Introduction
Osteosarcoma is a primary cancerous tumor in bones
and has an incidence rate of 3.4/1,000,000 per year worldwide
(1,2). In the last 100 years, the 5-year
survival rate of classical osteosarcoma has been ~20% (2). Surgical and/or en bloc resection and
radiotherapy are the primary options for the treatment of
osteosarcoma. However, patients with advanced osteosarcoma often
suffer from metastasis or other complications. The underlying
molecular mechanism of cell migration is still unclear in
osteosarcoma.
Wnt ligands are a group of autocrine proteins
binding to LDL receptor-related proteins (LRPs), Frizzled (Fzd)
receptors and/or receptor tyrosine kinase-like orphan receptors
(RORs) (3,4). Wnt5a, a non-canonical Wnt ligand,
binds to Fzd2 and subsequently inhibits Wnt3a-induced LRP6
activation and β-catenin signaling (5). The Fzd5 receptor can transduce Wnt5a
signaling to β-catenin and induce the formation of a secondary axis
in Xenopus embryos (6,7).
Previous studies have demonstrated that Wnt5a
activates dishevelled 2/disheveled-associated activator of
morphogenesis 1 (DAAM1)/RhoA, and subsequently enhances cell
migration in breast cancer and cell invasion in glioblastoma
(8,9). Wnt5a and type IV collagen were
revealed to activate DAAM1 and promote cell migration and
haptotaxis in breast cancer, respectively (8,10).
Wnt5a enhanced leukemia cell chemotaxis and proliferation by
inducing the oligomerization of ROR1 and ROR2 and activating
RhoA/Rac1 (11). Although it has
been reported that Wnt5a/ROR2 phosphorylates PI3K/Akt, activates
RhoA and promotes cell migration in osteosarcoma (12,13),
the role of ROR1 in the Wnt5a-induced migration of osteosarcoma
cells is still unknown.
The present study aimed to reveal whether ROR1
activates DAAM1 and promotes the migration of osteosarcoma cells in
response to Wnt5a. It was demonstrated that ROR1/ROR2-dependent
DAAM1 and parallel PI3Kα/Akt signaling activated RhoA and regulated
Wnt5a-induced osteosarcoma cell migration. These data indicated
ROR1/ROR2 may be novel clinical targets in inhibiting osteosarcoma
migration and decreasing mortality.
Materials and methods
Cell lines
MG-63 and U2OS human osteosarcoma cells were
purchased from Cell Bank (Shanghai, China) and cultured in DMEM
supplemented with 10% bovine serum (HyClone; GE Healthcare Life
Sciences), 0.5 µg/ml penicillin and streptomycin. All cell lines
were verified monthly to be mycoplasma-negative.
Short hairpin (sh)RNAs targeted to ROR2
(5′-AACTCTGAAAGGTTACTTTCTGA-3′) and DAAM1
(5′-GCCACTTTGTATCCTATCAGG-3′), GFP-RhoA-V14 and wild-type (WT) ROR2
constructs were transfected into osteosarcoma cells using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.).
Western blotting
MG-63 and U2OS cells, incubated in medium containing
100 ng/ml Wnt5a, were treated with 1 µmol/l Rho inhibitor CCG-1423
(Selleck), 1 nmol/l PI3Kα inhibitor HS-173 (APExBIO), 1 µg/ml
anti-ROR1 mAb or vehicle for 1 h. Then, the total protein of
osteosarcoma cells was extracted using ice-cold RIPA lysis buffer
(14). Cellular lysates were
centrifuged at 12,000 × g for 30 min in a cooler. The protein
concentration was determined by BCA method. Proteins were separated
in 10% gels and then transferred to polyvinylidene difluoride
membranes. The membranes were blocked in 5% BSA for 30 min at room
temperature. The primary antibodies employed were as follows:
Rabbit anti-Akt antibody (60 kDa; dilution 1:1,000; product #4691)
and rabbit anti-phospho-Akt (p-Ser473) antibody (60 kDa; dilution
1:1,000; product #4058, both from Cell Signaling Technology, Inc.;
Fig. 4A), mouse anti-phospho-Akt
(60 kDa; dilution 1:1,000; p-Ser473) antibody (cat. no. 66444;
Fig. 4D), anti-β-actin (45 kDa;
dilution 1:5,000; cat. no. 60008) and anti-ROR1 (130 kDa; dilution
1:1,000; cat. no. 20629; all from ProteinTech Group, Inc.),
anti-ROR2 (135 kDa; dilution 1:1,000; product no. 88639), anti-flag
(product no. 14793, dilution 1:1,000), anti-GST (product no. 2624;
dilution 1:1,000; all from Cell Signaling Technology, Inc.) and
anti-DAAM1 (122 kDa; dilution 1:1,000; cat. no. sc-100942; Santa
Cruz Biotechnology, Inc.) antibodies. Quantitative analysis of each
blot was performed with densitometric scanning. The bands were
visualized using enhanced chemiluminescent (ECL) kit (Tanon, China)
and densitometry analysis was performed using Tanon software
(Shanghai, China).
Pulldown assays and
immunoprecipitation (IP)
The measurement of DAAM1 activation was performed as
previously described (10). The
bacterially-expressed flag-ROR2 and GST-ROR1 were purified using
Pierce Anti-flag Magnetic Agarose Beads (Thermo Fisher Scientific,
Inc.) and GST-Sepharose 4B (GE Healthcare), respectively. Beads
that had captured the targeted proteins were subjected to western
blot analysis, which was performed according to the western
blotting protocol aforementioned.
Equal volumes of total cellular protein extractions
were subjected to Rho GTPase activation assays (Cytoskeleton,
Inc.). The detailed procedure for the G-LISA assays was previously
described (10).
The lysates of MG-63 cells were centrifuged at
12,000 × g for 15 min. The supernatants of cellular lysates were
incubated with lgG or ROR2 antibodies for 12 h. Protein
A/G-conjugated agarose beads (Pierce; Thermo Fisher Scientific,
Inc.) were mixed with the cell lysates and ROR2 antibodies and
maintained under agitation for 2 h. After washing with lysis buffer
four times, the beads were subjected to western blotting.
Boyden chamber assays
A Boyden chamber system (8.0-µm; Costar; Corning,
Inc.) was used to perform the cell migration assays. A total of
1×105 cells with 5 µg/ml BSA was seeded into the Boyden
chambers and allowed to migrate for 5 h. Subsequently, the
migratory cells in the lower compartments of the Boyden chambers
were stained with 0.2% crystal violet for 20 min. The number of
migratory cells was counted under a brightfield microscope (Mshot
MF53; Micro-shot Technology Co., Ltd.).
Actin cytoskeleton staining
Cells grown on glass slides were fixed in 4%
paraformaldehyde for 15 min. Then, the cells were permeabilized by
0.2% Triton X-100 and blocked in 1% BSA for 1 h. Next,
TRITC-labeled phalloidin (5 µg/ml; Sigma-Aldrich; Merck KGaA) was
used to stain the actin cytoskeleton for 1 h. The nucleus was
stained with DAPI. Fluorescent images were captured using a Zeiss
confocal microscope (LSM710; Zeiss AG).
Statistical analysis
One-way ANOVA or a Student's t-test were used to
perform statistical analyses, with SPSS 22.0 software (IBM Corp.).
Bar and scatter charts present the mean ± SD of five independent
replicates. P<0.05 was considered to indicate a statistically
significant difference.
Results
ROR1 directly binds to ROR2 and
mediates Wnt5a-induced cell migration
Wnt5a can induce ROR1 and ROR2
hetero-oligomerization in leukemia cells (11). The present study verified whether
ROR1 was able to physically bind to ROR2 in osteosarcoma cells. The
IP assay demonstrated that ROR2 bound to ROR1 in MG-63 osteosarcoma
cells (Fig. 1A). Moreover, it was
revealed that purified GST-tagged ROR1 was directly bound to
flag-tagged ROR2 (Fig. 1B).
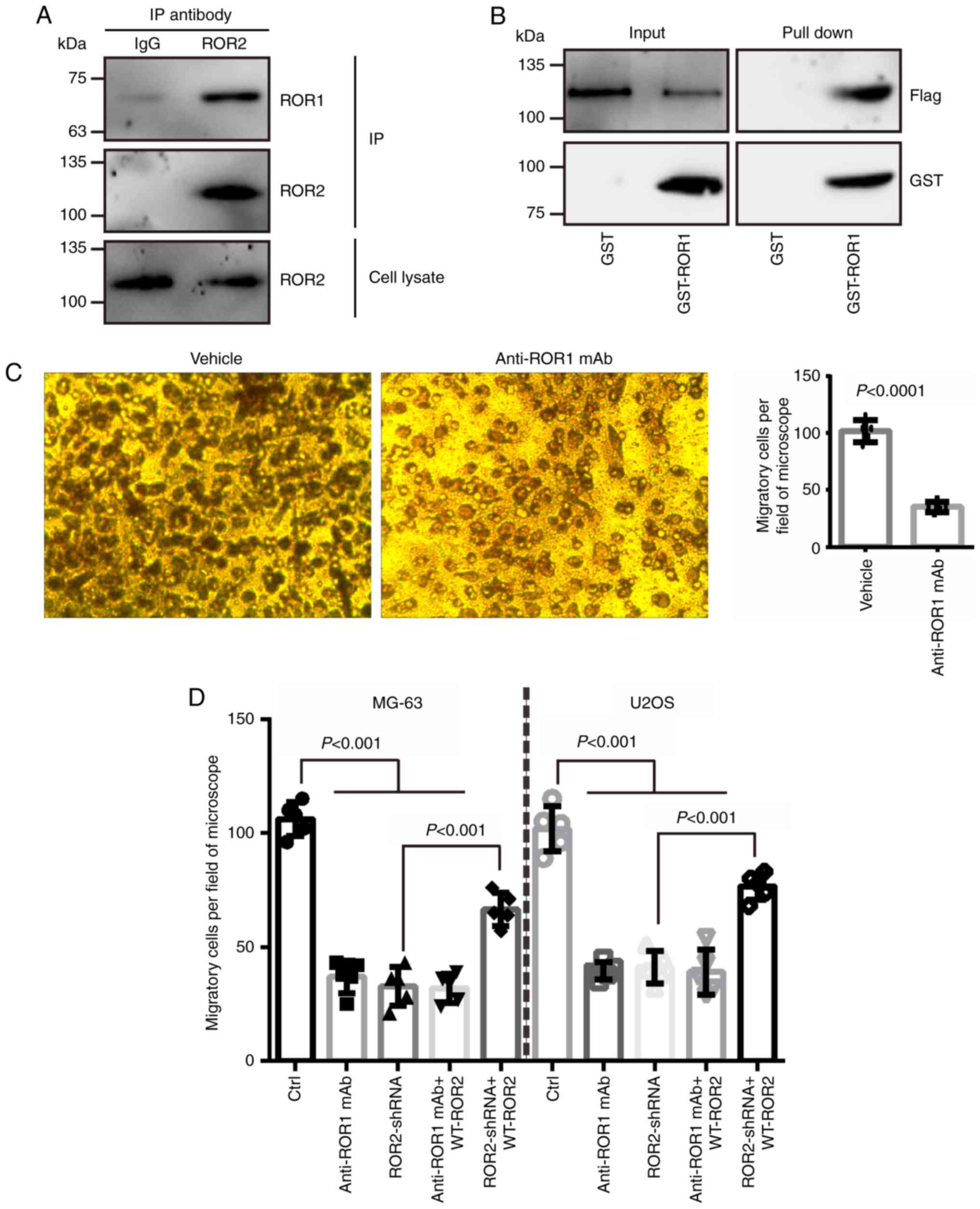 | Figure 1.ROR1 directly binds to ROR2 and
mediates Wnt5a-induced osteosarcoma cell migration. (A) The lysate
of MG-63 osteosarcoma cells was subjected to immunoprecipitation
with an antibody against ROR2, followed by immunoblotting with ROR1
or ROR2 antibodies. (B) ROR1 directly bound to ROR2. Purified GST
or GST-ROR1 was incubated with purified Flag-tagged full-length
ROR2. The amounts of Flag-ROR2 co-purified with GST or GST-ROR1
(pulldown) were analyzed by immunoblotting for anti-Flag or
anti-GST. (C) Cell migration was significantly abolished by ROR1
mAb. MG-63 cells were incubated with ROR1 mAb and then subjected to
cell migration assays. The cells incubated in the medium containing
100 ng/ml Wnt5a were allowed to migrate for 5 h. Magnification,
×20. (D) MG-63 and U2OS osteosarcoma cells were treated with ROR1
mAb, transfected with ROR2 shRNA, treated with combination ROR1-mAb
treatment with WT-ROR2 transfection, or treated with combination
ROR2 shRNA with shRNA-resistant WT-ROR2 transfection, and then
subjected to cell migration assays. The cells incubated in medium
containing 100 ng/ml Wnt5a were allowed to migrate for 5 h. The
number of migratory cells/field of microscope was counted. ROR1,
receptor tyrosine kinase-like orphan receptor 1; ROR2, receptor
tyrosine kinase-like orphan receptor 2; mAb, monoclonal antibody;
shRNA, short hairpin RNA; WT, wild-type. |
Since Wnt5a/ROR2 has been revealed to promote cell
migration in osteosarcoma (13), it
was hypothesized that ROR1 may play a similar role to that of ROR2
in osteosarcoma cell migration. As anticipated, the inhibition of
ROR1 signaling by ROR1 mAb treatment abolished the migration of
Wnt5a-treated osteosarcoma cells (Fig.
1C). Overexpression of shRNA-resistant WT ROR2 rescued the
ROR2-shRNA-mediated, but not the ROR1 mAb-induced, decrease in cell
migration (Fig. 1D). The treatment
with the ROR1 mAb, the transfection of ROR2-shRNA, and ROR2-shRNA
plus ROR1 mAb resulted in an equal significant decrease in cell
migration (Fig. 1D). These results
demonstrated that ROR1 and ROR2 receptors mediated the
Wnt5a-induced migration of osteosarcoma cells.
ROR1/ROR2 activates RhoA and mediates
cell migration
The finding that RhoA mediates Wnt5a-induced cell
migration in osteosarcoma (12)
prompted the study of whether Wnt5a/ROR1/ROR2 signaling activated
RhoA in osteosarcoma. G-LISA assays revealed a significant decrease
in the activation of RhoA, but not that of Rac1 or Rac2, in
osteosarcoma cells treated with ROR1 mAb and knockdown of ROR2 by
ROR2-shRNA transfection (Fig. 2A and
B). Overexpression of shRNA-resistant WT ROR2 rescued
ROR2-shRNA-mediated, but not ROR1 mAb-induced, decrease of RhoA
activation (Fig. 2A). ROR1 mAb,
ROR2-shRNA, and ROR2-shRNA plus ROR1 mAb resulted in an equal
significant reduction of RhoA activity (Fig. 2A).
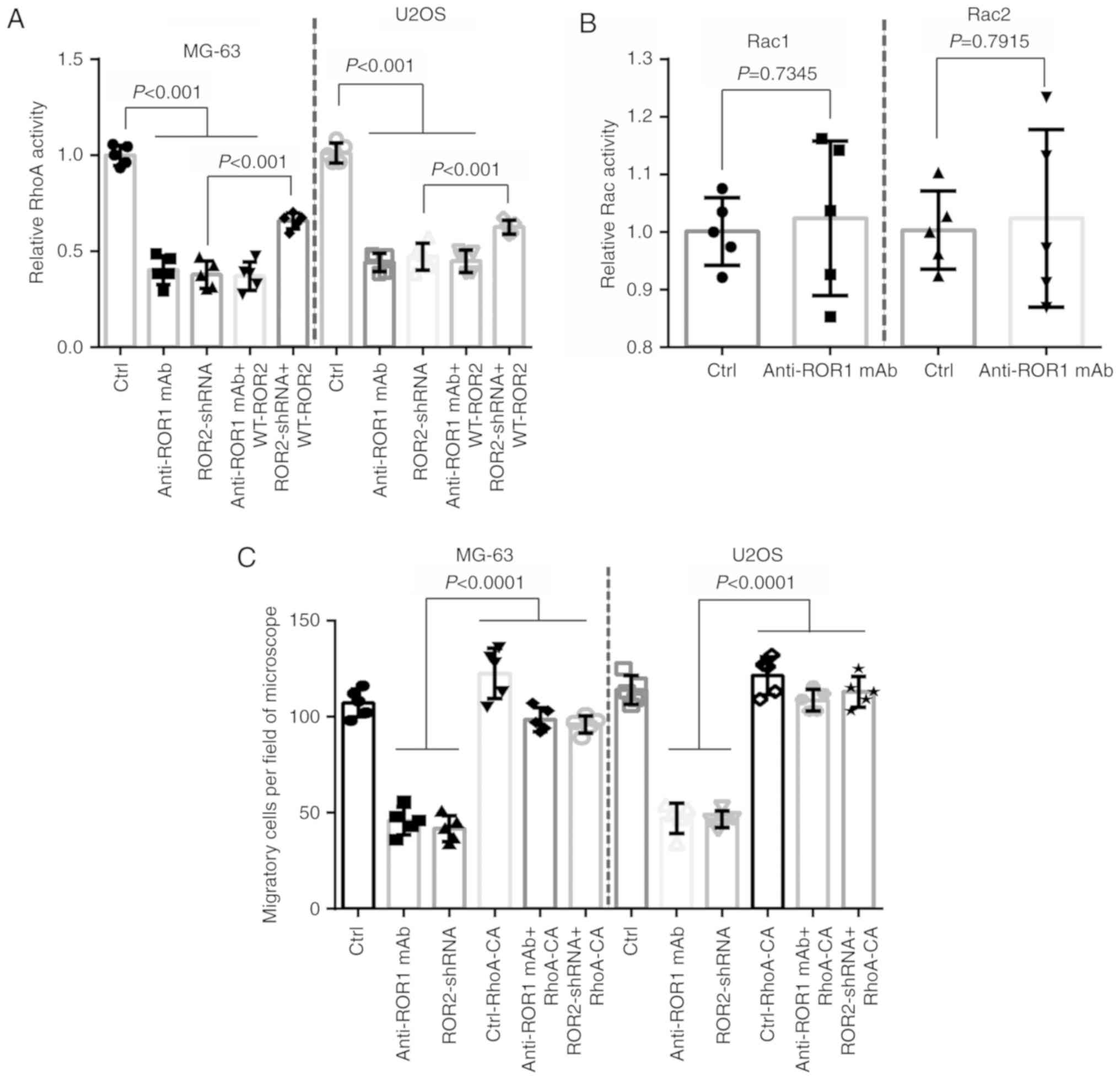 | Figure 2.Wnt5a-induced RhoA activation is
regulated by ROR1/ROR2, and required for osteosarcoma cell
migration. (A) MG-63 and U2OS osteosarcoma cells were treated with
ROR1 mAb, transfected with ROR2 shRNA, treated with combination
ROR1-mAb treatment with WT-ROR2 transfection, or treated with
combination ROR2-shRNA with shRNA-resistant WT-ROR2 transfection,
and then the RhoA activation was assessed using G-LISA assays. The
cells were incubated in medium containing 100 ng/ml Wnt5a. (B)
MG-63 cells were treated with ROR1 mAb, and then the activation of
Rac1 and Rac2 was assessed using G-LISA assays. The cells were
incubated in medium containing 100 ng/ml Wnt5a. (C) MG-63 and U2OS
osteosarcoma cells were treated with ROR1 mAb, transfected with
ROR2 shRNA, transfected with constitutively RhoA-CA, treated with
combination ROR1-mAb treatment with RhoA-CA transfection, or
treated with combination ROR2-shRNA with RhoA-CA transfection, and
then subjected to cell migration assays. The cells incubated in
medium containing 100 ng/ml Wnt5a were allowed to migrate for 5 h.
The number of migratory cells/field of microscope was counted.
ROR1, receptor tyrosine kinase-like orphan receptor 1; ROR2,
receptor tyrosine kinase-like orphan receptor 2; mAb, monoclonal
antibody; shRNA, short hairpin RNA; WT, wild-type; RhoA-CA,
activated RhoA. |
Constitutively activated RhoA (RhoA-CA) was used to
verify the accelerated effect of RhoA activity on cellular
motility. Overexpression of RhoA-CA completely rescued the ROR1
mAb- or ROR2-shRNA-mediated decrease of cell migration in
osteosarcoma cells (Fig. 2C). These
results demonstrated that RhoA was activated by Wnt5a/ROR1/ROR2 and
mediated osteosarcoma cell migration.
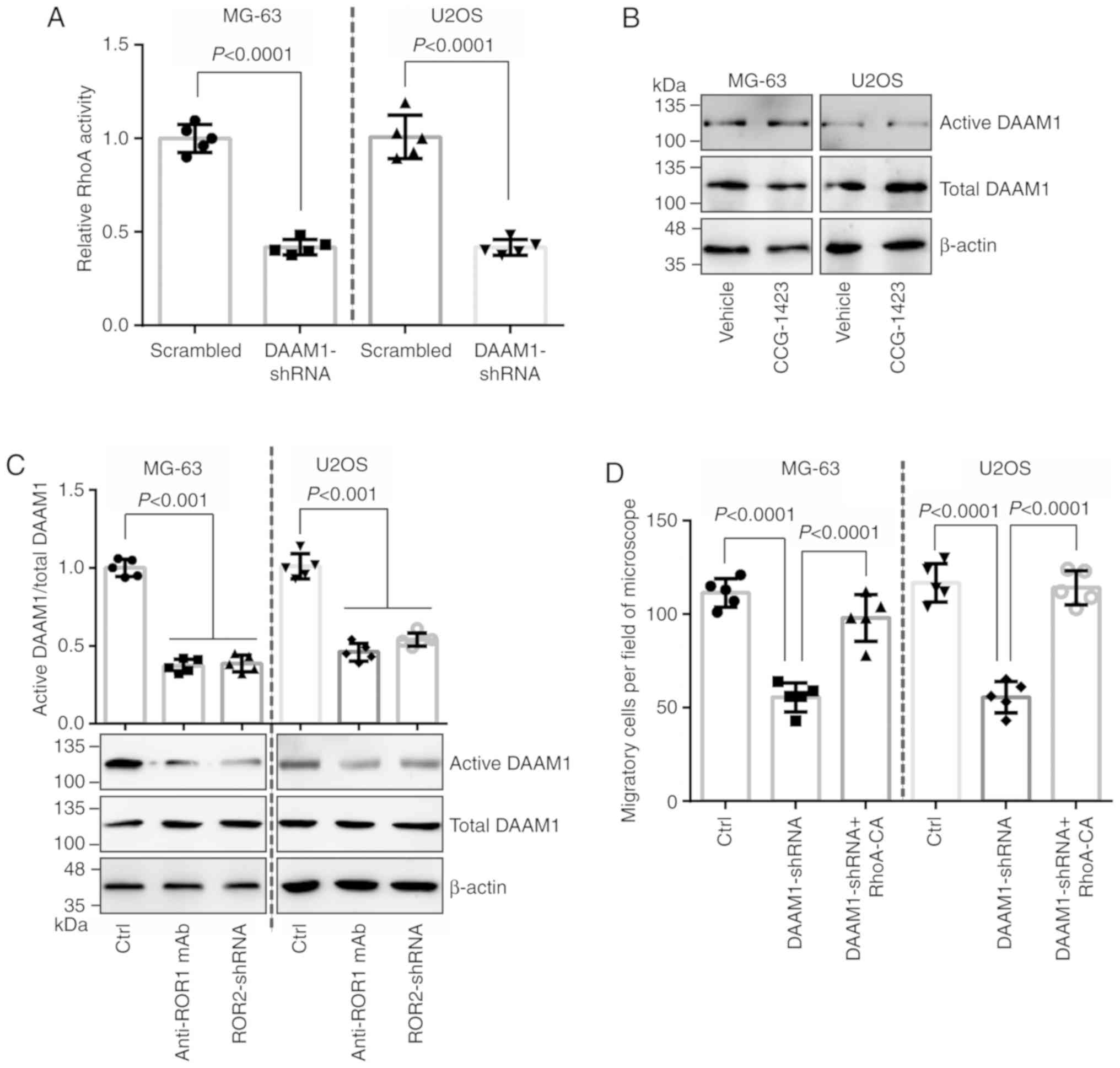
DAAM1 mediates the migration of
osteosarcoma cells
The activation of RhoA was assessed in
DAAM1-silenced osteosarcoma cells. An ~0.5-fold decrease was
observed in DAAM1-silenced osteosarcoma cells (Fig. 3A). CCG-1423 blocked the RhoA
activity, but failed to alter DAAM1 activity (Fig. 3B). These data indicated that RhoA
acted as the downstream target of DAAM1.
The present study verified whether the activation of
DAAM1 was dependent on ROR1 and ROR2 receptors in osteosarcoma
cells. The activation of DAAM1 was decreased after ROR1 mAb
treatment or ROR2 downregulation (Fig.
3C). The migration of DAAM1-silenced osteosarcoma cells was
significantly delayed, which was reversed by RhoA-CA overexpression
(Fig. 3D). These results indicated
that ROR1 and ROR2 may activate DAAM1/RhoA and promote cell
migration in osteosarcoma.
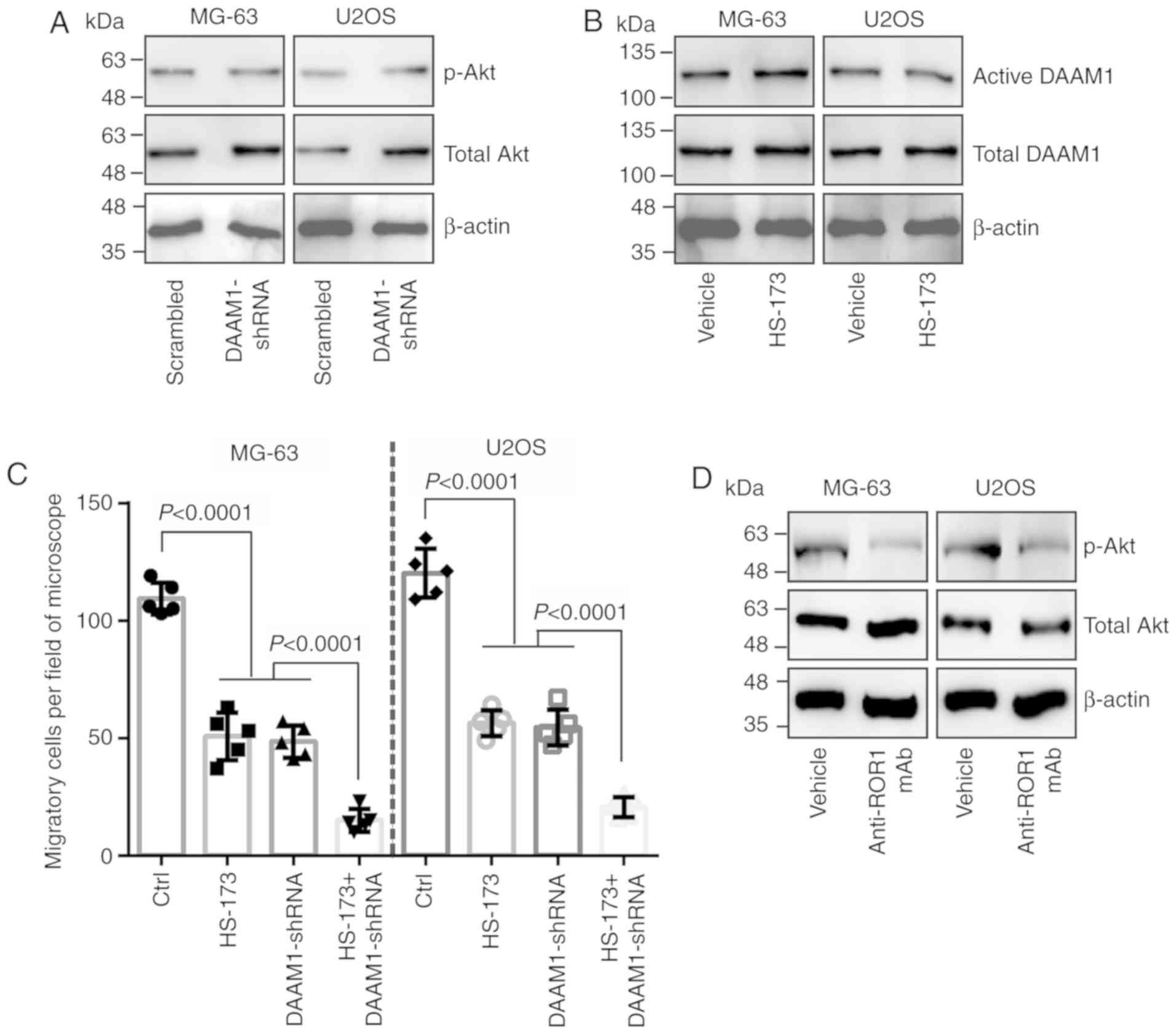
DAAM1 and PI3Kα/Akt are parallel
signaling pathways mediating Wnt5a-induced cell migration
The pulldown assay and immunoblotting demonstrated
that knockdown of DAAM1 did not alter PI3Kα/Akt activity (Fig. 4A). Conversely, the inhibition of
PI3Kα/Akt activity by treatment with HS-173 also did not change
DAAM1 activity (Fig. 4B). The
combination of HS-173 treatment with DAAM1-shRNA transfection
further delayed Wnt5a-induced cell migration compared with HS-173
treatment or DAAM1-shRNA transfection alone (Fig. 4C). Anti-ROR1 mAb markedly blocked
the phosphorylation of Akt in osteosarcoma cells (Fig. 4D). Thus, these results indicated
that DAAM1 and PI3Kα/Akt are parallel signaling pathways mediating
Wnt5a-induced cell migration.
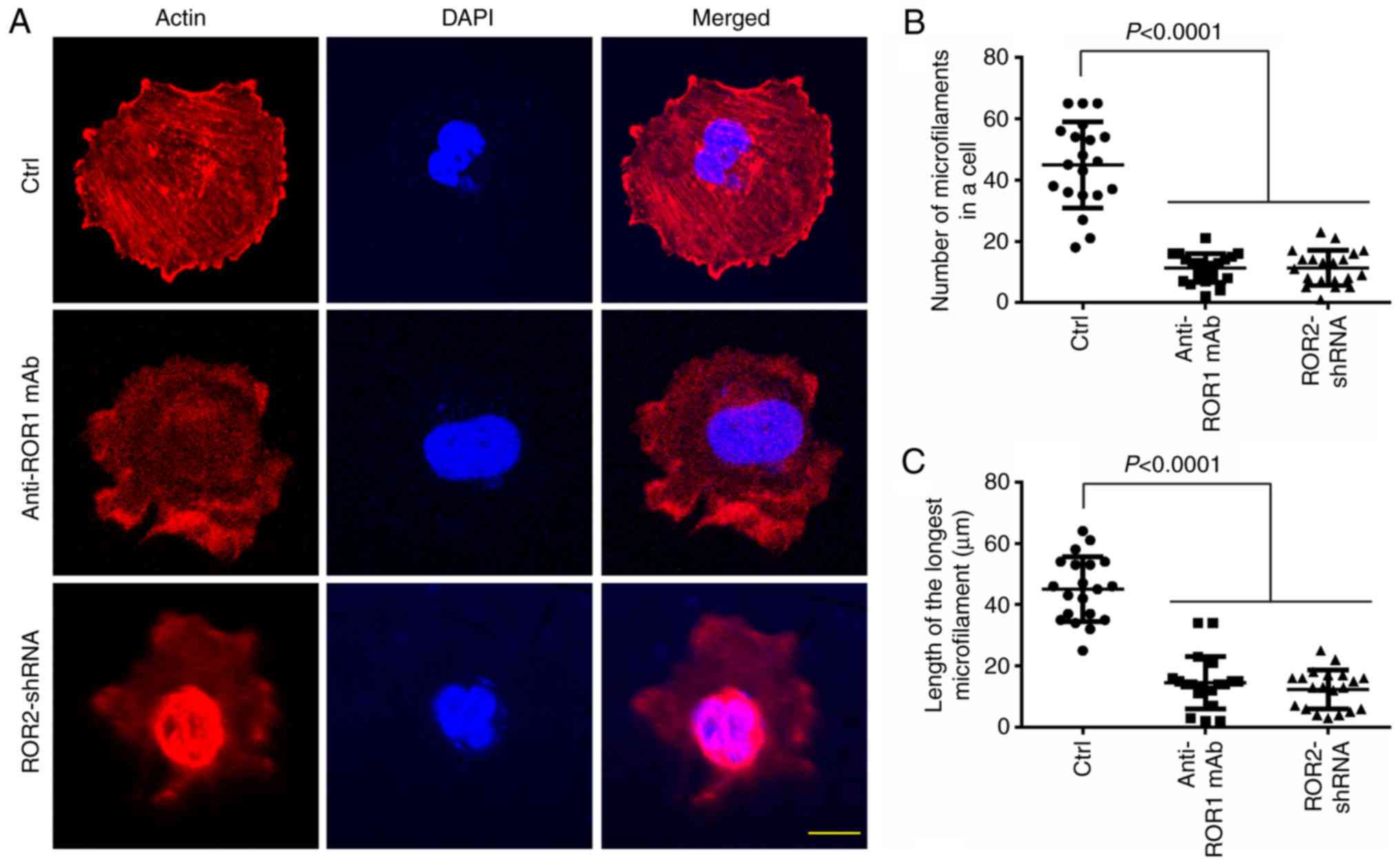
ROR1/ROR2 regulates the microfilament
assembly in osteosarcoma cells
Lastly, the present study validated whether ROR1/2
signaling was involved in the assembly of microfilaments in
osteosarcoma cells. Fluorescent phalloidin was used to stain
filamentous actin, thereby displaying its distribution in MG-63
cells. ROR1 mAb eliminated the assembly of microfilaments and
reduced microfilament length (Fig.
5). Moreover, ROR2-shRNA disrupted the formation of
microfilaments and shortened microfilament length (Fig. 5). These results indicated that
ROR1/ROR2 may modulate the assembly of microfilaments of
osteosarcoma cells.
Discussion
ROR1 and ROR2, two confirmed receptors for the Wnt5a
ligand, mediate non-canonical Wnt signaling regulating embryo/organ
development and tumor metastasis (15). UC-961 (cirmtuzumab) is a humanized
IgG1 monoclonal antibody that directly binds to ROR1 and completely
blocks ROR1 signaling (16–19), and has been demonstrated to be
effective and safe in a phase I trial for patients with
progressive, relapsed or refractory chronic lymphocytic leukemia
(20). The present study revealed
that ROR1 mAb markedly blocked the activation of DAAM1 and RhoA,
the formation of microfilaments and the migration of osteosarcoma
cells. ROR1 may be a novel therapeutic target to delay osteosarcoma
metastasis.
Wnt5a has been revealed to facilitate the
dimerization of ROR1/ROR2, and thus enhance leukemia metastasis and
proliferation (11,15). ROR2 was revealed to mediate
Wnt5a-induced cell migration through PI3K/Akt phosphorylation and
RhoA activation in osteosarcoma (12,13,21).
In the present study, it was revealed that the silencing of ROR2
significantly suppressed the activation of DAAM1 and RhoA, the
formation of microfilaments, and cell migration in osteosarcoma.
The equivalent effect of ROR1 mAb, ROR2-shRNA and ROR1 mAb plus
ROR2-shRNA on cell motility indicated that ROR1/ROR2 dimers may
mediate tumor cell migration in response to Wnt5a. Further studies
are required to consolidate the potential role of ROR1/ROR2 in
vivo. The PI3K/Akt signaling pathway involves a group of
phosphatases, which regulate diverse cellular functions including
metabolism, growth, proliferation, survival, transcription and
protein synthesis (22,23). Wnt5a has been revealed to activate
RhoA and subsequently promotes the invasion/migration of
osteosarcoma, gastric cancer, breast cancer and glioblastoma cells
(8,9,12,13,24,25).
PI3K/Akt activated RhoA activity and subsequently enhanced
Wnt5a-induced cell migration in both gastric cancer and
osteosarcoma cells (12,25). The present results demonstrated that
anti-ROR1 mAb blocked the signaling of PI3Kα/Akt of osteosarcoma
cells. A limitation of the present study is that the knockdown of
ROR1 on the PI3Kα/Akt pathway was not performed. The knockdown of
DAAM1 downregulated RhoA activity and cell migration in
osteosarcoma. The downregulation of RhoA activity, microfilament
formation and cell migration in DAAM1-silenced cells indicated that
DAAM1 played a relevant in Wnt5a/ROR1/ROR2 by activating RhoA.
Moreover, it was demonstrated that DAAM1 and PI3Kα/Akt are parallel
signaling pathways transducing ROR1/2 signaling and mediating
Wnt5a-induced cell migration.
In summary, the present study clarified the
association of Wnt5a/ROR1 signaling and osteosarcoma migration.
Wnt5a/ROR1/2 can activate RhoA via DAAM1 and the parallel signaling
pathway PI3Kα/Akt, and regulate the migration of osteosarcoma
cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from
Jiangsu Province's 333 Research Project to BD (BRA2016208), the
Science and Technology Bureau and Commission of Health and Family
Planning of Yancheng City to BD (YK2017062), the Science and
Technology Foundation of Nanjing Medical University (2017NJMU001)
to TY, and the Project funded by China Postdoctoral Science
Foundation (2018M630602) and the Science and Technology Bureau of
Changzhou (CJ20190061) to AZ.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
AZ designed the project and wrote the paper. BD and
YS performed the experiments. TY analyzed the data. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ROR1
|
receptor tyrosine kinase-like orphan
receptor 1
|
|
ROR2
|
receptor tyrosine kinase-like orphan
receptor 2
|
|
DAAM1
|
disheveled-associated activator of
morphogenesis 1
|
|
LRP
|
lipoprotein receptor-related
protein
|
|
mAb
|
monoclonal antibody
|
|
shRNA
|
short hairpin RNA
|
References
|
1
|
Mirabello L, Troisi RJ and Savage SA:
International osteosarcoma incidence patterns in children and
adolescents, middle ages and elderly persons. Int J Cancer.
125:229–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Misaghi A, Goldin A, Awad M and Kulidjian
AA: Osteosarcoma: A comprehensive review. SICOT J. 4:122018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nusse R, van Ooyen A, Cox D, Fung YK and
Varmus H: Mode of proviral activation of a putative mammary
oncogene (int-1) on mouse chromosome 15. Nature. 307:131–136. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nusse R: Wnt signaling in disease and in
development. Cell Res. 15:28–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sato A, Yamamoto H, Sakane H, Koyama H and
Kikuchi A: Wnt5a regulates distinct signalling pathways by binding
to Frizzled2. EMBO J. 29:41–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He X, Saint-Jeannet JP, Wang Y, Nathans J,
Dawid I and Varmus H: A member of the Frizzled protein family
mediating axis induction by Wnt-5A. Science. 275:1652–1654. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishikawa T, Tamai Y, Zorn AM, Yoshida H,
Seldin MF, Nishikawa S and Taketo MM: Mouse Wnt receptor gene Fzd5
is essential for yolk sac and placental angiogenesis. Development.
128:25–33. 2001.PubMed/NCBI
|
|
8
|
Zhu Y, Tian Y, Du J, Hu Z, Yang L, Liu J
and Gu L: Dvl2-dependent activation of Daam1 and RhoA regulates
Wnt5a-induced breast cancer cell migration. PLoS One. 7:e378232012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu G, Yan T, Li X, Sun J, Zhang B, Wang H
and Zhu Y: Daam1 activates RhoA to regulate Wnt5a-induced
glioblastoma cell invasion. Oncol Rep. 39:465–472. 2018.PubMed/NCBI
|
|
10
|
Yan T, Zhang A, Shi F, Chang F, Mei J, Liu
Y and Zhu Y: Integrin αvβ3-associated DAAM1 is essential for
collagen-induced invadopodia extension and cell haptotaxis in
breast cancer cells. J Biol Chem. 293:10172–10185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu J, Chen L, Cui B, Widhopf GF II, Shen
Z, Wu R, Zhang L, Zhang S, Briggs SP and Kipps TJ: Wnt5a induces
ROR1/ROR2 heterooligomerization to enhance leukemia chemotaxis and
proliferation. J Clin Invest. 126:585–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang A, Yan T, Wang K, Huang Z and Liu J:
PI3Kα isoform-dependent activation of RhoA regulates Wnt5a-induced
osteosarcoma cell migration. Cancer Cell Int. 17:272017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai B, Yan T and Zhang A: ROR2 receptor
promotes the migration of osteosarcoma cells in response to Wnt5a.
Cancer Cell Int. 17:1122017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiong H, Yan T, Zhang W, Shi F, Jiang X,
Wang X, Li S, Chen Y, Chen C and Zhu Y: miR-613 inhibits cell
migration and invasion by downregulating Daam1 in triple-negative
breast cancer. Cell Signal. 44:33–42. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hasan MK, Yu J, Widhopf GF II, Rassenti
LZ, Chen L, Shen Z, Briggs SP, Neuberg DS and Kipps TJ: Wnt5a
induces ROR1 to recruit DOCK2 to activate Rac1/2 in chronic
lymphocytic leukemia. Blood. 132:170–178. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu J, Chen L, Cui B, Wu C, Choi MY, Chen
Y, Zhang L, Rassenti LZ, Widhopf Ii GF and Kipps TJ: Cirmtuzumab
inhibits Wnt5a-induced Rac1 activation in chronic lymphocytic
leukemia treated with ibrutinib. Leukemia. 31:1333–1339. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu J, Chen Y, Chen L, Zhang L, Rassenti
LZ, Widhopf GF II and Kipps TJ: Cirmtuzumab inhibits
ibrutinib-resistant, Wnt5a-induced Rac1 activation and
proliferation in mantle cell lymphoma. Oncotarget. 9:24731–24736.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi MY, Widhopf GF II, Wu CC, Cui B, Lao
F, Sadarangani A, Cavagnaro J, Prussak C, Carson DA, Jamieson C and
Kipps TJ: Pre-clinical specificity and safety of UC-961, a
first-in-class monoclonal antibody targeting ROR1. Clin Lymphoma
Myeloma Leuk (15 Suppl). S167–S169. 2015. View Article : Google Scholar
|
|
19
|
Wu X, Yan T, Hao L and Zhu Y: Wnt5a
induces ROR1 and ROR2 to activate RhoA in esophageal squamous cell
carcinoma cells. Cancer Manag Res. 11:2803–2815. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi MY, Widhopf GF II, Ghia EM, Kidwell
RL, Hasan MK, Yu J, Rassenti LZ, Chen L, Chen Y, Pittman E, et al:
Phase I trial: Cirmtuzumab inhibits ROR1 signaling and stemness
signatures in patients with chronic lymphocytic leukemia. Cell Stem
Cell. 22:951.e3–959.e3. 2018. View Article : Google Scholar
|
|
21
|
Hasegawa D, Wada N, Yoshida S, Mitarai H,
Arima M, Tomokiyo A, Hamano S, Sugii H and Maeda H: Wnt5a
suppresses osteoblastic differentiation of human periodontal
ligament stem cell-like cells via Ror2/JNK signaling. J Cell
Physiol. 233:1752–1762. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fritsch R and Downward J: SnapShot: Class
I PI3K isoform signaling. Cell. 154:940–940.e1. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Linnerth-Petrik NM, Santry LA, Moorehead
R, Jücker M, Wootton SK and Petrik J: Akt isoform specific effects
in ovarian cancer progression. Oncotarget. 7:74820–74833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu Y, Shen T, Liu J, Zheng J, Zhang Y, Xu
R, Sun C, Du J, Chen Y and Gu L: Rab35 is required for
Wnt5a/Dvl2-induced Rac1 activation and cell migration in MCF-7
breast cancer cells. Cell Signal. 25:1075–1085. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu J, Zhang Y, Xu R, Du J, Hu Z, Yang L,
Chen Y, Zhu Y and Gu L: PI3K/Akt-dependent phosphorylation of GSK3β
and activation of RhoA regulate Wnt5a-induced gastric cancer cell
migration. Cell Signal. 25:447–456. 2013. View Article : Google Scholar : PubMed/NCBI
|