STAT3 inhibitor
that was identified through docking simulations that relied on the
X-ray crystal structure of the STAT3β homodimer bound to DNA to
screen the National Cancer Institute's chemical libraries (27). GLG-302 is an inhibitor of STAT3
DNA-binding activity in vitro with an IC50 of
86±33 µM (although it also shows low activity toward STAT1 and
STAT5), and it suppresses the growth of cells containing
constitutively active STAT3 (27–29).
Previous studies have shown that treatment with GLG-302 induces
apoptosis in breast cancer cell lines through the repression of
STAT3-mediated cyclin D1, Bcl-xL, and survivin expression, and that
it can inhibit the growth of pre-established breast cancer tumors
in xenograft mouse models (27).
In the present study, we investigated the ability of
orally-administered GLG-302 and its trizma salt derivative to
prevent the development of mammary cancers in female MMTV/Neu mice
and 7,12-dimethylbenz[a]anthracene (DMBA)-exposed Sprague-Dawley
(SD) rats. The MMTV/Neu (ErbB2+/−) model of breast
cancer was initially developed by Muller and colleagues (30–32). It
employs the overexpression of wild-type Neu and develops
ER-negative mammary carcinomas that overexpress Neu. The absence of
the ER and the overexpression of wild-type Neu are characteristics
of approximately 15% of all human breast cancers. There is evidence
that the expression of STAT3 is modulated by EGFR family members
including Neu (EGFR2) (24), and
preliminary studies in our laboratory demonstrated that activated
STAT3 was present in the normal mammary tissue of female MMTV/Neu
mice (unpublished data). The DMBA-induced rat model of breast
cancer was first described by Huggins et al in 1961
(33). Similar to approximately 70%
of all human breast cancers, rat mammary tumors that arise
following a single dose of the carcinogen DMBA are ER-positive and
PR-positive, and are thus strongly hormone dependent. Furthermore,
our laboratory has shown that activated STAT3 is highly expressed
in the mammary tissue of these female animals. Taken together,
these data indicate that Neu-overexpressing and DMBA-induced tumors
are good candidates for testing the efficacy of a STAT3 inhibitor,
and support the selection of the MMTV/Neu and DMBA-treated SD
models to evaluate the chemopreventive activity of GLG-302.
Materials and methods
Female MMTV/Neu (ErbB2+/−) mice were
generated in the Chemoprevention Center at the University of
Alabama at Birmingham by crossing ErbB2+/+ mice with
female FVB mice. Mice were genotyped by tail clips prior to being
placed on test. Female Sprague-Dawley rats were obtained from
Envigo (Madison, WI, USA) at 28 days of age. Animals received
Teklad (4% fat) diet purchased from Envigo. All mice were housed
(5/cage) in a room artificially lighted 12 h/day and maintained at
23±2°C. Access to food and water was ad libitum. All mice
with large tumors (as defined by IACUC guidelines) were sacrificed.
GLG-302 was provided by GLG Pharma (Jupiter, FL, USA). The high
doses of GLG-302 used resulted in a fairly dense mixture that
created difficulties during the gavage process. In order to improve
drug handling and delivery and to increase the bioavailability and
efficacy of the compound, GLG-302 was reformulated so that it could
be dissolved in an aqueous medium. Several derivatives of GLG-302
were synthesized, and a trizma salt form of the compound
(GLG-302/trizma salt) was ultimately selected because it was water
soluble at concentrations as high as 50 mg/ml and it displayed
acceptable stability when frozen until administered to the animals.
GLG-302/trizma salt was provided by the National Cancer
Institute/Division of Cancer Prevention Chemical Repository. For
the mouse studies, the vehicle for GLG-302 was 0.5%
carboxymethylcellulose (pH 6.5), and the vehicle for GLG-302/trizma
salt was water. For both agents, the volume was 0.2 ml/mouse, and
the agents were given daily, 5×/week. For administration of GLG-302
to the rats, the agent was incorporated with the diet using a
Patterson-Kelly blender with intensifier bar. Fresh diet was
provided to the rats 3×/week. 7,12-Dimethylbenz[a]anthracene (DMBA)
was purchased from Sigma-Aldrich Corporation/Merck KGaA. For the
mice, DMBA was dissolved in corn oil and administered by gavage
(0.2 ml). For the rats, 1.0 ml of the DMBA solution was given. All
animal experiments were conducted in AAALAC-approved facilities
following procedures approved by the Institutional Animal Care and
Use Committee at the University of Alabama at Birmingham (project
number IACUC-20269). All animals were weighed 1×/week and palpated
for mammary tumors 2×/week. At the end of the study, animals were
sacrificed using CO2 asphyxiation followed by a double
pneumothorax. All mammary tumors were evaluated by a
board-certified pathologist (MMJ) and weighed.
Mouse chemoprevention studies
In the first study, female mice (15/group) were
randomized into the following groups: GLG-302 [500 mg/kg body
weight (BW)/day], GLG-302 (250 mg/kg BW/day), GLG-302 (125 mg/kg
BW/day) and no treatment. The doses were selected based on a
3-month maximun tolerated dose (MTD) study (Table SI), which revealed no signs of
toxicity or changes in animal body weight. (Table SII). For the chemoprevention studies,
the mice received the agents beginning at 65 days of age and
continuing for the duration of the study (10 months of
treatment).
In the second study, female mice (25/group) were
randomized as follows: GLG-302/trizma salt (500 mg/kg BW/day),
GLG-302/trizma salt (250 mg/kg BW/day), GLG-302/trizma salt (125
mg/kg BW/day), and no treatment. These doses were also selected
based on a previous six-week study in our laboratory. The mice
received the agents beginning at 50 days of age and continuing for
the duration of the study. DMBA was initially given at 57 days of
age (1×/week for 4 weeks) to accelerate tumor development. The
study was terminated 19 weeks after the initial DMBA treatment. All
deaths in both studies were due to gavage errors.
Rat chemoprevention study
Female rats (15/group) were randomized into the
following groups: GLG-302 (8 g/kg diet) and no treatment. The rats
received the agents beginning at 43 days of age and continuing for
the duration of the study. DMBA was administered at 50 days of age
(50 mg/kg BW by gavage). The study was terminated 126 days after
DMBA treatment.
Proliferation and p-STAT3
measurements
In separate studies, the effects of GLG-302 and
GLG-302/trizma salt on normal mammary epithelial cell proliferation
and p-STAT3 levels were evaluated. Beginning at 7–8 weeks of age,
MMTV/Neu mice or SD rats (5/group) received the STAT3 inhibitors
for 2 weeks by gavage (5×/week). For GLG-302, dose levels of 500,
200 and 100 mg/kg BW/day were administered to the mice, while for
GLG-302/trizma salt, dose levels of 500, 250, and 125 mg/kg BW/day
were given. The rats received 500 mg/kg BW/day GLG-302. All animals
were sacrificed one day after the last treatment with the agents.
Mammary tissue was excised from an area in the abdominal/inguinal
glands (adjacent to the linea alba) that contains a high
concentration of epithelial cells. Mammary cancers from mice were
excised at the end of the study that used GLG-302/trizma salt. The
mammary tissues were fixed in 10% formalin for 24 h at room
temperature and were then transferred to 70% ethanol until
histologically processed.
After embedding in paraffin blocks, sections (4-µm
thick) were placed on positive microscope slides. The tissues were
de-paraffinized with xylene and placed in ethanol. Antigen
retrieval employed boiling in sodium citrate (pH 6.0) for 20 min.
Slides were then covered with peroxidase block for 3 h and washed
with Tris buffer. The tissues were incubated with primary antibody
p-STAT3 (cat no. 9145S; Cell Signaling Technology, Danvers, MA,
USA) or Ki-67 (cat. no. AB1667; Abcam, Cambridge, MA, USA) for 1 h
at room temperature. The dilution factor for p-STAT antibody was
1:200, while that for Ki-67 was 1:100. Processing and staining of
tissue were performed according to the manufacturer's procedures
(DAKO Envision + Kits; Agilent Technologies, Inc.). Tissues were
then washed and dehydrated in ethanol and xylene. The images were
captured and counted using the Aperio Scan Scope imaging system
(Aperio Imaging, Visa, CA, USA). For counting the cells, each area
containing mammary ductal epithelial cells was randomly analyzed
(stained cells + total cells counted) by a program within
ScanScope. A total of 1,000-1,500 cells were usually counted. This
varied depending on the degree of proliferation resulting from
treatment of the animal with the agent.
Statistical analysis
Final mammary tumor incidence was compared using
Chi-square or Fisher's exact tests. Mammary cancer latency was
analyzed with a Kaplan-Meier estimate and compared with a log-rank
test. Tumor multiplicity was compared using a Cochran-Armitage
trend test for mouse studies and Poisson regression for the rat
study. Tumor weights were analyzed via Chi-square for mouse studies
and Mann-Whitney U test for the rat study. Proliferation indices
were analyzed using one-way ANOVA. Due to the longitudinal nature
of the studies, experiments were not duplicated. Data are presented
as mean ± standard error. All statistical analyses were performed
using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). P<0.05 was
assigned as indicative of a statistically significant
difference.
Results
Effect of GLG-302 on spontaneous
mammary cancers in female MMTV/Neu mice
GLG-302 was evaluated at various doses for efficacy
in the prevention of spontaneous mammary cancers occurring in
female MMTV/Neu mice. There were no gross signs of toxicity, and
the body weights of the mice were not significantly altered during
the study. Because of the viscosity of the GLG-302 mixture
(particularly at the higher doses), several deaths occurred in the
various groups due to gavage errors (i.e., not related to drug
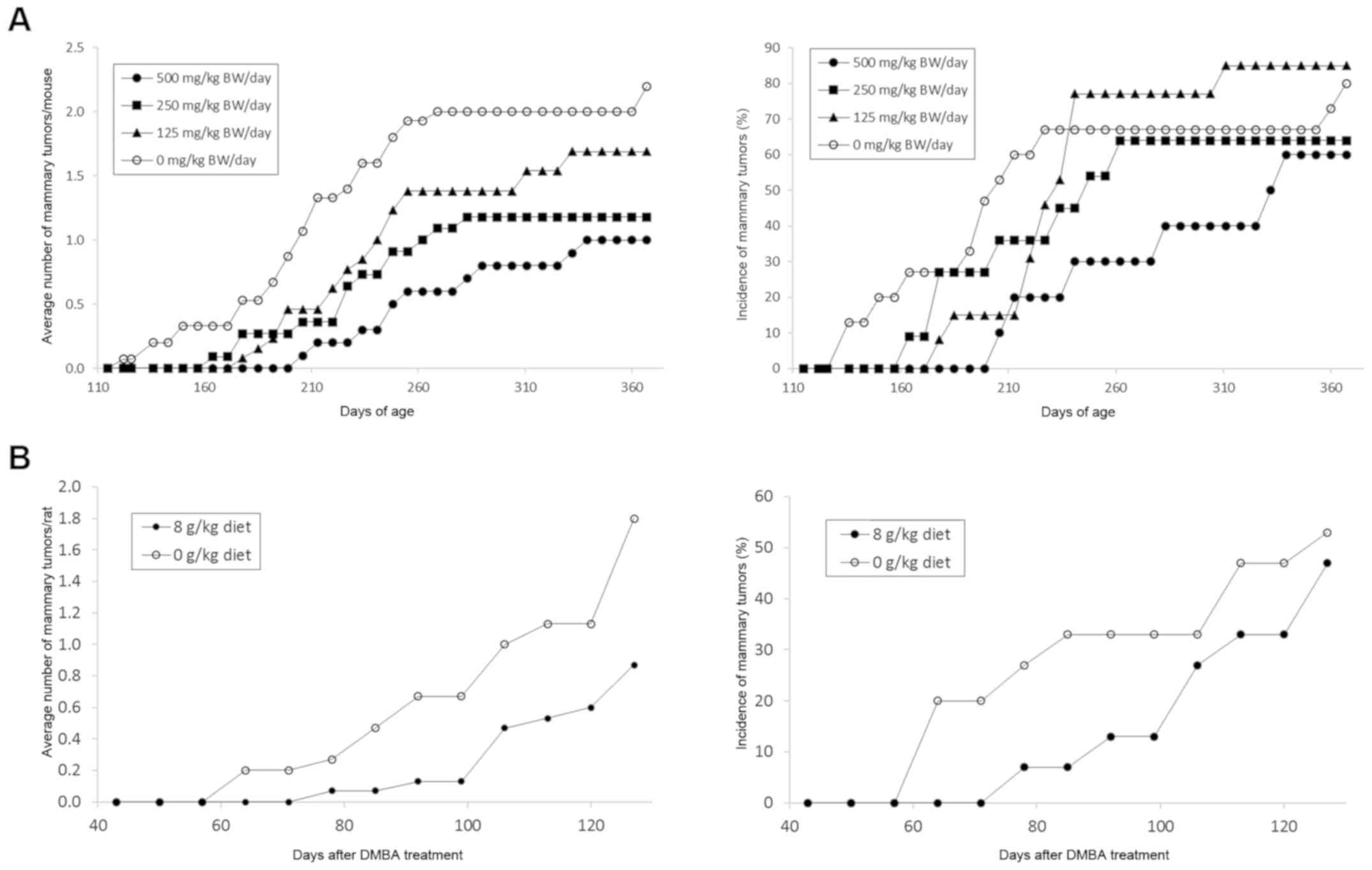
toxicity). GLG-302 at dose levels of 500 and 250 mg/kg BW/day
significantly decreased mammary cancer multiplicity by 55 and 46%,
respectively (Table IA and Fig. 1A). The highest dose also significantly
reduced the weight of the mammary cancers (77%) (Table IA). The effects of short-term
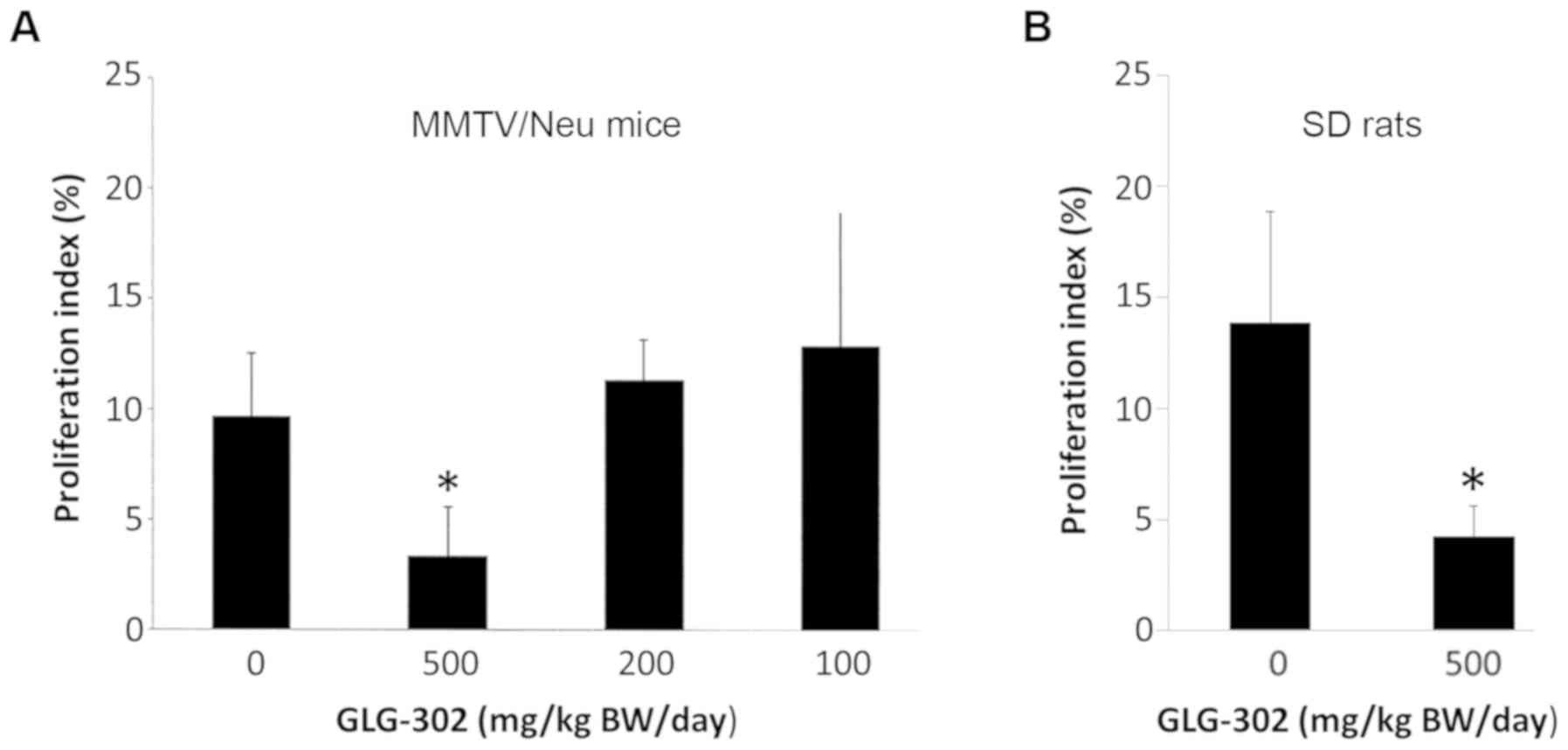
(2-week) treatment with various doses of GLG-302 on the rate of
proliferation of normal mammary epithelial cells is shown in
Fig. 2A. Only the highest dose (500
mg/kg BW/day) was significantly effective in reducing the
proliferation index. The effect of the highest dose of GLG-302 on
normal epithelial cell proliferation was correlated with the
significant effect of this dose in the prevention of mammary
cancers.
 | Table I.Effect of GLG-302 (STAT3 antagonist)
on mammary tumor incidence, multiplicity, and weight in female
MMTV/Neu mice and DMBA-treated SD rats. |
Table I.
Effect of GLG-302 (STAT3 antagonist)
on mammary tumor incidence, multiplicity, and weight in female
MMTV/Neu mice and DMBA-treated SD rats.
| A, MMTV/Neu
mice |
|---|
|
|---|
| GLG-302 (mg/kg
BW/day) | Incidence (%) | Multiplicity
(tumors/mouse) | Tumor weight
(g) |
|---|
| 500 | 60 (25%↓) | 1.00
(55%↓)a | 0.40
(77%↓)a |
| 250 | 64 (20%↓) | 1.18
(46%↓)a | 1.26 (28%↓) |
| 125 | 85 (6%↑) | 1.69 (23%↓) | 1.50 (14%↓) |
| 0 | 80 | 2.20 | 1.75 |
Effect of GLG-302 on
carcinogen-induced mammary cancers in female Sprague-Dawley
rats
A single dose of GLG-302 was also evaluated for
efficacy in the prevention of spontaneous mammary cancers occurring
in female DMBA-treated rats. GLG-302 was administered in the diet
for 4 months, beginning one week prior to carcinogen treatment at
50 days of age. Rats fed 8 g GLG-302/kg diet displayed no gross
signs of toxicity and maintained body weights comparable to
vehicle-treated rats throughout the study period (data not shown).
Although statistical significance levels were lower than our usual
standard (P<0.05) due to the unexpectedly low yields of tumors
in control animals and consequent effects on standard deviations
and small absolute differences, treatment with GLG-302 resulted in
a 52% decrease in tumor multiplicity (P=0.1) and an 87% decrease in
tumor weight (P=0.07) (Table IB and
Fig. 1B). In agreement with this
data, the short-term (2-week) administration of 500 mg/kg BW/day
GLG-302 by gavage reduced the rate of proliferation of normal
mammary epithelial cells by 80% (Fig.
2B).
| B, DMBA-treated SD
rats |
|---|
|
|---|
| GLG-302 (g/kg
diet) | Incidence (%) | Multiplicity
(tumors/rat) | Tumor weight
(g) |
|---|
| 8 | 47 (11%↓) | 0.87
(52%↓)b | 0.14
(87%↓)b |
| 0 | 53 | 1.80 | 1.10 |
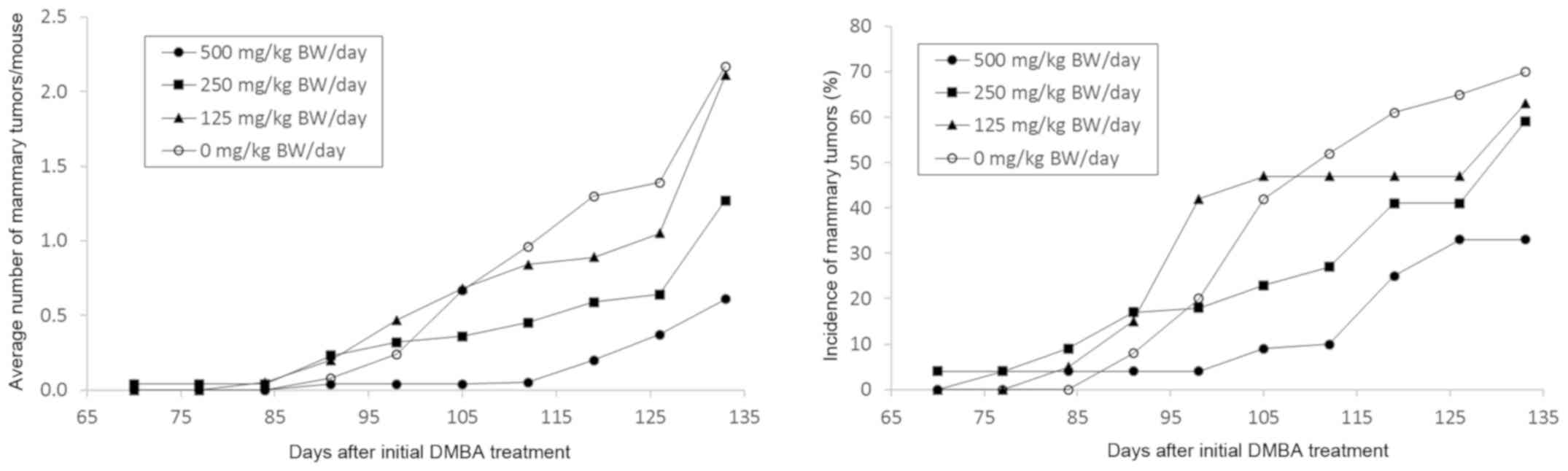
Effect of GLG-302/trizma salt on
mammary cancers in female MMTV/Neu mice
The examination of the pharmacokinetic properties of
GLG-302 indicated that the absorption of the compound was
sub-optimal. We therefore developed a trizma base formulation
(referred to as GLG-302/trizma salt) and evaluated its
chemopreventive activity in MMTV/Neu mice. Because the previous
study had resulted in a relatively long tumor latency period in
untreated mice (a 50% incidence of palpable lesions was not
obtained until 206 days of age in the untreated control group), the
mice in this study received DMBA beginning at 57 days of age to
reduce the length of the study from approximately 10 months to 4
months. As in the previous mouse study, the mice received the STAT3
inhibitor by gavage, but beginning one week prior to the
carcinogen. As with GLG-302, treatment with the trizma salt
derivative resulted in a dose-dependent decrease in the
multiplicity of mammary cancers; significant decreases of 72 and
41% were observed at the 500 and 250 mg/kg BW/day dose levels,
respectively (Table II and Fig. 3). The two highest doses also decreased
the weight of the mammary cancers by approximately 70–80% (Table II), and the 500 mg/kg BW/day dose
significantly increased tumor latency (P=0.012) and reduced tumor
incidence by 53% (Table II and
Fig. 3). The GLG-302/trizma salt did
not alter body weights or induce gross toxicity during the study
(data not shown).
 | Table II.Effect of GLG-302/trizma salt on
mammary tumor incidence, multiplicity and weight in female
DMBA-treated MMTV-Neu mice. |
Table II.
Effect of GLG-302/trizma salt on
mammary tumor incidence, multiplicity and weight in female
DMBA-treated MMTV-Neu mice.
| GLG-302/trizma salt
(mg/kg BW/day) | Incidence (%) | Multiplicity
(tumors/mouse) | Tumor weight
(g) |
|---|
| 500 | 33
(53%↓)a | 0.61
(72%↓)a | 0.24
(81%↓)a |
| 250 | 59 (16%↓) | 1.27
(41%↓)a | 0.37
(71%↓)a |
| 125 | 63 (10%↓) | 2.11 (3%↓) | 1.03 (20%↓) |
| 0 | 70 | 2.17 | 1.28 |
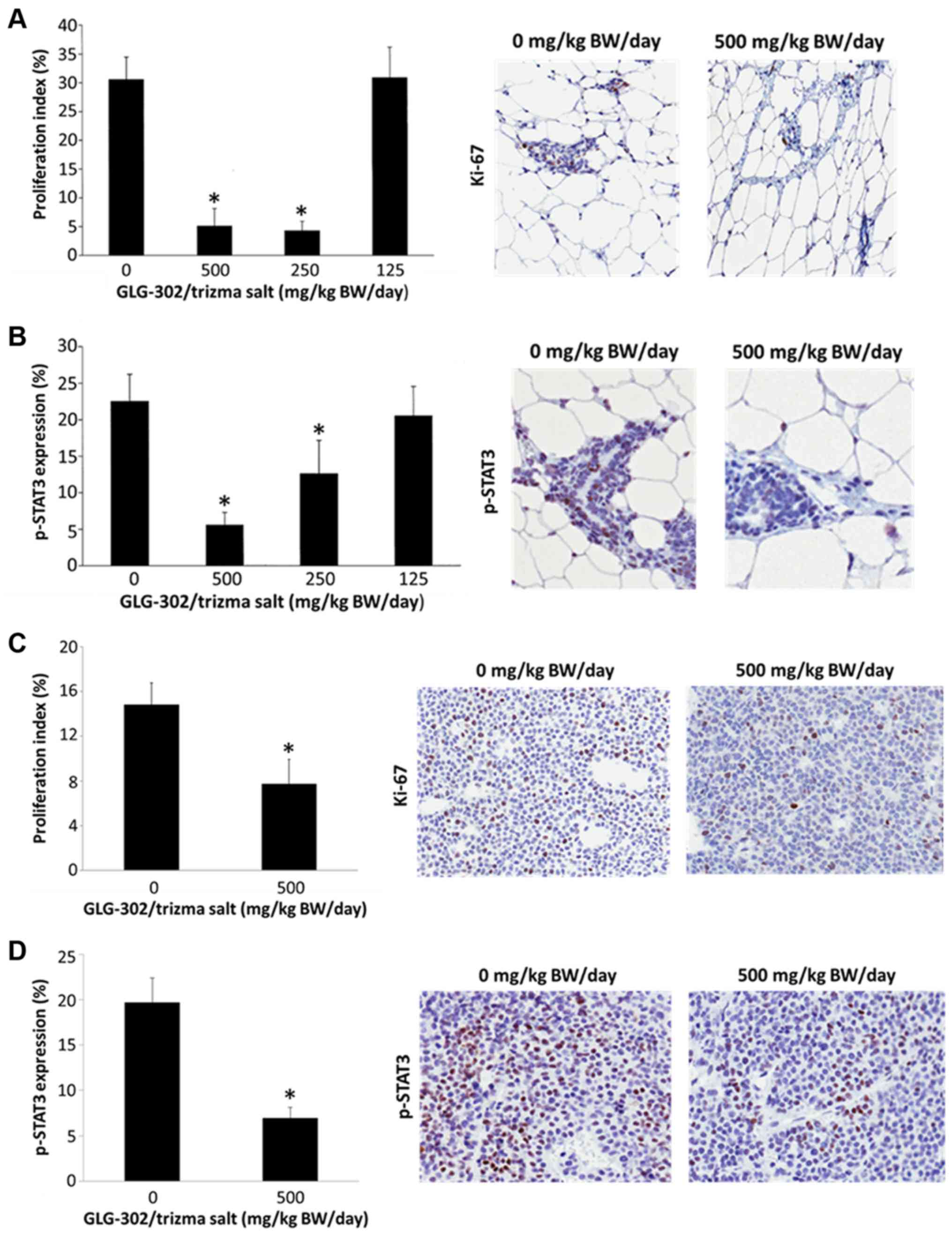
The epithelial cells of the normal mammary glands of
female MMTV/Neu mice showed decreased proliferation after two weeks
of treatment with GLG-302/trizma salt as determined by Ki-67
staining (Fig. 4A). In contrast to
what was observed with GLG-302 (Fig.
2), the two highest doses of GLG-302/trizma salt both resulted
in over 80% reductions in the proliferation index (Fig. 4A), suggesting that the new formulation
may be more potent than its parent compound. The normal mammary
epithelial cells also showed dose-dependent decreases in
phosphorylated STAT3 (p-STAT3) levels after two weeks of treatment
with GLG-302/trizma salt (Fig. 4B),
suggesting that the chemopreventive effects of GLG-302/trizma salt
were associated with STAT3 inhibition and a subsequent decrease in
cell proliferation. To confirm this, we measured Ki-67 and pSTAT3
in the tumors harvested from the mice upon completion of the study.
In agreement with the lower tumor weights of the GLG-302-treated
animals and the staining results in the normal mammary glands,
Ki-67 (Fig. 4C) and p-STAT3 (Fig. 4D) were significantly reduced in the
cancers of the mice that had received 500 mg/kg BW/day
GLG-302/trizma salt for 4 months.
Discussion
In the present study, we provide support for the use
of STAT3 inhibitors as breast cancer chemopreventive agents by
demonstrating that GLG-302 has an effect on cancer formation in the
ER-negative, Neu-overexpressing MMTV/Neu mouse and DMBA-induced
ER-positive rat models of breast cancer by preventing STAT3
activation and subsequently reducing cell proliferation. The doses
of GLG-302 used in this study are those that can be achieved in the
human. For example, using a standard conversion factor that
converts mg/kg in mouse to mg/kg in humans, one multiplies the dose
of 500 mg/kg BW/day in the mouse by 0.08 to obtain a dose of 40
mg/kg BW for a human. Thus, this dose can have clinical
significance.
Although distinct from triple-negative and
BRCA1 mutant mammary cancers, the MMTV/Neu mouse is clearly
a relevant model for Neu-overexpressing breast cancer in humans.
Neu-overexpressing tumors account for approximately 15% of total
human breast tumors, and although they are most commonly
ER-negative, they also represent a significant percentage of
ER-positive (Luminal B) tumors that are highly proliferative, have
low ER levels, and fail to respond to hormonal agents such as SERMs
and aromatase inhibitors (34–36).
Furthermore, Neu-overexpressing tumors comprise a high percentage
of pre-invasive DCIS lesions, which are potential targets for
prevention studies (37,38). The results achieved here with GLG-302
and GLG-302/trizma salt in this model are profound and reduce
mammary cancer multiplicity to approximately the same extent as
EGFR inhibitors and retinoid X receptor (RXR) agonists, which have
heretofore been the most effective agents in this model (39,40).
In terms of histology, the carcinogen-treated rat
model is most relevant to hormone receptor-positive breast cancers,
which make up 70% of all cases of the disease; approximately 76% of
the mammary cancers in the DMBA-treated rat model are of the
Luminal A subtype, whereas 24% have been characterized as Luminal B
(41). Since ER and PR expression is
found in the majority of DCIS lesions (42), the carcinogen-induced rat has become
one of the most commonly used animal models for breast cancer
chemoprevention studies. Although the preventive efficacy of
GLG-302/trizma salt could not be determined due to the failure of
DMBA to sufficiently induce tumors in the control animals (data not
shown), GLG-302 showed a distinct trend toward reducing tumor
multiplicity and weight in this model, despite its poor
bioavailability.
Over the years, many other STAT3 inhibitors in
addition to GLG-302 have been identified and developed. These
include compounds that directly prevent STAT3 phosphorylation,
dimerization, translocation, or DNA binding by targeting the SH2,
N-terminal, or DNA binding domains of the protein, and compounds
that indirectly interfere with STAT3 activity by blocking its
upstream regulators (43,44). Although potentially useful in a
chemotherapeutic setting where compound toxicity is generally
tolerated as a trade-off for increased patient survival, most of
these STAT3 inhibitors are not suitable for the prevention of
cancer in healthy individuals, as they produce numerous side
effects including fatigue, nausea, diarrhea, anemia, and infection.
It remains to be determined if the employment of GLG-302 as a
clinical chemopreventive agent will be hampered by the same side
effects that have restricted the use of many other STAT3
inhibitors. However, our preclinical studies suggest that this will
not be the case, since as much as 500 mg GLG-302/kg BW/day did not
significantly affect the body weights of the animals or induce
other signs of toxicity during this study. Similarly, GLG-302 was
well-tolerated in rats and dogs in pilot safety studies conducted
by GLG Pharma.
The design of and testing of new GLG-302 analogs is
currently underway to improve agent efficacy. Several of these
derivatives, including S3I-201.1066, BP-1-102, S3I-1757, and
SH5-07, have improved potencies compared to GLG-302 and have shown
activity in vitro and in xenograft models of breast cancer
(45–48). However, further studies are needed to
determine if these small molecules, similar to their parent
compound GLG-302, could also be used as chemopreventive agents.
It is also feasible that GLG-302 or its derivatives
could be used in combination with other agents that act
synergistically or additively with the STAT3 inhibitor to further
suppress mammary tumor development. For example, GLG-302 has been
shown to act synergistically with metformin to decrease cell growth
and induce apoptosis in triple-negative breast cancer cell lines
(49). Although some success has been
achieved in the area of combinatorial chemoprevention (50), toxic interactions between the agents
are a serious concern. A safer and more effective alternative may
be to use GLG-302 in combination with a cancer vaccine to directly
inhibit tumor development while simultaneously altering the
immunologic environment in favor of immunoprevention.
Supplementary Material
Supporting Data
Acknowledgements
GLG Pharma supplied the drug substance for the study
as well as advice on dosing and formulation. This study is
dedicated to the memory of the late Dr Michael Lovell of GLG
Pharma.
Funding
Formulation work (generation of the trizma salt of
GLG-302) was conducted at MRI Global, Kansas City, MO, USA under
NCI contract HHSN261201100046C. All animal studies were carried out
under contracts HHSN261201200021 I and HHSN2612015000361 from the
Division of Cancer Prevention, National Cancer Institute.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Author's contributions
RHS and CJG designed the experiments. CJG, MMJ, FLM
and JTF performed the experiments and/or analyzed the data. RHS,
CJG and JTF wrote the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All animal experiments were conducted in
AAALAC-approved facilities following procedures approved by the
Institutional Animal Care and Use Committee at the University of
Alabama at Birmingham (project number IACUC-20269).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DCIS
|
ductal carcinoma in situ
|
|
DMBA
|
7,12-dimethylbenz[a]anthracene
|
|
EGFR
|
epidermal growth factor receptor
|
|
ER
|
estrogen receptor
|
|
HER2/Neu
|
human epidermal growth factor receptor
2
|
|
IL6-R
|
interleukin 6 receptor
|
|
JAK
|
Janus kinase
|
|
PDGFR
|
platelet-derived growth factor
receptor
|
|
PIAS
|
protein inhibitor of activated
STAT
|
|
PR
|
progesterone receptor
|
|
RXR
|
retinoid X receptor
|
|
SD
|
Sprague-Dawley
|
|
SERM
|
selective estrogen receptor
modulator
|
|
SH2
|
src-homology 2
|
|
SOCS
|
suppressor of cytokine signaling
|
|
STAT
|
signal transducer and activator of
transcription
|
|
VEGFR
|
vascular endothelial growth factor
receptor
|
References
|
1
|
Al Rabadi L and Bergan R: A way forward
for cancer chemoprevention: Think local. Cancer Prev Res (Phila).
10:14–35. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Port ER, Montgomery LL, Heerdt AS and
Borgen PI: Patient reluctance toward tamoxifen use for breast
cancer primary prevention. Ann Surg Oncol. 8:580–585. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ropka ME, Keim J and Philbrick JT: Patient
decisions about breast cancer chemoprevention: A systematic review
and meta-analysis. J Clin Oncol. 28:3090–3095. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aggarwal BB, Kunnumakkara AB, Harikumar
KB, Gupta SR, Tharakan ST, Koca C, Dey S and Sung B: Signal
transducer and activator of transcription-3, inflammation, and
cancer: How intimate is the relationship? Ann N Y Acad Sci.
1171:59–76. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Leslie K, Lang C, Devgan G, Azare J,
Berishaj M, Gerald W, Kim YB, Paz K, Darnell JE, Albanese C, et al:
Cyclin D1 is transcriptionally regulated by and required for
transformation by activated signal transducer and activator of
transcription 3. Cancer Res. 66:2544–2552. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bowman T, Broome MA, Sinibaldi D, Wharton
W, Pledger WJ, Sedivy JM, Irby R, Yeatman T, Courtneidge SA and
Jove R: Stat3-mediated Myc expression is required for Src
transformation and PDGF-induced mitogenesis. Proc Natl Acad Sci
USA. 98:7319–7324. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bromberg JF, Wrzeszczynska MH, Devgan G,
Zhao Y, Pestell RG, Albanese C and Darnell JE Jr: Stat3 as an
oncogene. Cell. 98:295–303. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gritsko T, Williams A, Turkson J, Kaneko
S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, et al:
Persistent activation of stat3 signaling induces survivin gene
expression and confers resistance to apoptosis in human breast
cancer cells. Clin Cancer Res. 12:11–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsareva SA, Moriggl R, Corvinus FM,
Wiederanders B, Schütz A, Kovacic B and Friedrich K: Signal
transducer and activator of transcription 3 activation promotes
invasive growth of colon carcinomas through matrix
metalloproteinase induction. Neoplasia. 9:279–291. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niu G, Wright KL, Huang M, Song L, Haura
E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al:
Constitutive Stat3 activity up-regulates VEGF expression and tumor
angiogenesis. Oncogene. 21:2000–2008. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Niu G, Briggs J, Deng J, Ma Y, Lee H,
Kortylewski M, Kujawski M, Kay H, Cress WD, Jove R and Yu H: Signal
transducer and activator of transcription 3 is required for
hypoxia-inducible factor-1alpha RNA expression in both tumor cells
and tumor-associated myeloid cells. Mol Cancer Res. 6:1099–1105.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kinjyo I, Inoue H, Hamano S, Fukuyama S,
Yoshimura T, Koga K, Takaki H, Himeno K, Takaesu G, Kobayashi T and
Yoshimura A: Loss of SOCS3 in T helper cells resulted in reduced
immune responses and hyperproduction of interleukin 10 and
transforming growth factor-beta 1. J Exp Med. 203:1021–1031. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Garcia R, Yu CL, Hudnall A, Catlett R,
Nelson KL, Smithgall T, Fujita DJ, Ethier SP and Jove R:
Constitutive activation of Stat3 in fibroblasts transformed by
diverse oncoproteins and in breast carcinoma cells. Cell Growth
Differ. 8:1267–1276. 1997.PubMed/NCBI
|
|
14
|
Huang M, Page C, Reynolds RK and Lin J:
Constitutive activation of stat 3 oncogene product in human ovarian
carcinoma cells. Gynecol Oncol. 79:67–73. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mora LB, Buettner R, Seigne J, Diaz J,
Ahmad N, Garcia R, Bowman T, Falcone R, Fairclough R, Cantor A, et
al: Constitutive activation of Stat3 in human prostate tumors and
cell lines: Direct inhibition of Stat3 signaling induces apoptosis
of prostate cancer cells. Cancer Res. 62:6659–6666. 2002.PubMed/NCBI
|
|
16
|
Corvinus FM, Orth C, Moriggl R, Tsareva
SA, Wagner S, Pfitzner EB, Baus D, Kaufmann R, Huber LA, Zatloukal
K, et al: Persistent STAT3 activation in colon cancer is associated
with enhanced cell proliferation and tumor growth. Neoplasia.
7:545–555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo C, Yang G, Khun K, Kong X, Levy D, Lee
P and Melamed J: Activation of Stat3 in renal tumors. Am J Transl
Res. 1:283–290. 2009.PubMed/NCBI
|
|
18
|
Schaefer LK, Ren Z, Fuller GN and Schaefer
TS: Constitutive activation of Stat3alpha in brain tumors:
Localization to tumor endothelial cells and activation by the
endothelial tyrosine kinase receptor (VEGFR-2). Oncogene.
21:2058–2065. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei D, Le X, Zheng L, Wang L, Frey JA, Gao
AC, Peng Z, Huang S, Xiong HQ, Abbruzzese JL and Xie K: Stat3
activation regulates the expression of vascular endothelial growth
factor and human pancreatic cancer angiogenesis and metastasis.
Oncogene. 22:319–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan KS, Sano S, Kataoka K, Abel E,
Carbajal S, Beltran L, Clifford J, Peavey M, Shen J and Digiovanni
J: Forced expression of a constitutively active form of Stat3 in
mouse epidermis enhances malignant progression of skin tumors
induced by two-stage carcinogenesis. Oncogene. 27:1087–1094. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blando JM, Carbajal S, Abel E, Beltran L,
Conti C, Fischer S and DiGiovanni J: Cooperation between Stat3 and
Akt signaling leads to prostate tumor development in transgenic
mice. Neoplasia. 13:254–265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kataoka K, Kim DJ, Carbajal S, Clifford JL
and DiGiovanni J: Stage-specific disruption of Stat3 demonstrates a
direct requirement during both the initiation and promotion stages
of mouse skin tumorigenesis. Carcinogenesis. 29:1108–1114. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Andrea M, Ritta M, Landini MM, Borgogna
C, Mondini M, Kern F, Ehrenreiter K, Baccarini M, Marcuzzi GP,
Smola S, et al: Keratinocyte-specific stat3 heterozygosity impairs
development of skin tumors in human papillomavirus 8 transgenic
mice. Cancer Res. 70:7938–7948. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Banerjee K and Resat H: Constitutive
activation of STAT3 in breast cancer cells: A review. Int J Cancer.
138:2570–2578. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ishii Y, Waxman S and Germain D: Tamoxifen
stimulates the growth of cyclin D1-overexpressing breast cancer
cells by promoting the activation of signal transducer and
activator of transcription 3. Cancer Res. 68:852–860. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marotta LL, Almendro V, Marusyk A,
Shipitsin M, Schemme J, Walker SR, Bloushtain-Qimron N, Kim JJ,
Choudhury SA, Maruyama R, et al: The JAK2/STAT3 signaling pathway
is required for growth of CD44(+)CD24(−) stem cell-like breast
cancer cells in human tumors. J Clin Invest. 121:2723–2735. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Siddiquee K, Zhang S, Guida WC, Blaskovich
MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence
NJ, et al: Selective chemical probe inhibitor of Stat3, identified
through structure-based virtual screening, induces antitumor
activity. Proc Natl Acad Sci USA. 104:7391–7396. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin L, Amin R, Gallicano GI, Glasgow E,
Jogunoori W, Jessup JM, Zasloff M, Marshall JL, Shetty K, Johnson
L, et al: The STAT3 inhibitor NSC 74859 is effective in
hepatocellular cancers with disrupted TGF-beta signaling. Oncogene.
28:961–972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen W, Shen X, Xia X, Xu G, Ma T, Bai X
and Liang T: NSC 74859-mediated inhibition of STAT3 enhances the
anti-proliferative activity of cetuximab in hepatocellular
carcinoma. Liver Int. 32:70–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Muller WJ, Sinn E, Pattengale PK, Wallace
R and Leder P: Single-step induction of mammary adenocarcinoma in
transgenic mice bearing the activated c-neu oncogene. Cell.
54:105–115. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bouchard L, Lamarre L, Tremblay PJ and
Jolicoeur P: Stochastic appearance of mammary tumors in transgenic
mice carrying the MMTV/c-neu oncogene. Cell. 57:931–936. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guy CT, Cardiff RD and Muller WJ:
Activated neu induces rapid tumor progression. J Biol Chem.
271:7673–7678. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huggins C, Morii S and Grand LC: Mammary
cancer induced by a single dose of polynuclear hydrocarbons: Routes
of administration. Ann Surg. 154 (Suppl 6):S315–S318. 1961.
View Article : Google Scholar
|
|
34
|
Ariga R, Zarif A, Korasick J, Reddy V,
Siziopikou K and Gattuso P: Correlation of her-2/neu gene
amplification with other prognostic and predictive factors in
female breast carcinoma. Breast J. 11:278–280. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dowsett M, Allred C, Knox J, Quinn E,
Salter J, Wale C, Cuzick J, Houghton J, Williams N, Mallon E, et
al: Relationship between quantitative estrogen and progesterone
receptor expression and human epidermal growth factor receptor 2
(HER-2) status with recurrence in the arimidex, tamoxifen, alone or
in combination trial. J Clin Oncol. 26:1059–1065. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Konecny G, Pauletti G, Pegram M, Untch M,
Dandekar S, Aguilar Z, Wilson C, Rong HM, Bauerfeind I, Felber M,
et al: Quantitative association between HER-2/neu and steroid
hormone receptors in hormone receptor-positive primary breast
cancer. J Natl Cancer Inst. 95:142–153. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bartkova J, Barnes DM, Millis RR and
Gullick WJ: Immunohistochemical demonstration of c-erbB-2 protein
in mammary ductal carcinoma in situ. Hum Pathol. 21:1164–1167.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lodato RF, Maguire HC Jr, Greene MI,
Weiner DB and LiVolsi VA: Immunohistochemical evaluation of
c-erbB-2 oncogene expression in ductal carcinoma in situ and
atypical ductal hyperplasia of the breast. Mod Pathol. 3:449–454.
1990.PubMed/NCBI
|
|
39
|
Lu C, Speers C, Zhang Y, Xu X, Hill J,
Steinbis E, Celestino J, Shen Q, Kim H, Hilsenbeck S, et al: Effect
of epidermal growth factor receptor inhibitor on development of
estrogen receptor-negative mammary tumors. J Natl Cancer Inst.
95:1825–1833. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Zhang Y, Hill J, Shen Q, Kim HT, Xu
X, Hilsenbeck SG, Bissonnette RP, Lamph WW and Brown PH: The
Rexinoid LG100268 prevents the development of preinvasive and
invasive estrogen receptor negative tumors in MMTV-erbB2 mice. Clin
Cancer Res. 13:6224–6231. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Russo J: Significance of rat mammary
tumors for human risk assessment. Toxicol Pathol. 43:145–170. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lari SA and Kuerer HM: Biological markers
in DCIS and risk of breast recurrence: A systematic review. J
Cancer. 2:232–261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xiong A, Yang Z, Shen Y, Zhou J and Shen
Q: Transcription factor STAT3 as a novel molecular target for
cancer prevention. Cancers (Basel). 6:926–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yue P and Turkson J: Targeting STAT3 in
cancer: How successful are we? Expert Opin Investig Drugs.
18:45–56. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang X, Yue P, Fletcher S, Zhao W,
Gunning PT and Turkson J: A novel small-molecule disrupts Stat3 SH2
domain-phosphotyrosine interactions and Stat3-dependent tumor
processes. Biochem Pharmacol. 79:1398–1409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang X, Yue P, Page BD, Li T, Zhao W,
Namanja AT, Paladino D, Zhao J, Chen Y, Gunning PT and Turkson J:
Orally bioavailable small-molecule inhibitor of transcription
factor Stat3 regresses human breast and lung cancer xenografts.
Proc Natl Acad Sci USA. 109:9623–9628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang X, Sun Y, Pireddu R, Yang H, Urlam
MK, Lawrence HR, Guida WC, Lawrence NJ and Sebti SM: A novel
inhibitor of STAT3 homodimerization selectively suppresses STAT3
activity and malignant transformation. Cancer Res. 73:1922–1933.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yue P, Lopez-Tapia F, Paladino D, Li Y,
Chen CH, Namanja AT, Hilliard T, Chen Y, Tius MA and Turkson J:
Hydroxamic acid and benzoic acid-based STAT3 inhibitors suppress
human glioma and breast cancer phenotypes in vitro and in vivo.
Cancer Res. 76:652–663. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Deng XS, Wang S, Deng A, Liu B, Edgerton
SM, Lind SE, Wahdan-Alaswad R and Thor AD: Metformin targets Stat3
to inhibit cell growth and induce apoptosis in triple-negative
breast cancers. Cell Cycle. 11:367–376. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Meyskens FL Jr, McLaren CE, Pelot D,
Fujikawa-Brooks S, Carpenter PM, Hawk E, Kelloff G, Lawson MJ,
Kidao J, McCracken J, et al: Difluoromethylornithine plus sulindac
for the prevention of sporadic colorectal adenomas: A randomized
placebo-controlled, double-blind trial. Cancer Prev Res (Phila).
1:32–38. 2008. View Article : Google Scholar : PubMed/NCBI
|