Introduction
Epidermal growth factor receptor (EGFR) mutations
have been reported to play a vital role in the oncogenesis of
non-small cell lung cancer (NSCLC). Exon 19 deletion and exon 21
L858R point mutations are the most common mutations associated with
a positive response to first- and second-generation EGFR-tyrosine
kinase inhibitors (EGFR-TKIs), and improved progression-free
survival, compared with conventional chemotherapy (1). However, although patients show an
initial response to EGFR-TKIs, the development of acquired
resistance may appear after 9–14 months (2). The mechanisms underlying acquired
resistance to first- and second-generation EGFR-TKIs include
EGFR T790M secondary mutation, MET amplification,
IGF1R activation, HGF overexpression, BRAF
V600E mutation and epithelial-mesenchymal transition (EMT)
(3–5). Among them, the acquired T790M
mutation is the most common mechanism that accounts for more than
50% of resistant cases. Osimertinib, a third-generation EGFR-TKI,
was developed to overcome T790M-positive NSCLC that obtained
acquired resistance to EGFR-TKIs. Currently, osimertinib is the
only drug approved by the Food and Drug Administration for
T790M-positive NSCLC treatment, but with high financial cost
(6). On the other hand, resistance
to osimertinib has been reported (7). Such findings weaken the support for
the use of osimertinib for T790M-positive NSCLC treatment and limit
the efficacy and application of this drug. Therefore, it is
necessary to search for an anti-T790M-positive NSCLC agent that
exhibits high efficacy and low economic cost to the patient.
The development of anticancer agents from herbs has
emerged as a novel strategy for potential cancer treatment and
these agents show desirable efficacy with fewer adverse effects and
low cost. These agents have been reported to present specific
anti-proliferative, chemo-sensitizing or radio-sensitizing effects
in various types of malignancies by targeting multiple signaling
pathways (8–18). Hyperoside, a flavonol glycoside
compound, extracted from Hypericum perforatum, is cultivated
worldwide. Hyperoside has been studied extensively due to its
anti-inflammatory, anti-oxidative, analgesic and anticancer
activities. Hyperoside has been reported to inhibit the growth of a
variety of malignancies, including lung cancer, colorectal cancer,
pancreatic cancer, renal cancer, ovarian cancer, prostate cancer
and osteosarcoma, without severe side effects and drug resistance
(19–25).
Recent studies have demonstrated that hyperoside
inhibited lung cancer cell proliferation by inducing cell cycle
arrest, autophagy and apoptosis through multiple signaling pathways
(26,27). However, to the best of our
knowledge, the anticancer effect of hyperoside on NSCLC with
T790M mutation, and the underlying molecular mechanisms,
have not been previously investigated. The present study
investigated the anticancer activity of hyperoside in
T790M-positive NSCLC cells and a xenograft model, and aimed to
elucidate the underlying molecular mechanisms. In addition, the
anticancer potential of hyperoside as a novel candidate for
T790M-positive NSCLC treatment was investigated, and the associated
target signaling pathway was identified.
Materials and methods
Drugs and cell lines
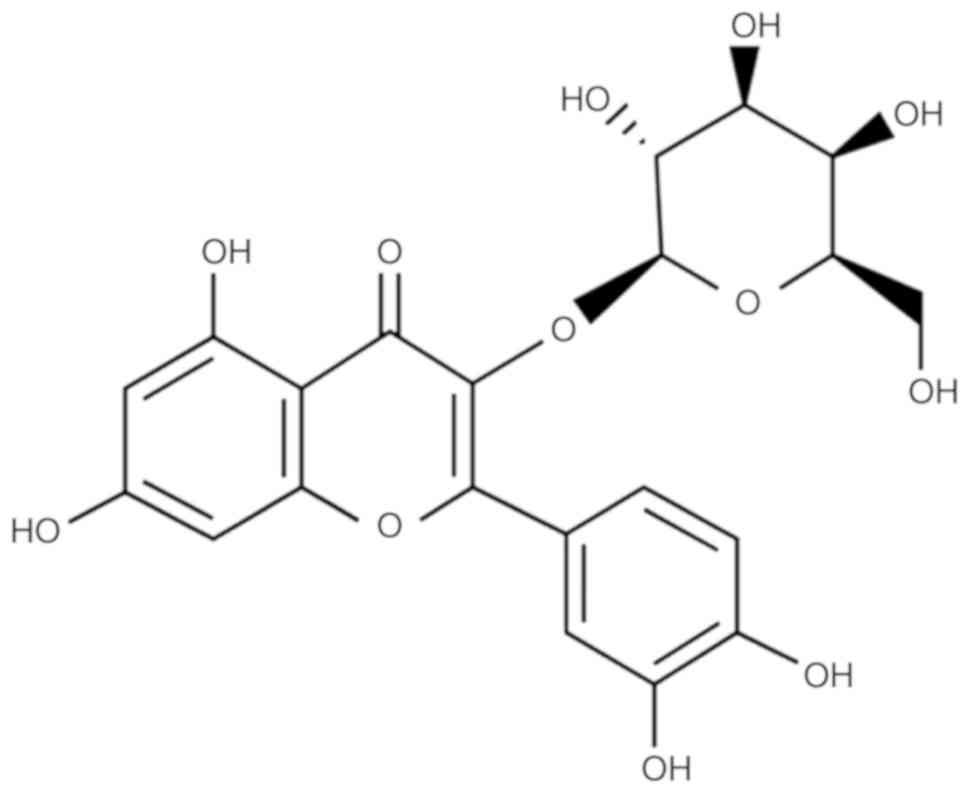
Hyperoside
(C21H20O12) (Fig. 1) was obtained from Sigma-Aldrich;
Merck KGaA (batch no. 00180585). The adenocarcinoma lung cancer
cell line PC-9 and the T790M-positive NSCLC cell line NCI-H1975
were obtained from the Cell Bank of the Chinese Academy of Science
(Shanghai, China). Cells were maintained in RPMI-1640 medium
supplemented with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc.) in a humidified atmosphere containing 5%
CO2 at 37°C (seeding density: 4×104
cells/cm2, subculture every 4–5 days, 1:5 split).
Cell viability assay
Cells were plated into 96-well plates at a density
of 5×103 cells per well. After incubation with different
concentrations of hyperoside (0, 30, 60, 90, 120 and 150 µM) for
24, 48 and 72 h, MTT reagent (Sigma-Aldrich; Merck KGaA) was added
and the cells were incubated for another 4 h. The supernatant was
then replaced by dimethyl sulfoxide and the absorbance was detected
at 490 nm using a microplate reader (Thermo Fisher Scientific,
Inc.). Cell viability curves were generated.
For the clonogenic assay, cells were plated into
6-well plates at a density of 1×103 cells per well.
Cells were treated with hyperoside (0, 30, 60, 90, 120 and 150 µM)
for 48 h and further cultured in a humidified atmosphere containing
5% CO2 at 37°C for 14 days. The colonies were then fixed
with paraformaldehyde prior to 0.5% crystal violet staining for 30
min at room temperature. Colonies were counted using an inverted
light microscope (magnification, ×200; Olympus Corp.).
Apoptosis analysis
Cells were plated into 6-well plates at a density of
1×104 cells per well. After incubation with different
concentrations of hyperoside (0, 30, 60, 90, 120 and 150 µM) for 48
h, the cells were trypsinized, washed and collected for apoptosis
detection by flow cytometry. Annexin V-FITC and propidium iodide
(Sigma-Aldrich; Merck KGaA) were added and the cells were incubated
in the dark at 37°C for 15 min. Cell apoptotic rates were detected
using a FACSCalibur flow cytometer.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells and xenograft
tumor specimens using TRIzol® reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
total RNA was reverse transcribed into complementary DNA using a
PrimeScript RT reagent kit (Takara). PCR amplification was
performed using a SYBR premix Taq kit (Takara). The primer
sequences used were as follows: CCAT1: Forward,
5′-CATTGGGAAAGGTGCCGAGA-3′ and reverse, 5′-ACGCTTAGCCATACAGAGCC-3′;
FoxO1: Forward, 5′-AGGATCCGATGTCACCATGGCCG-3′ and reverse,
5′-AAAGGATCCACCATGGCCG-3′. Amplification conditions for relative
expression analysis were as follows: Denaturation at 95°C for 2
min; 40 cycles of 98°C for 20 sec, 55°C for 20 sec, and 68°C for 30
sec, and finally extension at 72°C for 4 min. All RT-qPCR reactions
were performed using the ABI StepOne™ Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The relative gene
expression was calculated using the 2−ΔΔCq method and
normalized to β-actin (28).
Western blot analysis
Total protein was extracted from cells with RIPA
buffer. The protein concentration was quantified using a BCA
Protein Assay kit. Equal amounts of protein were separated by 10%
SDS-PAGE gel electrophoresis and transferred to polyvinylidene
difluoride membranes. The membranes were blocked in TBST with 5%
non-fat milk at 37°C for 2 h, followed by incubation with primary
antibody (FoxO1; C29H4; rabbit monoclonal antibody; cat. no. 2880;
dilution 1:1,000; Cell Signaling Technology, Inc.) at 4°C
overnight. The membranes were washed with TBST and further
incubated with horseradish peroxidase-conjugated secondary antibody
(anti-rabbit IgG, cat. no. 7074; dilution 1:10,000; Cell Signaling
Technology, Inc.) at 37°C for 1 h. Enhanced chemiluminescence was
used to detect protein bands (Image Lab version 5.2; Bio-Rad
Laboratories, Inc.). GAPDH was used as the endogenous control.
Cell transfection
Short hairpin RNA (shRNA) that specifically targets
lncRNA colon cancer associated transcript 1 (CCAT1) or forkhead box
protein O1 (FoxO1) (shCCAT1, shFoxO1) and amplified full-length
CCAT1 or FoxO1 cDNA for overexpression (CCAT1, FoxO1) were
synthesized by Genechem. The primer sequences used were as follows:
shCCAT1-1, CCATTCCATTCATTTCTCTTTCCTA and shCCAT1-2,
CAUACCAAUUGAACCGAGCCUUGUA; shFoxO1, GCTGCATGCTACCACCTTACA. The
cells in the logarithmic growth phase were collected and then
cultured in 6-well plates for transfection. Cell transfection was
performed using lentivirus according to the manufacturer's
protocols. Cells were transfected with lentiviral plasmid and
particles (Sino Biological, Inc.), and then harvested for further
evaluation at 48 h after transfection. RT-qPCR was performed in
order to determine the transfection efficiency.
Animal experiment
Ten nude male mice of 4 weeks of age (weight 20±1 g)
were purchased from SLAC Laboratory Animal, Co. H1975 cells, at a
density of 1×106, were collected and subcutaneously
injected into the flank of mice to form xenograft tumors. The mice
were were randomly divided into a hyperoside group and a control
group, and injected with hyperoside (25 mg/kg) once daily for 3
weeks, or injected with saline intraperitoneally once daily. The
tumor volume was calculated as: Volume (mm3) =
width2 (mm2) × length (mm) ÷ 2. Mice were
sacrificed by cervical dislocation after 3 weeks, and the tumors
were removed and weighed. The animal experimental procedures were
approved by the Ethics Committee of Zhejiang Hospital and were in
accordance with the National Institutes of Health Guidelines for
Animal Care and Use (https://www.nap.edu/read/5140/chapter/1).
Immunohistochemistry
Briefly, tumor specimen sections were cut into
4-µm-thick sections and used for immunohistochemistry staining
according to the manufacturer's protocol (Cell Signaling
Technology, Inc.). Sections were deparaffinized and rehydrated.
Incubation with the primary antibody (FoxO1; C29H4; rabbit
monoclonal antibody; cat. no. 2880; dilution 1:1,00; Cell Signaling
Technology, Inc.) was performed at 4°C overnight, and the sections
were then incubated with secondary antibody
[SignalStain® Boost IHC Detection Reagent (HRP, rabbit)
cat. no. 8114; Cell Signaling Technology, Inc.)] for 30 min at room
temperature, followed by DAB staining. Positive cells that
exhibited brownish-yellow or tan coloring were scored (the staining
intensity was scored as 0 (negative-weak), 1 (medium), 2 (strong),
or 3 (very strong). The percentage of the staining area was scored
as 0 (0–10%), 1 (11–50%), and 2 (51–100%) relative to the total
tumor area) and observed under light microscopy (magnification,
×200; Olympus Corp.).
Statistical analysis
The data are presented as the mean ± standard
deviation. One-way ANOVA followed by SNK-q post hoc test was
performed using SPSS software (version 17.0; SPSS Inc.). P<0.05
was considered to indicate a statistically significant
difference.
Results
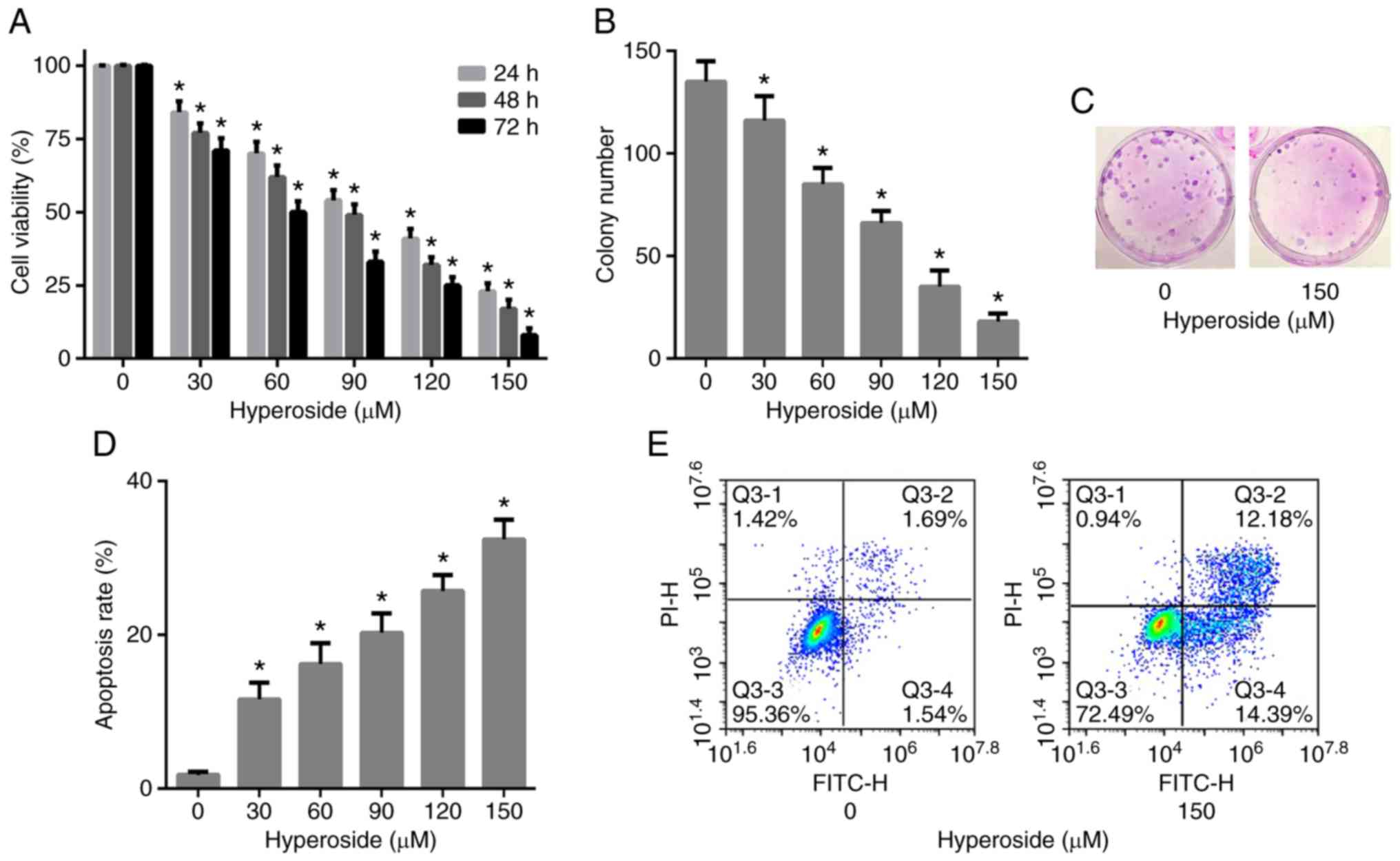
Hyperoside inhibits the proliferation
of T790M-positive NSCLC cells
H1975 cells were exposed to increasing doses of
hyperoside (0–150 µM) for 24, 48 and 72 h and the cell viability
was assessed by MTT assay. The viability of H1975 cells was
significantly inhibited following hyperoside treatment in a dose-
and time-dependent manner (Fig.
2A). The IC50 values of hyperoside at 24, 48 and 72
h were 104.1, 87.4 and 70.6 µM, respectively. Clonogenic assay was
further performed to confirm the anti-proliferative activity of
hyperoside, and the results revealed that hyperoside significantly
inhibited the clonogenic ability of H1975 cells in a dose-dependent
manner at 48 h (Fig. 2B and C).
These data indicated that hyperoside effectively inhibited the
growth of T790M-positive NSCLC.
Hyperoside induces the apoptosis of
T790M-positive NSCLC cells
Flow cytometric analysis was performed in order to
quantify the cellular apoptosis of H1975 cells induced by
hyperoside. H1975 cells were exposed to increasing doses of
hyperoside (0–150 µM) for 48 h, stained with Annexin V and PI and
subjected to flow cytometry. The results revealed that hyperoside
treatment led to a significant increase in the apoptosis rate in a
dose-dependent manner when compared with the control group
(Fig. 2D and E). These findings
indicated that cell proliferation suppression by hyperoside was
associated with the induction of apoptosis.
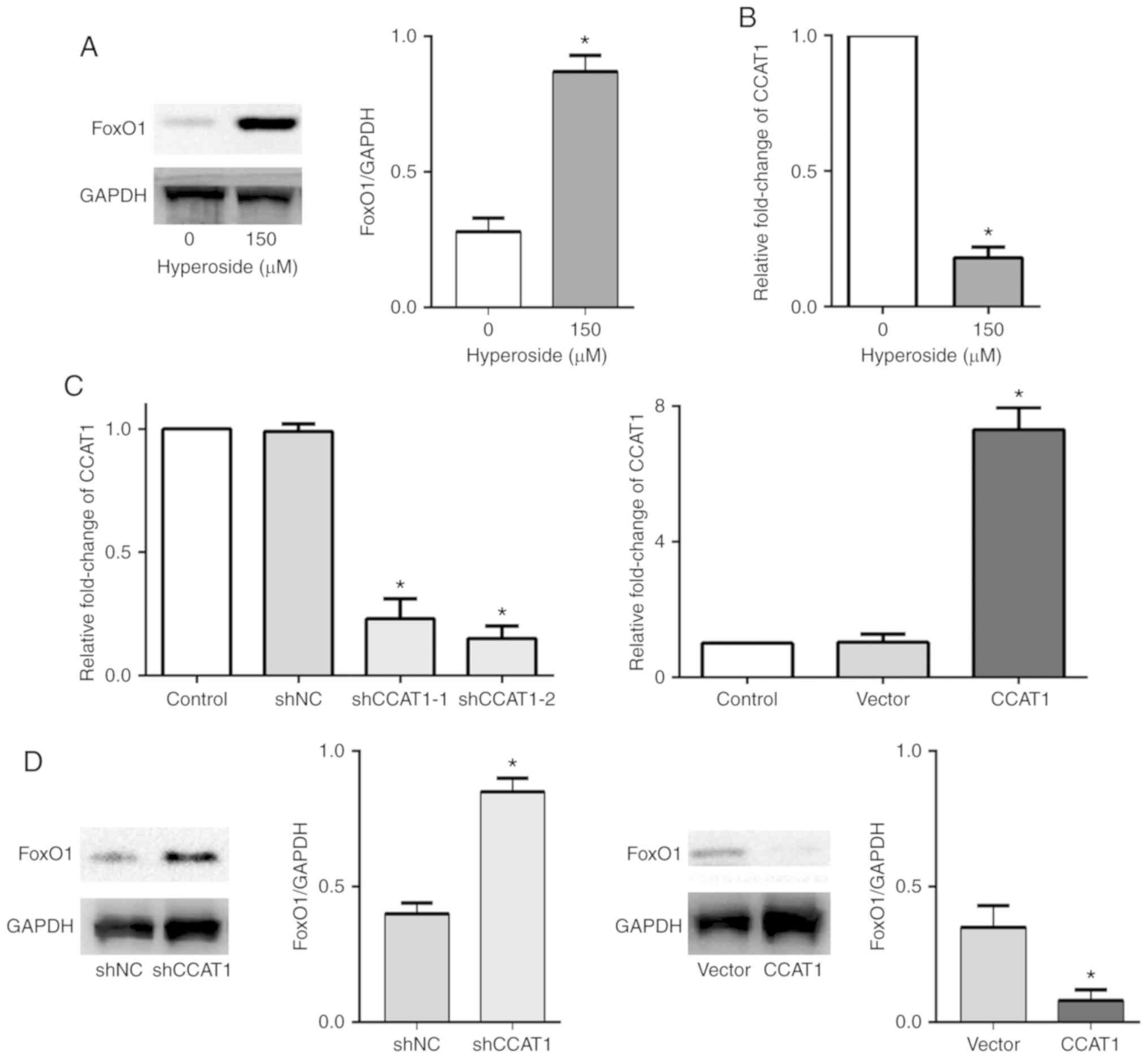
Hyperoside upregulates FoxO1
expression and downregulates the level of CCAT1 in T790M-positive
NSCLC cells
In order to investigate the potential mechanisms
underlying hyperoside in suppressing proliferation and inducing
apoptosis in H1975 cells, western blotting and RT-qPCR assays were
performed to examine the forkhead box protein O1 (FoxO1) protein
expression levels and the level of long non-coding RNA (lncRNA)
colon cancer associated transcript 1 (CCAT1). FoxO1 is a key
protein that plays a crucial role in tumor cell apoptosis, and the
results of the present study revealed that the expression of FoxO1
was downregulated and CCAT1 was upregulated in the T790M-positive
H1975 cells compared with the wild-type PC-9 cells (Fig. S1). However, FoxO1 protein
expression was observed to be upregulated following hyperoside (150
µM) treatment (Fig. 3A) in the
H1975 cells, demonstrating that hyperoside-induced apoptosis was
associated with FoxO1 upregulation. Meanwhile, hyperoside (150 µM)
significantly downregulated the level of CCAT1 expression at 48 h
(Fig. 3B). The present study
further investigated whether CCAT1 regulates FoxO1 expression in
T790M-positive H1975 cells. CCAT1 knockdown or overexpressing H1975
cells were established and the CCAT1 expression level was
determined (Fig. 3C). It was
revealed that FoxO1 protein expression was upregulated in the
CCAT1-knockdown H1975 cells, while FoxO1 protein expression was
downregulated in the CCAT1-overexpressing H1975 cells (Fig. 3D).
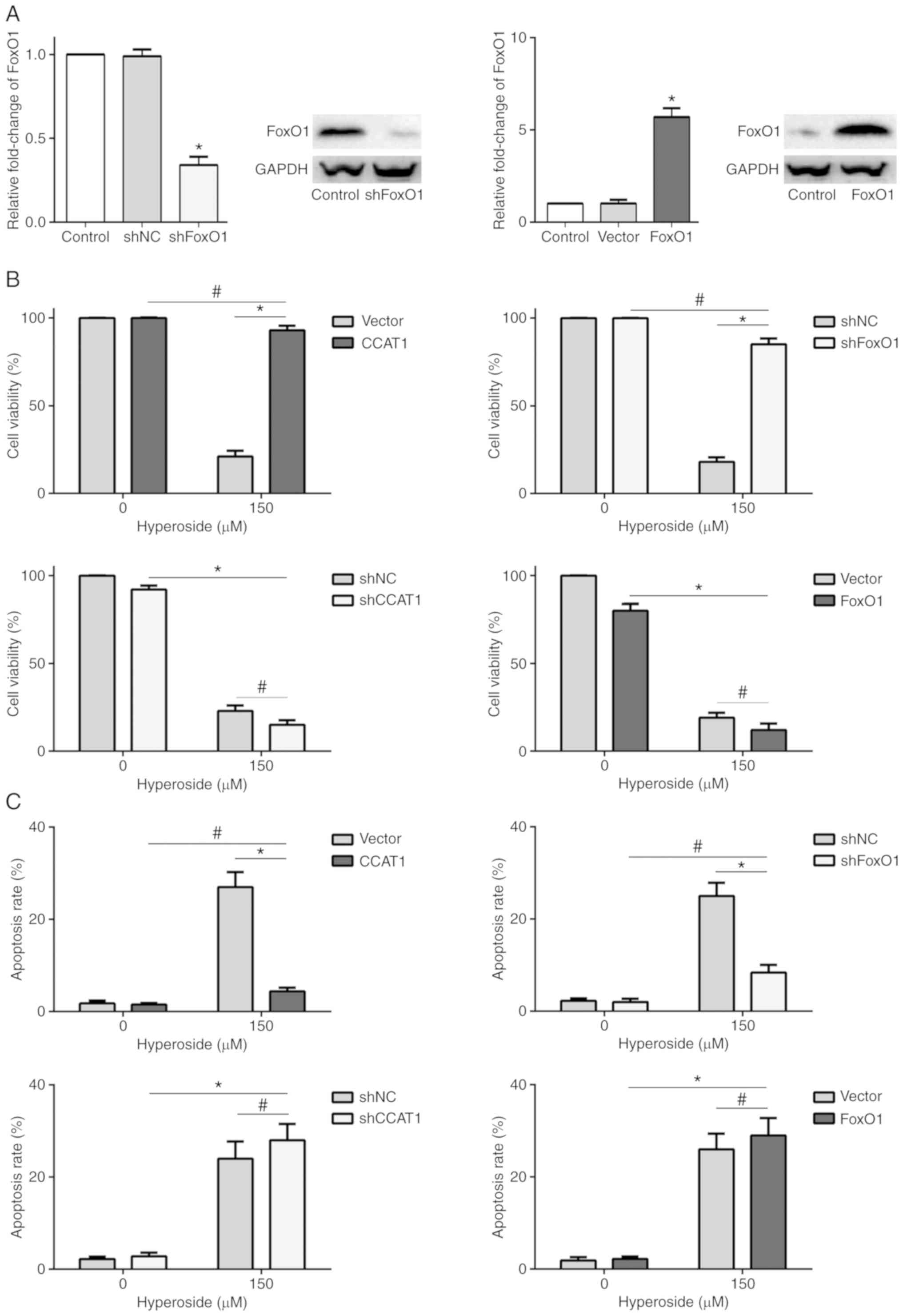
Hyperoside inhibits proliferation and
induces apoptosis through upregulation of FoxO1 via CCAT1 in
T790M-positive NSCLC cells
MTT assay and Annexin V/PI apoptosis analysis were
performed in order to investigate the anticancer activity of
hyperoside in CCAT1-knockdown or -overexpressing and
FoxO1-knockdown or -overexpressing H1975 cells (Figs. 3C and 4A). The results revealed that hyperoside
did not decrease the cell proliferation or increase the apoptosis
rate in the CCAT1-overexpressing or FoxO1-knockdown H1975 cells
(Fig. 4B and C), suggesting that
CCAT1-mediated FoxO1 signaling was essential for hyperoside in
treating T790M-positive NSCLC.
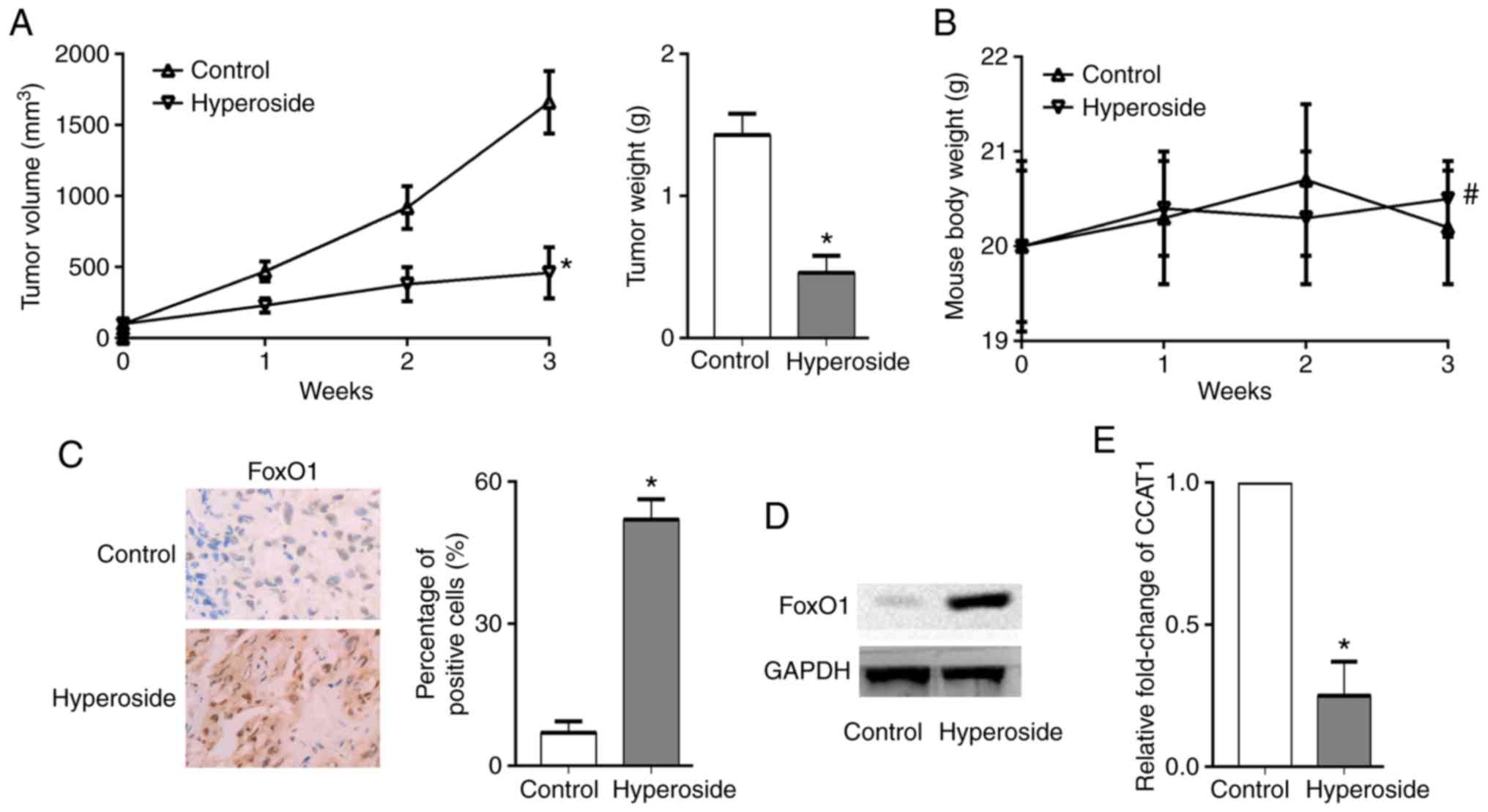
Hyperoside inhibits the growth of
T790M-positive NSCLC xenografts
A xenograft tumor model was established by
transplanting H1975 cells into nude mice in order to investigate
the anticancer effect of hyperoside in vivo. Hyperoside
significantly inhibited the growth of H1975 ×enograft tumors
(Fig. 5A), and the nude mice did
not exhibit significant weight loss in the hyperoside group
compared with the control group (Fig.
5B). Finally, tumor tissues were removed and prepared for
RT-qPCR analysis, immunohistochemistry staining and western blot
analysis. The results revealed that FoxO1was highly expressed in
the hyperoside group compared with the control group, and the level
of CCAT1 was significantly downregulated by hyperoside treatment
(Fig. 5C-E).
Discussion
Previous studies have demonstrated that hyperoside
exhibits anticancer effects in various types of cancer cell lines
by modulating multiple signaling pathways. Hyperoside was found to
exert an inhibitory effect on lung cancer growth by inducing
apoptosis and cell cycle arrest through phosphorylation of p38
mitogen-activated protein kinase (MAPK) and c-Jun N-terminal kinase
(JNK), activation of P53 signaling and caspase-3 and −9, and
inhibition of NF-κB transcriptional activity (19,29,30).
Hyperoside induced both autophagy and apoptosis in non-small cell
lung cancer (NSCLC) cells by inhibiting the phosphorylation of Akt,
mTOR, p70S6K and 4E-BP1, but increased the phosphorylation of
ERK1/2 (27). Hyperoside was found
to regulate microRNAs such as miR-21 or miR-27 to inhibit prostate
or renal cancer growth and metastasis (23,24).
However, to the best of our knowledge, the molecular mechanisms
underlying hyperoside in treating T790M-positive NSCLC have not yet
been elucidated.
Apoptosis is considered to be an important
biological process in cell survival, and resisting apoptosis is one
of the main hallmarks of carcinogenesis. Apoptosis is controlled by
a variety of apoptotic-associated genes, and current evidence
supports the fact that forkhead box protein O1 (FoxO1) is critical
for cell survival (31). FoxO1,
regarded as a tumor-suppressing factor, can inhibit carcinogenesis,
while FoxO1 disruption may promote carcinogenesis. Activation of
FoxO1 was found to trigger cancer cell apoptosis, leading to
inhibition of tumor growth (32).
The present study demonstrated that hyperoside inhibited
proliferation and induced apoptosis in H1975 cells, and the
suppression of cell proliferation by hyperoside was associated with
the induction of apoptosis. Further investigation revealed that
FoxO1 protein expression was upregulated as a result of hyperoside
treatment, suggesting that the anticancer activity of hyperoside
was associated with FoxO1 upregulation.
An increasing amount of evidence has demonstrated
that lncRNAs, >200 nucleotides in length, play an important role
in cellular biological processes, including carcinogenesis.
lncRNAs, exerting gene transcription regulatory function, have been
increasingly studied in cancer diagnosis and therapy. Notably, the
aberrant expression of lncRNAs has been demonstrated to contribute
to the development of cancer. lncRNA colon cancer associated
transcript 1 (CCAT1), located on chromosome 8q24.21, was first
observed as highly expressed in colorectal cancer. However, CCAT1
has been reported to be an oncogenic lncRNA and is upregulated in a
variety of human cancer types, including lung cancer, gastric
cancer, hepatocellular cancer, breast cancer, gallbladder cancer,
ovarian cancer and acute myeloid leukemia (33). Particularly in NSCLC, it has been
reported that aberrant CCAT1 expression may induce
epithelial-to-mesenchymal transition (EMT) by regulating the
expression levels of E-cadherin, N-cadherin and vimentin (34). CCAT1 was found to be upregulated in
cisplatin-resistant NSCLC and contributed to cisplatin-resistance
by downregulation of miR-130a-3p (35). CCAT1 was also found to contribute to
docetaxel-resistance in lung adenocarcinoma, and CCAT1
downregulation decreased chemoresistance, promoted apoptosis and
reversde the EMT phenotype of docetaxel-resistant cells (36). However, the expression levels of
CCAT1 in T790M-positive NSCLC and whether this lncRNA is involved
in the anticancer effects of hyperoside remain unclear. In the
present study, it was revealed that hyperoside notably
downregulated the level of CCAT1 expression. CCAT1 regulated the
FoxO1 expression in H1975 cells. Hyperoside could not decrease cell
proliferation or increase the apoptosis rate in
CCAT1-overexpressing or FoxO1-knockdown H1975 cells, demonstrating
that hyperoside inhibited T790M-positive NSCLC tumor growth and
promoted apoptosis by upregulating FoxO1 via CCAT1.
In conclusion, the present study demonstrated that
hyperoside inhibited proliferation and induced apoptosis by
upregulating FoxO1 via CCAT1 in T790M-positive NSCLC cells,
providing a theoretical basis for hyperoside in treating
T790M-positive NSCLC. Further studies are required in order to
apply hyperoside to the clinical setting.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Medical
Technology Planning Program of Zhejiang Province (grant no.
2019KY507).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
HX conceived and designed the study. ZH and PZ
performed the experiments. ZH wrote the paper. HX reviewed and
edited the manuscript. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The animal experimental procedures were approved by
the Ethics Committee of Zhejiang Hospital and were in accordance
with the National Institutes of Health Guidelines for Animal Care
and Use.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shigematsu H and Gazdar AF: Somatic
mutations of epidermal growth factor receptor signaling pathway in
lung cancers. Int J Cancer. 118:257–262. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou C and Yao LD: Strategies to improve
outcomes of patients with EGRF-mutant non-small cell lung cancer:
Review of the literature. J Thorac Oncol. 11:174–186. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kobayashi S, Boggon TJ, Dayaram T, Jänne
PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG and Halmos
B: EGFR mutation and resistance of non-small-cell lung cancer to
gefitinib. N Engl J Med. 352:786–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohashi K, Maruvka YE, Michor F and Pao W:
Epidermal growth factor receptor tyrosine kinase
inhibitor-resistant disease. J Clin Oncol. 31:1070–1080. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Remon J, Steuer CE, Ramalingam SS and
Felip E: Osimertinib and other third-generation EGFR TKI in
EGFR-mutant NSCLC patients. Ann Oncol. 29 (Suppl 1):i20–i27. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Z, Yang N, Ou Q, Xiang Y, Jiang T, Wu
X, Bao H, Tong X, Wang X, Shao YW, et al: Investigating novel
resistance mechanisms to third-generation EGFR tyrosine kinase
inhibitor osimertinib in non-small cell lung cancer patients. Clin
Cancer Res. 24:3097–3107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang H, Zhao PJ, Su D, Feng J and Ma SL:
Paris saponin I induces apoptosis via increasing the Bax/Bcl-2
ratio and caspase-3 expression in gefitinib-resistant non-small
cell lung cancer in vitro and in vivo. Mol Med Rep. 9:2265–2272.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jiang H, Zhao P, Feng J, Su D and Ma S:
Effect of Paris saponin I on radiosensitivity in a
gefitinib-resistant lung adenocarcinoma cell line. Oncol Lett.
7:2059–2064. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao P, Jiang H, Su D, Feng J, Ma S and
Zhu X: Inhibition of cell proliferation by mild hyperthermia at
43°C with Paris saponin I in the lung adenocarcinoma cell line
PC-9. Mol Med Rep. 11:327–332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu X, Jiang H, Li J, Xu J and Fei Z:
Anticancer effects of Paris saponins by apoptosis and PI3K/AKT
pathway in gefitinib-resistant non-small cell lung cancer. Med Sci
Monit. 22:1435–1441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao PJ, Song SC, Du LW, Zhou GH, Ma SL,
Li JH, Feng JG, Zhu XH and Jiang H: Paris Saponins enhance
radiosensitivity in a gefitinib-resistant lung adenocarcinoma cell
line by inducing apoptosis and G2/M cell cycle phase arrest. Mol
Med Rep. 13:2878–2884. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song S, Du L, Jiang H, Zhu X, Li J and Xu
J: Paris Saponin I sensitizes gastric cancer cell lines to
cisplatin via cell cycle arrest and apoptosis. Med Sci Monit.
22:3798–3803. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng R, Rao Y, Jiang H, Liu X, Zhu X, Li
J and Xu J: Therapeutic potential of ginsenoside Rg3 via inhibiting
Notch/HES1 pathway in lung cancer cells. Transl Cancer Res.
5:464–469. 2016. View Article : Google Scholar
|
|
15
|
Zheng R, Jiang H, Li J, Liu X and Xu H:
Polyphyllin II restores sensitization of the resistance of PC-9/ZD
cells to gefitinib by a negative regulation of the PI3K/Akt/mTOR
signaling pathway. Curr Cancer Drug Targets. 17:376–385. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Fei Z and Jiang H: Polyphyllin VII
increases sensitivity to gefitinib by modulating the elevation of
P21 in acquired gefitinib resistant non-small cell lung cancer. J
Pharmacol Sci. 134:190–196. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Q, Chen W, Xu Y, Lv X, Zhang M and
Jiang H: Polyphyllin I modulates MALAT1/STAT3 signaling to induce
apoptosis in gefitinib-resistant non-small cell lung cancer.
Toxicol Appl Pharmacol. 356:1–7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong F, Gu W, Jiang J, Liu X and Jiang H:
Anticancer activity of polyphyllin I in nasopharyngeal carcinoma by
modulation of lncRNA ROR and P53 signalling. J Drug Target.
27:806–811. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Y, Tantai J, Sun Y, Zhong C and Li Z:
Effect of hyperoside on the apoptosis of A549 human non-small cell
lung cancer cells and the underlying mechanism. Mol Med Rep.
16:6483–6488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guon TE and Chung HS: Hyperoside and rutin
of Nelumbo nucifera induce mitochondrial apoptosis through a
caspase- dependent mechanism in HT-29 human colon cancer cells.
Oncol Lett. 11:2463–2470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boukes GJ and van de Venter M: The
apoptotic and autophagic properties of two natural occurring
prodrugs, hyperoside and hypoxoside, against pancreatic cancer cell
lines. Biomed Pharmacother. 83:617–626. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu X, Ji M, Han Y, Guo Y, Zhu W, Gao F,
Yang X and Zhang C: PGRMC1-dependent autophagy by hyperoside
induces apoptosis and sensitizes ovarian cancer cells to cisplatin
treatment. Int J Oncol. 50:835–846. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li W, Liu M, Xu YF, Feng Y, Che JP, Wang
GC and Zheng JH: Combination of quercetin and hyperoside has
anticancer effects on renal cancer cells through inhibition of
oncogenic microRNA-27a. Oncol Rep. 31:117–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang FQ, Liu M, Li W, Che JP, Wang GC and
Zheng JH: Combination of quercetin and hyperoside inhibits prostate
cancer cell growth and metastasis via regulation of microRNA-21.
Mol Med Rep. 11:1085–1092. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang N, Ying MD, Wu YP, Zhou ZH, Ye ZM,
Li H and Lin DS: Hyperoside, a flavonoid compound, inhibits
proliferation and stimulates osteogenic differentiation of human
osteosarcoma cells. PLoS One. 9:e989732014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li JP, Liao XH, Xiang Y, Yao A, Song RH,
Zhang ZJ, Huang F, Dai ZT and Zhang TC: Hyperoside and let-7a-5p
synergistically inhibits lung cancer cell proliferation via
inducing G1/S phase arrest. Gene. 679:232–240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu T, Wang L, Jin XN, Sui HJ, Liu Z and
Jin Y: Hyperoside induces both autophagy and apoptosis in non-small
cell lung cancer cells in vitro. Acta Pharmacol Sin. 37:505–518.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu YH, Liu GH, Mei JJ and Wang J: The
preventive effects of hyperoside on lung cancer in vitro by
inducing apoptosis and inhibiting proliferation through Caspase-3
and P53 signaling pathway. Biomed Pharmacother. 83:381–391. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lü P: Inhibitory effects of hyperoside on
lung cancer by inducing apoptosis and suppressing inflammatory
response via caspase-3 and NF-κB signaling pathway. Biomed
Pharmacother. 82:216–225. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xing YQ, Li A, Yang Y, Li XX, Zhang LN and
Guo HC: The regulation of FOXO1 and its role in disease
progression. Life Sci. 193:124–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cosimo E, Tarafdar A, Moles MW, Holroyd
AK, Malik N, Catherwood MA, Hay J, Dunn KM, Macdonald AM, Guichard
SM, et al: AKT/mTORC2 inhibition activates FOXO1 function in CLL
cells reducing B-cell receptor-mediated survival. Clin Cancer Res.
25:1574–1587. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo X and Hua Y: CCAT1: An oncogenic long
noncoding RNA in human cancers. J Cancer Res Clin Oncol.
143:555–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin H, Cheng W, Yan H and Zhang X:
Overexpression of the long noncoding RNA CCAT1 promotes metastasis
via epithelial-to-mesenchymal transition in lung adenocarcinoma.
Oncol Lett. 16:1809–1814. 2018.PubMed/NCBI
|
|
35
|
Hu B, Zhang H, Wang Z, Zhang F, Wei H and
Li L: LncRNA CCAT1/miR-130a-3p axis increases cisplatin resistance
in non-small-cell lung cancer cell line by targeting SOX4. Cancer
Biol Ther. 18:974–983. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen J, Zhang K, Song H, Wang R, Chu X and
Chen L: Long noncoding RNA CCAT1 acts as an oncogene and promotes
chemoresistance in docetaxel-resistant lung adenocarcinoma cells.
Oncotarget. 7:62474–62489. 2016.PubMed/NCBI
|