Introduction
Gastric cancer (GC) is the third leading cause of
cancer-related death in the world (1). A deeper understanding of the
pathogenesis and biological features of GC is necessary to further
enhance early detection and treatment methods. The identification
of novel biomarkers, which could become both potent new tools for
the diagnosis of GC and targets for treatment, is one of the main
strategies for improving the diagnosis and treatment of GC
(2). In addition to the Lauren
histology-based classification (3),
GC can be subdivided into four phenotypes according to mucin
expression (2). Accumulating
evidence suggests that the gastric/intestinal mucin phenotypes of
GC have different clinical characteristics and specific genetic and
epigenetic changes (4). Thus, the
classification of the subtype of GC is important for clinical
decision-making.
Annexin A10 (ANXA10), a
calcium-/phospholipid-binding protein, is a member of the Annexin
family. It has been reported that Annexin family proteins play
important roles in calcium signaling, cell motility,
differentiation and proliferation (5,6).
Expression of ANXA10 is observed in foveolar and glandular cells of
the normal antral or the body-type gastric mucosa in the stomach
(7,8). Other normal organs, including the
duodenum and urothelial epithelium, also show the robust expression
of ANXA10 (8). With regard to
cancer tissue, the expression of ANXA10 has been observed in
several types of cancer, including GCs, but the clinical
significance of ANXA10 is still controversial in various types of
cancer (8–13). In colorectal adenocarcinomas,
several studies that have confirmed the induction of the expression
of gastric mucins in serrated neoplasms have shown that the
aberrant expression of ANXA10 is significantly correlated with
gastric differentiation (14–17).
The expression of ANXA10 is frequently found to be
downregulated in GC, and its downregulation is associated with a
poor prognosis (18). However, the
role of the expression of ANXA10 in tumor progression depends on
the morphologic classification of GC; in diffuse-type GC, the
expression of ANXA10 is associated with better survival, whereas in
intestinal-type GC, the expression of ANXA10 is associated with a
poorer prognosis (in comparison to the lack of expression of
ANXA10) (7). With regard to
biological functions, a small number of studies have reported that
ANXA10 plays a tumor-suppressive role through the regulation of
cellular proliferation and apoptosis in GC cell lines (18,19).
Taken together with these data, ANXA10 is more likely to be
involved in the differentiation of the stomach and it is presumed
that ANXA10 basically acts as a tumor suppressor as long as the
cancer cells retain the expression of ANXA10. However, no studies
have reported a correlation between the expression of ANXA10 and
the mucin phenotype of GC, and only a few studies have described
the functional role of ANXA10 in GC.
Pancreatic and duodenal homeobox-1 (PDX1) is one of
the transcription factors that belongs to the homeobox gene family,
which is important in the differentiation and development of the
antrum of the stomach, duodenum and pancreas (20). In the adult, the expression of PDX1
is selectively observed in pancreatic β cells, Brunner's glands of
the duodenum and pyloric glands of the stomach. In cancerous
tissues, PDX1 is aberrantly expressed in ductal adenocarcinoma of
the pancreas (21) and functions as
a tumor suppressor in GC (22). In
addition, we recently found that PDX1 is frequently expressed in
serrated adenocarcinoma of the colon and ANXA10 is also a valuable
diagnostic marker for serrated adenocarcinoma of the colon
(23). Although aberrant expression
of PDX1 has been reported mainly in cancers of the digestive organs
and somehow seems to have a role in the pathogenesis of carcinomas,
it has not been irrefutably ascertained how the expression of PDX1
is regulated.
In the present study, we examined the expression and
distribution of ANXA10 in GC by immunohistochemistry (IHC), and
investigated the relationship between the expression of ANXA10 and
the gastric/intestinal mucin phenotypes of GC in order to test the
abovementioned hypothesis. Furthermore, we also aimed to identify
the effect of ANXA10 on the expression of other molecules,
especially focusing on the molecules related to the differentiation
of the gastrointestinal organs. Among these molecules, it was
decided to focus on PDX1. In order to analyze the relationship
between ANXA10 and PDX1 in GC tissues and the effect of the
deregulation of ANXA10 on the expression of PDX1, both GC cell
lines and organoids were used since organoids may be useful to
reveal the correlation between ANXA10 and PDX1 in the minimum unit
of glands from non-cancerous and GC tissue, which could potentially
elucidate the actual correlation of these two molecules in
vivo.
Materials and methods
Tissue samples
Samples from 130 primary tumors that had been
collected from patients diagnosed with GC who underwent surgery at
Hiroshima University Hospital (Hiroshima, Japan) between 2003 and
2007 (range of age, 38–82 years; male, n=82; female, n=48) were
retrospectively analyzed. The patients were followed up by their
physicians until their death or the date of the last documented
contact. For the immunohistochemical analyses (IHC), archival
formalin-fixed, paraffin-embedded tissues from 130 GC patients who
had undergone surgical excision as treatment for GC were
consecutively collected. One representative tumor block from each
patient, including the tumor center, invasive front, and the
tumor-associated non-neoplastic mucosa, was evaluated by IHC. Tumor
staging was determined according to the TNM classification system.
The histological classifications were determined based on the
guidelines of the Japanese Research Society for Gastric Cancer.
Written informed consent for the establishment of organoids was
obtained from all of the patients. This study was approved by the
Ethics Committee for Human Genome Research of Hiroshima University,
Hiroshima (E-597-01). This study was conducted in accordance with
the Ethical Guidance for Human Genome/Gene Research of the Japanese
Government.
IHC
IHC was performed with a Dako EnVision+ mouse
peroxidase detection system (cat. no. K5007; DakoCytomation).
Antigen retrieval was performed by heating citrate buffer (pH 6.0)
in a microwave for 30 min. Peroxidase activity was blocked with 3%
H2O2-methanol for 10 min, and sections were
incubated with normal goat serum (cat. no. X090710; DakoCytomation)
for 20 min to block nonspecific antibody binding sites. Sections
were incubated with a rabbit monoclonal anti-ANXA10 antibody
(dilution 1:1,000, cat. no. NBP1-90156; Novus Biologicals) or a
rabbit monoclonal anti-PDX1 antibody (dilution 1:1,000, cat. no.
ab134150; Abcam) for 1 h at room temperature, followed by
incubation with EnVision+ anti-rabbit peroxidase for 1 h. For a
color reaction, sections were incubated with the DAB
substrate-chromogen solution (cat. no. K3468; DakoCytomation) for
10 min. Sections were counterstained with 0.1% hematoxylin.
Negative controls were created by omission of the primary
antibody.
The expression of ANXA10 was scored in all tumors as
positive or negative. When >10% of tumor cells were stained, the
immunostaining was considered positive for ANXA10. Using these
definitions, 3 surgical pathologists (AI, NS and DT), with no
knowledge of the clinical and pathologic parameters or the outcomes
of the patients, independently reviewed the immunoreactivity of
each specimen. Interobserver differences were resolved by consensus
review at a double-headed microscope after an independent
review.
Sample collection from the
database
We downloaded all of the GC transcriptome profiles
and clinical data from The Cancer Genome Atlas (TCGA) database
(https://xenabrowser.net/datapages/?cohort=TCGA%20Stomach%20Cancer%20(STAD)&removeHub=https%3A%2F%2Fxena.treehouse.gi.ucsc.edu%3A443).
Up until September 2018, the public database included the
expression profiles of 375 GC tissues and 32 normal tissues
obtained by RNA-seq (level 3). Our research conformed to the
guidelines published in TCGA. We took the logarithm of the
expression difference between normal and tumor samples.
Phenotypic analysis
GCs were classified into 4 phenotypes: Gastric (G),
intestinal (I), gastric and intestinal mixed (GI) and null (N)
types according to the mucin expression pattern. To analyze the
phenotypic expression of GC, we performed IHC (as described above)
with 4 antibodies, all from Novocastra Laboratories, Inc.:
Anti-MUC5AC (NCL-MUC-5AC), anti-MUC6 (NCL-MUC-6), anti-MUC2
(PA0155) and anti-CD10 (NCL-L-CD10-270). The criteria for the
classification of G- and I-type GCs have been described previously
(2).
Cell lines
Five cell lines derived from human GC (MKN-1, MKN-7,
MKN-45, MKN-74 and HSC-57) were used. MKN1, MKN7, MKN45 and MKN74
were purchased from the Japanese Collection of Research
Bioresources Cell Bank (Osaka, Japan) and HSC-57 was established by
Dr Kazuyoshi Yanagihara (Exploratory Oncology Research and Clinical
Trial Center, National Cancer Center) (24). All cell lines were maintained in
RPMI-1640 medium (cat. no. 05918; Nissui Pharmaceutical Co., Ltd.)
containing 10% fetal bovine serum (cat. no. 14-501F; BioWhittaker)
in a humidified atmosphere of 5% CO2 and 95% air at
37°C.
Western blotting
Western blotting was performed as described
previously (25). Immunocomplexes
were visualized with an Amersham ECL Western blot detection system
(cat. no. RPN2109; GE Healthcare). β-actin (cat. no. A5316;
Sigma-Aldrich; Merck KGaA) was also stained as a loading
control.
Lentiviral and retroviral vectors
For constitutive expression of human ANXA10, cDNA
was PCR amplified from MKN-74 cells and subcloned into pDON5neo
(Takara Bio) using a retrovirus vector with psPAX2 envelope and
pMD2.G packaging plasmids, according to the manufacturer's
instructions.
pTRIPZ-short hairpin (sh)ANXA10 (cat. no. 11199; GE
Healthcare) was constructed as follows: sh-ANXA10-1,
TTGCTGATTAGATAGTAGG; sh-ANXA10-2, TAATTGCTGATTAGATAGT; sh-ANXA10-3,
TAATTGCTGATTAGATAGT. ANXA10 was amplified from MKN-74 cDNA by a
PCR. The PCR fragment was ligated into a pDON-5 Neo plasmid (cat.
no. 3655; Takara) at the BamHI sites. All of the above were
cloned into the lentiviral transfer vector pTRIPZ (cat. no.
RHS4740-EG11199; GE Healthcare) and were verified by sequencing. A
lentivirus was produced by cotransfecting the transfer vector
containing the gene of interest, the VSVG envelope glycoprotein,
and the lentiviral packaging vector, into 293T cells (#HCL4517;
Dharmacon). The virus supernatant was concentrated by
ultrafiltration using Millex-HV (Millipore). In parallel,
transduced cells were selected in 3.0 µg/ml puromycin (cat. no.
P7255; Sigma-Aldrich; Merck KGaA) or 250 µg/ml G418 (cat. no.
11811031; Life Technologies)-containing media, corresponding to the
minimum time taken for untransduced cells to completely die in the
selection media. Protein knockdown or overexpression was verified
by western blotting.
Cell growth assay
To examine cell growth, an MTT assay was performed
as described previously (26). Cell
growth was monitored after 1, 2, 4 and 8 days, and OD570 was used
for the detection of absorbance.
Organoid culture and viral infection
of organoids
Organoids were derived from the stomach of patients
who underwent gastrectomy. Gastric organoids were generated and
propagated using the previously described ‘TMDU protocol’ (27), with minor modifications. The stomach
and tumor lesions were removed, minced into small pieces, and
suspended in 5 ml of DMEM (Thermo Fisher Scientific, Inc.)
supplemented with 100 U/ml penicillin (Thermo Fisher Scientific,
Inc.), 100 mg/ml streptomycin (Thermo Fisher Scientific, Inc.), 50
mg/ml gentamicin (Thermo Fisher Scientific, Inc.) and 1% FBS
(complete DMEM), to which 15 mM EDTA was added. The mixture was
shaken for 1 h at 4°C. The released crypts were washed extensively,
pelleted, and resuspended in 200 µl of the Matrigel (Corning, Inc.)
and placed in 3 wells of 24-well plates. After polymerization, 500
µl of ‘stomach’ and ‘colon’ media were added to each well to
produce the respective organoids. The details of ingredients in
each medium are summarized in Table
SI. The medium was changed every 2–3 days. The organoids were
split at a ratio of 1:3 every 7–8 days. Previously described
protocols (with minor modifications) were used for the gene
knockdown and forced-expression studies (28).
RT-qPCR
Total RNA was isolated from frozen samples or cancer
cell lines using Isogen (Nippon Gene), and 1 µg of total RNA was
converted to cDNA with a First Strand cDNA Synthesis Kit (GE
Healthcare). Real-time detection of the emission intensity of SYBR
Green bound to double-stranded DNA was performed with a CFX Connect
Real-Time System (Bio-Rad Laboratories). The thermocycling
conditions consisted of denaturation at 95°C for 10 sec and
annealing at 60°C for 30 sec repeated for 40 cycle.
ACTB-specific PCR products, which were amplified from the
same RNA samples, served as internal controls. Relative
quantification was determined using the ∆∆Cq method (29). The primer sequences are summarized
in Table SII.
Statistical analysis
Correlations between clinicopathological
parameters/markers of mucin phenotype and ANXA10 expression were
analyzed by Chi-square test, Fisher's exact test and two-sided
test. Kaplan-Meier survival curves were constructed for
ANXA10-positive or ANXA10-negative patients. Differences between
survival curves were tested for statistical significance using a
log-rank test. Expression levels of ANXA10 and PDX1 were analyzed
by Student's t-test. The data concerning cell growth assay was also
analyzed by Tukey's test or Student's t-test. The data obtained
from TCGA was analyzed by Spearman's Rho as for the correlation
between ANXA10 and PDX1 expression.
Results
Expression and the clinicopathological
significance of ANXA10 in GC
We analyzed the expression of ANXA10 in 130 GC cases
by immunohistochemical staining. In the non-neoplastic gastric
mucosa, the expression of ANXA10 was observed on the nucleoli of
the foveolar cells (Fig. 1A). No
expression of ANXA10 was observed on epithelial cells with
intestinal metaplasia (Fig. 1B). In
GC tissue, the robust nuclear staining of ANXA10 was detected in
both the well-differentiated (Fig.
1C) and poorly differentiated (Fig.
1D) types of GC. Since the median value of the positivity of
the GC cells in the sections was ~9.6%, the GC cases with staining
of >10% of tumor cells were regarded as positive cases. IHC
staining of PDX1 was conducted for specimens from 130 GC cases; the
expression of PDX1 was detected in the nucleoli of GC cells
(Fig. 1F) along with the expression
of ANXA10 (Fig. 1E). Correlations
were analyzed between the expression of ANXA10 and the
clinicopathological characteristics of the GC cases. A loss of
ANXA10 expression was associated with pN stage (P=0.020), pM stage
(P<0.001) and pStage (P=0.008) (Table I). The association between ANXA10
and PDX1 expression in GC was also investigated as previously
expression of both ANXA10 and PDX1 was frequently found in serrated
adenocarcinoma of the colon (23).
We found that the expression of ANXA10 was significantly associated
with that of PDX1 in GC cases (P=0.016) (Table I). The correlation between ANXA10
and PDX1 was also determined in the cohort that was uploaded in the
TCGA. A significant correlation was found between them (r=0.29,
P<0.001; Fig. 1G). Kaplan-Meier
analysis showed that a loss of ANXA10 expression was significantly
associated with poor survival in GC patients (P=0.010; Fig. 1H). These results suggest that ANXA10
plays an important role in the progression of GC.
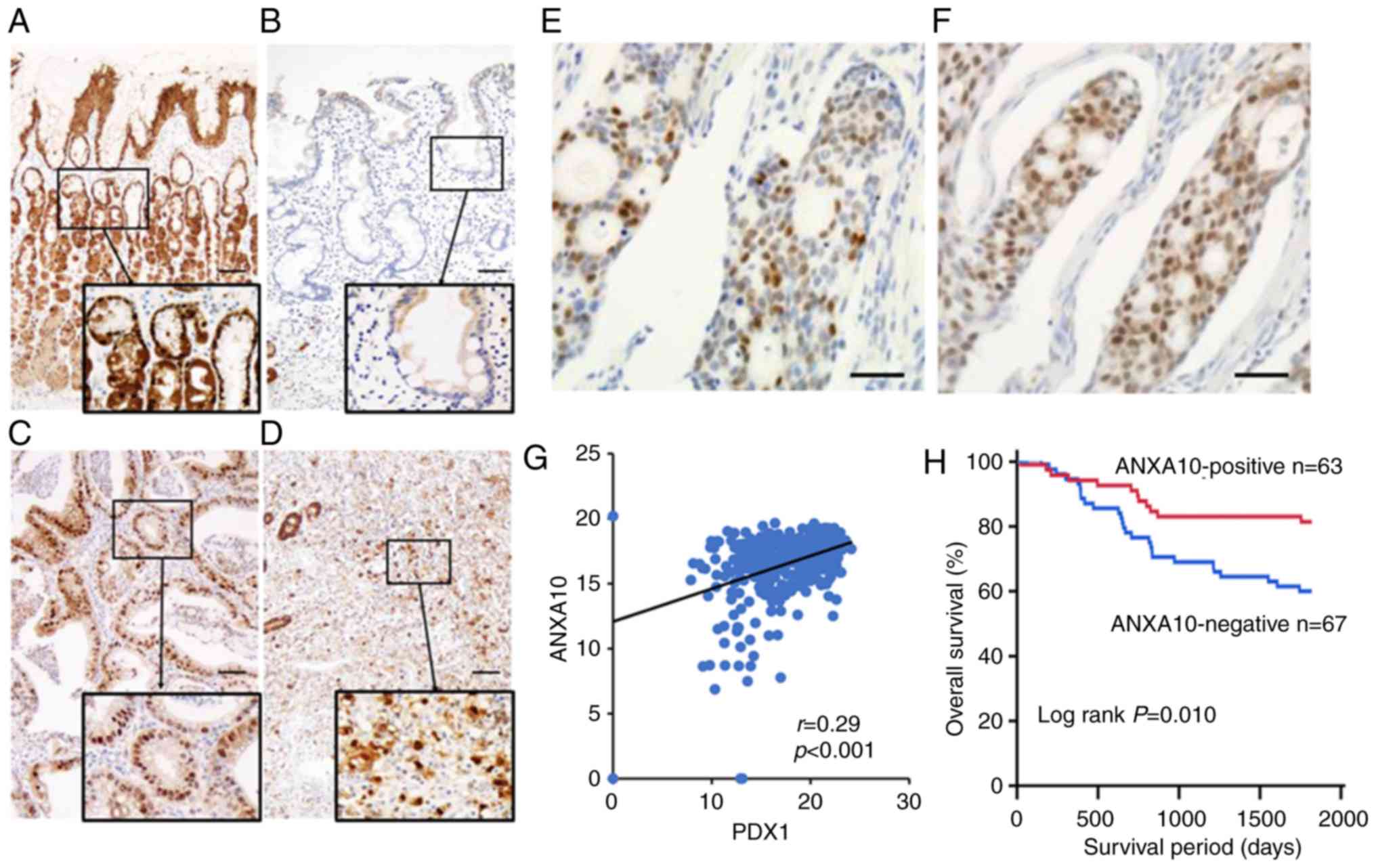 | Figure 1.Expression of ANXA10 in GC. (A)
Immunohistochemical staining of ANXA10 in normal gastric glands.
The nucleoli of normal cells of the gastric mucosa were positive
for ANXA10. Original magnification, ×100; scale bars, 100 µm. (B)
Immunohistochemical staining of ANXA10 in intestinal metaplasia. (C
and D) Immunohistochemical staining of ANXA10 in
well-differentiated type (C) and poorly differentiated type (D) GC.
Original magnification, ×100; scale bars, 100 µm. Staining of the
nucleoli revealed ANXA10 positivity (E and F) Expression of ANXA10
(E) and PDX1 (F) in a GC patient. Original magnification, ×400;
scale bars, 100 µm. (G) Correlation between ANXA10 and PDX1
expression in the GC dataset in TCGA. (H) A Kaplan-Meier plot of
survival based on ANXA10 expression in the group of 130 GC
patients. GC, gastric cancer; ANXA10, annexin A10; PDX1, pancreatic
and duodenal homeobox-1; TCGA, The Cancer Genome Atlas. |
 | Table I.Association between ANXA10 expression
and clinicopathological characteristics of the patients with GC
(N=130). |
Table I.
Association between ANXA10 expression
and clinicopathological characteristics of the patients with GC
(N=130).
|
| Annexin A10
expression |
|
|---|
|
|
|
|
|---|
|
Characteristics | Positive (%) | Negative |
P-valuea |
|---|
| Age (years) |
|
| 0.074 |
|
<65 | 39 (56) | 31 |
|
|
≥65 | 24 (40) | 36 |
|
| Sex |
|
| 0.174 |
|
Male | 36 (44) | 46 |
|
|
Female | 27 (56) | 21 |
|
| pT stage |
|
| 0.084 |
|
pT1 | 33 (57) | 25 |
|
|
pT2/3/4 | 30 (42) | 42 |
|
| pN stage |
|
| 0.020 |
|
pN0 | 41 (58) | 30 |
|
|
pN1/2/3 | 22 (37) | 37 |
|
| pM stage |
|
| <0.001 |
|
pM0 | 59 (57) | 44 |
|
|
pM1 | 4
(15) | 23 |
|
| pStage |
|
| 0.008 |
| pStage
I | 40 (60) | 27 |
|
| pStage
II/III/IV | 23 (37) | 40 |
|
| Histology |
|
| 0.112 |
|
Intestinal-type | 26 (41) | 37 |
|
|
Diffuse-type | 37 (55) | 30 |
|
| PDX1 |
|
|
|
|
Positive | 45 (57) | 34 | 0.016 |
|
Negative | 18 (35) | 33 |
|
Association of ANXA10/PDX1 with mucin
phenotypes of GC
The comparison between ANXA10 and markers of the
mucin phenotype revealed that ANXA10 was more frequently expressed
in 44 (66%) of the 67 MUC5AC-positive GC cases than in 19 (30%) of
the 63 MUC5AC-negative GC cases, and that it was less frequently
expressed in 3 (19%) of the 16 CD10-positive GC cases than in 60
(53%) of the 114 CD10-negative GC cases (Table II). Representative expression
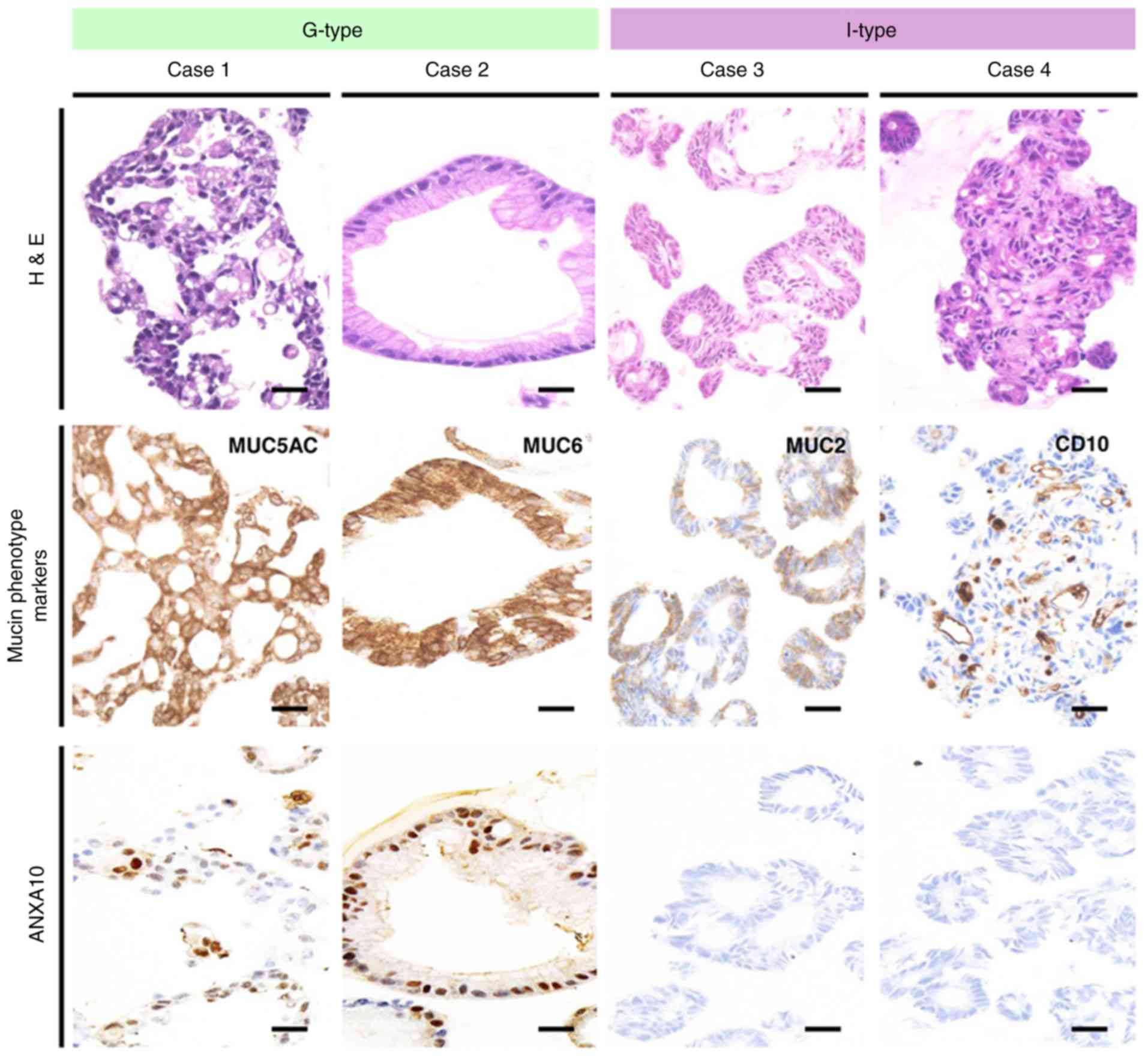
patterns of ANXA10 and other mucin markers are shown in Fig. S1. We used these 4 markers to
phenotypically classify the GC cases as follows: G-type (n=53;
41%), GI-type (n=20; 15%), I-type (n=25; 19%) and N-type (n=32;
25%). The expression of ANXA10 was more frequently observed in
G-type GC in comparison to the other types of GC (GI-, I- and
N-type) (P<0.001, Table II). We
also examined the correlation between PDX1 and markers of the mucin
phenotype as PDX1 is a transcription factor that plays critical
roles in the histogenesis of the stomach (19). In the comparison, neither of the
markers of mucin phenotype showed significant correlation with the
expression of PDX1 in the GC cases (Table III). Taken together with these
findings, only ANXA10 was more likely to be expressed in GC cases
with a gastric mucin phenotype and PDX1 did not show any
preferences in GC cases with the various specific mucin
phenotypes.
 | Table II.Expression of ANXA10 and markers of
the mucin phenotype in GC/mucin phenotypes of GC (N=130). |
Table II.
Expression of ANXA10 and markers of
the mucin phenotype in GC/mucin phenotypes of GC (N=130).
|
| ANXA10
expression |
|
|
|---|
|
|
|
|
|
|---|
| Markers | Positive (%) | Negative |
P-valuea |
P-valueb |
|---|
| Gastric
markers |
|
|
|
|
|
MUC5AC |
|
| <0.001 | <0.05 |
|
Positive | 44 (66) | 23 |
|
|
|
Negative | 19 (30) | 44 |
|
|
|
MUC6 |
|
| 0.485 | NS |
|
Positive | 6 (40) | 9 |
|
|
|
Negative | 57 (50) | 58 |
|
|
| Intestinal
markers |
|
|
|
|
|
MUC2 |
|
| 0.836 | NS |
|
Positive | 15 (47) | 17 |
|
|
|
Negative | 48 (49) | 50 |
|
|
|
CD10 |
|
| 0.011 | NS |
|
Positive | 3 (19) | 13 |
|
|
|
Negative | 60 (53) | 54 |
|
|
|
|
| ANXA10
expression |
|
|
|
|
|
|
|
| Mucin
phenotypes | Positive
(%) |
Negative |
P-valuec (G-types vs. others) |
|
| G-type | 34 (51) | 19 (30) | <0.001 |
| GI-type | 12 (18) | 8 (13) |
|
|
| I-type | 5
(7) | 20 (32) |
|
|
| N-type | 16 (24) | 16 (25) |
|
|
 | Table III.Expression of PDX1 and markers of the
mucin-phenotype in GC (N=130). |
Table III.
Expression of PDX1 and markers of the
mucin-phenotype in GC (N=130).
|
| PDX1
expression |
|
|---|
|
|
|
|
|---|
| Markers | Positive (%) | Negative |
P-valuea |
|---|
| Gastric
markers |
|
|
|
|
MUC5AC |
|
| 0.537 |
|
Positive | 39 (58) | 28 |
|
|
Negative | 40 (63) | 23 |
|
|
MUC6 |
|
| 0.234 |
|
Positive | 7 (46) | 8 |
|
|
Negative | 72 (62) | 43 |
|
| Intestinal
markers |
|
|
|
|
MUC2 |
|
| 0.852 |
|
Positive | 19 (59) | 13 |
|
|
Negative | 60 (61) | 38 |
|
|
CD10 |
|
| 0.880 |
|
Positive | 10 (62) | 6 |
|
|
Negative | 69 (60) | 45 |
|
Effects of ANXA10 on the expression of
PDX1 in GC
Although our results indicated that ANAXA10 and PDX1
were preferentially co-expressed in GC tissues, it remained unclear
whether there is an interaction between ANAX10 and PDX1. We
examined the expression of ANXA10 and PDX1 by western blotting
using 5 GC cell lines. The level of PDX1 expression was
undetectable in 1 cell line (MKN-1); however, the expression of
PDX1 was not detectable in any of the GC cell lines in which the
expression of ANXA10 was undetectable (Fig. 2A). We also evaluated the expression
of ANXA10 and PDX1 by RT-qPCR, and similar expression patterns were
found as those for the western blot analysis (Fig. 2B and C). We examined the transition
of ANXA10 expression using MKN-74 and MKN-45 cells transfected with
ANXA10-specific shRNAs. All of the 3 different shRNAs
(sh-ANXA10-1-3) repressed the expression of ANXA10 to some extent;
accordingly, the expression of PDX1 was decreased in the MKN-74
cells (Fig. 2D) and MKN-45 cells
(Fig. 2E). To further investigate
the direct correlation between ANXA10 and PDX1, HSC-57 cells were
stably transfected with pDON-ANXA10. HSC-57-ANXA10 expressed ANXA10
as expected and provided a significantly higher level of PDX1 in
comparison to the HSC-57-empty vector cells (Fig. 2F). We also performed an MTT assay in
order to examine the effect of ANXA10 on cell growth. The cell
growth of MKN-74-shRNA cells was significantly increased in
comparison to the MKN-74-scramble cells from day 2 (Fig. 2G). The same trend was observed in
the MKN-45 cells (Fig. 2H).
Conversely, the cell growth of HSC-57-ANXA10 cells was
significantly inhibited in comparison to the HSC-57-empty scramble
cells on day 4 and 6 (Fig. 2I). We
also examined the effect of overexpression of ANXA10 on the
expression of the markers for mucin phenotype using HSC-57-ANXA10
cells, and found that expression of any of the markers was not
affected by ANXA10 forced-expression (Fig. S2). These results demonstrated that
ANXA10 could play an important role in the regulation of PDX1
expression in GC cells.
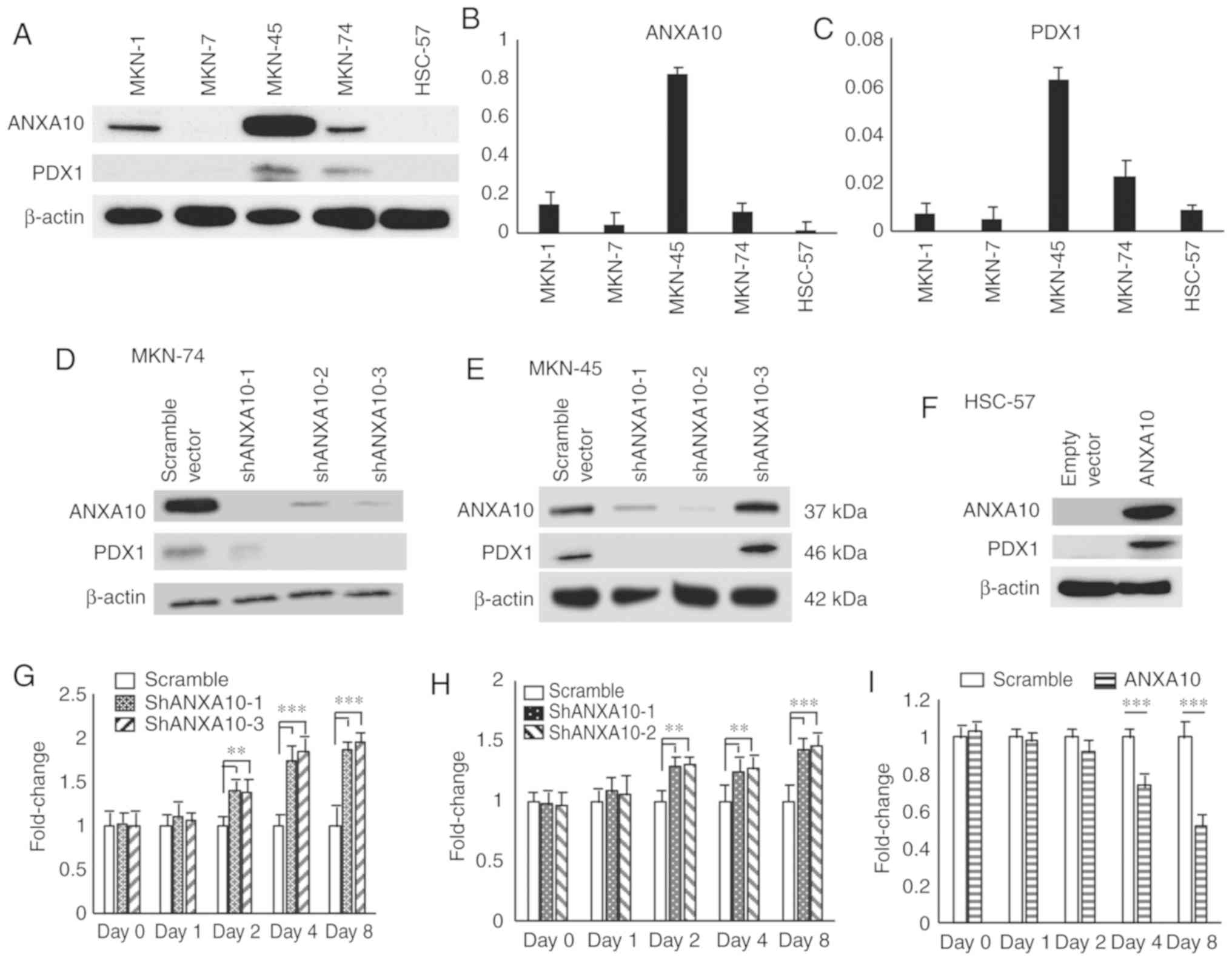 | Figure 2.Effects of ANXA10 on the expression
of PDX1. (A) Western blot analysis of ANXA10 and PDX1 proteins in 5
GC cell lines. (B and C) RT-qPCR for (B) ANXA10 and (C)
RT-qPCR for PDX1 expression in 5 GC cell lines. Bars and error bars
represent the mean and SD, respectively, of 3 independent
experiments. (D and E) Western blot analysis of the ANXA10 and PDX1
proteins in (D) MKN-74 and (E) MKN-45 cells transfected with ANXA10
shRNA (shANXA10-1-3) or scramble vector. The β-actin levels were
measured as a loading control. (F) Western blot analysis of ANXA10
and PDX1 proteins in HSC-57 cells transfected with ANXA10
expression vector or empty vector. (G and H) Cell proliferation
assays of (G) MKN-74 and (H) MKN-45 cells transfected with ANXA10
shRNA vector or scramble vector. Cell growth was assessed by MTT
assays at 1, 2, 4 and 8 days after seeding on 96-well plates. Bars
and error bars represent the mean and SD, respectively, of 3
independent experiments (**P<0.01, ***P<0.001). (I) Cell
proliferation assay of HSC-57 cells transfected with ANXA10
expression vector or empty vector (***P<0.001). GC, gastric
cancer; ANXA10, annexin A10; PDX1, pancreatic and duodenal
homeobox-1. |
Establishment of GC organoids with the
gastric and intestinal mucin phenotypes
In order to further validate the relationship
between ANXA10 and PDX1, GC organoids that displayed a distinct
mucin phenotype were established. Pieces of the GC specimens were
divided into two and then cultured in stomach and colon media,
respectively. We successfully established GC organoids from 10
patients, and the mucin phenotypes varied with each case (Fig. 3 and Table SIII). It is noteworthy that we
successfully generated an organoid from scirrhous-type GC in case
1, which was characterized by diffuse/scattered clusters of cancer
cells and abundant stroma. While the primary tumor did not show any
glandular structures, the organoids somehow formed glands that were
composed by cancer cells with a high nucleus/cytoplasm ratio; akin
to the cancer cells of the primary tumor.
Effect of ANXA10 on the PDX1
expression in GC patient-derived organoids
To further verify the tight correlation between
ANXA10 and PDX1, we used organoids derived from the non-neoplastic
epithelium and GC tissue. Robust expression of ANXA10 and PDX1 was
found in the organoids from the non-neoplastic mucosa and GC
showing only the gastric mucin phenotype (Fig. 4A and B), which was consistent with
the data obtained from the immunohistochemical analysis using GC
samples. We investigated the transition of the ANXA10 and PDX1
expression by IHC staining using the abovementioned organoids
transfected with ANXA10 shRNAs (shANXA10) in order to ascertain
whether similar changes would be observed in the organoids as those
in the GC cell lines. In both organoids generated from GC tissue
and the non-neoplastic epithelium, the PDX1 expression was
significantly repressed by ANXA10 knockdown (Fig. 4A-C). In comparison to the GC
organoids transfected with shANXA10, the morphology of the
organoids transfected with shANXA10 was dramatically altered: Every
single component looked thin and monotonous with a high
nuclear-cytoplasmic ratio, which indicated that these cells could
potentially be stem/progenitor-like cells. Taken together, the
results suggest that organoids are likely to be useful for
revealing dynamic molecular alterations in specific organs, and our
results further suggest that ANXA10 is involved in the regulation
of the expression of PDX1 in both normal stomach epithelium and GC
and that ANXA10 could also play an essential role in the
differentiation of the normal gastric epithelium.
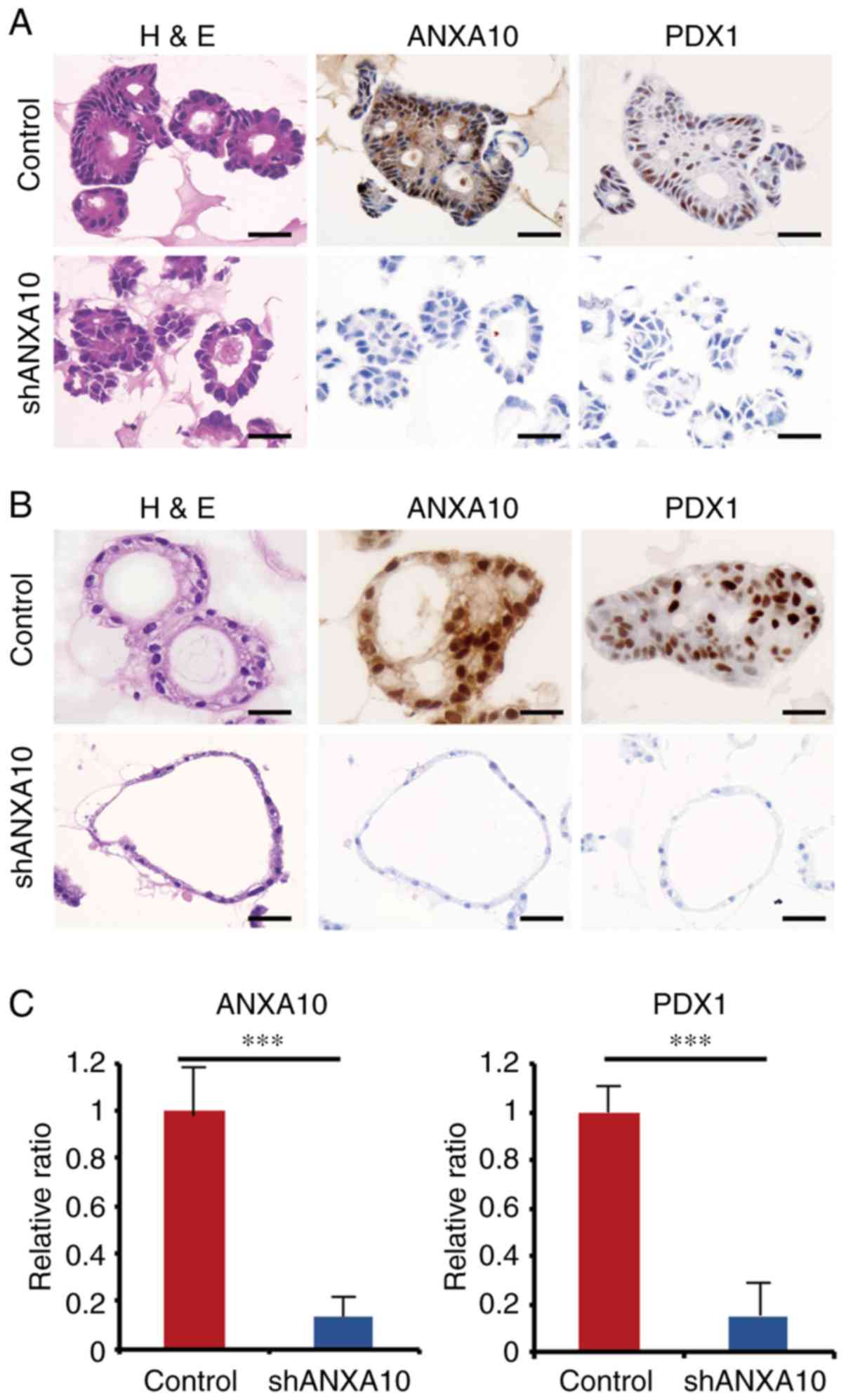 | Figure 4.(A) Expression of ANXA10 and PDX1 in
patient-derived GC organoids transfected with ANXA10 shRNA vector
(shANXA10) or scramble vector. Original magnification, ×400; scale
bars, 50 µm. (B) Expression of ANXA10 and PDX1 in patient-derived
normal gastric organoids transfected with ANXA10 shRNA vector
(shANXA10) or scramble vector. Original magnification, ×400; scale
bars, 50 µm. (C) RT-qPCR for ANXA10 and PDX1 in patient-derived GC
organoids transfected with ANXA10 shRNA vector (shANXA10) or
scramble vector. Bars and error bars represent the mean and SD,
respectively, of 3 independent experiments (***P<0.001). GC,
gastric cancer; ANXA10, annexin A10; PDX1, pancreatic and duodenal
homeobox-1; H&E, hematoxylin and eosin staining. |
Discussion
We immunohistochemically assessed the expression of
annexin A10 (ANXA10) in gastric cancer (GC) and confirmed the
clinicopathological significance and functional roles of ANXA10 in
GC using GC cell lines, tissues and patient-derived organoids. One
of the novel findings of this study is that ANXA10 regulates the
expression of pancreatic and duodenal homeobox-1 (PDX1); this was
verified in both GC cell lines and organoids. PDX1 plays an
important role in the differentiation and development of the
pancreas, duodenum and antrum (20)
and is regarded as a tumor suppressor (22); both of these roles are quite similar
to the roles of ANXA10. In light of the expression pattern revealed
by IHC, previous studies have observed the expression of ANXA10
(8) and PDX1 (30) in GC, and the PDX1 was localized in
nucleoli as well as ANXA10, supporting the correlation between
these two molecules. While we indicated various relationships
between ANXA10 and PDX1, it was not ascertained whether ANAX10
regulates PDX1 in a direct or indirect manner. One challenge in
resolving this issue is that the functional role of ANXA10 remains
unclear; there is no solid evidence of a key regulator or direct
target of ANXA10. Additional studies, perhaps using a proteome
analysis, will likely be needed to advance our understanding of the
mechanism involved in the regulation of ANXA10 and its target,
which could potentially reveal the fundamental mechanisms through
which ANXA10 contributes to the progression of GC.
As for the functional role of ANXA10 in GC, it was
verified that ANXA10 basically plays a tumor-suppressive role in
GC, which is consistent with the data in previous studies (18,19).
We observed expression of ANXA10 in normal stomach tissues and loss
of the expression of ANXA10 in some of the GC tissues, most of
which showed advanced clinicopathological features and/or poor
clinical outcome. In contrast, previous studies described that
normal squamous epithelium usually does not show expression of
ANXA10 and some squamous cell carcinoma tissues inversely exhibited
robust expression of ANXA10, which was found to significantly
contribute to the progression of squamous cell carcinoma (9,10).
These data indicate that the functional role of ANXA10 in human
cancers has a bilateral function displaying tumor-suppressive and
promotive roles depending on the original organs. Further studies,
such as constructing a library of the expression pattern of ANXA10
in human normal and cancer tissues, would be instrumental in
revealing the entire aspect of the variety of the functional roles
of ANXA10, which could potentially lead to a breakthrough in the
functional role of ANXA10 in human tissues. Another limitation of
our study is that we were not able to further validate our data
concerning the functional role of ANXA10 in an in vivo
study. These considerations point to the current issues concerning
the functional role of ANXA10 that could be further examined.
In the present study, we aimed to ascertain whether
overexpression of ANXA10 directly affects the mucin phenotypes of
GC cells using HSC-57-ANXA10 cells as shown in Fig. 2. However, the cells did not show any
differences regardless of the successful overexpression of ANXA10
and subsequent PDX1 expression. One of the reasons is that HSC-57
cells have been differentiated into an intestinal mucin phenotype
with expression of CDX2, which is a representative transcription
factor for the organogenesis and maintenance of the small intestine
and colon. We hypothesized that GC cells with N-type could be an
ideal model as they are more likely to represent an
undifferentiated status of GC, but unfortunately no GC cells with
N-type were currently available. Further studies using the models
that can recapitulate undifferentiated status of GC, such as GC
cells and organoids with N-type, could provide more solid evidence
concerning the functional roles of ANXA10 in regards to mucin
phenotypes of GC.
An epoch-making result of the present study is that
we successfully established a GC organoid library. Organoid
culturing is a novel 3D stem cell culture system that has been
developed by exploiting the understanding of stem cell niche
factors (31). These organoids
reproduce the histopathological grade and differentiation capacity
of their primary tumor (32). Only
one study has reported the establishment of GC organoids from
surgically resected samples (33);
however, that study only mentioned that the GC organoid represented
the p53 mutation. In the present study, we described a novel method
for recapitulating the mucin phenotypes of primary GC in each GC
organoid, which was quite useful for validating the specific
expression and function of ANXA10 in G-type GC. The mucin phenotype
of GC has mainly been examined by pathologists, and it has been
regarded as just one of the sub-classifications of GC. Recent
evidence suggests that GCs should be classified into 4 groups based
on data from comprehensive characterization by whole-genome
sequencing (34). When examining
the representative characteristics of each group proposed by TCGA,
we realized that the ‘Microsatellite instability (MSI) group’ and
GCs with gastric mucin phenotype share many genetic and phenotypic
features, including MLH1 silencing and hypermutations in
tumor-suppressor genes. In addition, a similar trend was observed
between the ‘Chromosomal instability (CIN) group’ and GCs with the
intestinal mucin phenotype, which is characterized by a high
frequency of p53 mutations (2,34).
Taking this information into account, it is believed that our GC
organoids, which exhibit a distinct mucin phenotype, will be highly
valuable for scrutinizing GC biology based on the TCGA
classification. Moreover, another cutting-edge use for organoid
libraries is for drug screening. van de Wetering et al
reported a screening strategy using a colorectal cancer organoid
library (35). Taken together, our
GC organoid library is considered to have great potential for
investigating drug sensitivity and/or resistance, as well as the
daisy chain-like genetic and phenotypic features of GC, and could
possibly be a powerful platform for establishing
‘personalized-medicine’ in GC treatment.
In conclusion, we demonstrated the clinical
significance of ANXA10 in GC. We also disclosed that ANXA10 is
involved in the regulation of PDX1; a finding that was verified in
GC tissues, cell lines and organoids. In addition, we highlighted
the usefulness of GC organoids in the detailed study of the mucin
phenotype of GC. Both longitudinal and cross-sectional studies
using our organoid library could potentially yield valuable
information on various issues concerning the pathogenesis and
clinical features of GCs.
Supplementary Material
Supporting Data
Acknowledgements
We thank Mr. Shinichi Norimura (Technical Center,
Hiroshima University) for his excellent technical assistance. This
research was carried out with the kind cooperation of the Research
Center for Molecular Medicine of the Faculty of Medicine of
Hiroshima University. We also thank the Analysis Center of Life
Science of Hiroshima University for the use of their facilities.
The R-spondin-producing cell line was a kind gift from Professor
Jeffery Whitsett (Cincinnati Children's Hospital Medical Center,
Cincinnati, OH, USA). We would like to thank Professor Eric Fearon
(University of Michigan, Ann Arbor, MI, USA) for providing
collaborative research resources and comments.
Funding
The present study was supported by Grants-in-Aid for
Scientific Research (JP15H04713, JP16K08691, JP16H06999) and for
Challenging Exploratory Research (26670175, JP16K15247) from the
Japan Society for the Promotion of Science.
Availability of data and materials
All data generated or analyzed during this study are
included either in this article or in the supplementary information
files.
Authors' contributions
NS designed the study. KS, NO, KT and HO collected
and analyzed the patient clinical data. AI, RH, DT, KF and TH
performed the experiments and collected and analyzed the data. NS
KS, NO, KY, KT, HO and WY interpreted and analyzed the results. AI,
NS and WY drafted and edited the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The Institutional Review Board of Hiroshima
University Hospital approved the present study (IRB# E-597-01).
Appropriate written informed consent was obtained from each
patient. The present study was conducted in accordance with the
Ethical Guidance for Human Genome/Gene Research of the Japanese
Government.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Profssor Wataru Yasui: ORCID: orcid.org/0000-0002-8647-8405.
Glossary
Abbreviations
Abbreviations:
|
GC
|
gastric cancer
|
|
ANXA10
|
annexin A10
|
|
PDX1
|
pancreatic and duodenal homeobox-1
|
|
IHC
|
immunohistochemistry
|
|
RT-qPCR
|
quantitative RT-PCR
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oue N, Sentani K, Sakamoto N and Yasui W:
Clinicopathologic and molecular characteristics of gastric cancer
showing gastric and intestinal mucin phenotype. Cancer Sci.
106:951–958. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Endoh Y, Sakata K, Tamura G, Ohmura K,
Ajioka Y, Watanabe H and Motoyama T: Cellular phenotypes of
differentiated-type adenocarcinomas and precancerous lesions of the
stomach are dependent on the genetic pathways. J Pathol.
191:257–263. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohnishi M, Tokuda M, Masaki T, Fujimura T,
Tai Y, Matsui H, Itano T, Ishida T, Takahara J and Konishi R:
Changes in annexin I and II levels during the postnatal development
of rat pancreatic islets. J Cell Sci. 107:2117–2125.
1994.PubMed/NCBI
|
|
6
|
Gerke V and Moss SE: Annexins: From
structure to function. Physiol Rev. 82:331–371. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu SH, Chen YL, Shun CT, Lai JN, Peng SY,
Lai PL and Hsu HC: Expression and prognostic significance of
gastric-specific annexin A10 in diffuse- and intestinal-type
gastric carcinoma. J Gastroenterol Hepatol. 26:90–97. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu SH, Yuan RH, Chen YL, Hsu HC and Jeng
YM: Annexin A10 is an immunohistochemical marker for adenocarcinoma
of the upper gastrointestinal tract and pancreatobiliary system.
Histopathology. 63:640–648. 2013.PubMed/NCBI
|
|
9
|
Kodaira H, Koma YI, Hosono M, Higashino N,
Suemune K, Nishio M, Shigeoka M and Yokozaki H: ANAX10 induction by
interaction with tumor-associated macrophages the growth of
esophageal squamous cell carcinoma. Pathol Int. 69:135–147. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimizu T, Kasamatsu A, Yamamoto A, Koike
K, Ishige S, Takatori H, Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H
and Uzawa K: Annexin A10 in human oral cancer: Biomarker for
tumoral growth via G1/S transition by targeting MAPK signaling
pathways. PLoS One. 7:e455102012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Masaki T, Tokuda M, Ohnishi M, Watanabe S,
Fujimura T, Miyamoto K, Itano T, Matsui H, Arima K, Shirai M, et
al: Enhanced expression of the protein kinase substrate annexin in
human hepatocellular carcinoma. Hepatology. 24:72–81. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim JH, Rhee YY, Kim KJ, Cho NY, Lee HS
and Kang GH: Annexin A10 expression correlates with serrated
pathway features in colorectal carcinoma with microsatellite
instability. APMIS. 122:1187–1195. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsai JH, Lin YL, Cheng YC, Chen CC, Lin
LI, Tseng LH, Cheng ML, Liau JY and Jeng YM: Aberrant expression of
annexin A10 is closely related to gastric phenotype in serrated
pathway to colorectal carcinoma. Mod Pathol. 28:268–278. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gonzalo DH, Lai KK, Shadrach B, Goldblum
JR, Bennett AE, Downs-Kelly E, Liu X, Henricks W, Patil DT, Carver
P, et al: Gene expression profiling of serrated polyps identifies
annexin A10 as a marker of a sessile serrated adenoma/polyp. J
Pathol. 230:420–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartley AN, Thompson PA, Buckmeier JA,
Kepler CY, Hsu CH, Snyder MS, Lance P, Bhattacharyya A and Hamilton
SR: Expression of gastric pyloric mucin, MUC6, in colorectal
serrated polyps. Mod Pathol. 23:169–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gibson JA, Hahn HP, Shahsafaei A and Odze
RD: MUC expression in hyperplastic and serrated colonic polyps:
Lack of specificity of MUC6. Am J Surg Pathol. 35:742–749. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Walsh MD, Clendenning M, Williamson E,
Pearson SA, Walters RJ, Nagler B, Packenas D, Win AK, Hopper JL,
Jenkins MA, et al: Expression of MUC2, MUC5AC, MUC5B, and MUC6
mucins in colorectal cancers and their association with the CpG
island methylator phenotype. Mod Pathol. 26:1642–1656. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim J, Kim MA, Jee CD, Jung EJ and Kim WH:
Reduced expression and homozygous deletion of annexin A10 in
gastric carcinoma. Int J Cancer. 125:1842–1850. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim JK, Kim PJ, Jung KH, Noh JH, Eun JW,
Bae HJ, Xie HJ, Shan JM, Ping WY, Park WS, et al: Decreased
expression of Annexin A10 in gastric cancer and its overexpression
in tumor cell growth suppression. Oncol Rep. 24:607–612.
2010.PubMed/NCBI
|
|
20
|
Brooke NM, Garcia-Fernàndez J and Holland
PW: The ParaHox gene cluster is an evolutionary sister of the Hox
gene cluster. Nature. 392:920–922. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koizumi M, Doi R, Toyoda E, Masui T,
Tulachan SS, Kawaguchi Y, Fujimoto K, Gittes GK and Imamura M:
Increased PDX-1 expression is associated with outcome in patients
with pancreatic cancer. Surgery. 134:260–266. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma J, Chen M, Wang J, Xia HH, Zhu S, Liang
Y, Gu Q, Qiao L, Dai Y, Zou B, et al: Pancreatic duodenal
homeobox-1 (PDX1) functions as a tumor suppressor in gastric
cancer. Carcinogenesis. 29:1327–1333. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sakamoto N, Feng Y, Stolfi C, Kurosu Y,
Green M, Lin J, Green ME, Sentani K, Yasui W, McMahon M, et al:
BRAFV600E cooperates with CDX2 inactivation to promote serrated
colorectal tumorigenesis. Elife. 6:e203312017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yanagihara K, Tanaka H, Takigahira M, Ino
Y, Yamaguchi Y, Toge T, Sugano K and Hirohashi S: Establishment of
two cell lines from human gastric scirrhous carcinoma that possess
the potential to metastasize spontaneously in nude mice. Cancer
Sci. 95:575–582. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yasui W, Ayhan A, Kitadai Y, Nishimura K,
Yokozaki H, Ito H and Tahara E: Increased expression of p34cdc2 and
its kinase activity in human gastric and colonic carcinomas. Int J
Cancer. 53:36–41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sakamoto N, Naito Y, Oue N, Sentani K,
Uraoka N, Oo HZ, Yanagihara K, Aoyagi K, Sasaki H and Yasui W:
MicroRNA-148a is downregulated in gastric cancer, targets MMP7, and
indicates tumor invasiveness and poor prognosis. Cancer Sci.
105:236–243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yui S, Nakamura T, Sato T, Nemoto Y,
Mizutani T, Zheng X, Ichinose S, Nagaishi T, Okamoto R, Tsuchiya K,
et al: Functional engraftment of colon epithelium expanded in vitro
from a single adult Lgr5+ stem cell. Nat Med.
18:618–623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Koo BK, Sasselli V and Clevers H:
Retroviral gene expression control in primary organoid cultures.
Curr Protoc Stem Cell Biol. 27:Unit 5A.6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim TH and Shivdasani RA: Stomach
development, stem cells and disease. Development. 143:554–565.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sato T, Vries RG, Snippert HJ, van de
Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters
PJ and Clevers H: Single Lgr5 stem cells build crypt-villus
structures in vitro without a mesenchymal niche. Nature.
459:262–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fujii M, Shimokawa M, Date S, Takano A,
Matano M, Nanki K, Ohta Y, Toshimitsu K, Nakazato Y, Kawasaki K, et
al: A colorectal tumor organoid library demonstrates progressive
loss of niche factor requirements during tumorigenesis. Cell Stem
Cell. 18:827–838. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bartfeld S, Bayram T, van de Wetering M,
Huch M, Begthel H, Kujala P, Vries R, Peters PJ and Clevers H: In
vitro expansion of human gastric epithelial stem cells and their
responses to bacterial infection. Gastroenterology.
148:126–136.e126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
van de Wetering M, Francies HE, Francis
JM, Bounova G, Iorio F, Pronk A, van Houdt W, van Gorp J,
Taylor-Weiner A, Kester L, et al: Prospective derivation of a
living organoid biobank of colorectal cancer patients. Cell.
161:933–945. 2015. View Article : Google Scholar : PubMed/NCBI
|