Introduction
Colorectal cancer (CRC) is one of the most malignant
tumors diagnosed in humans and CRC is the leading cause of
cancer-associated mortality worldwide. There were more than 145,600
patients diagnosed with CRC, and more than 50,000 individuals that
succumbed to the disease in the United States in 2019 (1,2). The
most common therapeutic interventions for patients with CRC include
surgery, neo-adjuvant radiotherapy and adjuvant chemotherapy (for
patients with stage III/IV and high-risk stage II colon cancer).
Although the prognosis of patients with CRC has slowly improved in
numerous countries due to the development of colonoscopy, the
5-year relative survival has remained <50% in low-income
countries (3–5). Therefore, it is extremely urgent to
elucidate the underlying molecular mechanisms and develop new
therapeutic strategies.
LHPP is located on chromosome 10q26.13 and
encodes a 29 kDa enzyme called phospholysine phosphohistidine
inorganic pyrophosphate phosphatase, which has been purified from
bovine liver (6–10). The protein is able to hydrolyze
imidodiphosphate, 3-phosphohistidine and 6-phospholysine.
Currently, LHPP has been demonstrated as a tumor suppressor,
which plays an essential role in inhibiting human hepatocellular
carcinoma (HCC) progression by regulating AKT expression
level and activity (9). After
analyzing The Cancer Genome Atlas (TCGA) and International Cancer
Genome Consortium (ICGC) databases, researchers also demonstrated
49 LHPP mutations that are predicted to be inactivating
mutations in other types of tumors, including esophageal cancer,
head and neck cancer and stomach cancer. The biological effects of
LHPP have also been identified in cervical cancer (11); the protein impedes cell growth,
proliferation and metastasis, and promotes cell apoptosis.
The phosphatidylinositol-3-kinase/protein kinase B
(PI3K/AKT) signaling pathway plays an extremely important role in
diverse cellular functions, such as cell proliferation,
differentiation, angiogenesis and autophagy (8,12).
Recently, a number of studies have demonstrated that the
PI3K/AKT signaling pathway is involved in the process of
epithelial-mesenchymal transition (EMT) either directly or
indirectly (13,14). There are three groups of the PI3K
family members, but only class IA PI3Ks play a role in
tumorigenesis (12,15). An increasing amount of evidence has
demonstrated that the PIK3CA mutation exists in various
types of tumor, including CRC. Of the patients with mutant CRC,
6–9% possess a double mutation of PIK3CA (16–18).
Thus, PI3K/AKT inhibitors (e.g., NVP-BEZ235, OSI-027 and BYL719)
are used as promising drugs in the treatment of CRC (19).
The present study used western blotting and
immunohistochemistry (IHC) to assess differences in LHPP expression
between normal mucosa tissues and cancer tissues. The results
revealed that LHPP expression was decreased in CRC tissues compared
with that noted in the adjacent normal tissues. The clinical
outcomes of patients with higher LHPP expression demonstrated
improved survival. Thus, the present study predicted that
LHPP could be a tumor suppressor in the progression of CRC.
Subsequently, in the present study both in vitro and in
vivo experiments were performed to investigate the role of
LHPP and its potential mechanisms. The results demonstrated
that LHPP could inhibit CRC cell growth and proliferation
via the PI3K/AKT signaling pathway. Therefore, LHPP
could be considered as a promising target to develop novel
therapeutic strategies against CRC.
Materials and methods
Bioinformatics prediction
The present study used gene data from The Cancer
Genome Atlas (TCGA; http://cancergenome.nih.gov/) in order to evaluate the
differences in LHPP mRNA expression between CRC tissues and
matched noncancerous tissues. The median mRNA expression of
LHPP was regarded as the cut-off value to distinguish
patients with high and low expression. The overall survival data
were collected for further analysis. A total of 407 patients were
selected in the present study (TCGA; http://cancergenome.nih.gov/).
Human samples
The present study obtained CRC tissues and their
corresponding adjacent non-tumor tissues (n=52) from patients (mean
age 65 years; range, 54–78) at the First Affiliated Hospital of
Xi'an Jiaotong University (Shaanxi, China) between June, 2016 and
March, 2019. Each tissue was immediately frozen and stored in
liquid nitrogen following surgery. All patients had not received
chemotherapy or radiotherapy prior to the primary surgery. Overall
survival was regarded as the primary point to evaluate the
association between LHPP expression and clinical outcomes of
patients with CRC. Other clinical parameters were selected for
further analysis. Informed consent was obtained from each patient.
The study protocol was approved by the Ethics Committee at the
First Affiliated Hospital of Xi'an Jiaotong University (Shaanxi,
China).
Immunohistochemistry (IHC)
The human CRC, adjacent normal tissues and
heterologous tumor tissues from nude mice were fixed in
formaldehyde and embedded in paraffin. Prior to the immunostaining,
4-µm-thick tissue sections were dewaxed in xylene and washed three
times in PBS. The sections were then autoclaved in 10 mM sodium
citrate buffer (pH 6.0) for 10 min at 120°C. The present study used
goat serum (10%) to block non-specific staining. The sections were
incubated with rabbit polyclonal antibodies to LHPP (dilution
1:200, catalog no. 15759-1-AP; Proteintech) or Ki-67 (dilution
1:200, catalog no. 27309-1-AP; Proteintech) overnight at 4°C. For
the diagnosis, two independent investigators who were blinded to
the study and the patient information performed the evaluations of
the staining. The semi-quantitative immunoreactive score (IRS) of
Remmele and Stegner was utilized to assess IHC scores (20,21).
The score was based on the percentage of positive cells (0 points,
absence of cells with positive reaction; 1 point, 1–10% positive
cells; 2 points, 11–50%; 3 points, 51–80%; 4 points, >80%) and
the intensity of reaction color (0, no reaction; 1, low intensity;
2, moderate intensity; 3, intense color). The final score was the
product of the two parameters (0–1 point, negative; 2–3 points,
weakly positive; 4–5 points, positive; >6 points, strongly
positive).
Cell lines and culture conditions
Human CRC cell lines (RKO, HT-29, SW480, CACO2 and
HCT 116) were purchased from the Cell Bank of the Chinese Academy
of Sciences (Shanghai). RKO, SW480 and CACO2 cells were cultured in
complete RPMI-1640 medium (Hyclone; GE Healthcare). HT-29 and
HCT116 cells were cultured in complete Dulbecco's modified Eagle's
medium (DMEM; Hyclone; GE Healthcare). The medium was supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.) and a 1% penicillin-streptomycin mixture. The cells were
maintained at 37°C in a humidified incubator with 5%
CO2.
Immunofluorescence (IF) assay
In order to identify the location of LHPP in cells,
SW480 and HT-29 cells (1×104) were seeded on slides in
24-well plates and cultured in an incubator containing 5%
CO2 at 37°C for 24 h with 500 µl complete medium. Cells
were fixed with 4% ice-cold paraformaldehyde for ~20 min, and 0.5%
Triton X-100/PBS was used to permeabilize the cancer cells for 30
min at room temperature. The cells were then blocked with 10% BSA
for 1 h and incubated with a primary antibody against LHPP
(dilution 1:30, catalog no. 15759-1-AP; Proteintech) overnight at
4°C. Goat anti-rabbit IgG/RBITC (catalog no. BS-0295G, Bioss) was
added for ~1 h at room temperature. Cell nuclei were
counter-stained with DAPI. Cells were observed under a fluorescence
microscope (magnification, ×200).
Cell transfection
A total 20–30% confluent SW480 cells were stably
transfected with LHPP lentiviruses (LV) or negative control
lentiviruses according to the manufacturer's protocols (viral
volume=MOI × cell numbers/viral titers; Shanghai GeneChem Co.,
Ltd.). We selected a multiplicity of infection (MOI) of 20 as the
final parameter after pre-experiments. Lentiviral vectors
overexpressing LHPP were generated using a GV358 System
(Shanghai China, GeneChem) and contained a U6 promoter-driven
multiple cloning site (MCS) combined with a cytomegalovirus
promoter-driven puromycin gene and green fluorescent protein. The
GV248 RNA interference (RNAi) system (Shanghai China, GeneChem)
contained a U6 promoter-driven multiple cloning site (MCS) combined
with a cytomegalovirus promoter-driven puromycin gene and green
fluorescent protein was used to generate lentiviruses expressing
short hairpin RNA (shRNA) targeting LHPP. The target sequence of
LHPP was 5′-GAGCAAGGCCUGCGACCAUTTAUGGUCGCAGGCCUUGCUCTT-3′.
The negative lentiviruses (LV-NC-RNAi) were also purchased from
Shanghai GeneChem. The negative sequence was
5′-UUCUCCGAACGUGUCACGUTT-3′. The HT-29 cell line was stably
transfected with LHPP-RNAi lentiviruses. After culturing for
2 days, the cells were purified with 3 µg/ml puromycin for 48 h,
and the concentration of puromycin was maintained with 1.5 µg/ml
for 5 days. The overexpression and knockdown efficiency of
LHPP were assessed using western blotting, RT-PCR and
fluorescence microscopy.
Western blot analysis
Total protein was extracted from cells or tissues
using RIPA buffer with protease inhibitors. Protein concentrations
were determined by using a BCA Protein assay kit (cat. no.
PA115-01; Tiangen Biotech Co. Ltd.). Equal amounts of protein
(20–30 µg) were separated via SDS-PAGE (10–12% gel) and then
transferred to PVDF membranes. The membranes were blocked with 5%
milk and incubated with primary antibodies overnight at 4°C,
followed by incubation with the secondary antibody (dilution
1:5,000) for 2 h at room temperature. The antibodies used were as
follows: LHPP (dilution 1:500, cat. no. 15759-1-AP; Proteintech),
P53 (dilution 1:500, cat. no. 10442-1-AP; Proteintech), total AKT
(dilution 1:1,000, cat. no. 10176-2-AP; Proteintech), p-AKT
(phospho-Ser 473; dilution 1:500, cat. no. 11054; SAB), GAPDH
(dilution 1:2,000, cat. no. 10494-1-AP; Proteintech), PCNA
(dilution 1:2,000; cat. no. 10205-2-AP; Proteintech), CDK4
(dilution 1:2,000; cat. no. 49132; SAB), cyclin D1 (dilution
1:1,000; cat. no. WL01435a; Wanleibio), NME1 (dilution 1:1,000,
cat. no. 11086-2-AP; Proteintech), PI3K (dilution 1:500, catalog
no. 41339; SAB), p-PI3K (phospho-Tyr467, dilution 1:500, cat. no.
11508; SAB). The bands were visualized using Immobilion Western
Chemilum HRP Substrate (cat. no. WBKLS0100; Millipore). The
immunoreactive membranes were scanned using GE Amersham Imager 600
system (GE Healthcare). ImageJ software (National Institutes of
Health) was used to examine gray values of each primary antibody
and GAPDH. The ratio of gray value (primary antibody/GAPDH) was
calculated using GraphPad Prism 6 software (GraphPad Software,
Inc.). Each experiment was performed in triplicate.
RNA extraction, reverse transcription
(RT) and RT-quantitative PCR (RT-qPCR)
Total RNA was extracted from cells using a Fastagen
200 kit (Fastagen cat. no. 220010; Shanghai Fastagen Biotechnology
Co., Ltd.) according to the manufacturer's protocol. cDNA was
synthesized using PrimeScript™ RT Master Mix (Takara
Biotechnology). qPCR was performed using the SYBR Green PCR kit
(Takara Biotechnology). Each experiment was performed in
triplicate. The expression level of LHPP was calculated
using the 2−ΔΔCq method (22). The housekeeping gene GAPDH
was applied as an internal control. The following primers were
used: GAPDH forward, 5′-TCCCCATTGGACTTACTTG-3′ and reverse,
5′-AGTACAGTCGCGATAAGAG-3′; LHPP forward,
5′-GCTTCAGAGGCTGGGATTTGAC-3′ and reverse,
5′-AATTACCACACAGTTTGGGTTGGA-3′. The thermal parameters were as
follows: 95°C for 10 min, followed by 40 cycles at 95°C for 15 sec
and 60°C for 1 min.
Cell viability analysis
Cells were seeded at 2,000–3,000 cells per well in
96-well plates and subsequently cultured for 7 days. CCK-8 solution
(10 µl) was added to each well and the cells were incubated for 2 h
under aseptic conditions in a 5% CO2 incubator at 37°C.
The spectrophotometric value of each sample was measured at 450
nm.
Colony formation analysis
Cells were seeded at 1,000–2,000 cells per well,
plated in 6-well plates, and incubated for 3–4 weeks at 37°C in a
humidified environment with 5% CO2. After three washes
with PBS, the colonies were fixed with 4% paraformaldehyde, and
then stained with crystal violet staining (1%) solution for 30 min
at room temperature. Then stained colonies were imaged using camera
and counted using microscope.
Cell cycle analysis
Cells (1–2×104) were plated in 6-well
plates and cultured for 24 h. Cultured cells were digested and
collected in 1.5 ml EP tubes and fixed with 70% cold ethanol at 4°C
overnight. Cells were then washed twice with cold PBS.
Subsequently, the cells were incubated with 500 µl propidium iodide
(PI) and RNase A (1:9) for 30 min in darkness. A FACSCalibur flow
cytometer (BD Bioscience) was used to analyze the results. Finally,
the cell proportions at different phases of the cell cycle were
calculated using Graphpad Prism 6 software (GraphPad Software,
Inc.).
Xenograft assays
Four-week-old female specific pathogen-free (SPF)
BALB/c nude mice (total number, 12) were purchased from Xian
Jiaotong University Animal Laboratory for the subcutaneous
xenograft experiments. LV-RNAi-LHPP and LV-SiNC HT-29 (HT-29
Sh-LHPP and HT-29 SiNC, respectively) cells (2–4×106/200
µl) were injected into mice subcutaneously (6 mice per group).
Tumor size was measured using a caliper every 3 days and calculated
using the following formula: Volume=length ×
(width2)/2(mm3) (23). Nude mice were humanely sacrificed
after 3–4 weeks using cervical dislocation and tumors were isolated
from the mice. Humane endpoints included i) tumor ulceration shows
no stabilization within 7 days of treatment; ii) ulcerated tumor is
actively bleeding; iii) ulcerated tumor shows visible signs of
infection; iv) animals show discomfort associated with tumor
ulceration such as biting/scratching; and v) tumor size did not
exceed 20 mm (2.0 cm) in mice (IACUC Guideline: Tumor Induction in
mice and rats) (24–26). All the conditions of the animal
laboratory conformed to the principles of Euroguide: On the
accommodation and care of animals used for experimental and other
scientific purposes (27). Tumor
tissues were fixed in 4% paraformaldehyde and cut into 10-µm
sections for IHC analysis.
Statistical analysis
All statistical data were analyzed using GraphPad
Prism 6 software (GraphPad Software, Inc.). Experiments were
performed in triplicate and data are expressed as the mean ±
standard error. The two-tailed Student's t test was used to analyze
the statistical significance in continuous variables between two
different groups. One-way or two-way analysis of variance (ANOVA)
followed by Bonferroni's Multiple Comparison Test was performed to
test differences between multiple groups. Next, the χ2
test was used to evaluate the association between LHPP expression
and clinical characteristics of the patients with CRC. Survival
curves were created using the Kaplan-Meier method, and the positive
difference between groups was analyzed using the log-rank test.
P<0.05 was consisted to indicate a statistically significant
difference.
Results
Expression of LHPP is lower in CRC
tissues compared with that in the matched normal tissues
LHPP is dysregulated in patients with CRC. The
present study used data published in TCGA to examine the expression
of LHPP in CRC. The results published or shown here are in
whole or part based upon data generated by the TCGA Research
Network: https://www.cancer.gov/tcga.
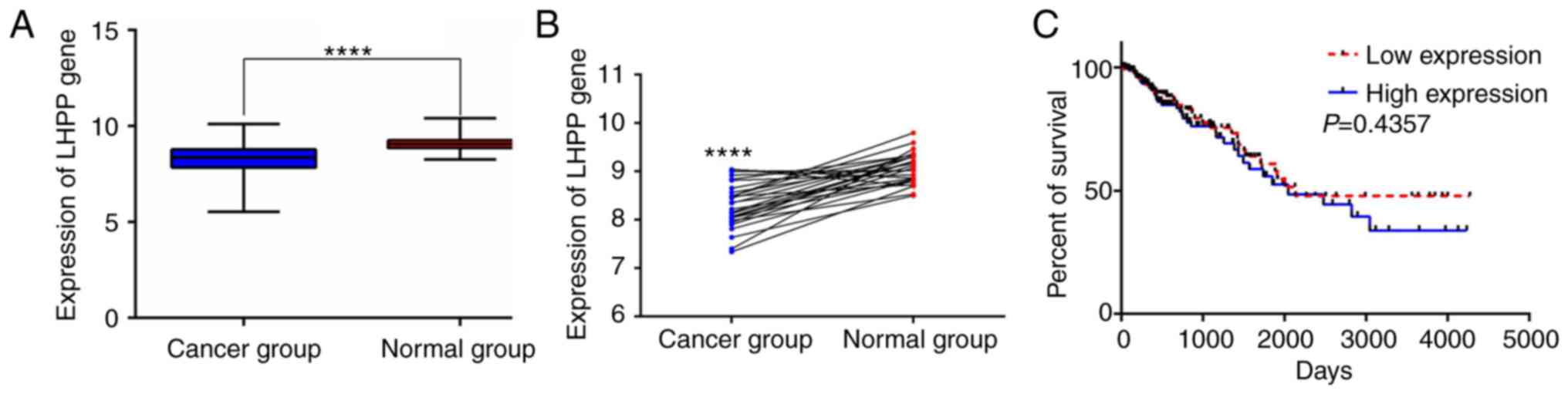
LHPP expression was markedly lower in CRC tissues compared
with that noted in the normal tissues (normal, 51; cancer, 384;
P<0.0001; Fig. 1A). The
LHPP expression was also significantly decreased in cancer
tissues when compared with the matched noncancerous tissues
(normal, 28; cancer, 28; P<0.0001; Fig. 1B). However, no significant
difference in the overall survival was observed between patients
with high and low expression of LHPP (median expression, 8.3611;
n=182; P=0.4357; Fig. 1C).
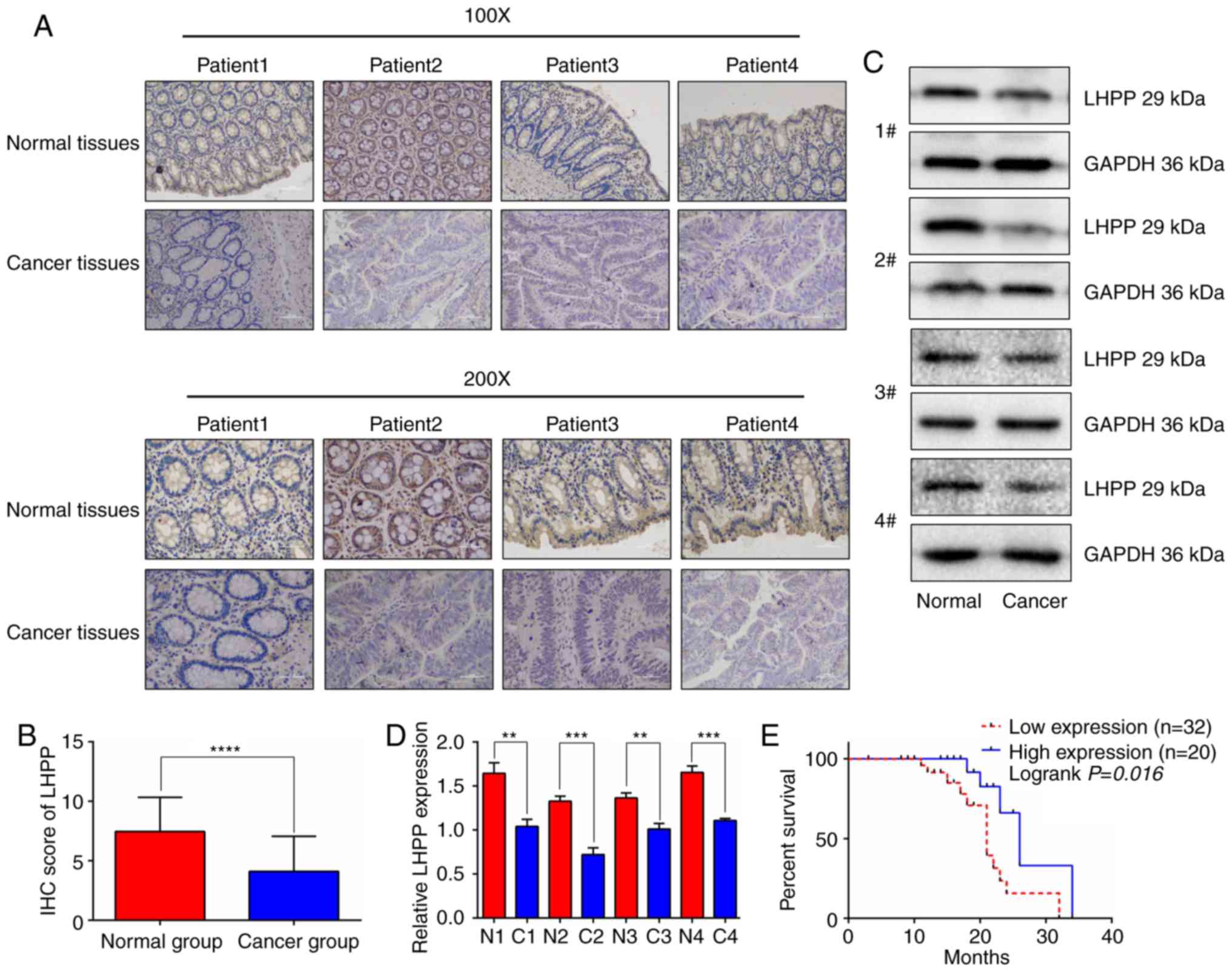
The present study then used IHC to identify the LHPP
expression in cancer samples. The results of the IHC staining are
presented in Fig. 2A; CRC tissues
exhibited a lower positive rate of LHPP staining compared with
adjacent normal tissues. GraphPad Prism 6 software was used to
calculate IHC scores (Fig. 2B). The
data suggested that cancer tissues had markedly lower IHC scores
when compared with the adjacent normal tissues (n=52; P<0.0001).
Moreover, the protein levels of LHPP in CRC samples were
significantly lower than these levels in the matched noncancerous
samples (Fig. 2C and D). After
further analysis, the data from the present study revealed that
patients with higher expression levels of LHPP were in the early
stages of disease (Stage I+II; P<0.05). In addition, lower
expression levels of LHPP were positively associated with advanced
T classifications (P<0.05), lymph node metastasis (P<0.05)
and long dimension of CRC (P<0.01) (Table I). There were no significant
differences observed between LHPP expression level and other
pathological data. The present study then selected an IHC score of
3 points as the cut-off value to distinguish patients in the two
groups. Notably, the results demonstrated that patients with higher
expression levels of LHPP exhibited extended overall survival
(Fig. 2E). The median survival of
patients in the higher expression group was 26.0 months, which was
significantly better than patients in the lower expression group
(median survival, 21.0 months; P<0.05).
 | Table I.Correlation between LHPP expression
and clinicopathological characteristics of the patients with
colorectal cancer (n=52). |
Table I.
Correlation between LHPP expression
and clinicopathological characteristics of the patients with
colorectal cancer (n=52).
|
| LHPP gene
expression (no. of patients) |
|
|
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | Low | High | Total | χ2 | P-value |
|---|
| Age (years) |
|
|
| 2.507 | 0.113 |
|
≤60 | 12 | 12 | 24 |
|
|
|
>60 | 20 | 8 | 28 |
|
|
| Sex |
|
|
| 0.430 | 0.624 |
|
Male | 18 | 9 | 27 |
|
|
|
Female | 14 | 11 | 25 |
|
|
| T
classification |
|
|
| 5.967 | 0.015 |
|
T1+T2 | 7 | 11 | 16 |
|
|
|
T3+T4 | 25 | 9 | 36 |
|
|
| N
classification |
|
|
| 5.609 | 0.018 |
| N0 | 15 | 16 | 31 |
|
|
|
N1+N2 | 17 | 4 | 20 |
|
|
| M
classification |
|
|
| 0.797 | 0.372 |
| M0 | 28 | 19 | 47 |
|
|
| M1 | 4 | 1 | 5 |
|
|
| Stage
classification |
|
|
| 5.852 | 0.016 |
| Stage
I+II | 13 | 15 | 28 |
|
|
| Stage
III+IV | 19 | 5 | 24 |
|
|
| Longest tumor
dimension (cm) |
|
|
| 6.857 | 0.009 |
|
≤1.5 | 9 | 13 | 22 |
|
|
|
>1.5 | 23 | 7 | 30 |
|
|
| Survival
status |
|
|
| 1.328 | 0.249 |
|
Living | 13 | 5 | 18 |
|
|
|
Deceased | 19 | 15 | 34 |
|
|
Cell transfection results
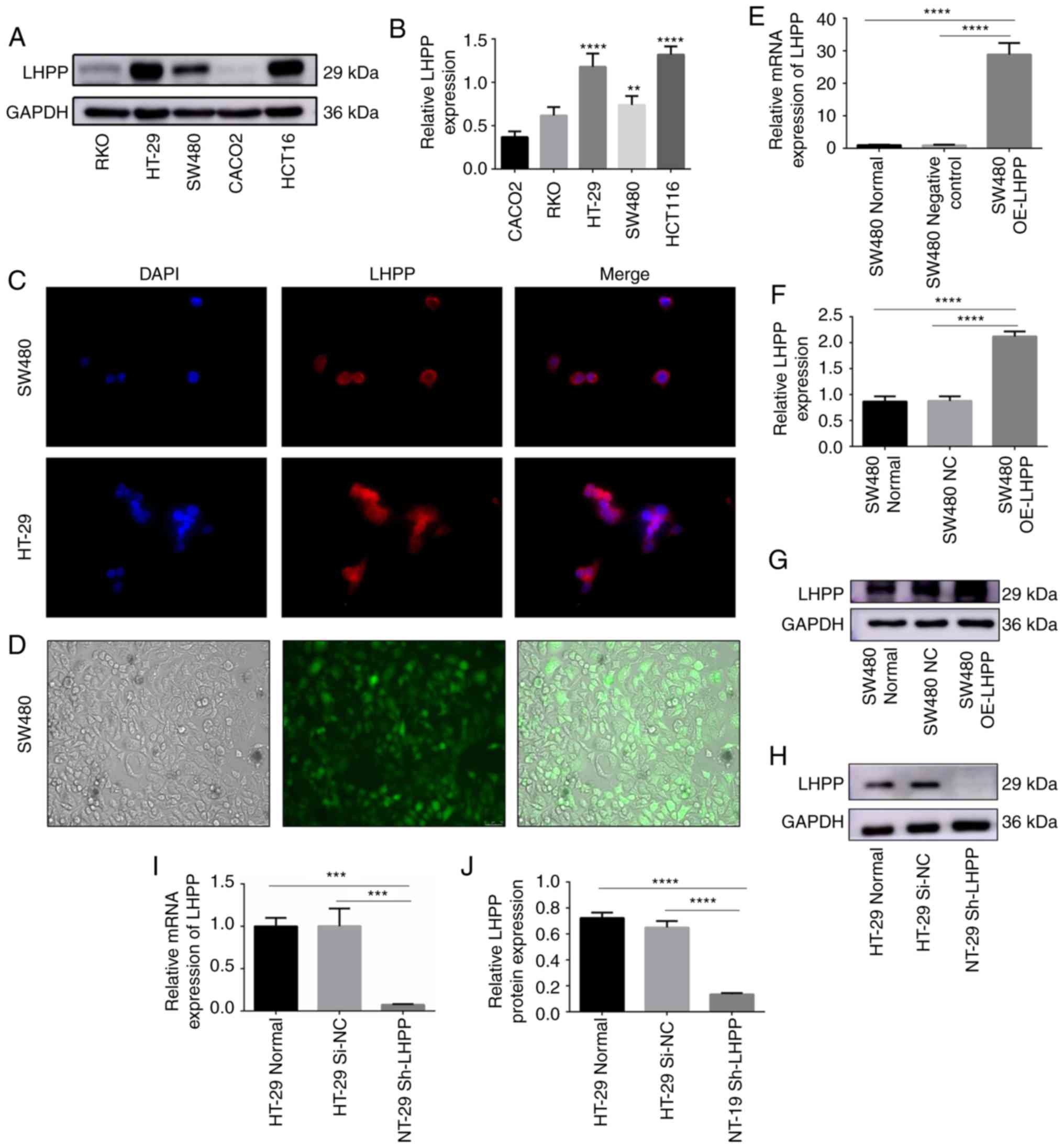
The present study analyzed the expression levels of
LHPP in different CRC cell lines (RKO, HT29, SW480, CACO2 and
HCT116) via western blotting (Fig. 3A
and B). The data suggested that HT-29 and HCT116 had higher
expression levels of LHPP. Lower expression levels of LHPP were
observed in CACO2 and RKO cell lines. The SW480 cell line presented
with a median expression level of LHPP among the cell lines. Thus,
the present study selected SW480 and HT-29 cell lines for the
subsequent analyses. It was revealed that the LHPP protein was
located in the cytoplasm in SW480 and HT-29 cells when using
immunofluorescence (Fig. 3C).
In order to investigate the biological functions of
LHPP, SW480 cells were stably transfected with LHPP
lentiviruses according to the manufacturer's protocol (GeneChem).
As illustrated in Fig. 3D, SW480
cells expressed green fluorescent protein; these results suggested
that the transfection efficiency was >80%. As presented in
Fig. 3E-G, the relative mRNA and
protein expression levels of LHPP were markedly higher in
the SW480 OE-LHPP groups when compared with the negative
control (SW480 NC) groups (P<0.0001).
In order to further investigate the role of LHPP in
CRC, HT-29 cells were transfected with LHPP shRNA lentiviruses or
negative control lentiviruses. The results are presented in
Fig. 3. HT-29 cells in the
shRNA-LHPP (HT-29 Sh-LHPP) groups exhibited significantly
lower LHPP mRNA (P<0.001) and protein expression
(P<0.0001) levels when compared with the negative control groups
(HT-29 Si-NC) (Fig. 3H-J).
Overexpression of LHPP suppresses CRC
cell growth and proliferation, and decreases p-PI3K/p-AKt
expression levels
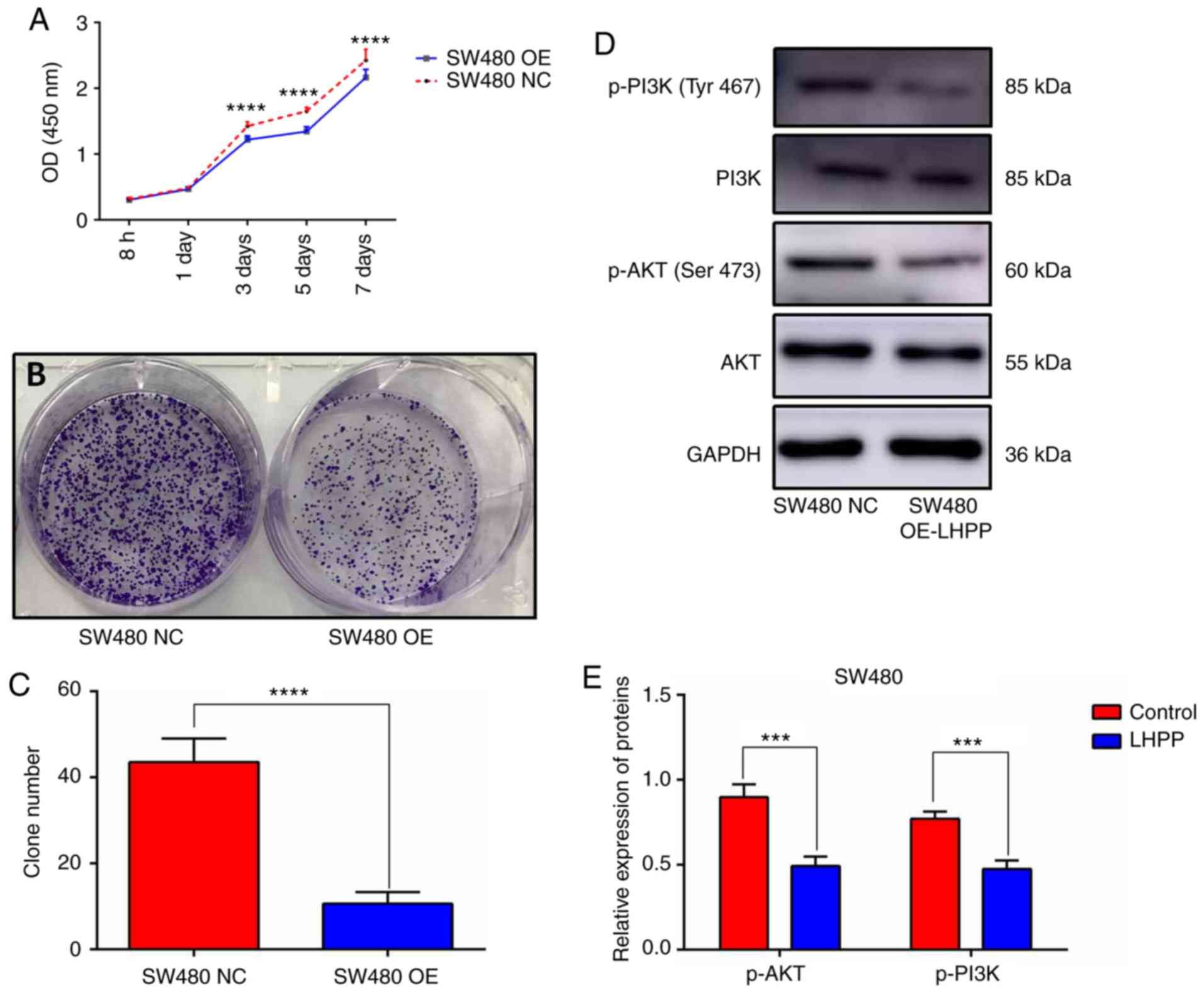
The CCK-8 assay and colony formation assay were used
to investigate cell viability in the present study. The viability
of SW480 cells was decreased in the SW480 OE-LHPP group
after transfection for 3, 5 and 7 days (Fig. 4A). The colony formation assay also
demonstrated that overexpression of LHPP could significantly
suppress CRC cell proliferation (Fig.
4B and C; SW480 OE-LHPP group vs. NC group;
P<0.0001). It is already well established that the PI3K/AKT axis
plays a pivotal role in the development of cancer; therefore, the
expression levels of AKT, PI3K, p-AKT (Ser473) and
p-PI3K (Tyr467) were detected via western blotting in
the present study. The expression levels of p-AKT and p-PI3K were
significantly decreased in the SW480 OE-LHPP groups compared with
the SW480 NC groups (Fig. 4D and E;
P<0.001). These results suggest that upregulation of LHPP
may inhibit the activity of the PI3K/AKT signaling pathway.
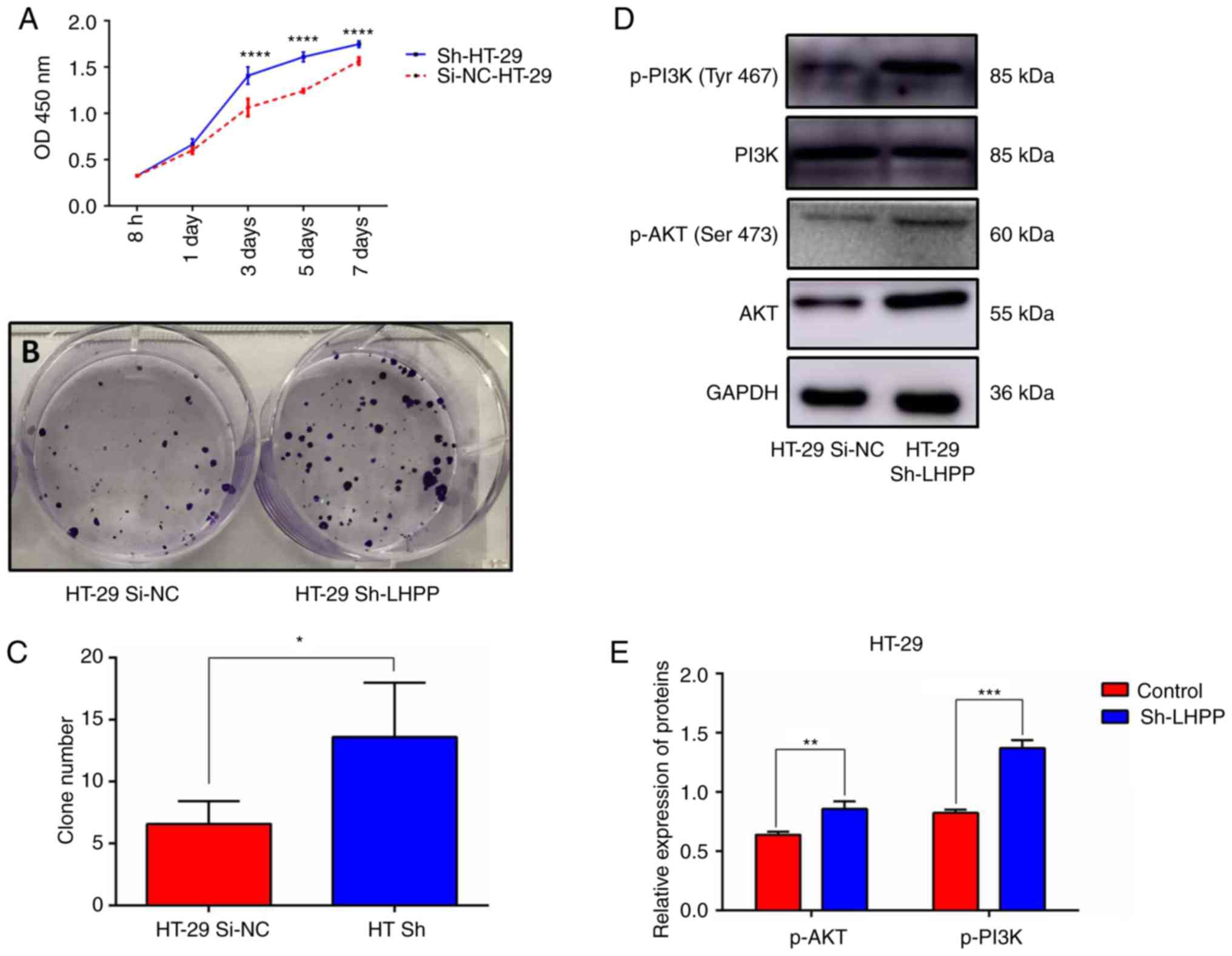
Knockdown of LHPP promotes CRC growth
and proliferation through the PI3K/AKT pathway
Both the CCK-8 and colony formation assays indicated
that knockdown of LHPP promoted cell growth and
proliferation (Fig. 5A-C), which
was consistent with the results in the LHPP overexpression
groups. Subsequently, AKT, PI3K, p-AKT and p-PI3K proteins were
also examined via western blotting. It was revealed that HT-29
cells in the HT-29 Sh-LHPP groups had significantly higher
expression levels of p-AKT and p-PI3K than these levels noted in
the negative control (HT-29 Si-NC) groups (Fig. 5D and E; P<0.01 and P<0.001,
respectively).
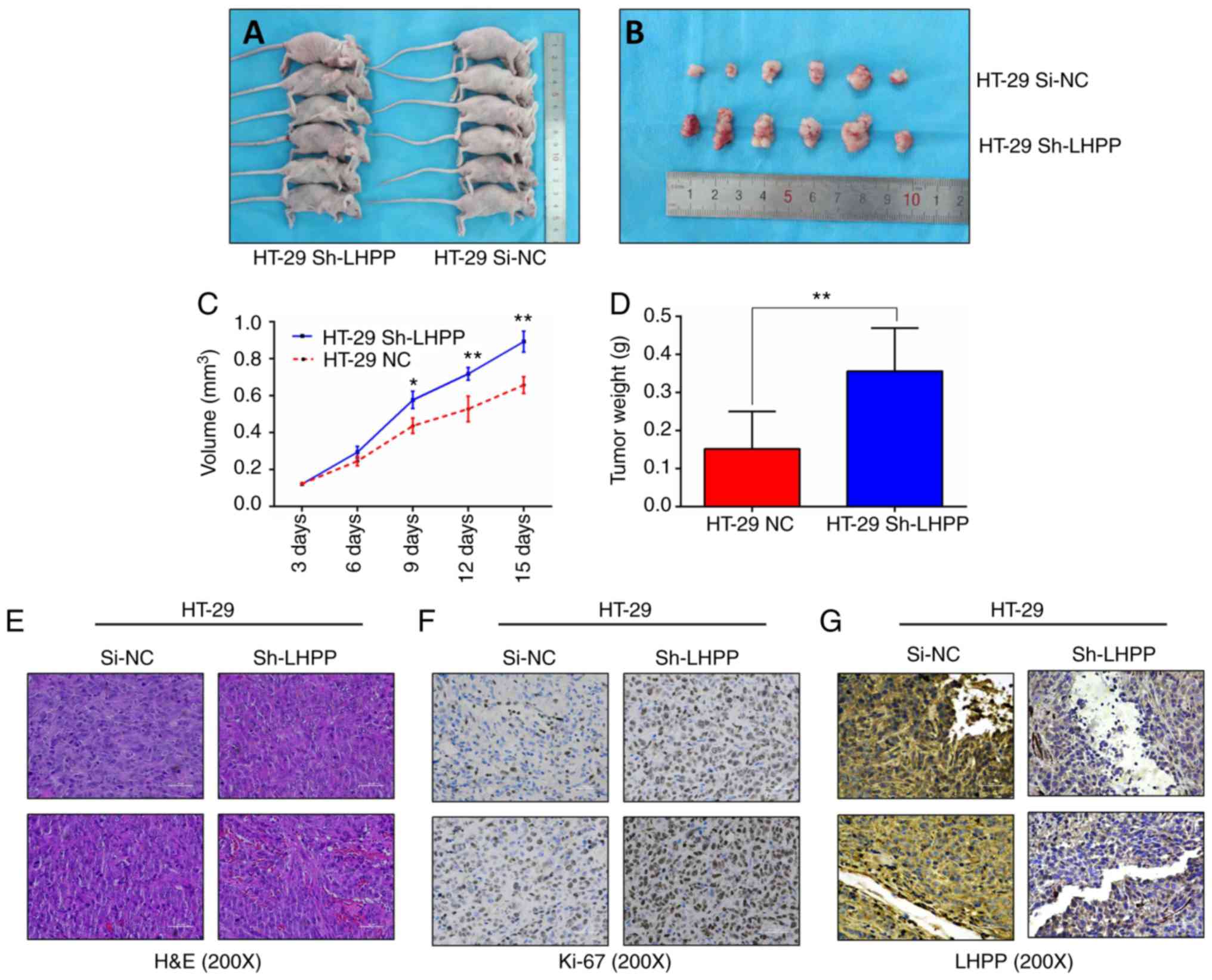
LHPP influences tumor growth in
vivo
In order to confirm whether LHPP expression
could impact the progression of CRC in vivo, HT-29 cells
stably transfected with shRNA-LV (HT-29 Sh-LHPP) and
negative control lentiviruses (HT-29 Si-NC) were injected into nude
mice; each group contained six mice. As presented in Fig. 6A-D, tumors in the HT-29 Sh-LHPP
group grew significantly larger and heavier than tumors in the
si-NC group (P<0.01). The expression of LHPP was confirmed via
IHC (Fig. 6G), and tumor tissues
were confirmed by H&E (Fig.
6E). Ki-67 is a biomarker of cell proliferation in the
diagnosis of CRC. Therefore, the proportions of Ki-67-positive
cells were assessed using IHC. The results revealed that positive
Ki-67 staining was much stronger in the HT-29 Sh-LHPP group
(Fig. 6F). These in vivo
data demonstrated that LHPP may act as a tumor suppressor to
decrease cancer growth.
Cell cycle is influenced by LHPP
expression
CCK-8 assay and colony formation indicated that
LHPP overexpression could inhibit cell proliferation and
tumor growth. In order to reveal the molecular mechanisms
underlying the suppressive effect of LHPP on cell
proliferation and tumor growth, the present study used flow
cytometry to analyze the cell cycle after PI staining. Notably, the
flow cytometry results revealed that LHPP overexpression
contributed to an increase in the G0/G1-phase
and a decrease in S-phase of SW480 cells (Fig. 7A and B). In addition, the percentage
of cells in the G2 phase was elevated in the
OE-LHPP group. On the contrary, the knockdown of LHPP
caused a marked decrease in the cell proportions of
G0/G1-phase and a significant increase in
S-phase (Fig. 7C and D). These data
were consistent with previous experiments.
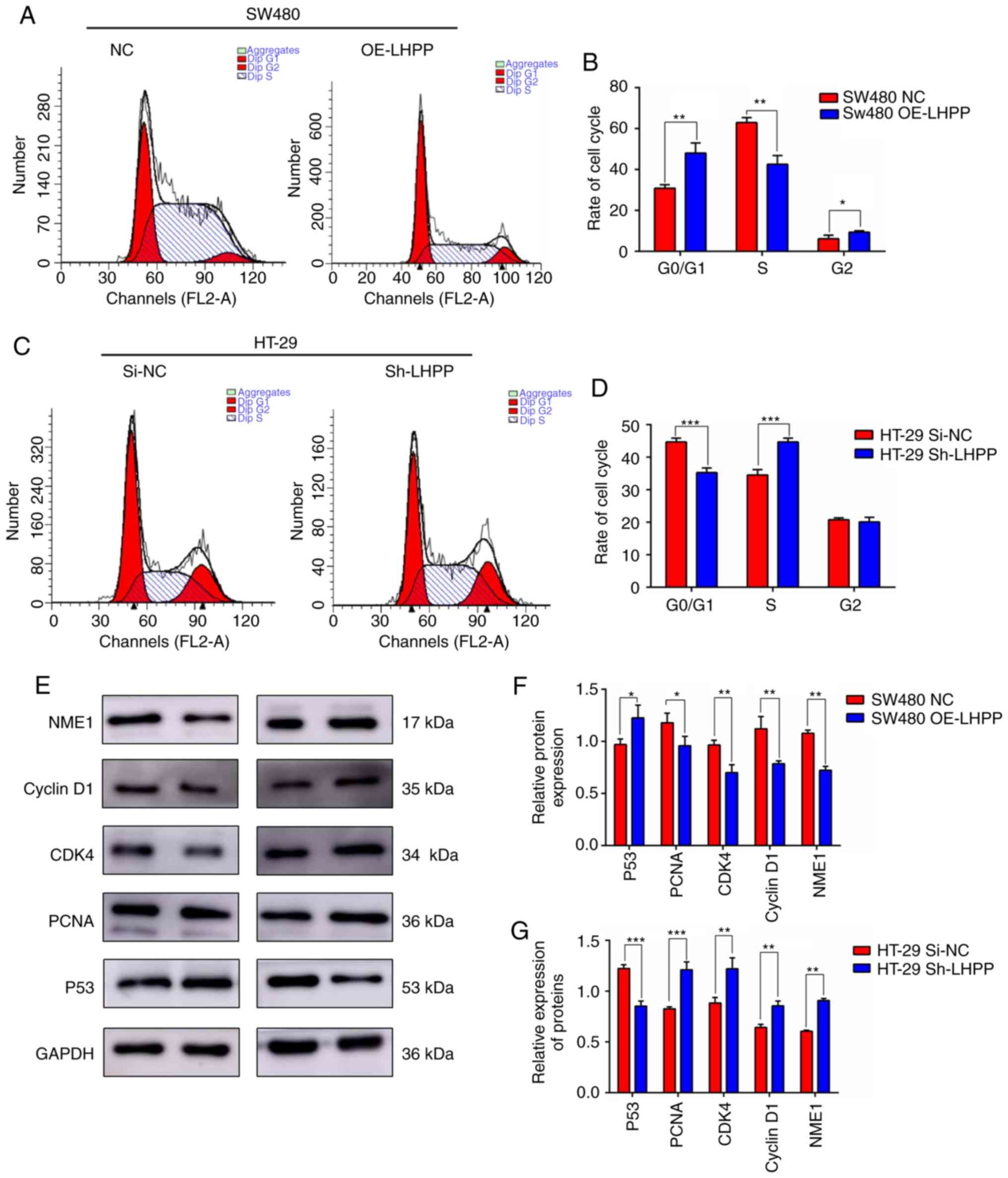 | Figure 7.(A) Effect of LHPP overexpression on
cell cycle distribution; representative images of DNA content
determined by PI staining and flow cytometric analysis. (B) The
percentages of cells in the G0/G1, S and M
phases in the LHPP overexpression SW480 cell line (SW480 OE-LHPP)
compared with the negative control (SW480 Si-NC). (C) The effect of
LHPP downregulation on cell cycle distribution; representative
images of DNA content determined by PI staining and flow cytometry
analysis. (D) The percentage of cells at the
G0/G1, S and M phases in the LHPP-depleted
HT-29 cell line (HT-29 Sh-LHPP) compared with the negative control
(HT-29 Si-NC). (E) Expression of proteins (NME1-1, cyclin D1, CDK4,
PCNA and P53) was examined via western blotting. (F) Relative
expression of proteins was calculated using GraphPad Prism 6 in the
OE-LHPP groups. (G) Relative expression of proteins was calculated
using GraphPad Prism 6 in the Sh-LHPP groups. *P<0.05,
**P<0.01, ***P<0.001. LHPP, phospholysine phosphohistidine
inorganic pyrophosphate phosphatase; PCNA, proliferating cell
nuclear antigen (PCNA); CDK4, cyclin-dependent kinase 4; NME1-1,
nucleoside diphosphate kinase A 1. |
It has been well established that cyclin D1/CDK4 and
P53 are crucial regulators of G1-S transition. The
present study assessed expression changes of cyclinD1/CDK4 and P53
following LHPP upregulation and downregulation. As presented in
Fig. 7E and F, LHPP markedly
promoted the expression of P53 (P<0.05) and suppressed the
expression of cyclinD1/CDK4 (P<0.01), while depletion of LHPP
significantly upregulated cyclinD1/CDK4 expression (P<0.01) and
inhibited P53 expression (P<0.001) (Fig. 7E and G). These data demonstrated
that cell cycle arrest in the G0/G1 phase
mediated by LHPP may be associated with the expression levels of
P53 and cyclinD1/CDK4.
Discussion
Phospholysine phosphohistidine inorganic
pyrophosphate phosphatase (LHPP) is a novel protein histidine
phosphatase that is associated with protein homodimerization and
inorganic diphosphatase activity (6,10). It
hydrolyzes P-N bonds in synthetic substrates in vitro with
low efficiency. LHPP is conserved from the worm to the human and is
poorly characterized (28,29). Recently, low expression of LHPP was
observed in a hepatocellular carcinoma (HCC) mouse model generated
by deletion of PTEN and TSC1 (9).
Moreover, a decrease in LHPP was associated with tumor severity and
worse overall survival in patients with HCC. Consistently, these
results were also identified in cervical cancer. Zheng et al
(11) suggested that upregulation
of LHPP could inhibit cell proliferation, metastasis and promote
apoptosis in cervical cancer cells by modulating AKT level.
Furthermore, patients with low expression levels of LHPP exhibited
markedly larger tumor size, advanced FIGO stage and lymph node
metastasis. Thus, LHPP may act as a tumor suppressor to inhibit the
development of tumors.
The present study focused on the role of LHPP
in colorectal cancer (CRC) proliferation. It was revealed that LHPP
expression was clearly lower in patients with CRC compared with
matched adjacent normal tissues. Patients with lower LHPP
expression levels exhibited significantly larger tumor size,
advanced-stage disease and lymph node metastasis. Furthermore, LHPP
expression was also associated with clinical outcomes. Patients in
the high LHPP expression group had a prolonged overall survival
compared with patients in the low group (median survival time, 26.0
months vs. 21.0 months; P<0.01). These results were not
consistent with data from the The Cancer Genome Atlas (TCGA)
database. Three reasons may be attributed to the difference between
the two clinical research findings. First, the limited sample size
may have had a crucial impact on the clinical research in the
present study (n=52); second, genetic mutations in the Chinese Han
population were slightly different from mutations found in foreign
population. Third, surgical skill may play a crucial role in the
final outcome of patients with CRC. Therefore, future studies
should utilize an increased number of patient cases. In order to
investigate the functions of LHPP in the development of CRC,
cell lines (SW480 and HT-29) were stably transfected with
OE-LHPP and shRNA-LHPP lentiviruses. The data
revealed that overexpression of LHPP suppressed CRC cell growth and
proliferation both in vitro and in vivo. The cell
cycle was arrested in the G0/G1 phase after
increasing the expression of LHPP, whereas knockdown of LHPP
exhibited the opposite effects.
Cyclin D1 and CDK4 (cyclin-dependent kinase 4) have
been confirmed as pivotal regulators in the cell cycle. CDK4/cyclin
D1 complex phosphorylates and inactivates the retinoblastoma (Rb)
protein family (p107, pRb and p130) (30,31).
Phosphorylation of Rb proteins contributes to the activation and
upregulation of E2F target genes, such as E-type cyclins, which
promotes cell proliferation through from the G1 phase to
S phase (32). To date, evidence
have demonstrated that upregulation of the cyclinD1/CDK4 axis is
extremely important in tumor growth. The present study also
confirmed that an increased LHPP level inhibited the expression of
cyclinD1/CDK4 in CRC cells. On the contrary, upregulation of
cyclinD1 and CDK4 was observed following LHPP knockdown. These
results suggest that LHPP may influence activation of the
cyclinD1/CDK4 axis to inhibit tumor proliferation. Proliferating
cell nuclear antigen (PCNA) (33)
is a bio-marker of proliferation in the diagnosis of tumors. Its
expression is significantly increased during the S phase of the
cell cycle (34). PCNA promotes DNA
synthesis of the leading strand and replication of the lagging
strand (35). In the present study,
the expression level of PCNA was decreased by LHPP activation.
Thus, CRC cells were arrested in the G0/G1
phase. These findings indicate that LHPP impedes cancer cell growth
and proliferation by modulating various target proteins.
Tumor suppressor p53 is a key negative regulator of
the cell cycle, as well as cell apoptosis, invasion and migration
(36). Under normal conditions, the
expression level of p53 is low. However, as a transcription factor,
activated p53 could bind to a number of target genes and lead to
various functions, such as cell cycle arrest and apoptosis, in
response to stress signals (36,37).
For instance, p53 promotes the activation of mitochondrial and
death receptor-induced apoptotic pathways to mediate cell
apoptosis. Cell cycle arrest induced by p53 requires p21, which is
the downstream gene of p53 and a type of cyclin-dependent kinase
inhibitor (38). Thus, p53 acts as
a critical barrier against tumor growth. Consistently, the results
from the present study indicated that cell cycle arrest in the
G0/G1 phase was also associated with p53
expression. Knockdown of LHPP markedly decreased p53 expression
level, contributing to the progression of CRC cells.
Histidine phosphorylation is a common but poorly
characterized method of post-translational modification of proteins
(39). NME1 and NME2 are homologous
proteins (88% identical) that, to the best of our knowledge, are
currently the only known mammalian histidine kinases (40). Hindupur et al indicated that
NME1/NME2 activation, in a background of low LHPP expression, is a
crucial event in the development of liver cancer (9). Proteomic analyses of tumors from HCC
mouse models revealed that the expression levels of nucleoside
diphosphate kinase A and B (NME1 and NME2) were upregulated when
compared with the control group. The data also revealed that the
expression level of NME1 was opposite to that of LHPP activity
(9). Thus, histidine
phosphorylation modulated by NME1 and LHPP may be highly associated
with tumorigenesis. Notably, NME1 was first identified as a tumor
metastasis suppressor in melanoma (41), which contributes to genome stability
by possessing three main enzymatic activities, including nucleoside
diphosphate kinase (NDPK), histidine kinase (hisK) and
3′-5′exonuclease (3′-5′ EXO) functions (40,42).
Thus, the functions of NME1 may potentially differ between tumor
types. Investigation of the association between NME1 and LHPP is a
key point for future studies.
The phosphatidylinositol-3-kinase/protein kinase B
(PI3K/AKT) signaling pathway is one of the most classical pathways
involved in tumorigenesis. It is aberrantly activated by
extracellular signals, such as the insulin receptor tyrosine kinase
(InsR), epidermal growth factor (EGF), the associated insulin-like
growth factor 1 receptor (IGF-1R), platelet-derived growth factor
receptors (PDGF-R), and plays an extremely important part in
regulating cell proliferation and maintaining the biological
features of malignant cells (12,13,16).
PI3K can generate PIP3 in the plasma membrane, then PIP3 causes the
aggregation of AKT by interacting with the PH domain of AKT
(12). Subsequently, an increase in
AKT expression or activity becomes the first step for the
progression of various types of tumor. For instance, the PI3K/AKT
signaling pathway promotes metastasis of CRC (16). Activation of AKT is essential for
decreasing apoptosis in breast cancer (17). In addition, PI3K/AKT contributes to
drug resistance by inhibiting the expression of p53 in ovarian
cancer cells (43). Consistently,
the present study observed that the expression levels of p-AKT and
p-PI3K were positively decreased by LHPP overexpression in CRC
cells. On the contrary, knockdown of LHPP increased p-AKT and
p-PI3K expression levels and activity. Furthermore, LHPP-induced
p53 was markedly opposite from the activation of p-AKT and p-PI3K.
Thus, it was speculated that LHPP suppresses the activity of the
PI3K/AKT signaling pathway to promote p-53 expression, contributing
to suppression of cell growth and proliferation.
In conclusion, the present study demonstrated that
the expression level of LHPP was deregulated in CRC tissues
compared with matched noncancerous tissues, which indicated that
LHPP may be a potential tumor suppressor. Further experiments
demonstrated that LHPP could inhibit cell growth and proliferation,
and promote apoptosis by modulating PI3K/AKT expression and
activation. Therefore, the data from the present study may provide
reliable and effective evidence towards developing novel CRC
therapy options. In addition, the present study investigated the
association between LHPP expression and CRC metastasis and
apoptosis. Additional potential mechanisms underlying the
biological functions of LHPP are currently under research.
Acknowledgements
Not applicable.
Funding
This research was supported by grants from the
Science and Technology Foundation of Shaanxi Province
(S2018-YF-ZDSF-0102) and the Shaanxi Province Key Scientific and
Technological Innovation Team Project (2014KCT-24).
Availability of data and materials
The data used to support the findings of this study
are included within the article.
Authors' contributions
DC and BH designed the study. BH, WL, ZL, QZ and XZ
performed the experiments. BH wrote the manuscript. PX, JL and JM
contributed to the experiments, revised and edited the manuscript
and revised it critically for important intellectual content. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The Fifth Affiliated Hospital of Xi'an Jiaotong
University and all the participants signed informed consent. The
protocol of the present study conformed to the ethical guidelines
of the 1975 Declaration of Helsinki (No. XJTU1AF2018LSF-121).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no potential
competing interests.
Reference
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller KD, Nogueira L, Mariotto AB,
Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL and Siegel
RL: Cancer treatment and survivorship statistics, 2019. CA Cancer J
Clin. 69:363–385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sankaranarayanan R, Swaminathan R, Brenner
H, Chen K, Chia KS, Chen JG, Law SC, Ahn YO, Xiang YB, Yeole BB, et
al: Cancer survival in Africa, Asia, and Central America: A
population-based study. Lancet Oncol. 11:165–173. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brenner H, Bouvier AM, Foschi R, Hackl M,
Larsen IK, Lemmens V, Mangone L and Francisci S; EUROCARE Working
Group, : Progress in colorectal cancer survival in Europe from the
late 1980s to the early 21st century: The EUROCARE study. Int J
Cancer. 131:1649–1658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yokoi F, Hiraishi H and Izuhara K:
Molecular cloning of a cDNA for the human phospholysine
phosphohistidine inorganic pyrophosphate phosphatase. J Biochem.
133:607–614. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hiraishi H, Ohmagari T, Otsuka Y, Yokoi F
and Kumon A: Purification and characterization of hepatic inorganic
pyrophosphatase hydrolyzing imidodiphosphate. Arch Biochem Biophys.
341:153–159. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El Sheikh SS, Domin J, Tomtitchong P, Abel
P, Stamp G and Lalani EN: Topographical expression of class IA and
class II phosphoinositide 3-kinase enzymes in normal human tissues
is consistent with a role in differentiation. BMC Clin Pathol.
3:42003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hindupur SK, Colombi M, Fuhs SR, Matter
MS, Guri Y, Adam K, Cornu M, Piscuoglio S, Ng CKY, Betz C, et al:
The protein histidine phosphatase LHPP is a tumour suppressor.
Nature. 555:678–682. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hiraishi H, Yokoi F and Kumon A:
3-phosphohistidine and 6-phospholysine are substrates of a 56-kDa
inorganic pyrophosphatase from bovine liver. Arch Biochem Biophys.
349:381–387. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng J, Dai X, Chen H, Fang C, Chen J and
Sun L: Down-regulation of LHPP in cervical cancer influences cell
proliferation, metastasis and apoptosis by modulating AKT. Biochem
Biophys Res Commun. 503:1108–1114. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Noorolyai S, Shajari N, Baghbani E,
Sadreddini S and Baradaran B: The relation between PI3K/AKT
signalling pathway and cancer. Gene. 698:120–128. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bakin AV, Tomlinson AK, Bhowmick NA, Moses
HL and Arteaga CL: Phosphatidylinositol 3-kinase function is
required for transforming growth factor beta-mediated epithelial to
mesenchymal transition and cell migration. J Biol Chem.
275:36803–36810. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu W, Yang Z and Lu N: A new role for the
PI3K/Akt signaling pathway in the epithelial-mesenchymal
transition. Cell Adhes Migr. 9:317–324. 2015. View Article : Google Scholar
|
|
15
|
Bellacosa A, Kumar CC, Di Cristofano A and
Testa JR: Activation of AKT kinases in cancer: Implications for
therapeutic targeting. Adv Cancer Res. 94:29–86. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnson SM, Gulhati P, Rampy BA, Han Y,
Rychahou PG, Doan HQ, Weiss HL and Evers BM: Novel expression
patterns of PI3K/Akt/mTOR signaling pathway components in
colorectal cancer. J Am Coll Surg. 210:767–778. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Campbell IG, Russell SE, Choong DY,
Montgomery KG, Ciavarella ML, Hooi CS, Cristiano BE, Pearson RB and
Phillips WA: Mutation of the PIK3CA gene in ovarian and breast
cancer. Cancer Res. 64:7678–7681. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Samuels Y, Wang Z, Bardelli A, Silliman N,
Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al:
High frequency of mutations of the PIK3CA gene in human cancers.
Science. 304:5542004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bahrami A, Khazaei M, Hasanzadeh M,
ShahidSales S, Joudi Mashhad M, Farazestanian M, Sadeghnia HR,
Rezayi M, Maftouh M, Hassanian SM and Avan A: Therapeutic potential
of targeting PI3K/AKT pathway in treatment of colorectal cancer:
Rational and progress. J Cell Biochem. 119:2460–2469. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wojnar A, Pula B, Piotrowska A, Jethon A,
Kujawa K, Kobierzycki C, Rys J, Podhorska-Okolow M and Dziegiel P:
Correlation of intensity of MT-I/II expression with Ki-67 and MCM-2
proteins in invasive ductal breast carcinoma. Anticancer Res.
31:3027–3033. 2011.PubMed/NCBI
|
|
21
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
22
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lv Q, Wang G, Zhang Y, Han X, Li H, Le W,
Zhang M, Ma C, Wang P and Ding Q: FABP5 regulates the proliferation
of clear cell renal cell carcinoma cells via the PI3K/AKT signaling
pathway. Int J Oncol. 54:1221–1232. 2019.PubMed/NCBI
|
|
24
|
Euhus DM, Hudd C, LaRegina MC and Johnson
FE: Tumor measurement in the nude mouse. J Surg Oncol. 31:229–234.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wallace J: Humane endpoints and cancer
research. ILAR J. 41:87–93. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Workman P, Aboagye EO, Balkwill F, Balmain
A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DA,
Glennie MJ, et al: Committee of the National Cancer Research
Institute: Guidelines for the welfare and use of animals in cancer
research. Br J Cancer. 102:1555–1577. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Forbes D, Blom HJM, Kostomitsopoulos N,
Moore G and Perretta G: FELASA EUROGUIDE. On the accomodation and
care of animals used for experimental and other scientific
purposes. The Royal Society of Medicine Press Ltd.; 2007
|
|
28
|
Panda S, Srivastava S, Li Z, Vaeth M, Fuhs
SR, Hunter T and Skolnik EY: Identification of PGAM5 as a mammalian
protein histidine phosphatase that plays a central role to
negatively regulate CD4(+) T cells. Mol Cell. 63:457–469. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Klumpp S, Hermesmeier J, Selke D,
Baumeister R, Kellner R and Krieglstein J: Protein histidine
phosphatase: A novel enzyme with potency for neuronal signaling. J
Cereb Blood Flow Metab. 22:1420–1424. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kudo Y, Kitajima S, Ogawa I, Miyauchi M
and Takata T: Down-regulation of Cdk inhibitor p27 in oral squamous
cell carcinoma. Oral Oncol. 41:105–116. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Peurala E, Koivunen P, Haapasaari KM,
Bloigu R and Jukkola-Vuorinen A: The prognostic significance and
value of cyclin D1, CDK4 and p16 in human breast cancer. Breast
Cancer Res. 15:R52013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang H, Chen X, Chen Y, Sun L, Li G, Zhai
M, Zhai W, Kang Q, Gao Y and Qi Y: Antitumor activity of novel
chimeric peptides derived from cyclinD/CDK4 and the protein
transduction domain 4. Amino Acids. 44:499–510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Juríková M, Danihel Ľ, Polák Š and Varga
I: Ki67, PCNA, and MCM proteins: Markers of proliferation in the
diagnosis of breast cancer. Acta Histochem. 118:544–552. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Celis JE and Celis A: Cell cycle-dependent
variations in the distribution of the nuclear protein cyclin
proliferating cell nuclear antigen in cultured cells: Subdivision
of S phase. Proc Natl Acad Sci USA. 82:3262–3266. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Prelich G and Stillman B: Coordinated
leading and lagging strand synthesis during SV40 DNA replication in
vitro requires PCNA. Cell. 53:117–126. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang X, Simpson ER and Brown KA: p53:
Protection against tumor growth beyond effects on cell cycle and
apoptosis. Cancer Res. 75:5001–5007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stewart ZA and Pietenpol JA: p53 Signaling
and cell cycle checkpoints. Chem Res Toxicol. 14:243–263. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Waldman T, Kinzler KW and Vogelstein B:
p21 is necessary for the p53-mediated G1 arrest in human cancer
cells. Cancer Res. 55:5187–5190. 1995.PubMed/NCBI
|
|
39
|
Fuhs SR, Meisenhelder J, Aslanian A, Ma L,
Zagorska A, Stankova M, Binnie A, Al-Obeidi F, Mauger J, Lemke G,
et al: Monoclonal 1- and 3-phosphohistidine antibodies: New tools
to study histidine phosphorylation. Cell. 162:198–210. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hartsough MT, Morrison DK, Salerno M,
Palmieri D, Ouatas T, Mair M, Patrick J and Steeg PS: Nm23-H1
metastasis suppressor phosphorylation of kinase suppressor of Ras
via a histidine protein kinase pathway. J Biol Chem.
277:32389–32399. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Leonard MK, Novak M, Snyder D, Snow G,
Pamidimukkala N, McCorkle JR, Yang XH and Kaetzel DM: The
metastasis suppressor NME1 inhibits melanoma cell motility via
direct transcriptional induction of the integrin beta-3 gene. Exp
Cell Res. 374:85–93. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Freije JM, Blay P, MacDonald NJ, Manrow RE
and Steeg PS: Site-directed mutation of Nm23-H1. Mutations lacking
motility suppressive capacity upon transfection are deficient in
histidine-dependent protein phosphotransferase pathways in vitro. J
Biol Chem. 272:5525–5532. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guo SL, Ye H, Teng Y, Wang YL, Yang G, Li
XB, Zhang C and Yang X, Yang ZZ and Yang X: Akt-p53-miR-365-cyclin
D1/cdc25A axis contributes to gastric tumorigenesis induced by PTEN
deficiency. Nat Commun. 4:25442013. View Article : Google Scholar : PubMed/NCBI
|