Introduction
The synthetic nucleoside analog
1-β-D-ribofuranosyl-1H-1,2,4-triazole-3-carboxamide (ribavirin), is
frequently used in combination with interferon (IFN)-α for the
treatment of hepatitis C virus (HCV) infection (1,2) and is
being repurposed as a therapeutic agent in the treatment of cancer
(3). Ribavirin has demonstrated
clinical activity in patients with relapsed or refractory acute
myeloid leukemia of subtypes M4 and M5, and other subtypes with
increased eukaryotic translation initiation factor 4E (eIF4E)
levels. Of note, these clinical effects were reported in response
to 5–36 µM ribavirin in the plasma (4). Our previous study revealed that
ribavirin at clinically achievable concentrations (<50 µM)
exerted growth inhibitory effects in vitro upon certain
cancer cell lines (3). Additionally,
ribavirin inhibits eIF4E and inosine-5-monophosphate dehydrogenase
(IMPDH) (5,6). Ribavirin was determined to downregulate
the expression of enhancer of zeste homolog 2 (EZH2), an epigenetic
enzyme of the polycomb complex, at the RNA and protein levels, but
also inhibited its activity, as demonstrated by reductions in
histone 3, lysine 27 trimethylated (H3K27) trimethylation (3,7,8). Gain-of-function heterozygous point
mutations in the su(var)3-9, enhancer-of-zeste and trithorax-coding
domain of the histone methyltransferase gene EZH2 has been
reported in a subset of lymphomas. Increased expression or activity
of EZH2 has been associated with lymphomagenesis; thus, EZH2
inhibition may be considered as a novel anticancer strategy
(9,10). Clinical studies with at least three
EZH2 inhibitors are in progress against EZH2-mutant
lymphomas (11).
Evidence has suggested that ribavirin may exhibit
certain effects on lymphomas. Peveling-Overhag et al
reported the overall response rate (ORR) of 254 patients (based on
20 studies) with B-cell non-Hodgkin lymphoma and HCV infection
receiving antiviral therapy. The overall lymphoma response rate was
73%, and a strong association between sustained serological viral
response and lymphoma response (83% ORR) was reported compared with
those that failed to achieve a viral serological response (53%).
Improved response was observed in HCV-associated marginal zone
lymphomas compared with that of non-marginal zone origin; however,
the anti-lymphoma role of ribavirin remains uncertain as seven of
these studies investigated IFN treatment only, while 12 studies
analyzed the effects of IFN plus ribavirin, and one study evaluated
the combination of rituximab, IFN and ribavirin (12).
To further investigate the effectiveness of
ribavirin against lymphoma, the present study analyzed the
antitumor properties of ribavirin in lymphoma cancer cell lines
with and without EZH2 mutations.
Materials and methods
Cell lines, culture and ribavirin
treatment
The following cell lines were used in the present
study: Pfeiffer and Toledo, which are diffuse large B cell lymphoma
(DLBCL) cells, and Hut78, which corresponds to a cutaneous T-cell
lymphoma (CTCL). Cells were obtained from the American Type Culture
Collection (ATCC; Manassas VA, USA). The Pfeiffer cell line carries
the A677G mutation in EZH2, while Toledo cells are
wild-type. Cells were cultured at 37°C in a humidified atmosphere
containing 5% CO2 in complete medium, comprising
RPMI-1640 medium supplemented with 10% fetal bovine serum and 1%
antibiotic-antimycotic solution (all from Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Ribavirin was obtained from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany); 3-deazaneplanocin A
(DZNep) served as the control and was purchased from Calbiochem
(cat. no. 120964-45-6). Ribavirin and DZNep were dissolved in
RPMI-1640 medium, stored at −20°C and thawed before use. Stock
solutions were thawed/frozen no more than three times. Cell lines
were treated with 10, 15, 20, 25 and 50 µM of ribavirin, or 0.1 µM
DZNep for 24, 48 and 120 h.
Cell viability assay
Cells were seeded in 25-cm2 culture
flasks (Corning Inc., Corning, NY, USA) at a density of
2×105 cells in 5 ml of complete medium. Cells were then
treated with ribavirin at the indicated concentrations; the medium
containing the drug was replaced daily. DZNep at a concentration of
0.1 µM was used as a positive control. After 24, 48 and 120 h,
cells were stained with 0.4% trypan blue to assess cell viability
and counted using a TC10 Automated Cell Counter (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). All assays were performed
in triplicate. The viability of cells under each treatment
condition was expressed as a percentage of cell count relative to
the untreated control cells.
Clonogenic assay
Exponentially proliferating cells (2×105)
were plated in a 25-cm2 cell culture flask and incubated
in RPMI-1640 medium and treated with ribavirin (10, 25 and 50 µM)
or DZNep (0.1 µM) for 120 h. Untreated cells served as the negative
control. After 120 h of treatment, the cells were collected;
2×103 cells were seeded into 25-cm2 culture
flasks for ≤16 days in complete drug-free medium. The medium was
discarded every 48 h and replaced with complete fresh medium. Cells
were cultured for 16 days, after which the viability of cells was
determined by a trypan blue exclusion assay using a TC10 Automated
Cell Counter (Bio-Rad Laboratories, Inc.).
Statistical analyses
Three independent experiments in triplicate were
performed, and data are expressed as the mean ± standard deviation.
Data were statistically analyzed using GraphPad Prism V6 software
(GraphPad Software Inc., La Jolla, CA, USA). Significant
differences were determined using one-way analysis of variance
(ANOVA) followed by Bonferroni correction to determine significant
differences between each experimental group against its respective
control. P<0.05 was considered to indicate a statistically
significant difference.
Protein extraction and western
blotting
Hut78 cells (2.5×105) were cultured in
25-cm2 flasks and treated with ribavirin for 120 h. Once
cells were washed with PBS and centrifuged for 120 × g for 5 min,
proteins were extracted using radioimmunoprecipitation buffer (150
mM NaCl; 1.0% IGEPAL CA630; 0.5% sodium deoxycholate; 0.1% SDS and
50 mM Tris, pH 8.0) in the presence of proteinase inhibitors (cat.
no. p8340; Sigma-Aldrich; Merck KGaA). The protein concentration
was determined using a bicinchoninic acid assay (BCA-1, Bio-Rad
Laboratories, Inc.) and the integrity was assessed by Coomassie
staining. A total of 30 µg protein was separated by 10% SDS PAGE
and transferred onto a polyvinylidene difluoride membrane (cat. no.
1620177; Bio-Rad Laboratories, Inc.). The membrane was blocked with
5% skim milk in PBS for 1 h at room temperature and subsequently
incubated with antibodies against EZH2 (cat. no. 36-6300; 1:500,
Invitrogen; Thermo Fisher Scientific, Inc.), STAT-1 (cat. no.
sc417; 1:200, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
SCD (cat. no. sc58420; 1:500,) (Santa Cruz Biotechnology, Inc.),
and anti-actin peroxidase (cat. no. A3854; 1:10,000, Sigma Aldrich;
Merck KGaA) in blocking solution (5% skim milk in TBS + 0.1%
Tween-20), overnight at 4°C. The following secondary antibodies
were used: for EZH2, anti-rabbit (cat. no. sc2370) and for STAT-1
and SCD, anti-mouse (cat. no. sc2371), which were obtained from
Santa Cruz Biotechnology, Inc. The secondary antibodies were
diluted 1:1,000 and the incubation was performed for 1 h at room
temperature. Protein bands were visualized using Clarity Western
Enhanced Chemiluminescence Substrate (cat. no. 1705060; Bio Rad
Laboratories, Inc.). Bands were quantified densitometrically using
ImageJ version 1.50f (National Institutes of Health, Bethesda, MD,
USA).
Extraction, purification, and analysis
of histones
Histone proteins were isolated via the sulfuric acid
extraction method. Briefly, the nuclear pellet was resuspended in
0.4 M H2SO4 for 4 h; following centrifugation
(10,000 × g, 20 min), acid-soluble proteins in the supernatant were
obtained via overnight precipitation with 20% trichloroacetic acid
and centrifugation (16,000 × g for 30 min). The pellets containing
histone proteins were washed once in ice-cold acetone containing 1%
HCl and followed by ice-cold acetone alone. The pellet was dried
under vacuum and stored at −80°C. The protein concentration was
determined by the Bradford method, and the integrity of the histone
proteins in the acid-soluble extract was evaluated using Coomassie
staining. Proteins were separated via 15% SDS-PAGE and then
transferred to a polyvinylidene difluoride membrane (Bio-Rad
Laboratories, Inc.). The membrane was incubated for 1 h with
blocking solution (TBS-Tween-20, 5% of non-fat milk) followed by
overnight incubation with antibodies, including anti-H3K27me3 (cat.
no. 07-449) and anti-H3 total (cat. no. 06-755) obtained from EMD
Millipore (Billerica, MA, USA). Anti-rabbit secondary antibody
(cat. no. sc2370; 1:1,000, Santa Cruz Biotechnology, Inc) was then
applied for 1 h at room temperature. Protein bands were visualized
using the chromogenic substrate Clarity Western Enhanced
Chemiluminescence Substrate (cat. no. 1705060, Bio-Rad
Laboratories, Inc.). Bands were quantified densitometrically using
ImageJ software.
Microarrays and gene expression
analysis
Hut78 cells were treated with 50 µM ribavirin for
120 h as aforementioned and then total RNA was isolated using
TRIzol (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocols. RNA quality was evaluated by capillary
electrophoresis (Agilent 2100 Bioanalyzer, Agilent Technologies,
Inc., Santa Clara, CA, USA); only RNA samples with an RNA integrity
number >8.0 were further processed for microarray analysis. A
total of 200 ng RNA from each experimental cell group was evaluated
using the Gene Chip Human Transcriptome Array 2.0 (Affymetrix;
Thermo Fisher Scientific, Inc.) to determine the whole
transcriptome expression profiles according to the manufacturer's
protocols. Briefly, the synthesis and amplification of cDNA, and
gene expression profiling were conducted using the WT PLUS Reagent
Kit for fresh samples (Affymetrix; Thermo Fisher Scientific, Inc.).
Washing and staining of the samples were performed using the Gene
chip hybridization wash and stain kit in the Gene Chip Fluidics
Station 450 system (Affymetrix; Thermo Fisher Scientific, Inc.).
The probe arrays were scanned using The Gene Chip Scanner 30007G
(Affymetrix; Thermo Fisher Scientific, Inc.). Signal intensities of
the array were analyzed with Affymetrix Expression Console software
(version 1.3). Briefly, raw data probes were normalized using
Signal Space Transformation-Robust Multichip Analysis for
background correction and to obtain the quantile algorithm. To
define the differential expression profiles of the different
conditions, two-way ANOVA was performed in the Affymetrix
Transcriptome Analysis Console software (version 3.0). Genes with a
fold change >2 or <2 and with an P≤0.05 (obtained via ANOVA)
were considered significantly altered between the conditions. All
data were uploaded in Gene Expression Omnibus: GSE118866, token:
Klujuowqpnsvpkl.
Bioinformatics analysis
Gene Ontology analysis was performed using Protein
Analysis Through Evolutionary Relationship (http://www.pantherdb.org). Molecular pathways and
interaction networks were analyzed by Ingenuity Pathway Analysis
(Ingenuity Systems Inc., Redwood City, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Hut78 cells were treated with ribavirin for 120 h,
and total RNA was isolated when cells attained ~70% confluence,
using TRIzol reagent according to the manufacturer's protocols. RNA
purity and integrity were assessed via spectrophotometric analysis
using a NanoDrop 2000c spectrophotometer (NanoDrop Technologies;
Thermo Fisher Scientific, Inc., Wilmington, DE, USA) and denaturing
2% agarose gel. Bands were visualized using a MiniBIS Pro
D-Transilluminator (DNR Bio-Imaging Systems Ltd., Neve Yamin,
Israel). A total of 1 µg total RNA was used for cDNA synthesis with
the GeneAmp RNA PCR Core kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). iQ SYBR Green SuperMix (Bio-Rad Laboratories,
Inc.) was used according to the manufacturer's protocols. qPCR
reactions were run in triplicate using an ABI PRISM 7000 (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions for qPCR were as follows: 10 min at 95°C; 40 cycles of
30 sec at 95°C and 30 sec at 60°C. Data were analyzed using the
2−ΔΔCq method (12), and
reported as the fold-change in gene expression normalized to the
endogenous control gene hypoxanthine phosphoribosyltransferase 1
(HPRT1), and relative to untreated cells. The primers used
were: HPRT1 forward, 5′-GAACCTCTCGGCTTTCCCG-3′ and reverse,
3′-CACTAATCACGACGCCAGGG-5′; STAT-1 forward,
5′-ATGCTGGCACCAGAACGAAT-3′ and reverse, 3′-GCTGGCTGACGTTGGAGATC-5′;
and SCD forward 5′-GGGATCCTTCAGCACAGGAA-3′ and reverse
3′-CACCGCTTCTCCAATGGATT-5′. Annealing temperatures were 60°C for
all reactions. Three independent triplicates were conducted.
P-values were calculated using a two-tailed t-test.
Signal transducer and activator 1
short hairpin (sh)RNA lentiviral gene silencing
Hut78 cells were plated (2×105) in a
6-well plate 24 h prior to viral infection with 1 ml of complete
optimal medium (with serum and antibiotics) and were incubated
overnight at 37°C and 5% CO2. Then, the medium was
removed from the wells and replaced with 1 ml of complete medium
with Polybrene (cat. no. sc-134220, Santa Cruz Biotechnology, Inc.)
at a final concentration of 2 µg/ml. Lentiviral particles were
thawed at room temperature and mixed gently before use.
Subsequently, cells were infected by adding STAT-1 shRNA
Lentiviral Particles (cat. no. sc-44123V, Santa Cruz Biotechnology,
Inc.) to the culture and centrifuged at 2,460 × g for 1 h, and then
mixed for 6 h at 37°C in 5% CO2. Control shRNA
Lentiviral Particles (cat. no. sc-108080, Santa Cruz Biotechnology,
Inc.) were used. The next day, the culture medium was removed and
replaced with 1 ml of complete medium (without Polybrene) and cells
were incubated overnight at 37°C in 5% CO2. Then, the
shRNA Lentiviral Particles were diluted 1:3 according to the
manufacturer's instructions and added to the cells; the cells were
incubated for 24–48 h in complete medium. The clones expressing the
shRNA were selected with puromycin, and the medium was replaced
with fresh puromycin-containing medium every 3–4 days until
resistant cells could be identified. Cell viability assays were
performed using cells treated with 50 µM ribavirin for 120 h as
aforementioned. The experiments were conducted with at least three
independent triplicates. P-values were calculated using one-way
ANOVA with Bonferroni correction.
Results
Ribavirin suppresses cell
viability
Lymphoma cells were exposed to different
concentrations of ribavirin and the cell viability was analyzed at
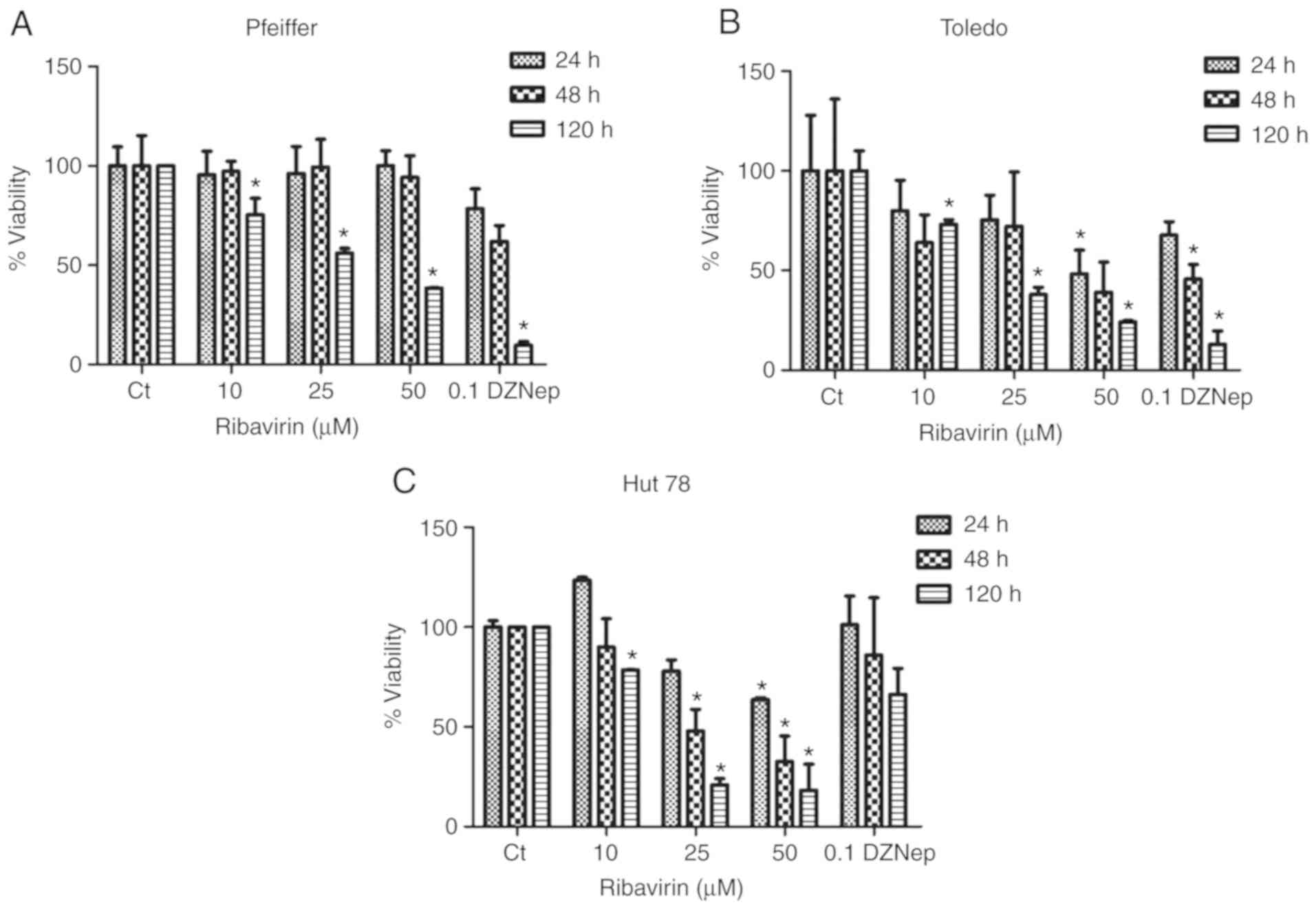
120 h post-treatment. As presented in Fig. 1A-C, a dose-dependent effect of
different extents was observed in the cell lines. For Pfeiffer
cells, no inhibition at 24 and 48 h was reported regardless of the
dose of ribavirin administered; however, a significant effect was
observed with the three concentrations at 120 h. Inhibition with
DZNep revealed a significant effect at 120 h. For Toledo cells,
suppressed cell viability was observed following treatment with 25
µM ribavirin for 120 h, and with 50 µM ribavirin at 24 and 120 h.
DZNep treatment also revealed a significant effect on cell
viability at 48 and 120 h. In the CTCL cell line Hut78, significant
inhibition was observed with 25 µM ribavirin at 48 h and 120 h as
well while 50 µM exhibited inhibitory effects at 24, 48 and 120 h.
Of note, this cell line appeared to be the most sensitive to
ribavirin, but less so to DZNep.
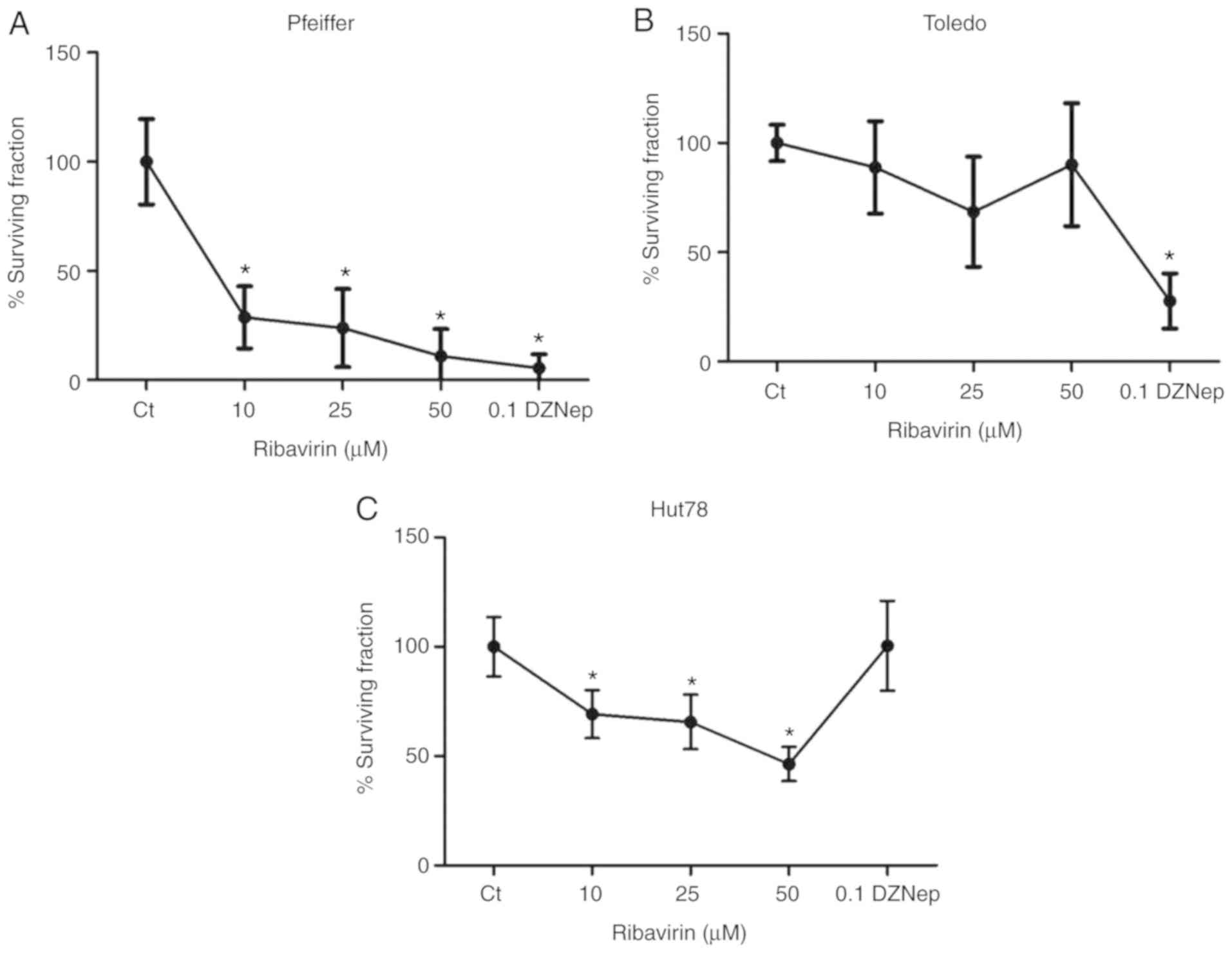
Ribavirin inhibits clonogenicity
A clonogenic assay was conducted to determine the
colony formation ability of cells. The results suggested that the
colony formation ability of Pfeiffer cells was significantly
inhibited with all doses of ribavirin. On the contrary, the colony
formation ability of Toledo cells was markedly unaffected in
response to ribavirin; however, significant inhibition was observed
following treatment with DZNep. In the CTCL cell line Hut78, a
significant decrease in clonogenicity was observed in response to
50 µM ribavirin, while DZNep did not change the clonogenicity of
this cell line (Fig. 2A-C).
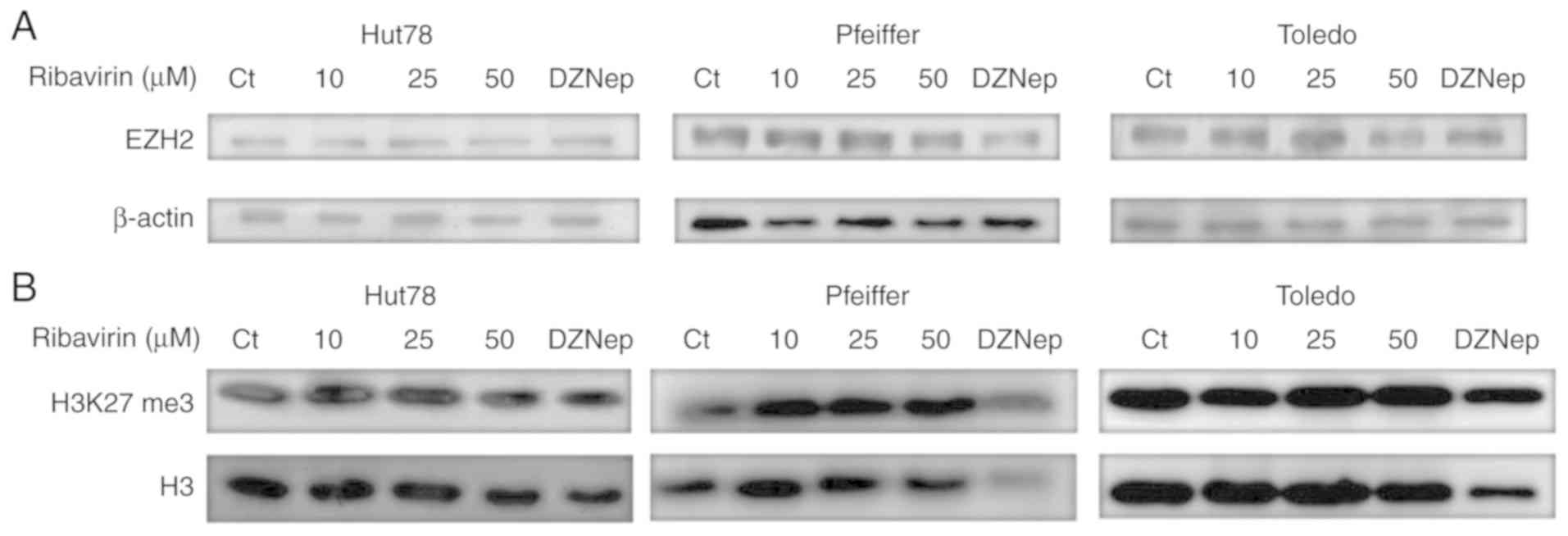
Effects of ribavirin on EZH2 protein
expression and H3K27 trimethylation
To determine whether the effects of ribavirin on the
viability and clonogenicity of the lymphoma cell lines were
associated with EZH2 and H3K27m3, cells were treated for 72 h with
the aforementioned doses of ribavirin and DZNep. The results
indicated that the expression of EZH2 was markedly unaffected in
response to ribavirin in the cell lines, whereas a notable
reduction in Pfeiffer cells was observed following treatment with
DZNep (Fig. 3A). Of note, no marked
reductions in H3K27 trimethylation were reported in response to
ribavirin (Fig. 3B).
Effects of ribavirin on gene
expression in Hut78 cells
A total of 978 genes were reported to be
differentially expressed. Among these, 629 were upregulated and 349
were downregulated. Ingenuity Pathway Analysis of the microarray
results (Table I) revealed that
KLHDC7B, PTGS2, GBP1, STAT1, GBP5, GBP2, GBP4, WARS, RGS1
and GBP1P1 were the top 10 most upregulated genes, while
SCD, TIMP2, GIPC3, DUSP9, CD5, TCF7, RFLNB, SBK1, LGMN and
SMIM24 were the most downregulated. The top canonical
pathways most significantly affected by ribavirin treatment
included ‘antigen presentation’, ‘communication between innate and
adaptive immune cells’, ‘cross-talk between dendritic and natural
killer cells’, ‘unfolded protein response’ and ‘allograft
rejection’. The top regulators of these pathways were determined to
be STAT1, IFN-γ, RELA, IFN-α and IFN-α2.
 | Table I.List of the 10 most upregulated genes
and the 10 most downregulated genes in Hut78 cells following
ribavirin treatment. |
Table I.
List of the 10 most upregulated genes
and the 10 most downregulated genes in Hut78 cells following
ribavirin treatment.
| Upregulated
molecules | Expression
fold-change value |
|---|
| KLHDC7B | ↑ 53.590 |
| PTGS2 | ↑ 52.680 |
| GBP1 | ↑ 28.730 |
| STAT1 | ↑ 28.330 |
| GBP5 | ↑ 21.080 |
| GBP2 | ↑ 18.550 |
| GBP4 | ↑ 12.990 |
| WARS | ↑ 11.170 |
| RGS1 | ↑ 10.200 |
| GBP1P1 | ↑ 9.950 |
|
| Downregulated
molecules | Expression
fold-change value |
|
| SCD | ↓ −11.150 |
| TIMP2 | ↓ −7.780 |
| GIPC3 | ↓ −5.610 |
| DUSP9 | ↓ −5.500 |
| CD5 | ↓ −5.240 |
| TCF7 | ↓ −4.820 |
| RFLNB | ↓ −4.490 |
| SBK1 | ↓ −4.450 |
| LGMN | ↓ −3.980 |
| SMIM24 | ↓ −3.900 |
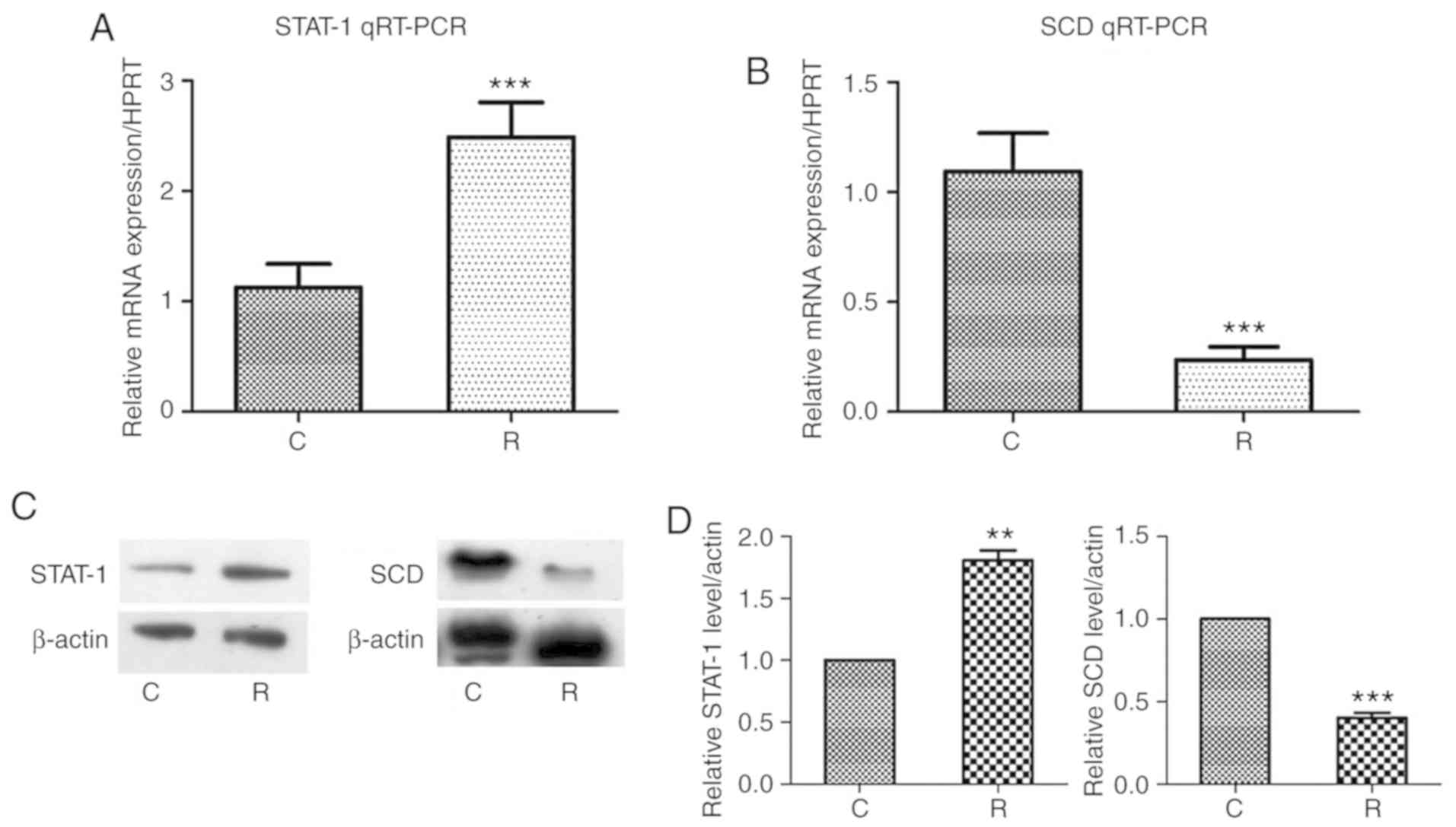
Gene validation of the transcriptional
effects and knockout of STAT1
STAT1 and SCD, which were determined
to be upregulated and downregulated, respectively, were analyzed by
RT-qPCR and western blotting. The results demonstrated that in both
cases, STAT1 expression was increased, while that of
SCD was decreased at the RNA and protein levels; these
differences were statistically significant (Fig. 4A-D). As STAT1 was proposed as a
top regulator of the central canonical pathways determined to be
altered in microarray analysis, this gene was deleted using shRNA.
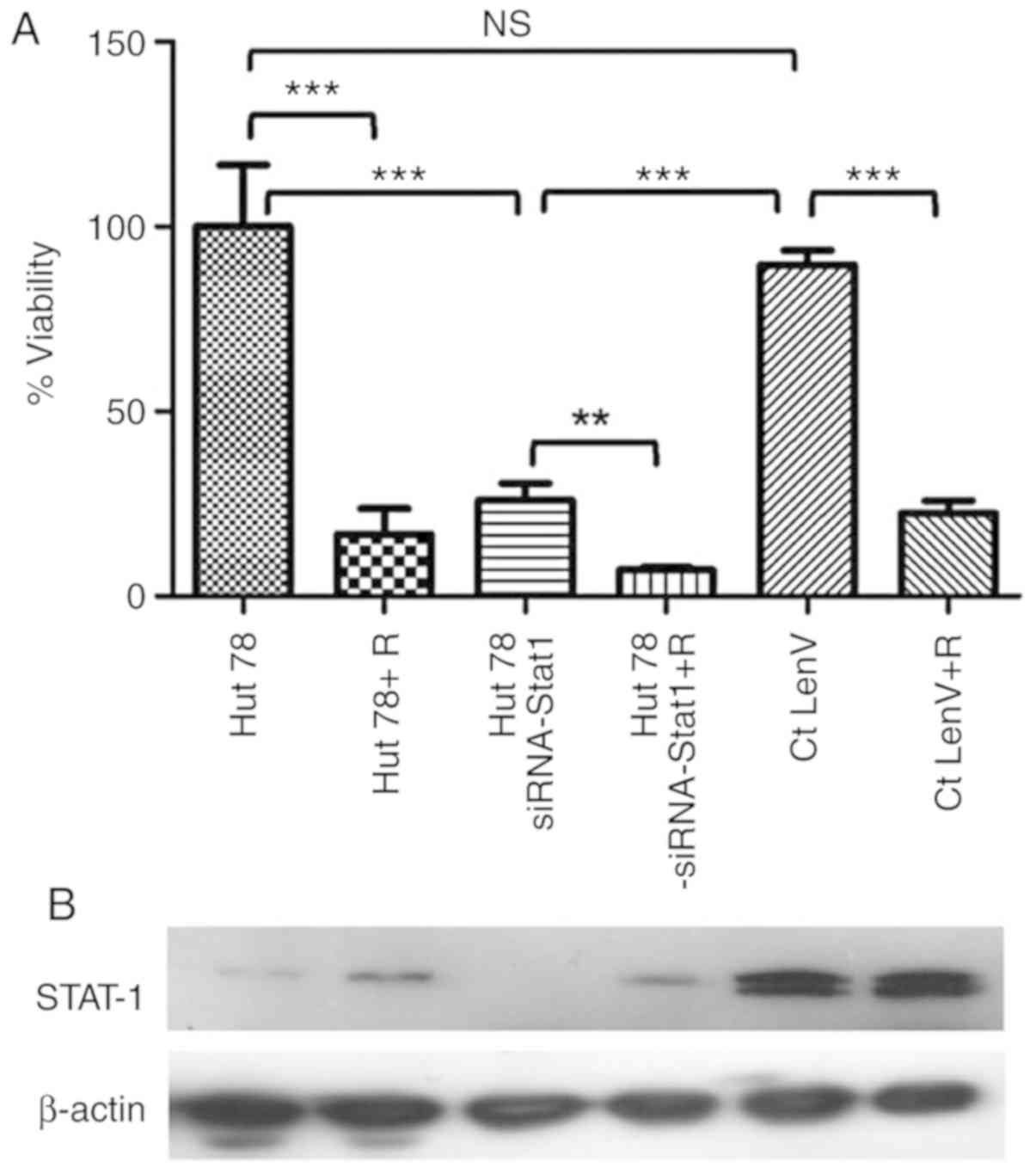
As presented in Fig. 5, an ~70%
reduction in cell viability was observed following
STAT1-shRNA Lentiviral Particles knockout; however, cell
viability was markedly unaffected in response to the scramble
control miRNA. Treatment with ribavirin of STAT1-depleted
cells further decreased cell viability; these differences were
statistically significant.
Discussion
The results of the present study revealed that
ribavirin inhibited the growth and clonogenicity of cells in a
dose-dependent manner in certain lymphoma cell lines. These effects
were not related to the mutational status of EZH2; the expression
and activity of EZH2 were markedly unaffected by ribavirin
as evaluated by RT-qPCR and H3K27 trimethylation analysis,
respectively. Furthermore, the results of transcriptome analysis
indicated that the majority of the canonical pathways affected by
ribavirin were associated with the immune system, including
‘antigen presentation’, ‘communication between innate and adaptive
immune cells’ and ‘cross-talk between dendritic and natural killer
cells’. Analysis of expression at the mRNA and protein levels
revealed that SCD was downregulated, while STAT1 was
upregulated. Depletion of STAT1, which was proposed as a top
regulator of the aforementioned pathways, exerted growth inhibitory
effects almost to the same extent as ribavirin.
Our results revealed the antitumor effects of
ribavirin. The inhibition of cell growth, differentiation and
migration by ribavirin has been observed in numerous cancer cell
lines, including breast, cervical, colon, brain, prostate, head and
neck, and lung cancer. These effects may occur by inhibiting eIF4E,
EZH2 and IMPDH (3,13–20);
however, the majority of studies into the antitumor effects of
ribavirin have focused on eIF4E, which is directly targeted by
ribavirin (5,21–23). In
addition, in models of aggressive double- and triple-hit diffuse
large B-cell lymphomas, eIF4E inhibition mediated by ribavirin
resulted in tumor-suppressive effects in cell lines and
patient-derived tumor grafts (24).
Based on the fact that anti-lymphoma activity in patients has been
reported following treatment with ribavirin (12,25), as
well as in light of our previous findings of downregulated EZH
expression and activity in MCF-7 cells (3), we investigated the effects of ribavirin
in lymphoma cell lines. The present study reported that, regardless
of EZH2 mutation, ribavirin exhibited inhibitory effects in
certain lymphoma cell lines. As notable inhibition with DZNep was
also observed, it was proposed that ribavirin is likely to exert
its effects independent of EZH2, at least in the cell lines
employed in the present study.
On the contrary, significant inhibition was observed
in the CTCL cell line following treatment with ribavirin, but not
with DZNep, suggesting that other mechanisms may be involved in
this phenomenon. Thus, global gene expression analysis was
conducted in the present study to further investigate the
mechanisms underlying the effects of ribavirin in T-cell lymphoma.
The present study determined that ribavirin affects the
transcription of genes as alterations in the expression of 978
genes were reported; the majority of genes were upregulated,
including STAT1 and IFN-γ. IFNs and other cell
signals have been proposed to activate STAT1. Following activation,
STAT1 translocates to the nucleus to induce IFN-stimulated genes
associated with antiviral defense, tumor-suppressive functions and
the immune surveillance of tumors (26–28).
Analysis of STAT1 knockout in the present study may provide
further insight into the role of STAT1 as the inhibitory effects
exerted on Hut78 cells were similar to the effects of
ribavirin.
To the best of our knowledge, no studies have
investigated the transcriptomic response of cancer cells to
ribavirin. However, this response was determined in the liver of
patients with chronic HCV infection. Gene expression analysis of
liver biopsies of patients treated with ribavirin revealed no
significant effects on hepatic gene expression in response to
ribavirin compared with controls. Similar results were obtained
following treatment with ribavirin combined with PEG-IFN than
PEG-IFN alone; however, significant downregulation in the
expression of IFN-stimulated genes was reported in the liver, but
not in peripheral blood cells (29).
The findings of the present study in a CTCL lymphoma cell line
supports the importance of the IFN-STAT1 signaling pathway in CTCL.
Sun et al investigated the development of the IFN-resistant
Hut78 cell line. Although the expression levels of the IFN receptor
and its binding affinity were comparable between parental and
resistant cells, IFN-α stimulation failed to induce IFN-stimulated
gene factor 3 complex formation in IFN-resistant Hut78R cells. This
may be associated with downregulated STAT1 protein or mRNA
expression, suggesting that at least in this model, STAT1 may be
required for the antitumor effects of IFN (30). IFN-γ was used in a phase II trial with
15 patients, 11 (73.3%) of whom achieved an objective response
(31). A recent study reported on 6
patients with CTCL (mycosis fungoides) and HCV infection who
received antiviral treatment; 3 patients received PEG-IFN plus
ribavirin. A total of 2 patients exhibited stabilization of skin
lesions with marked improvement in pruritus, while another had a
complete response that was maintained for 5-years at the time of
analysis (32). At present, the
majority of studies that reported non-Hodgkin lymphoma with IFN and
ribavirin treatment investigated B-cell lymphoma; the direct
inhibition of eIF4E has been notably evaluated (24,25). The
results of the present study on the DLBCL cell lines, Pfeiffer with
an EZH2 mutation and Toledo with no mutation, revealed inhibition
of cell growth in response to ribavirin. A limitation of this study
is that transcriptome analysis was limited to the CTCL cell line;
thus, it cannot be suggested that the same transcriptional response
to ribavirin may be observed in all B-cell lymphomas. Nevertheless,
in an analysis of 2,030 cases of DLBCL from 10 publicly available
gene expression datasets, polarized T-cell and cytotoxic gene
expression in association with the IFN-γ/STAT1/IRF1 axis was
reported. To note, such a response may be related to improved
outcome, particularly in the germinal center B-cell subsets of
DLBCL (33).
In conclusion, the results of the present study
suggest that EZH2, at least in certain B-cell lymphoma cell lines,
may participate in the pathogenesis of this disease as these cells
were notably affected by the EZH2 inhibitor DZNep. This supports
the reported inhibition by potent EZH2 inhibitors in Pfeiffer cells
(34); however, ribavirin was found
to not affect EZH2 expression or H3K27 trimethylation. This
indicates the potential weak inhibitory activity of ribavirin
against EZH2. On the contrary, our study demonstrated that
ribavirin notably inhibited the growth of lymphoma cell lines.
These effects were more potent in the T-cell lymphoma cell line,
which may depend, at least in part, on the activation of canonical
immune pathways regulated by the key factors STAT1 and IFN-γ. The
results of the present study provide insight into the effects of
ribavirin, which warrants its analysis in preclinical and clinical
studies to determine the potential of this drug as a candidate
anticancer agent for the treatment of lymphoma.
Acknowledgements
Not applicable.
Funding
The present study was funded by Consejo Nacional de
Ciencia y Tecnologia, grant 161915.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
GDG, DCP, ASC, ACB, AGF, JDC and LTC performed the
experiments and collected the data in regards to the cell culture,
western blotting and RT-PCR. FBA, ACT and AHM performed the
microarray experiments and analysis. ADG conceived and wrote the
manuscript. All authors contributed to the discussion of the
results and critically read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Sidwell RW, Robins RK and Hillyard IW:
Ribavirin: An antiviral agent. Pharmacol Ther. 6:123–416. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shepherd J, Jones J, Hartwell D, Davidson
P, Price A and Waugh N: Interferon alpha (pegylated and
non-pegylated) and ribavirin for the treatment of mild chronic
hepatitis C: A systematic review and economic evaluation. Health
Technol Assess. 111–205. (iii)2007.PubMed/NCBI
|
|
3
|
De la Cruz-Hernandez E, Medina-Franco JL,
Trujillo J, Chavez-Blanco A, Dominguez-Gomez G, Perez-Cardenas E,
Gonzalez-Fierro A, Taja-Chayeb L and Dueñas-Gonzalez A: Ribavirin
as a tri-targeted antitumor repositioned drug. Oncol Rep.
33:2384–2392. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Assouline S, Culjkovic B, Cocolakis E,
Rousseau C, Beslu N, Amri A, Caplan S, Leber B, Roy DC, Miller WH
Jr and Borden KL: Molecular targeting of the oncogene eIF4E in
acute myeloid leukemia (AML): A proof-of-principle clinical trial
with ribavirin. Blood. 114:257–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kentsis A, Topisirovic I, Culjkovic B,
Shao L and Borden KL: Ribavirin suppresses eIF4E-mediated oncogenic
transformation by physical mimicry of the 7-methyl guanosine mRNA
cap. Proc Natl Acad Sci USA. 101:18105–18110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Borroto-Esoda K, Myrick F, Feng J, Jeffrey
J and Furman P: In vitro combination of amdoxovir and the inosine
monophosphate dehydrogenase inhibitors mycophenolic acid and
ribavirin demonstrates potent activity against wild-type and
drug-resistant variants of human immunodeficiency virus type 1.
Antimicrob Agents Chemother. 48:4387–4394. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Casaos J, Huq S, Lott T, Felder R, Choi J,
Gorelick N, Peters M, Xia Y, Maxwell R, Zhao T, et al: Ribavirin as
a potential therapeutic for atypical teratoid/rhabdoid tumors.
Oncotarget. 9:8054–8067. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Volpin F, Casaos J, Sesen J, Mangraviti A,
Choi J, Gorelick N, Frikeche J, Lott T, Felder R, Scotland SJ, et
al: Use of an anti-viral drug, Ribavirin, as an anti-glioblastoma
therapeutic. Oncogene. 36:3037–3047. 2007. View Article : Google Scholar
|
|
9
|
Béguelin W, Popovic R, Teater M, Jiang Y,
Bunting KL, Rosen M, Shen H, Yang SN, Wang L, Wang L, et al: EZH2
is required for germinal center formation and somatic EZH2
mutations promote lymphoid transformation. Cancer Cell. 23:677–692.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sasaki D, Imaizumi Y, Hasegawa H, Osaka A,
Tsukasaki K, Choi YL, Mano H, Marquez VE, Hayashi T, Yanagihara K,
et al: Overexpression of Enhancer of zeste homolog 2 with
trimethylation of lysine 27 on histone H3 in adult T-cell
leukemia/lymphoma as a target for epigenetic therapy.
Haematologica. 96:712–719. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Soumyanarayanan U and Dymock BW: Recently
discovered EZH2 and EHMT2 (G9a) inhibitors. Future Med Chem.
8:1635–1654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peveling-Oberhag J, Arcaini L, Bankov K,
Zeuzem S and Herrmann E: The anti-lymphoma activity of antiviral
therapy in HCV-associated B-cell non-Hodgkin lymphomas: A
meta-analysis. J Viral Hepat. 23:536–544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pettersson F, Yau C, Dobocan MC,
Culjkovic-Kraljacic B, Retrouvey H, Puckett R, Flores LM, Krop IE,
Rousseau C, Cocolakis E, et al: Ribavirin treatment effects on
breast cancers overexpressing eIF4E, a biomarker with prognostic
specificity for luminal B-type breast cancer. Clin Cancer Res.
17:2874–2884. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pettersson F, Del Rincon SV, Emond A, Huor
B, Ngan E, Ng J, Dobocan MC, Siegel PM and Miller WH Jr: Genetic
and pharmacologic inhibition of eIF4E reduces breast cancer cell
migration, invasion, and metastasis. Cancer Res. 75:1102–1112.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sharma S, Baksi R and Agarwal M:
Repositioning of anti-viral drugs as therapy for cervical cancer.
Pharmacol Rep. 68:983–989. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xi C, Wang L, Yu J, Ye H, Cao L and Gong
Z: Inhibition of eukaryotic translation initiation factor 4E is
effective against chemo-resistance in colon and cervical cancer.
Biochem Biophys Res Commun. 503:2286–2292. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Richard SM and Martinez Marignac VL:
Sensitization to oxaliplatin in HCT116 and HT29 cell lines by
metformin and ribavirin and differences in response to
mitochondrial glutaminase inhibition. J Cancer Res Ther.
11:336–340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kosaka T, Nagamatsu G, Saito S, Oya M,
Suda T and Horimoto K: Identification of drug candidate against
prostate cancer from the aspect of somatic cell reprogramming.
Cancer Sci. 104:1017–1026. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Culjkovic B and Borden KL: Understanding
and targeting the eukaryotic translation initiation factor eIF4E in
head and neck cancer. J Oncol 2009. 9816792009.
|
|
20
|
Attar-Schneider O, Drucker L and Gottfried
M: Migration and epithelial-to-mesenchymal transition of lung
cancer can be targeted via translation initiation factors eIF4E and
eIF4GI. Lab Invest. 96:1004–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan K, Culjkovic B, Amri A and Borden KL:
Ribavirin targets eIF4E dependent Akt survival signaling. Biochem
Biophys Res Commun. 375:341–345. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Volpon L, Osborne MJ, Zahreddine H, Romeo
AA and Borden KL: Conformational changes induced in the eukaryotic
translation initiation factor eIF4E by a clinically relevant
inhibitor, ribavirin triphosphate. Biochem Biophys Res Commun.
434:614–619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Borden KL and Culjkovic-Kraljacic B:
Ribavirin as an anti-cancer therapy: Acute myeloid leukemia and
beyond? Leuk Lymphoma. 51:1805–1815. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Culjkovic-Kraljacic B, Fernando TM,
Marullo R, Calvo-Vidal N, Verma A, Yang S, Tabbò F, Gaudiano M,
Zahreddine H, Goldstein RL, et al: Combinatorial targeting of
nuclear export and translation of RNA inhibits aggressive B-cell
lymphomas. Blood. 127:858–868. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rutherford SC, Stewart EN, Chen Z,
Chadburn A, Wehrli NE, van Besien K, Martin P, Furman RR, Leonard
JP and Cerchietti L: The eIF4E inhibitor ribavirin as a potential
antilymphoma therapeutic: Early clinical data. Leuk Lymphoma.
59:256–258. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dunn GP, Koebel CM and Schreiber RD:
Interferons, immunity and cancer immunoediting. Nat Rev Immunol.
6:836–848. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferrantini M, Capone I and Belardelli F:
Interferon-alpha and cancer: Mechanisms of action and new
perspectives of clinical use. Biochimie. 89:884–893. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Belardelli F, Ferrantini M, Proietti E and
Kirkwood JM: Interferon-alpha in tumor immunity and immunotherapy.
Cytokine Growth Factor Rev. 13:119–134. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rotman Y, Noureddin M, Feld JJ, Guedj J,
Witthaus M, Han H, Park YJ, Park SH, Heller T, Ghany MG, et al:
Effect of ribavirin on viral kinetics and liver gene expression in
chronic hepatitis C. Gut. 63:161–169. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun WH, Pabon C, Alsayed Y, Huang PP,
Jandeska S, Uddin S, Platanias LC and Rosen ST: Interferon-alpha
resistance in a cutaneous T-cell lymphoma cell line is associated
with lack of STAT1 expression. Blood. 91:570–576. 1998.PubMed/NCBI
|
|
31
|
Sugaya M, Tokura Y, Hamada T, Tsuboi R,
Moroi Y, Nakahara T, Amano M, Ishida S, Watanabe D, Tani M, et al:
Phase II study of i.v. interferon-gamma in Japanese patients with
mycosis fungoides. J Dermatol. 41:50–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kyvernitakis A, Duvic M, Mahale P and
Torres HA: Interferon-based treatment for patients with mycosis
fungoides and hepatitis C virus infection: A case series. Am J Clin
Dermatol. 15:451–456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Care MA, Westhead DR and Tooze RM: Gene
expression meta-analysis reveals immune response convergence on the
IFNγ-STAT1-IRF1 axis and adaptive immune resistance mechanisms in
lymphoma. Genome Med. 7:962015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Woo J, Kim HY, Byun BJ, Chae CH, Lee JY,
Ryu SY, Park WK, Cho H and Choi G: Biological evaluation of
tanshindiols as EZH2 histone methyltransferase inhibitors. Bioorg
Med Chem Lett. 24:2486–2492. 2014. View Article : Google Scholar : PubMed/NCBI
|