Introduction
Endometrial cancer (EC) is the most common
malignancy of the female reproductive system, causing 76,000 deaths
every year worldwide (1). Despite
major advances in EC diagnosis and treatment strategies, metastasis
is a significant clinical challenge and represents the main cause
of EC mortality; in patients with EC without metastatic disease,
the 5-year overall survival ranges between 74 and 91% (2), whereas patients with stage III or IV
EC exhibit 5-year overall survival rates of 57–65 and 20–26%,
respectively (1). Thus,
understanding the underlying mechanisms of metastatic disease may
help in the development of more effective therapeutic strategies
for EC.
Eukaryotic translation initiation factor 4E (eIF4E)
is the most important component of the eukaryotic translation
initiation complex eIF4F. At the initiation of translation, eIF4E
binds to the 5′-7-methylguanosine cap structure of an mRNA,
connects it to the ribosome and enables translation (3). In addition to cap-dependent protein
synthesis, eIF4E also contributes to malignancy as mRNAs regulated
by eIF4E generally encode key proteins involved in cell
proliferation, angiogenesis, survival and malignant transformation
[e.g. cyclin D1, c-MYC, vascular endothelial growth factor and
matrix metallopeptidase 9 (MMP-9)] (4). As an oncogene, eIF4E has been
demonstrated to be upregulated in a variety of malignancies, such
as breast (5), colorectal (6) and prostate (4) cancer, but its role in EC remains to be
elucidated. A previous study has revealed that eIF4E is more
frequently upregulated in EC extending outside the uterus (FIGO
stage III/IV vs. I/II, and downregulation of eIF4E by small
interfering (si)RNA significantly reduced the proliferation of
HEC-1A cells (7). These findings
suggested that eIF4E may serve an important role in the metastasis
of EC and may represent a potential anti-metastatic therapeutic
target.
The epithelial-mesenchymal transition (EMT) is an
important mechanism in tumor metastasis that is triggered by the
activation of transcription factors such as Snail family
transcriptional repressor 1 (Snail), Twist-related protein 1, Snail
family transcriptional repressor 2, forkhead box C2, SOX4 and zinc
finger E-box-binding homeobox (8).
These activated transcription factors downregulate the epithelial
marker E-cadherin, as well as polarity-related proteins, such as
lethal giant larvae 2 (9,10), and upregulate mesenchymal markers
such as N-cadherin and vimentin (11). The transcriptional events
controlling EMT are well characterized, but the associated
post-transcriptional mechanisms have not been clearly elucidated
(12). A previous study has
demonstrated that phosphorylation of eIF4E promotes transforming
growth factor β1 (TGF-β1)-mediated EMT via Snail and MMP-3
translation activation of (9),
suggesting that eIF4E may serve an important role in the
translational control of EMT.
MicroRNAs (miRNAs) are an abundant class of small
regulatory RNAs in animals and plants that serve important
regulatory roles by interacting with the 3′-untranslated region
(3′-UTR) of target mRNAs for cleavage or translational repression
(13). A number of studies have
demonstrated that miRNAs serve important roles in cell
proliferation, apoptosis, migration, chemosensitivity and
radio-resistance (14–18), and recent findings have revealed
that miRNAs also regulate EMT in various tumor cells. In
hepatocellular carcinoma, miRNA (miR)-199b-5p attenuates
TGF-β1-induced EMT by directly targeting N-cadherin (19), whereas miR-190 suppresses
TGF-β1-induced EMT by targeting SMAD2 in breast cancer (20). Although several miRNAs have been
demonstrated to regulate EMT and metastasis of EC, the roles of
miR-320a and miR-340-5p in EC have not yet been fully
elucidated.
The present study aimed to investigate the
expression of eIF4E, miR-320a and miR-340-5p in EC and identify
their interactions. The effects of miR-320a and miR-340-5p on cell
metastatic potential and EMT were further investigated in EC
cells.
Materials and methods
eIF4E gene expression data from
patients with EC in the Oncomine database
The Oncomine database (https://www.oncomine.org/resource/login.html) was used
to mine the data of eIF4E gene expression in EC using the key words
‘eIF4E’ and ‘endometrial carcinoma’. For the Kaplan-Meier survival
analysis, patients with eIF4E expression values below the 20th
percentile were classified as having low eIF4E levels.
Endometrial cancer tissues
Between August 2016 and July 2017, eight pairs of EC
and adjacent normal tissues (≥2 cm from the tumor edge), were
collected from eight patients who underwent hysterectomy at the
Affiliated Hospital of Binzhou Medical University. All samples were
diagnosed by surgical-pathology or biopsy. The tissues were
snap-frozen in liquid nitrogen and stored at −80°C for later
experiments, including RNA extraction and western blotting. This
study was approved by the Medical Ethics Committee of Binzhou
Medical University (approval no. 2016-21). Informed consent was
obtained from all patients prior to the collection of samples.
Cell culture
The human EC cell line HEC-1A was purchased from the
Shanghai Institute of Cell Biology. The human EC cell lines
Ishikawa and RL95-2 were obtained from Dalian Medical University.
HEC-1A, RL-952 and Ishikawa cells were cultured in McCoy's 5A
(Beijing Macgene Biotechnology Co., Ltd.), DMEM/F12 (Gibco; Thermo
Fisher Scientific, Inc.) and RPMI-1640 (Gibco; Thermo Fisher
Scientific, Inc.) medium, respectively. The media were supplemented
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.)
and 100 U/ml penicillin-streptomycin (Sigma-Aldrich; Merck KGaA) at
37°C with 5% CO2 and saturated humidity.
miRNA transfections
EC cells (HEC-1A and RL95-2) at the logarithmic
growth phase were seeded in 6-well plates at 3×105
cells/well. Transfection was performed in triplicate at 50–60%
confluency using 1 µg miRNA mimics, mutation mimics (mu-320a or
mu-340-5p), siRNA or an eIF4E-encoding vector (Sino Biological,
Inc.) in 2.5 µl Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The sequences of the miRNA mimics were as
follows: miR-320a sense, 5′-AAAAGCUGGGUUGAGAGGGCG-3′ and antisense,
5′-GCCCUCUCAACCCAGCUUUUUU-3′; miR-340-5p sense,
5′-UUAUAAGCAAUGAGACUGAUU-3′ and antisense,
5′-UCAGUCUCAUUGCUUUAUAAUU-3′. The mimics were synthesized by
Shanghai GenePharma Co., Ltd. The sequences of si-eIF4E were sense,
5′-GCUUCUGUAUUCUAAUCUAAU-3′ and antisense,
5′-UAGAUUAGAAUACAGAAGCUU-3′, synthesized by Shanghai GeneChem Co.,
Ltd. Transfection was performed according to the manufacturer's
instructions at room temperature; the transfection complex were
replaced with complete medium 6–8 h post-transfection, and the
cells were incubated for 24–48 h at 37°C prior to subsequent
experiments. For experiments involving TGF-β1 treatment, various
concentrations of TGF-β1 (5, 10 and 20 ng/ml; Sino Biological,
Inc.) were added to treat EC cells for 48 h.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total miRNA of endometrial adenocarcinoma cells
(HEC-1A and RL95-2) was isolated by RNAiso Plus (Takara Bio, Inc.),
and polyA was added using a polyA polymerase (Ambion; Thermo Fisher
Scientific, Inc.). cDNA was synthesized using PrimeScriptÔ RT
reagent Kit with gDNA Eraser (Takara Bio, Inc.) with the RT primer
5′-AACATGTACAGTCCATGGATGd(T)30N(A, G, C or T)-3′ at 42°C for 15
min, and qPCR was performed to detect miR-320a and miR-340-5p.
Primers used for amplification were as follows: miR-320a forward,
5′-AAAAGCTGGGTTGAGAGG-3′ and reverse, 5′-AACATGTACAGTCCATGGATG-3′;
miR-340-5p forward, 5′-AAGCAATGAGACTGATT-3′ and reverse,
5′-AACATGTACAGTCCATGGATG-3′; human 5S rRNA forward,
5′-GCCATACCACCCTGAACG-3′ and reverse, 5′-AACATGTACAGTCCATGGATG-3′.
A SYBR® Premix Ex Taq kit (Takara Bio, Inc.) was used
according to the manufacturer's instructions. The expression levels
of the two miRNAs were measured by the RG3000 system (Corbett Life
Science; Qiagen, Inc.) using the following thermocycling
conditions: Initial denaturation at 95°C for 3 min, followed by 40
cycles of denaturation at 95°C for 20 sec, annealing at 56°C for 20
sec and an extension at 72°C for 20 sec. Fluorescence was detected
at 585 nm, and the cycle threshold (Ct) was recorded. Human 5S rRNA
served as a control. The relative expression of miR-320a and
miR-340-5p was normalized to that of 5S rRNA, and the experiments
were repeated three times in triplicate. The results ware
quantified using the 2−ΔΔCq method (21).
Wound-healing assay
HEC-1A cells were seeded into 12-well plates at
1.5×105 cells/well and cultured to 90% confluency the
next day. Subsequently, these cells were subjected to an in
vitro wound-healing assay; a sterile 10 µl pipette tip was used
to scratch the confluent cell monolayer, the cells were washed,
suspended in using PBS and incubated in serum-free McCoy's 5A
medium at 37°C. Images were captured using an inverted light
microscope (×100 magnification; Leica Microsystems GmbH) at 0, 24
and 48 h of incubation. The rate of migration was measured by
quantifying the distance that the HEC-1A cells moved from the edge
of the scratch toward the center of the scratch (marked by dotted
lines).
Transwell cell migration assays
HEC-1A or RL-952 cells were treated with miRNA
mimics for 24 h. A total of 100 µl cell suspension was added to the
upper chamber of the Transwell insert (Corning, Inc.) at a
concentration of 5×105 cells/ml diluted with serum-free
McCoy's 5A medium, whereas medium with 20% fetal calf serum was
added to the lower chamber. At 24 h, the liquid in the upper
chamber was removed, the surface was washed with PBS, the
non-migrated cells were removed with a cotton swab, 600 µl 4%
methanol was added to fix the cells (20 min at room temperature),
and 600 µl 0.1% crystal violet (Sigma-Aldrich; Merck KGaA) was
added to stain the cells (15 min at room temperature). The number
of migrated cells was counted under an inverted light microscope
(×200 magnification; Leica Microsystems GmbH); the average number
of migrated cells was determined by quantification in five random
fields. The migratory ability of the cells was determined based on
the number of transmembrane cells.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
For the MTT assay, 1×104 HEC-1A and
RL95-2 cells/well were cultured in 96-well plates. The following
day, cells were treated with the miR-320a or miR-340-5p mimics and
control oligomers for 48 h. Each group was tested in six
replicates. Subsequently, 10 µl MTT (5 mg/ml; Sigma-Aldrich; Merck
KGaA) was added to each well and incubated for 4 h, followed by the
addition of 100 µl DMSO (Sigma-Aldrich; Merck KGaA). The optical
density (OD) was measured using an auto-microplate reader (Thermo
Fisher Scientific, Inc.) at 490 nm.
Detection of apoptosis
Apoptosis was measured by fluorescence-activated
cell sorting (FACS). Cells (HEC-1A and RL95-2) were cultured in
6-well plates at 3×105 cells/well and treated with miRNA
mimics or control oligomers when the confluency reached 70% the
next day. Detection of apoptosis was performed at 48 h using an
Annexin V-FITC/PI apoptosis detection kit (BD Biosciences)
according to the manufacturer's instructions. The cells were
analyzed using a flow cytometer (Beckman Coulter, Inc.), and the
CytExpert 1.2.11.0 software (Beckman Coulter, Inc.) were used for
data analysis.
Construction of the
pcDNA-GFP-eIF4E-3′UTR vector
The sequence of the eIF4E 3′-UTR was obtained from
GenBank and was amplified by PCR from human genomic DNA (extracted
from whole human blood). The primer sequences were as follows:
eIF4E 3′-UTR forward, 5′-CCCAAGCTTTCATTCGCCTTTGTCTTGTA-3′ and
reverse, 5′-CGGGGTACCTGGCAGGTGCTTGTAGTC-3′. The eIF4E 3′-UTR was
then inserted into a pcDNA3.1-GFP-neo (+) (GenScript Biotech, Inc.)
expression vector.
Western blotting
Cells (HEC-1A or RL95-2) were lysed with RIPA lysis
buffer containing a protease inhibitor cocktail (cat. no. S8820;
Sigma-Aldrich; Merck KGaA) for 30 min on ice. The protein
concentrations were measured using the bicinchoninic acid assay,
and the protein (35 µg/lane) was subjected to SDS-PAGE (10%) and
transferred onto PVDF membranes. Subsequently, the membranes were
blocked with 7% fat-free milk and were immunoblotted overnight at
4°C with antibodies against eIF4E (1:1,000; cat. no. BS3432),
p-eIF4E (1:1,000; cat. no. BS5015), α-smooth muscle actin (α-SMA;
1:1,000; cat. no. BS70000; all from Biogot Technology Co., Ltd.),
MMP-3 (1:400; cat. no. bs-0413R; Bioss), MMP-9 (1:400; cat. no.
bs-4593R; Bioss), E-cadherin (1:1,000; cat. no. 20874-1-AP;
Proteintech Group, Inc) and Snail (1:1,000; cat. no. 13099-1-AP;
Proteintech Group, Inc). GAPDH (1:3,000; cat. no. AP0063; Biogot
Technology Co., Ltd.) was used as a control. Following washing with
TBS + Tween-20 (0.1%), the membranes were incubated with
horseradish peroxidase-labeled goat anti-rabbit IgG (1:5,000; cat.
no. ZB-2301; Beijing Zhongshan Golden Bridge Technology Co., Ltd.)
for the detection of primary antibodies. The membranes were
visualized with ECL (Shanghai Novland Co., Ltd.), and images were
captured using an automatic chemiluminescence image analysis system
(Tanon Science and Technology Co., Ltd.). Densitometric analysis of
the blots was performed using Gel Image System 4.2 software (Tanon
Science and Technology Co. Ltd.).
miRNA prediction
The online miRNA analysis software TargetScan
(http://www.targetscan.org/vert_72/)
was used to identify the miRNAs with potential binding sites in the
eIF4E 3′-UTR.
Statistical analysis
Statistical significance of experimental data was
evaluated with GraphPad Prism 5 (GraphPad Software, Inc.).
Quantitative results are presented as the mean ± standard
deviation. Paired Student's t-test was used to compare two groups.
Differences among three or more groups were compared using one-way
ANOVA followed by a Tukey's test. Correlations were calculated with
a Spearman rank test. Array data of eIF4E were obtained from the
Oncomine database (https://www.oncomine.org/resource/main.html; TCGA
Endometrium 2 dataset). Survival rates were analyzed using
Kaplan-Meier survival analysis by Gehan-Breslow-Wilcoxon tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
eIF4E is upregulated in EC tissues and
is associated with poor clinical outcomes, whereas miR-320a and
miR-340-5p are downregulated in EC tissues
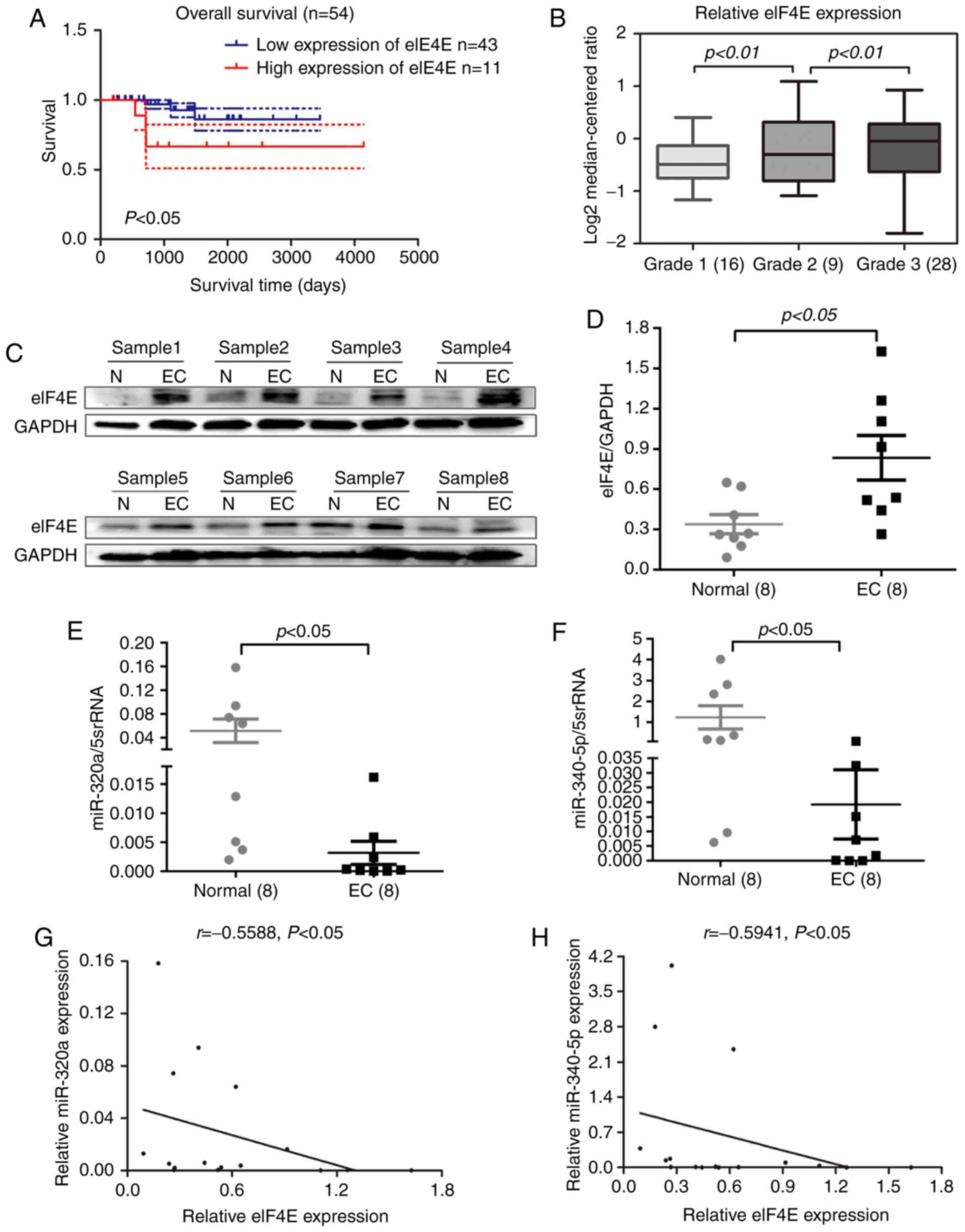
The expression profile of eIF4E in human EC was
investigated using patient datasets from the Oncomine database.
Data analysis revealed that high eIF4E expression levels in EC
tissues were associated with reduced overall survival (Fig. 1A) and a high pathological grade
(Fig. 1B). To validate this result,
eIF4E protein expression was determined in eight pairs of EC and
normal adjacent tissues by Western blotting. The results
demonstrated that eIF4E expression levels in EC tissues were
significantly higher compared with those in normal adjacent tissues
(Fig. 1C and D). To explore their
potential role in EC, the levels of miR-320a and miR-340-5p were
measured by RT-qPCR in eight paired EC and adjacent tissues. Of
note, miR-320a and miR-340-5p expression levels were significantly
decreased in EC tissues compared with those in adjacent normal
tissues (Fig. 1E and F).
Correlation analysis indicated that the expression levels of
miR-320a and miR-340-5p were inversely correlated with eIF4E
expression (Fig. 1G and H).
Collectively, these results suggested that eIF4E may function as a
tumor promoter in EC.
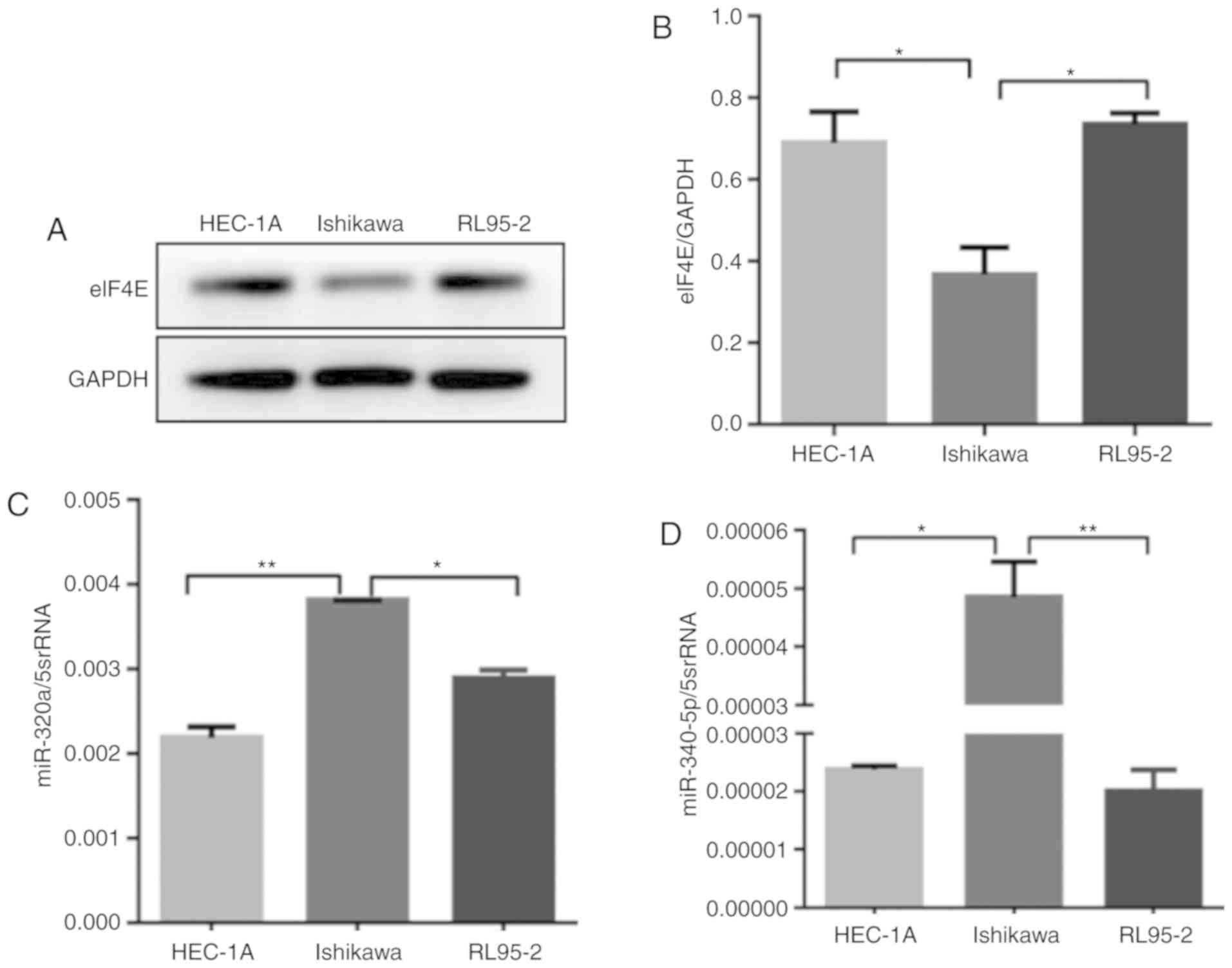
eIF4E is overexpressed, whereas
miR-320a and miR-340-5p are downregulated in HEC-1A and RL95-2 EC
cell lines
eIF4E expression was analyzed by western blotting in
three endometrial cancer cell lines: HEC-1A, Ishikawa and RL95-2
cells. The expression of eIF4E was high in HEC-1A and RL95-2 cells,
but low in Ishikawa cells (Fig. 2A and
B). The expression levels of miR-320a and miR-340-5p in the
three human EC cell lines were measured by RT-qPCR; miR-320a and
miR-340-5p were downregulated in HEC-1A and RL95-2 compared with
Ishikawa cells (Fig. 2C and D).
Based on these results, it was hypothesized that this heterogeneity
was due to the degree of differentiation in the three cell
lines.
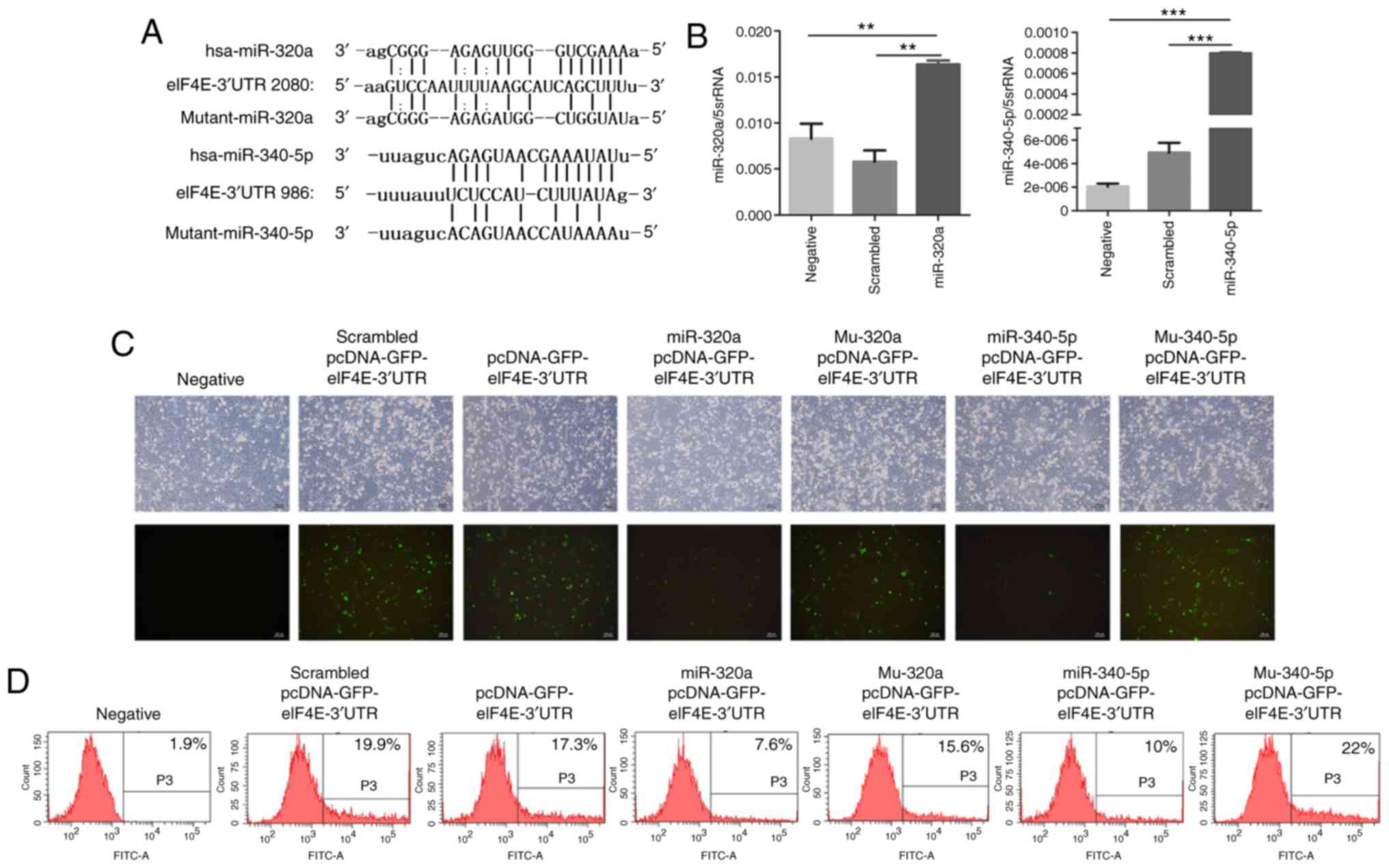
eIF4E is a direct target of miR-320a
and miR-340-5p
To investigate whether miR-320a and miR-340-5p were
involved in regulating eIF4E expression, the potential miRNAs that
target the 3′-UTR of eIF4E mRNA were determined. Based on the
results of the online miRNA analysis software TargetScan, target
sites for miR-320a and miR-340-5p were identified in the 3′-UTR of
eIF4E (Fig. 3A). Treatment of
HEC-1A cells with miR-320a or miR-340-5p mimics significantly
increased their corresponding miRNA levels, as determined by
RT-qPCR (Fig. 3B). Subsequently, a
pcDNA expression vector encoding GFP-eIF4E-3′-UTR was
co-transfected with miR-320a or miR-340-5p mimics into HEC-1A
cells. Fluorescence microscopy revealed that the fluorescence
intensity was significantly decreased in miR-320a and miR-340-5p
mimic-treated cells compared with that in controls (Fig. 3C). Flow cytometry also demonstrated
that the fluorescence decreased significantly in miR-320a and
miR-340-5p mimic-treated cultures compared with that in the control
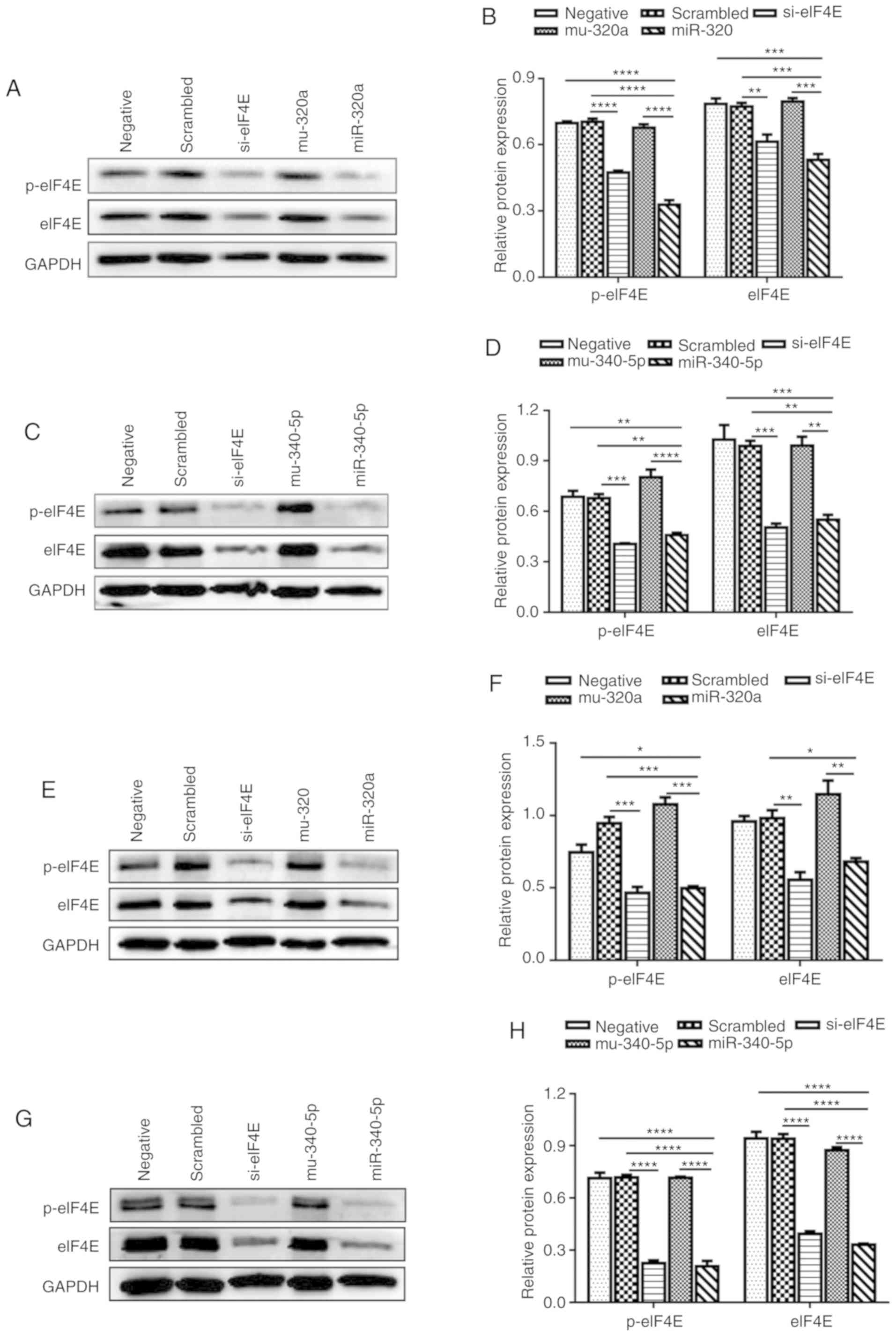
groups (Fig. 3D). Western blotting
confirmed that miR-320a and miR-340-5p mimics reduced the protein
expression levels of not only eIF4E, but also p-eIF4E in HEC-1A
cells (Fig. 4A-D). These
experiments were repeated in RL95-2 cells, which confirmed the
results obtained in HEC-1A cells (Fig.
4E-H). These results indicated that the eIF4E-3′UTR was
directly targeted by miR-320a and miR-340-5p.
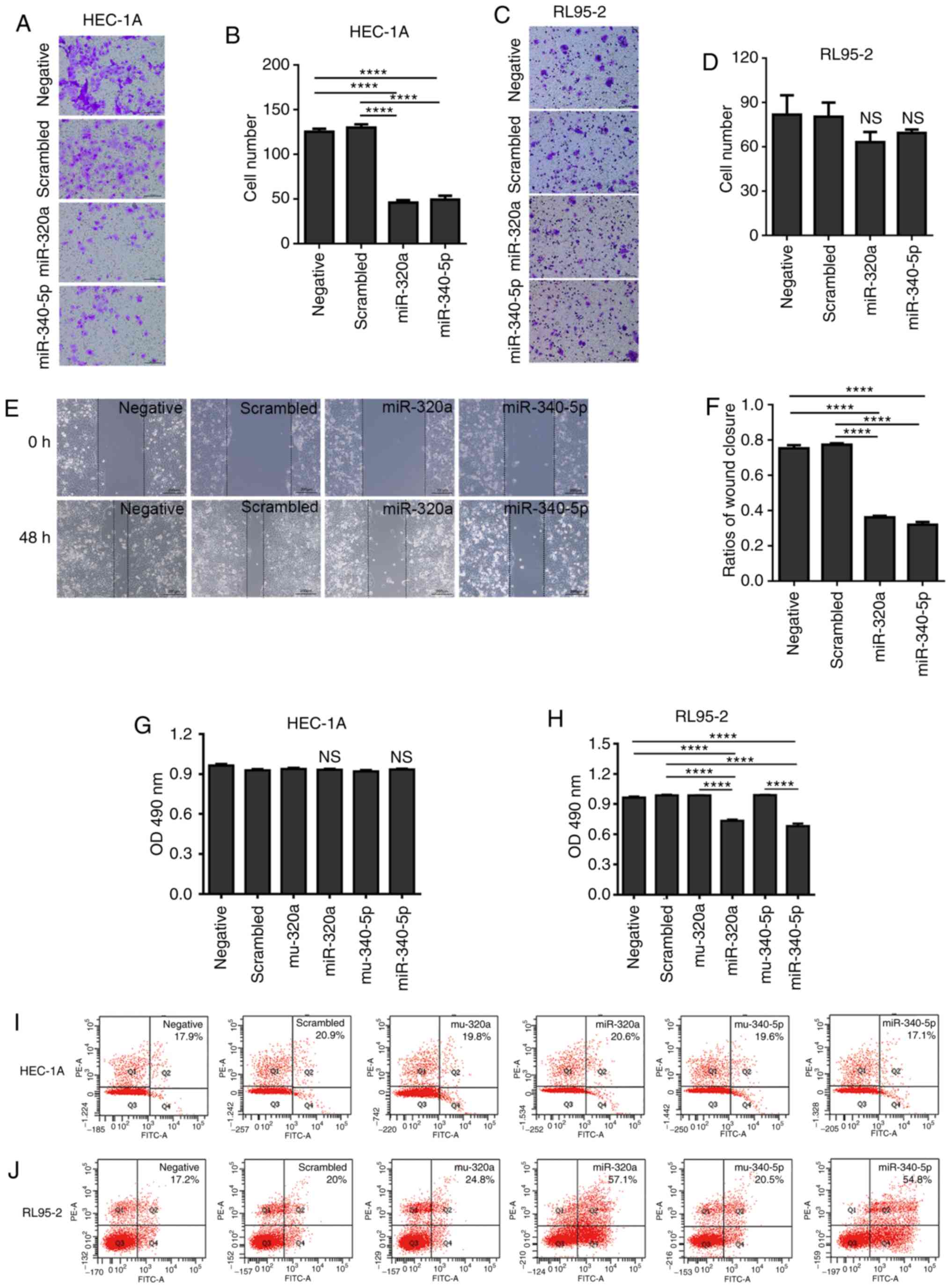
Overexpression of miR-320a or
miR-340-5p inhibits EC cell viability and migration
Considering that high expression of eIF4E is
associated with the prognosis and grade of EC, the present study
investigated whether miR-320a and miR-340-5p may reduce the
metastatic capability of EC cells. Either miR-320a or miR-340-5p
mimic treatment in HEC-1A cells reduced the number of cells that
migrated to the lower chamber in the Transwell assay; however, no
effect was observed in RL95-2 cells (Fig. 5A-D). The results of the
wound-healing assay also demonstrated that miR-320a or miR-340-5p
mimics significantly decreased the migration in HEC-1A cells
(Fig. 5E and F).
To further investigate the proliferation inhibitory
effect of miR-320a and miR-340-5p in EC cells, MTT and apoptosis
detection assays were performed. miR-320a and miR-340-5p mimics
inhibited RL95-2 cell proliferation, but did not affect HEC-1A
cells (Fig. 5G and H). In addition,
flow cytometric analysis of apoptosis indicated that miR-320a and
miR-340-5p mimics induced apoptosis in RL95-2 cells, but had no
effect on apoptosis in HEC-1A cells (Fig. 5I and J).
miR-320a and miR-340-5p mimics
suppress MMP-3 and MMP-9 expression in HEC-1A cells
MMP-3 and MMP-9 expression levels were determined to
explore the mechanisms involved in reduced cell migration and
invasion following either miR-320a or miR-340-5p mimic treatment.
As demonstrated in Fig. 6, MMP-3
and MMP-9 protein levels were attenuated, suggesting that the
reduced level of MMP-3 and MMP-9 following miR-320a or miR-340-5p
mimic treatment may account, at least in part, for the
anti-migratory effects of miR-320a and miR-340-5p mimics.
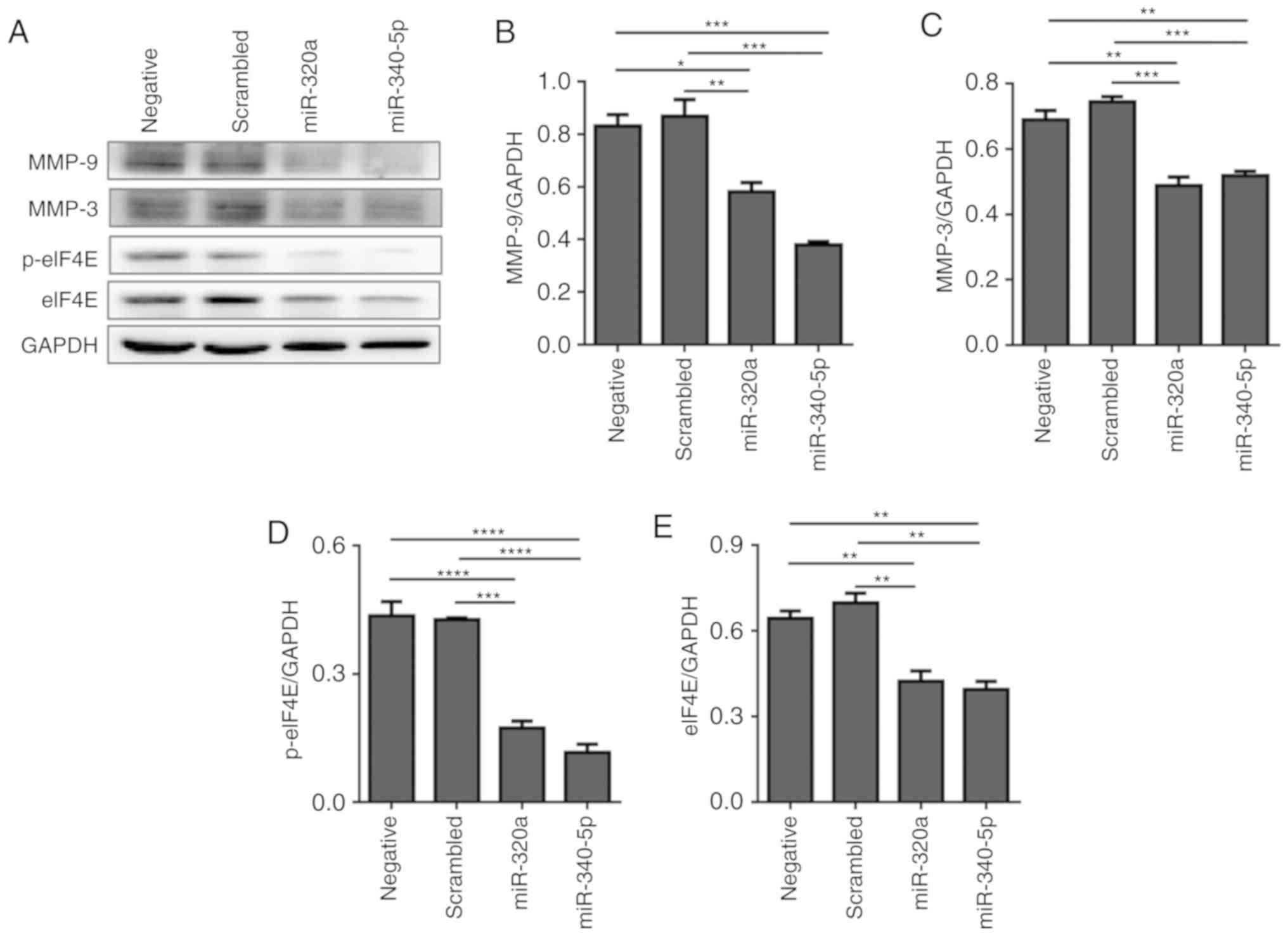 | Figure 6.miR-320a and miR-340-5p mimic
treatment suppresses the expression of MMP-3 and MMP-9 in HEC-1A
cells. (A) Western blotting analysis indicated that the expression
levels of MMP-3 and MMP-9 were downregulated following miR-320a or
miR-340-5p mimic treatment. (B-E) Quantification of (B) MMP-3, (C)
MMP-9, (D) p-eIF4E and (E) eIF4E relative expression levels (n=3).
*P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. eIF4E,
eukaryotic translation initiation factor 4E; p, phosphorylated;
miR, microRNA; MMP, matrix metallopeptidase; negative, mock
transfections; scrambled, cells treated with scrambled-oligomer
control RNA; miR-320a or miR-340-5p, cells treated with miR-320a or
miR-340-5p mimics. |
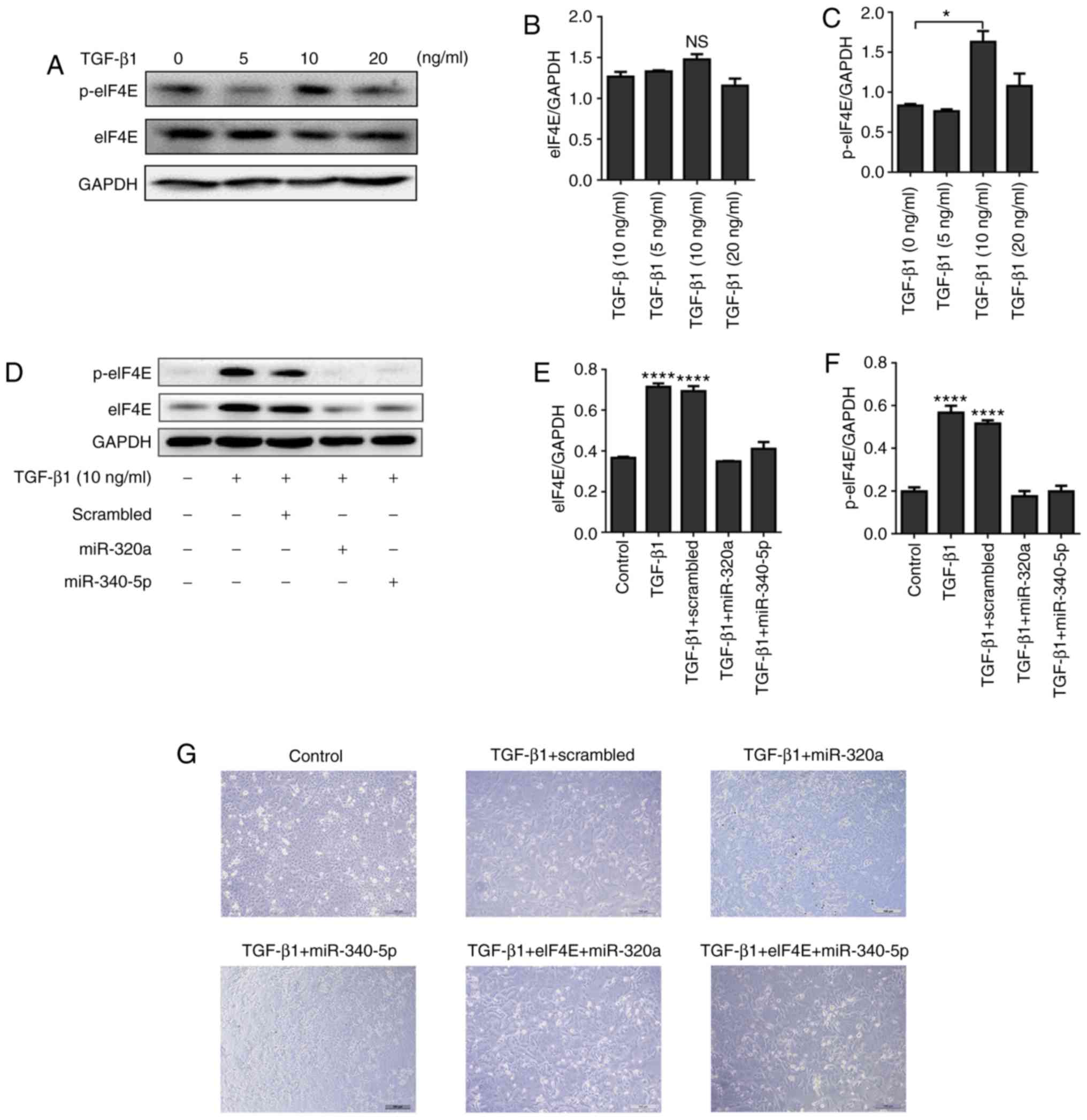
miR-320a and miR-340-5p mimics
suppress TGF-β1-induced EMT and change p-eIF4E expression in EC
cells
HEC-1A cells were treated with different
concentrations of TGF-β1 (0, 5, 10 and 20 ng/ml) for 48 h. The
expression of p-eIF4E was significantly enhanced by 10 ng/ml TGF-β1
(Fig. 7A-C), but this upregulation
was suppressed when the cells were treated with miR-320a or
miR-340-5p mimics (Fig. 7D-F). In
terms of cell morphology, following 10 ng/ml TGF-β1 treatment for
48 h, HEC-1A cells exhibited fibroblast-like features; by contrast,
a cobblestone-like appearance was observed in the control, miR-320a
mimic + TGF-β1 and miR-340-5p mimic + TGF-β1 groups (Fig. 7G). An eIF4E-encoding vector
co-transfected with either miR-320a or miR-340-5p into HEC-1A cells
blocked the effects of miR-320a and miR-340-5p on cell morphology
(Fig. 7G). To further assess the
effects of miR-320a and miR-340-5p on the biological outcomes of
EMT in HEC-1A cells, a wound-healing assay was performed; TGF-β1
promoted EC cell migration, whereas treatment with either miR-320a
or miR-340-5p mimics prevented TGF-β1-induced cell migration
(Fig. 7H and I).
miR-320a and miR-340-5p mimics
attenuate the TGF-β1- induced EMT marker expression in EC
cells
To further explore the effects of miR-320a and
miR-340-5p on the inhibition of EMT, HEC-1A cells transfected with
either miR-320a or miR-340-5p mimics were exposed to TGF-β1 for 48
h, and EMT markers were subsequently assessed by western blotting.
TGF-β1 suppressed the expression of the epithelial marker
E-cadherin and enhanced the expression of the mesenchymal markers
Snail and α-SMA (Fig. 8A-D). A
siRNA targeting eIF4E exhibited similar results to those observed
following miR-320a and miR-340-5p mimic treatments (Fig. 8E and F).
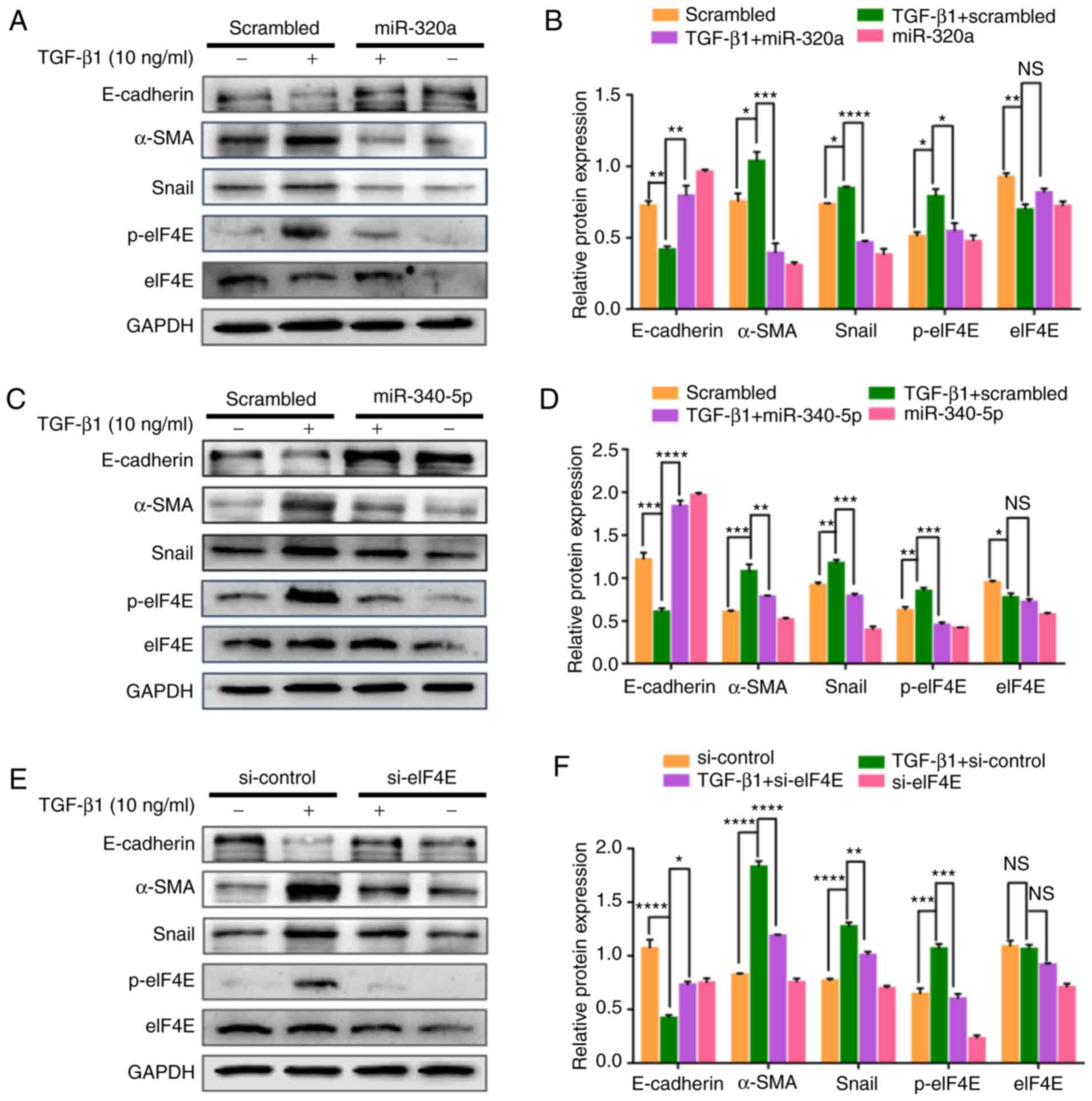 | Figure 8.miR-320a and miR-340-5p mimic
treatment suppresses TGF-β1-induced epithelial-mesenchymal
transition through the inhibition of p-eIF4E. (A, C and E) Western
blotting analysis was performed to detect E-cadherin, α-SMA, Snail,
p-eIF4E and eIF4E expression levels in HEC-1A cells transfected
with the (A) miR-320a mimic, (B) miR-340-5p mimic or (C) si-eIF4E
and treated with 10 ng/ml TGF-β1 for 48 h. (B, D and F)
Quantification of E-cadherin, α-SMA, Snail, p-eIF4E and eIF4E
relative expression levels in HEC-1A cells transfected with the (B)
miR-320a mimic, (D) miR-340-5p mimic or (E) si-eIF4E and treated
with 10 ng/ml TGF-β1 for 48 h (n=3). *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001; NS, not significant. TGF-β1,
transforming growth factor β1; eIF4E, eukaryotic translation
initiation factor 4E; miR, microRNA; α-SMA, α-smooth muscle actin;
p, phosphorylated; scrambled, cells treated with scrambled control
RNA; miR-320a or miR-340-5p, cells treated with miR-320a or
miR-340-5p mimics; si-eIF4E or si-control, cells treated with small
interfering RNA specific to eIF4E or siRNA-control. |
Discussion
EC is the most common type of female reproductive
system cancer and accounts for 4.8% of all cancers diagnosed in
women (1). The mechanisms
underlying metastasis, which is the main cause of EC treatment
failure, have not been elucidated. Thus, a clearer understanding of
metastasis is critical for the development of new therapeutic
strategies for treating EC.
The miRNA-mediated regulation has complex cellular
outcomes, as miRNAs can be involved in cell proliferation,
apoptosis, invasion and migration (22,23).
First identified as a potential modulator of aquaporin 1 and
aquaporin 4, miR-320a also serves a role in cerebral ischemia
(24). In metastatic colon cancer
tissues and cells within the liver, miR-320a is downregulated, and
its levels are associated with tumor progression in colorectal
cancer (25). Additionally,
miR-320a inhibits the proliferation of human colon cancer cells by
directly targeting β-catenin (26).
In non-small cell lung cancer, miR-320a suppresses cell migration
and invasion through the PI3K/Akt signaling pathway by inhibiting
the expression of E74-like ETS transcription factor 3 (16). Another miRNA that plays a role in a
variety of tumors is miR-340-5p (also termed miR-340). In cervical
cancer, miR-340 expression is downregulated compared with that in
normal tissues, and miR-340 inhibits cervical cancer metastasis
through targeting Ephrin type-A receptor 3 (27). In breast cancer, miR-340-5p inhibits
the proliferation and drug resistance and increases the apoptosis
of breast cancer cells by downregulating the expression of
leucine-rich repeat-containing G protein-coupled receptor 5 via the
Wnt/β-catenin pathway (28). In the
present study, miR-320a and miR-340-5p expression levels were
downregulated in EC tissues compared with those in adjacent normal
tissues, and miR-320a or miR-340-5p mimics inhibited the migration
and invasion of EC cells in vitro by downregulating eIF4E.
In addition, miR-320a and miR-340-5p mimics exhibited different
effects on different EC cell lines; HEC-1A migration was
significantly affected by miR-320a or miR-340-5p, whereas RL95-2
cell migration was not. By contrast, miR-320a or miR-340-5p mimics
exhibited effects on RL95-2 cell proliferation and apoptosis, which
may have been a result of the different degrees of differentiation
and different phenotypic characteristics of the two EC cell lines.
Upregulation of eIF4E, a translation initiation factor, has been
previously detected in many human tumors. Specifically, eIF4E has
been associated with disease progression, cellular transformation,
tumorigenesis and metastasis in experimental models (29). Choi et al (7) first reported that the positive rate of
eIF4E expression is higher in metastatic EC and promotes the
proliferation of HEC-1A cells in vitro. Additionally,
another study demonstrated that eIF4E levels were higher in EC
specimens compared with those in hyperplastic or normal endometrial
tissue specimens (30). In the
present study, analysis of the Oncomine database revealed that high
eIF4E expression was associated with a poor prognosis and a high
pathological grade of EC.
The results of the present study demonstrated that
either miR-320a or miR-340-5p mimics downregulated the expression
levels of MMP-3 and MMP-9 by targeting eIF4E. MMP-3 has been
reported to be involved in vascular invasion and metastasis of EC
through EMT (31). The translation
of MMP-9 mRNA has been demonstrated to be exceptionally dependent
on elevated eIF4F activity (32).
MMP-9 is a target of eIF4E, and its upregulation is associated with
vascular and lymphatic invasion in EC (33). Considering the results of the
present study, it may be hypothesized that the inhibition of MMP-3
and MMP-9 expression may represent one of the mechanisms by which
miR-320 and miR-340-5p prevent the invasion and migration of EC
cells. This process may also be related to other mechanisms; in
EMT, benign tumor cells acquire the capacity of invasion and
metastasis and can infiltrate the surrounding tissues and
eventually move to distant regions (34). During EMT and tumor invasion, eIF4E
has been identified to serve important roles, as it has been
demonstrated to regulate the expression of a number of proteins
involved in EMT and metastasis, such as MMP-3 and Snail (35). Smith et al (9) demonstrated that overexpression of
eIF4E induced EMT in lung epithelial cells. TGF-β is a major
regulator of the EMT (34). In the
present study, exogenous TGF-β1-induced EMT of HEC-1A cells was
accompanied by an upregulation of p-eIF4E. These results suggested
that eIF4E may promote a metastatic phenotype of EC, in part by
regulating EMT. It was thus predicted that suppression of eIF4E may
inhibit EMT in EC; transfection with either miR-320a or miR-340-5p
mimics in EC cells prevented TGF-β1-induced changes in cell
morphology and the upregulation of p-eIF4E. In addition, the
expression of Snail was attenuated by miR-320a and miR-340-5p
mimics. Snail, which is a key regulator of EMT, induces epithelial
cells with migratory and invasive properties during tumor
progression (36), and several
studies have confirmed that Snail stimulates invasion and
metastasis of EC (37,38). Robichaud et al (35) have demonstrated that Snail is
regulated by p-eIF4E. The results of the present study demonstrated
that downregulation of Snail by either miR-320a/p-eIF4E or
miR-40-5p/p-eIF4E may represent a part of the mechanism underlying
the prevention of TGF-β1-induced EMT. In addition, the
TGF-β1-induced downregulation of E-cadherin and upregulation of
α-SMA were prevented in miR-320a or miR-340-5p mimic-treated
cells.
In conclusion, the results of the present study
demonstrated that in EC, eIF4E was upregulated, whereas miR-320a
and miR-340-5p were downregulated. Specifically, miR-320a and
miR-340-5p mimics inhibited the proliferation and migration of EC
cells in vitro by downregulating MMP-3 and MMP-9 expression
and prevented the TGF-β1-induced EMT by targeting p-eIF4E. These
results suggested that miR-320a and miR-340-5p may be potential
therapeutic targets for EC treatment.
Acknowledgements
Not applicable.
Funding
This study was supported by National Natural
Scientific Grants (grant no. 31570798 and 31971209), Liaoning
Excellent Talents in University (grant no. LR2017042), Liaoning
Provincial Program for Top Discipline of Basic Medical Sciences and
the Shandong Science and Technology Committee (grant no.
2018GSF118056).
Availability of data and materials
The datasets used or analyzed in the present study
are available from the corresponding author upon reasonable
request.
Authors' contributions
HHZ and YK conceived and designed the experiments.
HHZ, RL, YJL, XXY, QNS, and AYL performed the experiments. HHZ and
YK analyzed the data and wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All experiments with human specimens were performed
in accordance with the relevant guidelines and were approved by the
Medical Ethics Committee of Binzhou Medical University (Yantai,
China). Prior to study inclusion, written informed consent was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
miRNA
|
microRNA
|
|
EC
|
endometrial cancer
|
|
eIF4E
|
eukaryotic translation initiation
factor 4E
|
|
3′-UTR
|
3′-untranslated region
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5diphenyltetrazolium bromide
|
|
TGF-β1
|
transforming growth factor β1
|
|
EMT
|
epithelial- mesenchymal transition
|
References
|
1
|
Van Nyen T, Moiola CP, Colas E, Annibali D
and Amant F: Modeling endometrial cancer: Past, present, and
future. Int J Mol Sci. 19:E23482018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morice P, Leary A, Creutzberg C,
Abu-Rustum N and Darai E: Endometrial cancer. Lancet.
387:1094–1108. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jackson RJ, Hellen CU and Pestova TV: The
mechanism of eukaryotic translation initiation and principles of
its regulation. Nat Rev Mol Cell Biol. 11:113–127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Benedetti A and Graff JR: eIF-4E
expression and its role in malignancies and metastases. Oncogene.
23:3189–3199. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pettersson F, Yau C, Dobocan MC,
Culjkovic-Kraljacic B, Retrouvey H, Puckett R, Flores LM, Krop IE,
Rousseau C, Cocolakis E, et al: Ribavirin treatment effects on
breast cancers overexpressing eIF4E, a biomarker with prognostic
specificity for luminal B-type breast cancer. Clin Cancer Res.
17:2874–2884. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berkel HJ, Turbat-Herrera EA, Shi R and de
Benedetti A: Expression of the translation initiation factor eIF4E
in the polyp-cancer sequence in the colon. Cancer Epidemiol
Biomarkers Prev. 10:663–666. 2001.PubMed/NCBI
|
|
7
|
Choi CH, Lee JS, Kim SR, Lee YY, Kim CJ,
Lee JW, Kim TJ, Lee JH, Kim BG and Bae DS: Direct inhibition of
eIF4E reduced cell growth in endometrial adenocarcinoma. J Cancer
Res Clin Oncol. 137:463–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng H and Kang Y: Multilayer control of
the EMT master regulators. Oncogene. 33:1755–1763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smith KA, Zhou B, Avdulov S, Benyumov A,
Peterson M, Liu Y, Okon A, Hergert P, Braziunas J, Wagner CR, et
al: Transforming growth factor-β1 induced epithelial mesenchymal
transition is blocked by a chemical antagonist of translation
factor eIF4E. Sci Rep. 5:182332015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spaderna S, Schmalhofer O, Wahlbuhl M,
Dimmler A, Bauer K, Sultan A, Hlubek F, Jung A, Strand D, Eger A,
et al: The transcriptional repressor ZEB1 promotes metastasis and
loss of cell polarity in cancer. Cancer Res. 68:537–544. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Xie Y, Cui D, Ma Y, Sui L, Zhu C,
Kong H and Kong Y: Osteopontin promotes invasion, migration and
epithelial-mesenchymal transition of human endometrial carcinoma
cell HEC-1A through AKT and ERK1/2 signaling. Cell Physiol Biochem.
37:1503–1512. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aparicio LA, Abella V, Valladares M and
Figueroa A: Posttranscriptional regulation by RNA-binding proteins
during epithelial-to-mesenchymal transition. Cell Mol Life Sci.
70:4463–4477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He M and Xue Y: MicroRNA-148a suppresses
proliferation and invasion potential of non-small cell lung
carcinomas via regulation of STAT3. Onco Targets Ther.
10:1353–1361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang HH, Pang M, Dong W, Xin JX, Li YJ,
Zhang ZC, Yu L, Wang PY, Li BS and Xie SY: miR-511 induces the
apoptosis of radioresistant lung adenocarcinoma cells by triggering
BAX. Oncol Rep. 31:1473–1479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao W, Sun Q, Yu Z, Mao S, Jin Y, Li J,
Jiang Z, Zhang Y, Chen M, Chen P, et al: MiR-320a-3p/ELF3 axis
regulates cell metastasis and invasion in non-small cell lung
cancer via PI3K/Akt pathway. Gene. 670:31–37. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ge X, Cui H, Zhou Y, Yin D, Feng Y, Xin Q,
Xu X, Liu W, Liu S and Zhang Q: miR-320a modulates cell growth and
chemosensitivity via regulating ADAM10 in gastric cancer. Mol Med
Rep. 16:9664–9670. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu X, Tang H, Liu G, Wang H, Shu J and Sun
F: miR-448 suppressed gastric cancer proliferation and invasion by
regulating ADAM10. Tumour Biol. 37:10545–10551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou SJ, Liu FY, Zhang AH, Liang HF, Wang
Y, Ma R, Jiang YH and Sun NF: MicroRNA-199b-5p attenuates
TGF-β1-induced epithelial-mesenchymal transition in hepatocellular
carcinoma. Br J Cancer. 117:233–244. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu Y, Luo W, Yang ZJ, Chi JR, Li YR, Ding
Y, Ge J, Wang X and Cao XC: miR-190 suppresses breast cancer
metastasis by regulation of TGF-β-induced epithelial-mesenchymal
transition. Mol Cancer. 17:702018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng AM, Byrom MW, Shelton J and Ford LP:
Antisense inhibition of human miRNAs and indications for an
involvement of miRNA in cell growth and apoptosis. Nucleic Acids
Res. 33:1290–1297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baranwal S and Alahari SK: miRNA control
of tumor cell invasion and metastasis. Int J Cancer. 126:1283–1290.
2010.PubMed/NCBI
|
|
24
|
Sepramaniam S, Armugam A, Lim KY, Karolina
DS, Swaminathan P, Tan JR and Jeyaseelan K: MicroRNA 320a functions
as a novel endogenous modulator of aquaporins 1 and 4 as well as a
potential therapeutic target in cerebral ischemia. J Biol Chem.
285:29223–29230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang Y, He X, Liu Y, Ye Y, Zhang H, He P,
Zhang Q, Dong L, Liu Y and Dong J: microRNA-320a inhibits tumor
invasion by targeting neuropilin 1 and is associated with liver
metastasis in colorectal cancer. Oncol Rep. 27:685–694.
2012.PubMed/NCBI
|
|
26
|
Sun JY, Huang Y, Li JP, Zhang X, Wang L,
Meng YL, Yan B, Bian YQ, Zhao J, Wang WZ, et al: MicroRNA-320a
suppresses human colon cancer cell proliferation by directly
targeting β-catenin. Biochem Biophys Res Commun. 420:787–792. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiao H, Yu L, Li F, Wang H, Li W and He X:
MiR-340 suppresses the metastasis by targeting EphA3 in cervical
cancer. Cell Biol Int. 42:1115–1123. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi S, Chen X, Liu H, Yu K, Bao Y, Chai J,
Gao H and Zou L: LGR5 acts as a target of miR-340-5p in the
suppression of cell progression and drug resistance in breast
cancer via Wnt/β-catenin pathway. Gene. 683:47–53. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Graff JR, Konicek BW, Carter JH and
Marcusson EG: Targeting the eukaryotic translation initiation
factor 4E for cancer therapy. Cancer Res. 68:631–634. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shi ZM, Liu YN, Fu B, Shen YF and Li LM:
Expression profile of eukaryotic translation initiation factor and
matrix metalloproteinase 9 in endometrial cancer tissue. J Biol
Regul Homeost Agents. 31:1053–1059. 2017.PubMed/NCBI
|
|
31
|
Mannelqvist M, Stefansson IM, Bredholt G,
Hellem Bø T, Oyan AM, Jonassen I, Kalland KH, Salvesen HB and
Akslen LA: Gene expression patterns related to vascular invasion
and aggressive features in endometrial cancer. Am J Pathol.
178:861–871. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Konicek BW, Dumstorf CA and Graff JR:
Targeting the eIF4F translation initiation complex for cancer
therapy. Cell Cycle. 7:2466–2471. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Karahan N, Guney M, Baspinar S, Oral B,
Kapucuoglu N and Mungan T: Expression of gelatinase (MMP-2 and
MMP-9) and cyclooxygenase-2 (COX-2) in endometrial carcinoma. Eur J
Gynaecol Oncol. 28:184–188. 2007.PubMed/NCBI
|
|
34
|
Lee JM, Dedhar S, Kalluri R and Thompson
EW: The epithelial-mesenchymal transition: New insights in
signaling, development, and disease. J Cell Biol. 172:973–981.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Robichaud N, del Rincon SV, Huor B, Alain
T, Petruccelli LA, Hearnden J, Goncalves C, Grotegut S, Spruck CH,
Furic L, et al: Phosphorylation of eIF4E promotes EMT and
metastasis via translational control of SNAIL and MMP-3. Oncogene.
34:2032–2042. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vega S, Morales AV, Ocaña OH, Valdes F,
Fabregat I and Nieto MA: Snail blocks the cell cycle and confers
resistance to cell death. Genes Dev. 18:1131–1143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dragomirescu M, Stepan AE, Margaritescu C
and Simionescu CE: The immunoexpression of p53 and snail in
endometrioid endometrial carcinomas. Rom J Morphol Embryol.
59:131–137. 2018.PubMed/NCBI
|
|
38
|
Xiong S, Klausen C, Cheng JC and Leung PC:
Activin B promotes endometrial cancer cell migration by
down-regulating E-cadherin via SMAD-independent MEK-ERK1/2-SNAIL
signaling. Oncotarget. 7:40060–40072. 2016. View Article : Google Scholar : PubMed/NCBI
|