Introduction
Renal cell carcinoma (RCC) represents the 6th most
frequently diagnosed cancer in men and is 10th in women, accounting
for 5 and 3% of all oncological diagnoses, respectively. According
to the most updated data provided by the World Health Organization,
there are more than 140,000 RCC-associated deaths yearly, with RCC
ranking as the 13th most common cause of cancer-related deaths
worldwide (1). The disease
encompasses >10 histological and molecular subtypes, with clear
cell RCC (ccRCC) accounting for approximately 75% of these subtypes
and most cancer-related deaths (2).
After recurrence or metastasis, ccRCC is largely incurable, and no
specific marker can effectively predict or treat advanced
cases.
Runt-related transcription factor 2 (RUNX2) belongs
to the RUNX family of metazoan transcription factors, which serve
as the major regulators of development with roles in proliferation,
differentiation, apoptosis, and cell lineage specification. RUNX2,
located on chromosome 6p21, is involved in bone formation and plays
an important role in bone development (3). The RUNX2 protein plays a vital role in
the pathogenesis of some cancers (4). Amplification of RUNX2 was revealed in
osteosarcoma and has been proposed as an early event in its
development (5). RUNX2 is also
highly expressed in metastatic prostate cancer cells and is
involved in promoting prostate cancer cell survival and bone tissue
invasion (6). In breast cancer,
RUNX2 can promote tumor invasion and metastasis, particularly
during bone metastasis (7).
The role of RUNX2 in ccRCC has not been reported.
Through bioinformatics and experimental verification, it was
revealed that RUNX2 plays an important role in the progression of
RCC. Combined with data from The Cancer Genome Atlas (TCGA) and
Gene Expression Omnibus (GEO), the pathways and mechanisms
involving RUNX2 were identified and evaluated. The functions of and
pathways mediated by RUNX2 were demonstrated via experiments by
silencing of RUNX2. These results may reveal the role of RUNX2 in
promoting cancer progression in ccRCC.
Materials and methods
Data downloading and
preprocessing
The RNA-sequencing (RNA-seq) results and
corresponding clinical data from 611 tissues and 530 cases of ccRCC
samples were obtained from TCGA database (https://portal.gdc.cancer.gov). These data were
current as of May 7, 2019. RNA-seq results of 72 normal samples and
539 cancer samples were combined into a matrix file using a merge
script in Perl language (http://www.perl.org/). Next, the Ensembl database
(http://asia.ensembl.org/index.html)
was used to convert gene names from the Ensembl IDs to a matrix of
gene symbols. The data was normalized using the R package edgeR and
genes with expression levels of greater than one were retained. In
the GEO database, GSE36133 (containing the expression profiles of
20 RCC cell lines determined by array) was selected and
downloaded.
Clinical significance of RUNX2
Using TCGA data downloaded in the previous step, the
information was grouped according to sample type, tumor stage, and
pathological grade to compare the expression of RUNX2. In the
comparison process, 3 samples without stage information and 6
samples without grade information were excluded. Four cases lacked
relevant follow-up information and were excluded, and the remaining
526 cases were divided into high and low groups according to the
RUNX2 expression level for survival analysis by the log-rank
test.
Gene set enrichment analysis
(GSEA)
Both tumor samples from TCGA and data from 20 RCC
cell lines were divided into two groups according to the median
expression of RUNX2. GSEA (http://software.broadinstitute.org/gsea/index.jsp) was
used to identify the potential functions correlated with RUNX2 by
assessing whether a series of a priori-defined biological
processes was enriched in the gene rank derived from whole genes
between the two groups. In the molecular signature database v6.2,
hallmark gene sets of curated gene sets were used. Terms with
|normalized enrichment score| >1, nominal P-value <0.05, and
false discovery rate of q-value <0.25 were identified.
Human samples
ccRCC and corresponding noncancerous tissues were
obtained from patients at the Department of Urology at the First
Hospital of China Medical University, between December 2016 and
January 2018. There was a total of 40 patients, 28 males and 12
females, aged 41–78 years, with mRNA samples from 14 patients (10
males, 4 females, age range 45–74 years) and protein samples from
26 other patients (18 males, 6 females, age range 41–78 years). The
protocols used in the study were approved by the Hospital's
Protection of Human Subjects Committee and written informed consent
was obtained from all patients. The collected tissue samples were
stored at −80°C prior to use.
Cell culture and reagents
Cells from the human RCC cell lines 769-P, 786-O and
OS-RC-2 were cultured in RPMI-1640 medium (HyClone; GE Healthcare
Life Sciences), ACHN cells were cultured in minimal essential
medium with Earle's balanced salts (HyClone; GE Healthcare Life
Sciences), and Caki-1 cells were cultured in McCoy's 5A medium
(Gibco; Thermo Fisher Scientific, Inc.). HK-2 cells (normal
cortex/proximal tubule cells) were cultured in Dulbecco's modified
Eagle's medium/F12 (HyClone; GE Healthcare Life Sciences). All
culture media were supplemented with 10% fetal bovine serum. All
cell lines were obtained from the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China) and cultured under
humidified air containing 5% CO2 at 37°C. When the cells
exceeded 80% confluence, they were washed with 1X
phosphate-buffered saline (PBS) and trypsinized at 37°C for a
specified amount of time for cell passage cultivation.
RNA preparation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using ice-cold TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. The concentration of total RNA was
measured using Thermo Fisher Scientific NanoDrop ND-100. Total RNA
was subjected to reverse transcription using the SYBR PrimeScript
RT-PCR kit (Perfect Real-Time) (Takara Bio, Inc.). Real-time PCR
was carried out using a Thermal Cycler Dice™ Real-Time system TP800
(Takara Bio, Inc.). The primer sequences designed for RUNX2 and
β-actin were as follows (5′-3′): RUNX2 forward,
GCGCATTCCTCATCCCAGTA and reverse, GGCTCAGGTAGGAGGGGTAA; and β-actin
forward, CATGTACGTTGCTATCCAGGC and reverse, CTCCTTAATGTCACGCACGAT.
The mRNA expression of the target gene was analyzed using the
2−ΔΔCq method (8).
Western blot analysis
Whole-cell lysates were extracted in
radioimmunoprecipitation assay buffer containing 1 mM
phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology), protease and phosphatase inhibitors. The
concentration of protein samples was measured using a bicinchoninic
acid assay (Beyotime Institute of Biotechnology). Equal amounts of
protein (30 µg/lane) were separated by 10% SDS-PAGE (140 V) and the
resolved proteins were transferred (350 mA) to polyvinylidene
fluoride membranes (0.2 µm) using a Mini-Trans-Blot apparatus
(Bio-Rad Laboratories, Inc.). The membranes were blocked with 5%
non-fat milk at 37°C for 1 h, incubated with the primary antibody
at 4°C overnight, and then incubated for 1 h with the appropriate
secondary antibody. Proteins were finally detected with the
EasySee® Western Blot kit (Beijing Transgen Biotech Co.,
Ltd.). The immunoblots were quantified using ImageJ software
(version 1.51; National Institutes of Health). The optical density
values from the immunoblots for samples and cells were normalized
to the density values acquired for β-actin and GAPDH, respectively.
The monoclonal primary antibodies were: Anti-E-cadherin (dilution
1:5,000; product code ab40772), anti-N-cadherin (dilution 1:5,000;
product code ab76011) and anti-vimentin (dilution 1:1,000; product
code ab92547; all from Abcam), anti-p44/42MAPK (anti-ERK; dilution
1:1,000; product no. 4695), anti-phospho-p44/42MAPK (anti-p-ERK;
dilution 1:2,000; product no. 4370), anti-RUNX2 (dilution 1:1,000;
product no. 12556) and anti-GAPDH (dilution 1:1,000; D16H11; all
from Cell Signaling Technology, Inc., and anti-β-actin (dilution
1:5,000; cat. no. 66009-1-Ig; ProteinTech Group, Inc.).
Small interfering RNA (siRNA)
transfection
The 786-O and Caki-1 cells (cultured to 70%
confluence) were seeded into 6-well plates at a density of
4×105 cells/well and transfected with two effective
RUNX2 siRNAs (JTS Scientific). siRNA transfection was performed
using Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the product's protocol. After 6 h, the medium
was replaced, and the cells were cultured for another 24 or 48 h
for further assays. The siRNA and negative control (NC) siRNA
sequences used were as follows (5′-3′): siRNA-1 sense,
CACGCUAUUAAAUCCAAAUTT and antisense, AUUUGGAUUUAAUAGCGUGTT; siRNA-2
sense, CAAGUCCUUUUAAUCCACATT and antisense, UGUGGAUUAAAAGGACUUGGT;
and NC sense, UUCUCCGAACGUGUCACGUTT and antisense,
ACGUGACACGUUCGGAGAATT.
Transwell assays
Cell migration was measured using Transwell chambers
with 8-µm pores in 24-well tissue culture plates (Corning Costar,
Corning, Inc.). For cell invasion assays, Transwell chambers coated
with Matrigel (BD Biosciences) were inserted into a 24-well plate.
After transfection of siRNA for 24 h, the cells were re-suspended
in medium without fetal bovine serum and 0.2 ml of cell suspension
(2×104 cells/well for cell migration; 4×104
cells/well for cell invasion) was seeded into the top chamber,
whereas the lower chamber of each well was filled with 0.6 ml of
medium containing 10% fetal bovine serum to act as a
chemoattractant. After 12–24 h of incubation at 37°C, the cells
that remained on the upper side of the filter were removed using
cotton swabs and those that had migrated to the lower side were
fixed with 4% paraformaldehyde for 10 min, and stained with 1.0%
crystal violet for 10 min at room temperature. Images were captured
with an EVOS™ XL Core Imaging system (Invitrogen; Thermo Fisher
Scientific, Inc.) and cells were counted using ImageJ software
(version 1.51; National Institutes of Health).
5-Ethynyl-2-deoxyuridine (EdU)
786-O and Caki-1 cells were seeded into 24-well
plates at a density of 1×104 cells/well and cultured for
24 h. The solution of an EdU Kit (BeyoClick™ EDU Cell Proliferation
Kit with Alexa Fluor 488; Beyotime Institute of Biotechnology) was
diluted 1:1,000 in cell medium. The cells were incubated with the
EdU solution for 2 h at 37°C. Following fixation in 4%
paraformaldehyde for 15 min at room temperature and treatment with
0.3% Triton-X for 15 min at room temperature, the cells were
incubated for 30 min with Click reaction cocktail in a dark room at
room temperature. Prior to observation under a fluorescence
microscope, nuclei were stained with Hoechst 33342.
Interaction of proteins
STRING (https://www.string-db.org) version 11.0 was used with
multiple protein modules for protein interaction network
analysis.
Gene co-expression analysis
The co-expression relationships between RUNX2 and
related genes were determined based on gene expression levels to
determine the strength of the relationships at the transcriptional
level. The R corrplot package was used to calculate the Pearson
correlation between genes.
Statistical analysis
All data were expressed as the mean ± standard
deviation (SD) and represented as the average of at least three
experiments with each experiment performed in triplicate.
Statistical significance was determined using the Student's
t-test (two-tailed) for 2 groups, one-way analysis of
variance or (and) Tukey's test for more than 2 groups. Correlations
between the expression levels of different genes were evaluated by
Spearman correlation analysis. In the bar graphs *, **, ***, ****,
and ns indicate P<0.05, P<0.01, P<0.001, P<0.0001, and
not significant, respectively. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
Expression and survival analysis
Box-and-Whisker plots revealed RUNX2 expression
levels (based on sample type) in normal and tumor samples (Fig. 1A). The expression was higher in all
individual cancer stages than in adjacent normal tissues, and stage
4 exhibited the highest RUNX2 expression (Fig. 1B). Comparisons of the grades
revealed that RUNX2 expression had a more evident increasing trend
(Fig. 1C). Survival analysis
revealed that the RUNX2 gene significantly affected prognosis
(Fig. 1D). The aforementioned
results indicated that RUNX2 is an important gene in ccRCC and its
mRNA expression is upregulated during tumor development.
GSEA
To identify the potential function of RUNX2, GSEA
was conducted to search for pathways enriched in the
higher-RUNX2-expressed samples. The functions and pathways
potentially enriched by RUNX2 were separately analyzed in the
previously downloaded TCGA cancer samples and GEO cell line data.
The purity of the cancer samples affected our analysis results,
however the cell lines were not influenced by purity, but had a
small total sample size. Therefore, the screening conditions for
the cell lines were relaxed and the results obtained to determine
the functions and pathways potentially affected by RUNX2 were
combined (Fig. 1E). The
IL6/JAK/STAT3 signaling axis in cancer drives the proliferation,
survival, invasiveness, and metastasis of tumor cells while
strongly suppressing the antitumor immune response (9). In the IL2/STAT5 signaling pathway,
STAT5 was revealed to play a critical role in the function and
development of regulatory T cells (Tregs), and consistently
activated STAT5 was associated with the suppression of antitumor
immunity and increased proliferation, invasion, and survival of
tumor cells (10).
Epithelial-mesenchymal transition (EMT) is associated with tumor
stemness, metastasis, and therapy resistance (11). The three pathways and mechanisms
aforementioned were correlated with RUNX2 and promoted tumor
progression. Overexpression of RUNX2 was related to the
inflammatory response and allograft rejection (Fig. 1F).
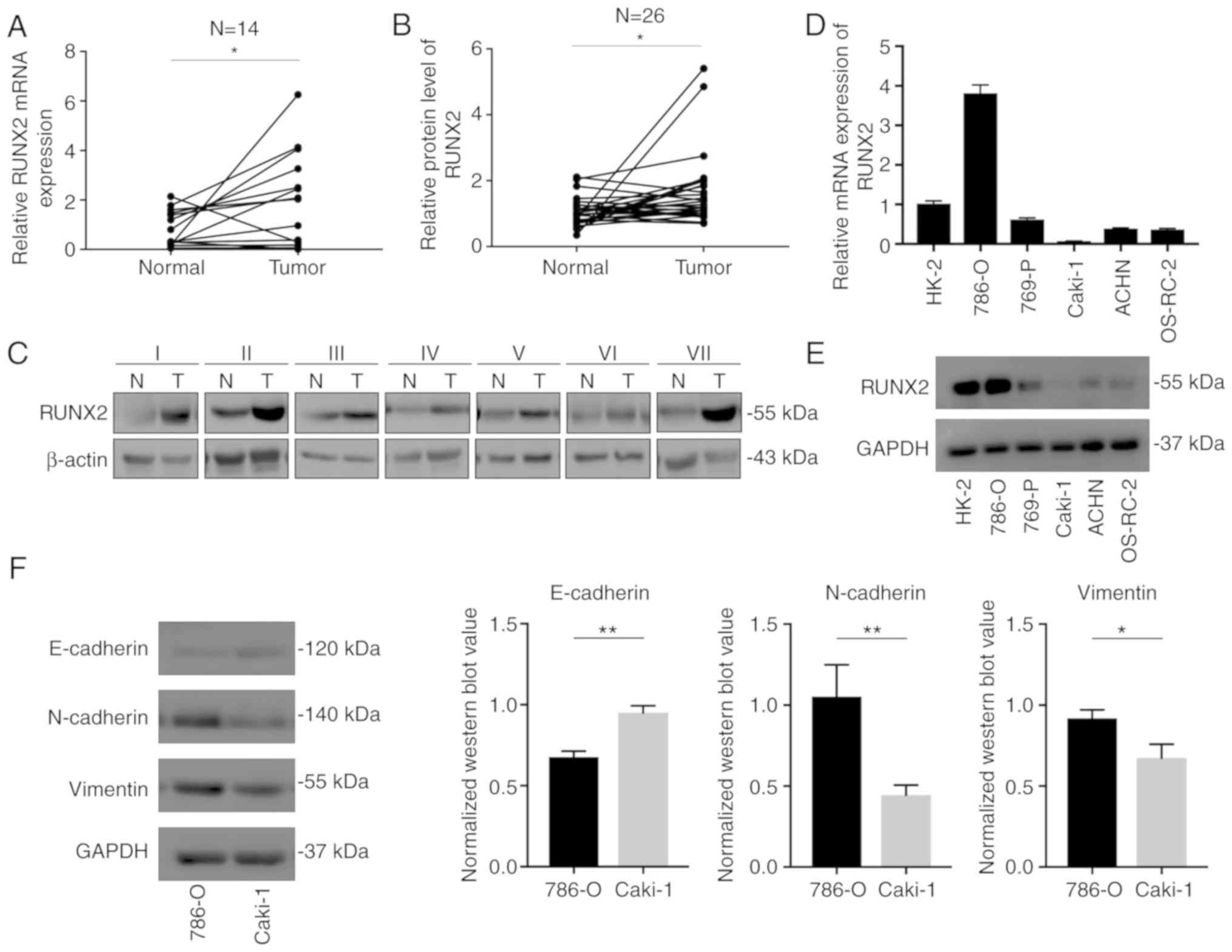
RUNX2 mRNA and protein levels in ccRCC
and RCC cell lines
The clinical tissue specimens collected from our
hospital were analyzed to verify the results of bioinformatics
analyses and it was confirmed that RUNX2 mRNA expression was
significantly upregulated in tumor tissues compared to normal
kidney tissues in 14 paired samples (Fig. 2A). To examine RUNX2 protein
expression in clinical ccRCC specimens, another 26 pairs of cancer
tissues and normal tissues were assessed. Western blot results
indicated a significant increase of RUNX2 protein expression in
tumor tissues compared to adjacent normal tissues (Fig. 2B and C). These data indicated that
RUNX2 was highly expressed in kidney tumors and may be a reliable
target for further investigation. Next, RUNX2 mRNA and protein
levels were assessed in RCC cell lines and the cell line with the
highest RUNX2 expression (780-O) and that with the lowest
expression (Caki-1) were selected for subsequent experiments
(Fig. 2D and E). Notably, the EMT
pathway-associated proteins in 786-O were significantly different
from those in Caki-1, consistent with the high activation of the
RUNX2-mediated EMT mechanism predicted by GSEA (Fig. 2F).
Migration and invasion
Transwell assays were performed to explore the
effect of RUNX2 on the migration and invasion of RCC cells.
Decreasing RUNX2 expression with siRNA in 786-O and Caki-1 cells
inhibited cell migration and invasion (Fig. 3A and B). Combined with upregulation
of E-cadherin and downregulation of N-cadherin, vimentin and
phosphorylated extracellular signal-regulated kinase (p-ERK)
proteins after transfection, RUNX2 was revealed to be associated
with the EMT pathway in renal clear cell carcinoma, which is
consistent with the previous results of GSEA. The gray value of the
western blot of RUNX2 in Caki-1 (Fig.
3A) was significantly higher than that of RUNX2 (Fig. 2E) in the previous cell line
comparison. This was because the sample load was increased by
3-fold and the exposure time was extended to 6 min (exposure time
in Fig. 3E is 2 min).
Proliferation
EdU assays revealed that cell proliferation was
decreased when RUNX2 was silenced, indicating that RUNX2 enhanced
tumor growth in vitro (Fig.
3C).
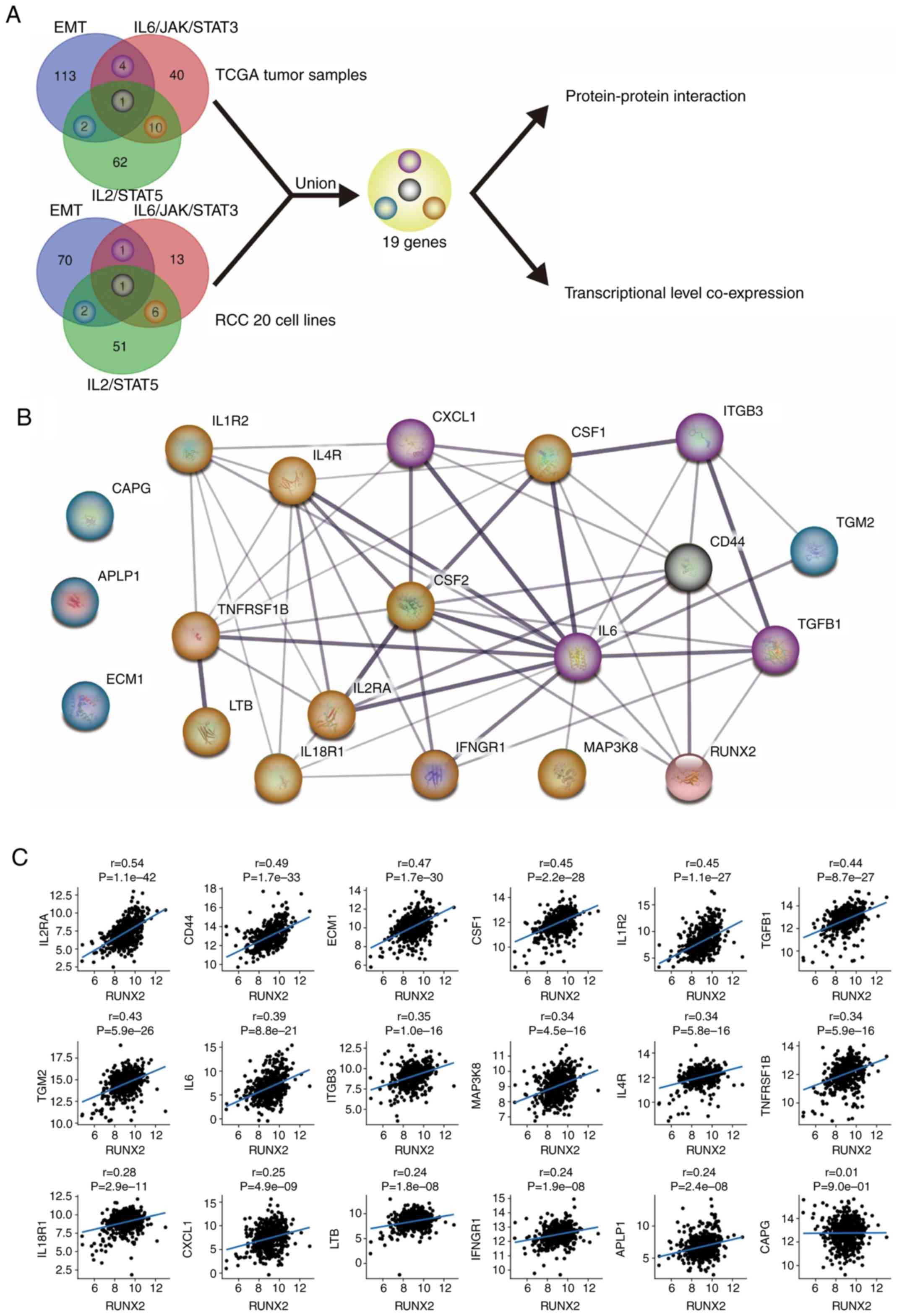
Identification of core-related
genes
Overexpression of RUNX2 is related to ccRCC
progression. Tumor progression is a complex biological process
involving multiple cellular pathways and mechanisms. RUNX2 has been
revealed to be involved in the EMT pathway, the IL6/JAK/STAT3
signaling pathway, and the IL2/STAT5 signaling pathway (screened by
GSEA), wherein the relationship with the core enrichment genes may
be direct or indirect. IL6/JAK/STAT signaling has been revealed to
promote EMT (12). These enriched
pathways and mechanisms have similar biological functions and thus
the genes shared among them may be critical. The core enrichment
genes of the EMT, IL6/JAK/STAT3 signaling, and IL2/STAT5 signaling
pathways were analyzed to identify the genes revealing significant
relationships with RUNX2, and then the genes were combined from
TCGA and cell lines for subsequent analysis (Fig. 4A).
Protein-protein interaction and
co-expression of core-related genes
In STRING, the proteins from the 19 core genes
mostly revealed protein interactions (Fig. 4B). Among them, IL6 exhibited the
most extensive effect, whereas RUNX2 exhibited protein interactions
with 5 other proteins. According to TCGA ccRCC tumor data, RUNX2
was also positively correlated with most core-related gene
transcriptional levels normalized by log2 (Fig. 4C). CSF2 was ruled out because its
expression was too low.
Discussion
RUNX2 was first demonstrated to promote cancer
progression in RCC. The RUNX2-related EMT pathway determined by
GSEA was related to tumor invasion and metastasis. The results of
the Transwell and EDU experiments confirmed the role of RUNX2 in
promoting tumor progression, and the EMT pathway-related proteins
were subsequently altered after silencing of RUNX2. The genes
shared by the GSEA screening pathways may be essential for RUNX2 to
promote tumor progression.
When searching for enriched pathways, data from RCC
cell lines were also included to more accurately identify cellular
pathways potentially involving RUNX2. RCC cell lines can be
considered as cancer cells extracted from the tumor environment,
where interference from other cells is excluded. Based on the
results of GSEA, RUNX2 may mediate EMT. 786-O cells with the high
expression of RUNX2 were compared to Caki-1 cells with low
expression of RUNX2, and their EMT-related proteins exhibited the
same trend. Silencing of RUNX2 could reduce the phosphorylation
level of ERK, which is a key coordinator of EMT (13).
Based on the GSEA results, it was revealed that
overexpression of RUNX2 was associated with IL6/JAK/STAT3
signaling, IL2/STAT5 signaling, and EMT and that overexpression may
be directly or indirectly related to these pathways or mechanisms
in promoting tumor progression. However, RUNX2 upregulation also
positively regulated the inflammatory response and allograft
rejection, indicating that the body is consistently undergoing an
immune response during tumor progression. However, the process of
immune clearance was strongly inhibited. STAT3 is frequently
overactivated in tumor-infiltrating immune cells, negatively
regulating neutrophils, natural killer (NK) cells, effector T
cells, and dendritic cells (DCs), suggesting that activation of
STAT3 results in downregulation of antitumor immunity (9). STAT5 plays a critical role in the
function and development of Tregs, and consistently activated STAT5
is associated with the suppression of antitumor immunity (10). EMT also promotes immune escape
(14), and thus activation of the
aforementioned pathway mechanisms may lead not only to tumor
progression, but also to resistance and evasion of the immune
system. Moreover, once the inflammatory response fails to control
the tumor, it is typically exploited by the tumor to promote its
own growth and progression to metastasis (15).
When searching for possible RUNX2-related genes,
correlation analysis was not performed for all genes. Moreover, the
expression level was first subjected to log2 transformation to
reduce the scale of expression over different orders of magnitude
and render the data more stable. We first searched for core genes
in the enrichment pathway through GSEA, and then conducted
correlation analysis to identify potentially associated genes. This
approach is more biologically meaningful.
Both CD44 and transforming growth factor beta 1
(TGFβ1) have a relatively strong relationship with RUNX2 as
revealed through protein interactions and co-expression. The
protein encoded by CD44 is a cell-surface glycoprotein involved in
cell-cell interaction, cell adhesion, and migration. CD44, the only
gene shared by all enrichment pathways, may have protein
interactions and be co-expressed with RUNX2. In prostate cancer PC3
cells, knockdown of CD44 reduced RUNX2 expression at the mRNA and
protein levels, thereby reducing RUNX2-mediated signaling (16). In addition, CD44 expression may play
an important role in the progression of RCC (17). RUNX2 was revealed to be a target of
TGFβ1 in C2C12 pluripotent mesenchymal precursor cells and was
involved in regulating the TGF-β pathway (18). In RCC, TGFβ1 was revealed to be
significantly associated with tumor stage T3-4, Fuhrman III and IV,
and tumor size >4 cm (19).
RUNX2-related IL6/JAK/STAT3 signaling, IL2/STAT5
signaling, and core-related proteins were not experimentally
verified. This will be evaluated in our future studies.
In general, RUNX2 plays a role in promoting tumor
progression in RCC. RUNX2 drives the EMT process to promote RCC
migration and invasion. Additional studies are required to
determine the mechanism by which RUNX2 promotes RCC progression.
RUNX2 may be a useful therapeutic target for RCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Fund (grant no. 81672525), the Liaoning Natural
Science Fund (grant no. 201602830), and China Medical University's
2017 discipline promotion program (grant no. 2017XK08).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. Publicly available datasets can be found here: https://portal.gdc.cancer.gov.
Authors' contributions
BL designed the study and drafted the manuscript. JL
and BL performed the experiments. BL collected, analyzed, and
interpreted the data. HY and CW collected tissue samples of the
patients with RCC and contributed to revision of the manuscript and
figures. CK acquired funding, established the urology laboratory,
provided the required equipment and instruments, contributed to the
critical reading of the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee on Human Research of the First Affiliated Hospital of
China Medical University (Shenyang, China), and written informed
consent was obtained from all patients.
Patient consent for publication
Consent for publication was obtained from all
participants.
Competing interests
The authors declare no competing financial
interests. The funding agency did not participate in the design of
the study and collection, analysis, and interpretation of data or
in writing of the manuscript.
References
|
1
|
Capitanio U, Bensalah K, Bex A, Boorjian
SA, Bray F, Coleman J, Gore JL, Sun M, Wood C and Russo P:
Epidemiology of renal cell carcinoma. Eur Urol. 75:74–84. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hsieh JJ, Purdue MP, Signoretti S, Swanton
C, Albiges L, Schmidinger M, Heng DY, Larkin J and Ficarra V: Renal
cell carcinoma. Nat Rev Dis Primers. 3:170092017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Z, Yao X, Yan G, Xu Y, Yan J, Zou W
and Wang G: Mediator MED23 cooperates with RUNX2 to drive
osteoblast differentiation and bone development. Nat Commun.
7:111492016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ito Y, Bae SC and Chuang LS: The RUNX
family: Developmental regulators in cancer. Nat Rev Cancer.
15:81–95. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martin JW, Zielenska M, Stein GS, van
Wijnen AJ and Squire JA: The role of RUNX2 in osteosarcoma
oncogenesis. Sarcoma. 2011:2827452011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akech J, Wixted JJ, Bedard K, van der Deen
M, Hussain S, Guise TA, van Wijnen AJ, Stein JL, Languino LR, et
al: Runx2 association with progression of prostate cancer in
patients: Mechanisms mediating bone osteolysis and osteoblastic
metastatic lesions. Oncogene. 29:811–821. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li XQ, Du X, Li DM, Kong PZ, Sun Y, Liu
PF, Wang QS and Feng YM: ITGBL1 Is a Runx2 transcriptional target
and promotes breast cancer bone metastasis by activating the TGFβ
signaling pathway. Cancer Res. 75:3302–3313. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rani A and Murphy JJ: STAT5 in cancer and
immunity. J Interferon Cytokine Res. 36:226–237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pastushenko I, Brisebarre A, Sifrim A,
Fioramonti M, Revenco T, Boumahdi S, Van Keymeulen A, Brown D,
Moers V, Lemaire S, et al: Identification of the tumour transition
states occurring during EMT. Nature. 556:463–468. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao J, Gong Y, Chen Y, Yu D, Wang X,
Zhang X, Dou Y, Liu D, Cheng G, Lu S, et al: IL-6 promotes
epithelial-to-mesenchymal transition of human peritoneal
mesothelial cells possibly through the JAK2/STAT3 signaling
pathway. Am J Physiol Renal Physiol. 313:F310–F318. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shin S, Buel GR, Nagiec MJ, Han MJ, Roux
PP, Blenis J and Yoon SO: ERK2 regulates epithelial-to-mesenchymal
plasticity through DOCK10-dependent Rac1/FoxO1 activation. Proc
Natl Acad Sci USA. 116:2967–2976. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nan X, Wang J, Liu HN, Wong STC and Zhao
H: Epithelial-mesenchymal plasticity in organotropism metastasis
and tumor immune escape. J Clin Med. 8:E7472019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dominguez C, David JM and Palena C:
Epithelial-mesenchymal transition and inflammation at the site of
the primary tumor. Semin Cancer Biol. 47:177–184. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gupta A, Cao W and Chellaiah MA: Integrin
αvβ3 and CD44 pathways in metastatic prostate cancer cells support
osteoclastogenesis via a Runx2/Smad 5/receptor activator of NF-κB
ligand signaling axis. Mol Cancer. 11:662012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee YM, Kim JM, Lee HJ, Seong IO and Kim
KH: Immunohistochemical expression of CD44, matrix
metalloproteinase2 and matrix metalloproteinase9 in renal cell
carcinomas. Urol Oncol. 37:742–748. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee KS, Kim HJ, Li QL, Chi XZ, Ueta C,
Komori T, Wozney JM, Kim EG, Choi JY, Ryoo HM and Bae SC: Runx2 is
a common target of transforming growth factor beta1 and bone
morphogenetic protein 2, and cooperation between Runx2 and Smad5
induces osteoblast-specific gene expression in the pluripotent
mesenchymal precursor cell line C2C12. Mol Cell Biol. 20:8783–8792.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lebdai S, Verhoest G, Parikh H, Jacquet
SF, Bensalah K, Chautard D, Rioux Leclercq N, Azzouzi AR and Bigot
P: Identification and validation of TGFBI as a promising prognosis
marker of clear cell renal cell carcinoma. Urol Oncol.
33:69.e11–e68. 2015. View Article : Google Scholar
|