Introduction
Osteosarcoma (OS) is a primitive mesenchymal
cell-derived bone tumor that most commonly occurs at the metaphyses
of long bones in adolescents (1).
Although the annual incidence of OS is three to four patients per
million (2), OS is ranked among the
leading causes of cancer-related deaths in the pediatric age group
(3). OS is rarely cured because it
spreads systemically, with the lung being the most common
metastatic hotspot followed by bone (4). The prognosis of OS patients has
greatly improved due to multi-agent chemotherapy, with long-term
survival rates improving from less than 20% to 65–70% (5). Aside from low prevalence and easy
metastasis, large tumor heterogeneity causes difficulty in
significantly improving the survival rates of patients (3). Therefore, there is an urgent research
aim to decipher the molecular mechanisms underlying the occurrence
and development of OS, and explore possible effective molecular
treatment strategies to halt its metastasis.
MicroRNAs (miRNAs) are endogenous ~22 nt, short,
non-coding RNAs that regulate gene expression through direct
post-transcriptional repression of mRNA targets (6,7). Since
the discovery of the first miRNA, lin-4, an increasing
number of miRNAs have been found, and a large number of target
genes have been validated. Mammalian miRNAs have been identified to
play multiple roles in diverse cellular (including cell death and
cell proliferation) and physiological processes (7,8). In
the field of cancer, miRNAs function as oncogenes or tumor
suppressors through their participation in tumor growth, invasion,
angiogenesis, and immune evasion (9). Although the molecular etiology of OS
remains to be further defined, the effects of miRNAs on the
pathogenesis and progression of OS have been widely investigated
(10). A previous study found that
miR-708-5p was downregulated in OS samples compared with
non-neoplastic bone samples (11).
In our bioinformatics analysis, we detected that miR-708-5p was
decreased in OS cells MG63 and SaOS-2 compared with human bone
marrow-derived mesenchymal stem cells (hMSCs). The differential
expression of miR-708-5p between normal and OS cells impelled us to
clarify the specific function and mechanism of miR-708-5p in
OS.
Epithelial cells converting to mesenchymal cells is
the basic process for embryonic development. This process consists
of important phenotypic changes, which include loss of cell-cell
adhesion, lack of cell polarity, and development of migratory and
invasive properties (12).
Carcinoma cells are able to acquire increased motility and impaired
intercellular adhesion to develop mesenchymal cell morphology and
infiltrate ambient tissues through epithelial-to-mesenchymal
transition (EMT) (13). EMT
transcription factors (EMT-TFs), such as Snail1, Twist1, ZEB1 and
ZEB2, activate EMT (14,15). EMT-TFs act as repressors of
E-cadherin genes by directly (Snail1, ZEB1 and ZEB2) or indirectly
(Twist1) binding to the E-boxes on the E-cadherin promoter to
repress its transcription. Moreover, EMT can be regulated by TGF-β,
hypoxia, Notch, and WNT pathways. MicroRNAs such as miR-200,
miR-103/107, and miR-181a can mediate EMT by targeting EMT-TFs
(14,15). However, the relationship between
miR-708-5p and EMT in OS has been insufficiently clarified.
Therefore, we investigated the specific impacts of miR-708-5p on
the metastasis of OS and EMT and explored its target genes in
OS.
Zinc finger E-box-binding homeobox 1 (ZEB1) and its
mammalian paralog ZEB2 belong to the ZEB family within the ZF (zinc
finger) class of homeodomain transcription factors. The ZEB family
plays an important role in normal embryonic development, including
inducing EMT, in which epithelial cells lose polarity and obtain
invasive properties, thus becoming mesenchymal cells (16). The main role of ZEB1 during EMT is
the inhibition of the expression of E-cadherin by binding to its
promoter region, which results in the cells losing their epithelial
properties (17). ZEB1 has been
reported to be associated with proliferation, apoptosis, migration,
invasion, and EMT of OS (18–21).
ZEB1 has been validated to be regulated by several miRNAs in OS.
miR-126 was testified to inhibit proliferation, migration,
invasion, and EMT in OS via targeting ZEB1 (19), miR-130a was reported to inhibit OS
growth and metastasis through directly targeting ZEB1 (20). Nonetheless, the relationship between
miR-708-5p and ZEB1 has not been fully explored in OS.
In the present study, we examined the expression of
miR-708-5p in OS cell lines and reintroduced miR-708-5p in OS cell
lines utilizing cell transfection. The effects of miR-708-5p on OS
cell migration and invasion were observed, the direct target gene
of miR-708-5p was validated, and the EMT of OS cells was explored.
The results will provide novel theoretical strategies for the
diagnosis and treatment of OS. In summary, this study will help us
further decipher the molecular etiology of OS.
Materials and methods
Cell lines and human bone marrow
mesenchymal stem cells (hMSCs)
293T and human OS cell lines MG63, SaOS-2 and normal
bone marrow cell line HS-5 were purchased from the American Type
Culture Collection (Manassas, VA, USA). Upon approval by the Ethics
Committee of the Children's Hospital of Chongqing Medical
University (Chongqing, China), human bone marrow-derived
mesenchymal stem cells (hMSCs) were obtained from three healthy
donors who provided informed consent. hMSCs were isolated from bone
marrow utilizing density gradient centrifugation as previous
described (22,23). In brief, Ficoll medium (TBD Science)
and bone marrow dilution solution (diluted with PBS of 1:1 ratio)
were gently added into a centrifuge tube at a 1:2 ratio and then
centrifuged for 30 min at 644 × g. The cloudiness interface layer
which contains monocytes were aspirated into another centrifuge
tube and mixed with PBS, and the mixture was centrifuged for 3 min
at 161 × g. The supernatant of the mixture was discarded, and then
complete culture medium was added to suspend the primary cells. The
primary cell suspension was transferred onto T-25 flasks, and the
culture medium was discarded to abandon unattached cells. All the
above cells were maintained in Dulbecco's modified Eagle's medium
(Hyclone, GE Healthcare) supplemented with 10% fetal bovine serum
(FBS; Lonsera) and 100 U/ml streptomycin/penicillin at 37°C in 5%
CO2.
Differentially expressed miRNAs
miRNA Expression data from human osteosarcoma (OS)
dataset GSE70367 was downloaded from the National Center for
Biotechnology Information (NCBI) Gene Expression Omnibus (GEO)
database (http://www.ncbi.nlm.nih.gov/geo). The dataset GSE70376
was based on GPL16384 platform. GSE70376 dataset includes miRNAs
expression data in five OS cell lines (MG63, Saos, HOS, NY and
Hu09) and human mesenchymal stem cells (hMSCs).
Cell transfection
miRNA mimics, scramble negative control mimics
(scramble NC mimic) and ZEB1-specific small interfering RNA
(si-ZEB1) were synthesized by GenePharma (Shanghai, China). The
ZEB1 overexpression plasmid pEZ-ZEB1 and empty plasmid pEZ-M35 were
purchased from GeneCopoeia. MicroRNA-708-5p mimic (20 nM) and
scramble NC mimic (20 nM) were transfected into OS cells using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The subsequent
experiments were performed after the cells were transfected for 48
h.
RNA extraction and RT-qPCR
Total RNA was extracted using TRIzol reagent
(Tiangen) in line with the manufacturer's instructions and
first-strand DNA was synthesized using the Reverse Transcriptase
(RT) M-MLV kit with random hexamer primers (Takara). miRNAs were
extracted utilizing Magen Hipure Universal RNA Kits (Magen,
Guangzhou, China) according to manufacturer's instructions.
Target-specific primers were used for reverse transcription [RT
primers (5′-3′) for miR-708-5p:
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCCCAGC and RT primers
(5′-3′) for U6:
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTTGCGC]. TB Green™
Premix Ex Taq™ II (Takara) was used to determine the
expression of miRNAs and mRNAs utilizing the 2−ΔΔCq
(24) method by CFX Connect
Real-Time PCR system (Bio-Rad Laboratories, Inc). GAPDH and U6 were
used as the internal references for mRNAs and miRNAs, respectively.
The sequence of primers for RT-qPCR are listed in Table I.
 | Table I.Primer sequences used for
RT-qPCR. |
Table I.
Primer sequences used for
RT-qPCR.
| Genes | Sequences
(5′-3′) |
|---|
|
miR-708-5p |
|
|
Forward |
GGCGCGAAGGAGCTTACAATCTA |
|
Reverse |
ATCCAGTGCAGGGTCCGAGG |
| U6 |
|
|
Forward |
GTGCTCGCTTCGGCAGCACA |
|
Reverse |
ATCCAGTGCAGGGTCCGAGG |
| GAPDH |
|
|
Forward |
CAGCGACACCCACTCCTC |
|
Reverse |
TGAGGTCCACCACCCTGT |
| MMP2 |
|
|
Forward |
AGACATACATCTTTGCTGGAGACA |
|
Reverse |
CTTGAAGAAGTAGCTGTGACCG |
| MMP7 |
|
|
Forward |
GGAGGAGATGCTCACTTCGAT |
|
Reverse |
AGGAATGTCCCATACCCAAAGA |
| MMP9 |
|
|
Forward |
GGGACGCAGACATCGTCATC |
|
Reverse |
TCGTCATCGTCGAAATGGGC |
|
E-cadherin |
|
|
Forward |
AATGAAGCCCCCATCTTTG |
|
Reverse |
CAGCCAGTTGGCAGTGTCT |
|
N-cadherin |
|
|
Forward |
CCATCAAGCCTGTGGGAATC |
|
Reverse |
GCCGCTTTAAGGCCCTCAT |
|
Vimentin |
|
|
Forward |
TGCTCAATGTTAAGATGGCCCT |
|
Reverse |
TTCAAGGTCATCGTGATGCTGA |
| Snail |
|
|
Forward |
CCATGTCCGGACCCACAC |
|
Reverse |
GCCGGACTCTTGGTGCTT |
| ZEB1 |
|
|
Forward |
CCAAGCTTATGAAAGTTACAAATTATAA |
|
Reverse |
CGGGATCCCTTCAAAGGACTTTGTAGAT |
Wound healing assay
To determine whether miR-708-5p affects the lateral
migration ability of OS cells, cells were digested and resuspended
in DMEM after 48 h of transfection, and then plated into 6-well
plates. When the cell confluence reached 80–90%, a straight scratch
was made using a 10-µl pipette tip. The floating debris were
removed by washing with PBS and the culture medium was replaced
with serum-free DMEM culture media. The final photomicrographs were
captured utilizing an inverted phase contrast microscope (Nikon,
Japan) at a magnification of ×100 at 24 h for MG63 cells or 48 h
for SaOS-2 cells.
Cell migration and invasion
assays
For the cell migration assay, 1.5×104 OS
cells were plated into 8-micron inserts in a 24-well plate
containing serum-free DMEM. For the invasion assay, the Transwell
chambers were pre-coated with 1:3 diluted Matrigel (Solarbio) and
3×104 OS cells were plated into 8-micron inserts. The
lower chambers were filled with 700 µl complete medium with 10%
FBS. Cells were allowed to migrate for 24 h (MG63) or 48 h
(SaOS-2). After migration, the inserts were washed twice with fresh
PBS and fixed with 4% paraformaldehyde for 10 min. After fixation,
the inserts were washed with ddH2O twice and stained
with 5% crystal violet solution for 15 min. After staining, the
inserts were washed with ddH2O and the non-migrated
cells were carefully removed with a cotton swap. The migrated cells
were captured and counted at ×100 magnification under an inverted
phase contrast microscope (Nikon, Japan) in at least five visual
fields.
Western blot analysis
Total protein was extracted after transfection for
at least 48 h. OS cells were lysed with RIPA buffer containing
protease inhibitors and phosphatase inhibitor (Roche Applied
Science), and then centrifuged at 12,000 × g for 25 min at 4°C and
the supernatants were collected. Concentration of protein was
measured by BCA assay and then the protein was boiled for 10 min in
loading buffer. Equal amounts (15 ng) of cell protein were loaded
onto 8–10% SDS-PAGE gels and subsequently transferred onto
polyvinylidene difluoride membranes (PVDF; Millipore) membranes.
The membranes were blocked with 5% bovine serum albumin (BSA;
Solarbio) diluted with TBST and then incubated with primary
antibodies overnight at 4°C. Western blotting was performed
utilizing primary antibodies specific for β-actin (1:1,000
dilution; cat. no. TA-09; Zhongshan Golden Bridge Biotechnology),
MMP2 (1:1,000 dilution; D8N9Y; Cell Signaling Technology), MMP7
(1:1,000 dilution; AF0218; Affinity Biosciences), MMP9 (1:1,000
dilution; AF5228; Affinity Biosciences), E-cadherin (1:500
dilution; sc-21791; Santa Cruz Biotechnology, Inc.), N-cadherin
(1:500 dilution; sc-53488; Santa Cruz Biotechnology, Inc.),
Vimentin (1:500 dilution; WL01960; Wanleibio), ZEB1 (1:1,000
dilution; YN3011; Immunoway) and Snail (1:1,000 dilution; WL01863;
Wanleibio). After incubation with the primary antibody at 4°C
overnight, the membranes were washed with TBST for 30 min, and then
the membranes were incubated with HRP-labelled IgG secondary
antibody (1:5,000 dilution; Zhongshan Goldenbridge Biotechnology)
at 37°C for 1 h. The protein bands were detected using ECL solution
kit (Immobilon Western, Millipore). The protein bands were
quantified using Image Lab software version 5.2 (Bio-Rad
Laboratories, Inc.), and the values are expressed relative to
β-actin.
Dual-luciferase reporter assay
We cloned the sequences from miR-708-5p binding
sites on 3′ UTRs of ZEB1, SEMAC and MAP3K3, respectively, into the
pGL6-miR (Beyotime Institute of Biotechnology) reporter plasmid.
The miRNA-mRNA binding sites were also mutated and cloned into the
reporter plasmid. When the 293T cells reached 70% confluence in
24-well plates, the cells were co-transfected with 400 ng wild-type
(or mutant) reporter plasmids plus miR-708-5p mimic (or scramble NC
mimic), together with 10 ng Renilla reporter plasmid
PRL-SV40 per well, and the mixture was diluted in serum-free
medium. The medium was replaced with complete medium containing 10%
FBS after 6 h, and the 293T cells were lysed to be measured 48 h
post-transfection. Luciferase activity assay was then performed
using the Dual-Luciferase Reporter Assay System (Promega), and
normalized with the Renilla activity.
Immunofluorescence
Cells cultured on crawling pieces were washed with
PBS and fixed with 4% paraformaldehyde for 20 min, and 0.25% Triton
X-100 (Solarbio) was used for permeabilization at 37°C for 15 min.
Next, 0.5% Triton X-100 was used for permeabilizing at room
temperature for 20 min. Goat serum (AR0009, Boster Biological
Technology) was used for blocking at 37°C for 30 min and then the
cells were incubated with primary antibodies specific for ZEB1
(1:200 dilution; sc-515797; Santa Cruz Biotechnology, Inc.) at 4°C
overnight. Then the crawling pieces were washed with PBS for 3
times and Rhodamine (TRITC)-conjugated goat anti-mouse IgG (ZF0313;
Zhongshan Goldenbridge Biotechnology, Beijing, China) was used to
incubate the cells at 37°C for 1 h. Nuclei were stained with
Hoechst 33258 (DA0011, Leagene Biotechnology) at room temperature
for 5 min. Images were captured using a fluorescence microscope at
×400 magnification (DM4B, Leica, Germany). ImageJ software (version
1.48; National Institutes of Health, Bethesda, MD, USA) was
utilized to quantify the cell fluorescence.
Statistical analysis
GraphPad Prism 6 (GraphPad Software, Inc.) was
utilized to analyze the data. All the data are presented as the
mean ± standard deviation from at least three independent
experiments. Unpaired t-test was used to compare two groups.
Comparisons between multiple groups (when >2 groups) were
performed by one-way analysis of variance and Tukey's multiple
comparision test in which pairwise comparisons between all groups
were performed. Statistical significance was set at
P<0.05.
Results
Lower expression of miR-708-5p in OS
cell lines
The microRNA expression data were obtained from Gene
Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo) (accession number:
GSE70367), which partly consists of five OS cell lines (MG63,
Saos-2, Hu09, NY, HOS) and normal control cells (hMSCs). These data
were analyzed using GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/), the
parameter was set as default. The top 50 differentially expressed
miRNAs are presented in a heatmap drawn using HemI (http://hemi.biocuckoo.org/), in which hsa-miR-708-5p
was decreased in the OS cell lines (MG63, SaOS-2, Hu09, NY, HOS)
compared with that noted in the human bone marrow-derived
mesenchymal stem cells (hMSCs) (Fig.
1A). Furthermore, the expression of miR-708-5p was
significantly lower in the MG63 and SaOS-2 cells in comparison with
the hMSCs and HS-5, as validated by RT-qPCR (Fig. 1B-E). Moreover, miR-708-5p was
reported to be downregulated in OS samples and was correlated with
OS prognosis and progression (3).
These findings suggest that miR-708-5p may serve as a therapeutic
miRNA for OS.
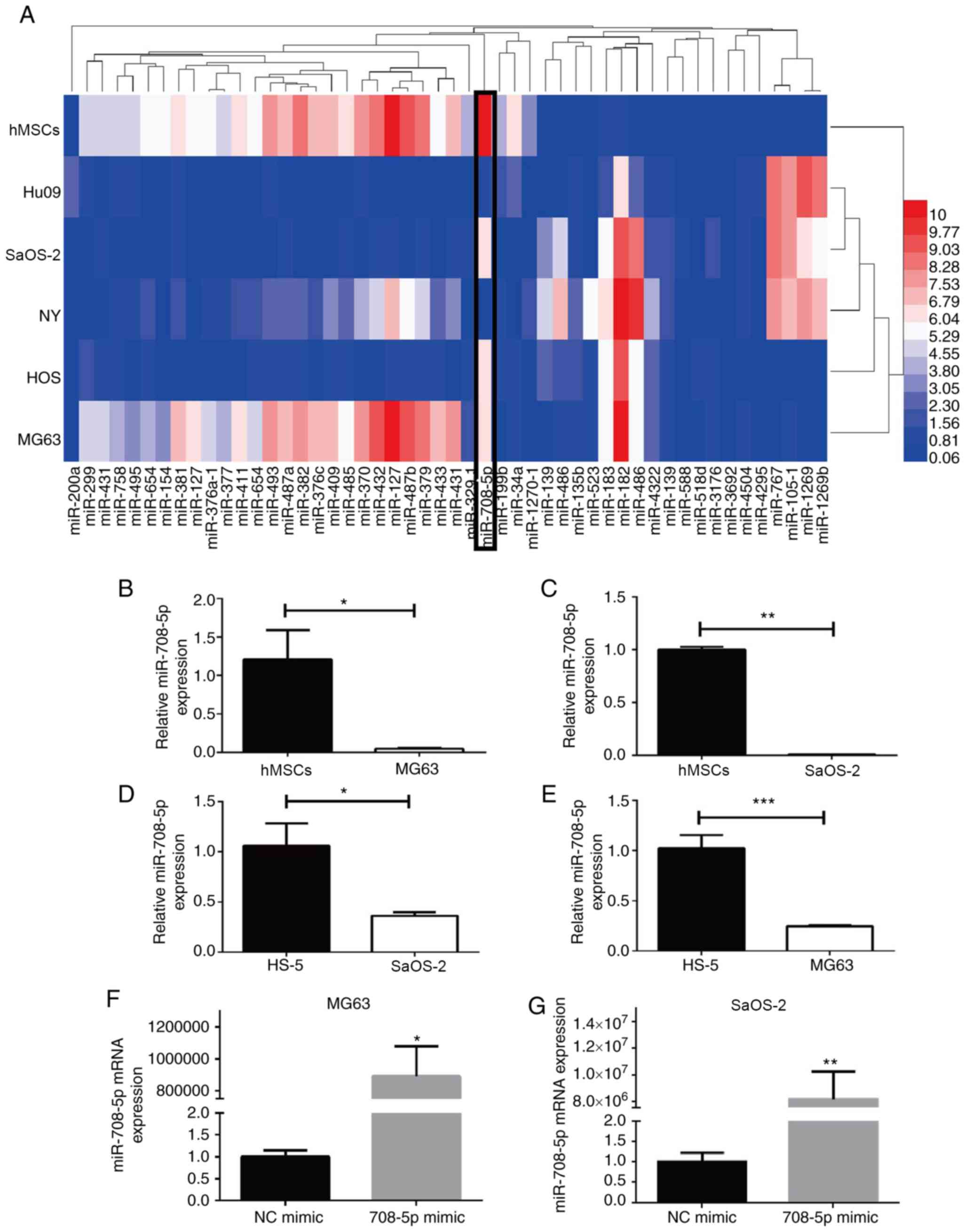 | Figure 1.Differentially expressed miRNAs in OS
cell lines and miR-708-5p expression in OS cell lines. (A) Top 50
differentially expressed miRNAs in OS cell lines (MG63, HOS, NY,
SaOS-2, Hu09) and normal hMSCs. (B-E) Expression of miR-708-5p in
MG63, SaOS-2, hMSC and HS-5 cells by RT-qPCR. All data are
presented as mean ± SD from at least three independent experiments.
*P<0.05, **P<0.01, ***P<0.001 vs. hMSCs or HS-5 cells. (F
and G) Expression of miR-708-5p in MG63 and SaOS-2 cells after
transfection with 20 nM miR-708-5p mimics (708-5p mimics) or the
scramble negative control (NC mimic). All data are presented as
mean ± SD from at least three independent experiments. *P<0.05,
**P<0.01 vs. the NC mimic. OS, osteosarcoma; hMSCs, human bone
marrow-derived mesenchymal stem cells. |
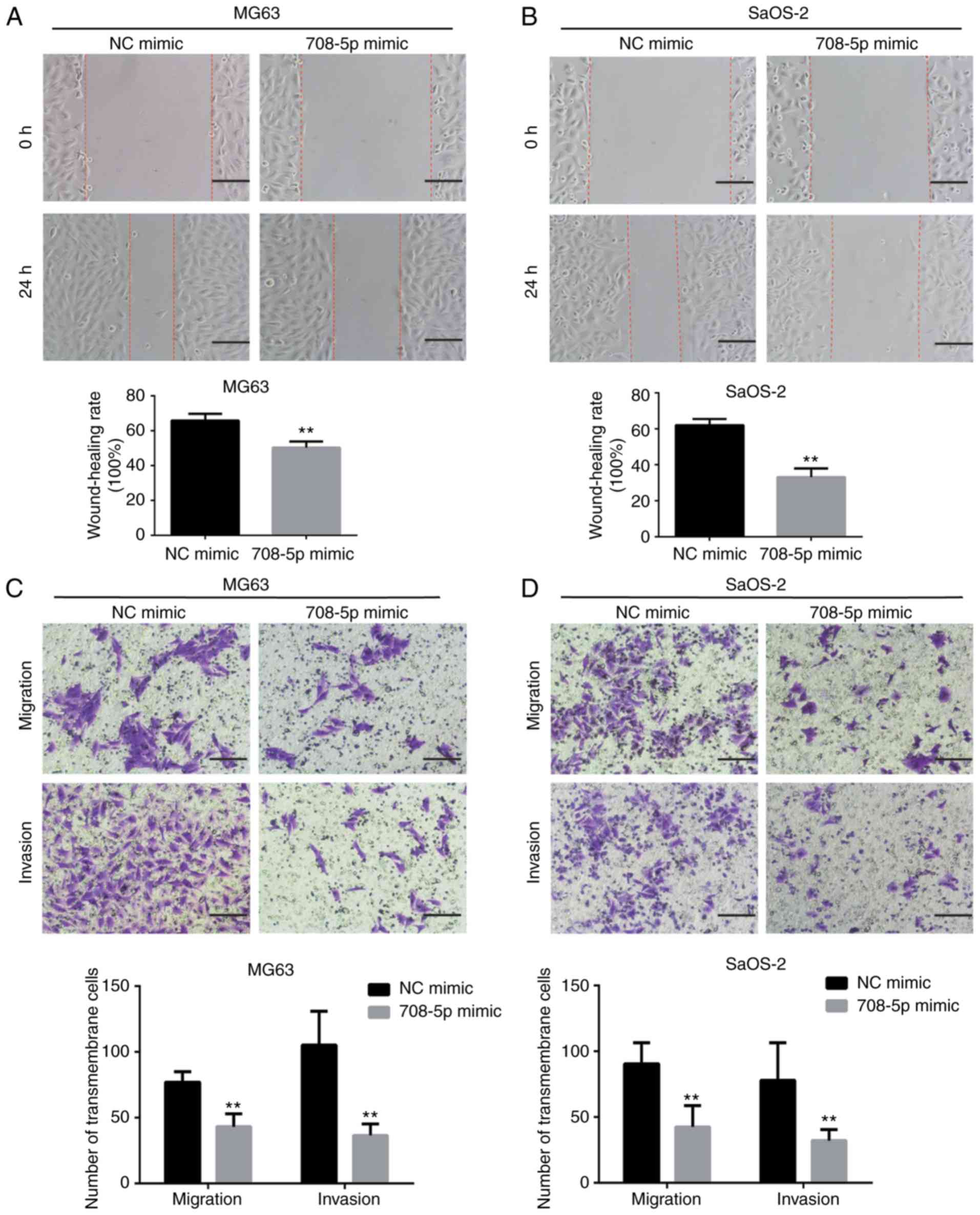
Overexpression of miR-708-5p impairs
the migration and invasion of OS cells
MicroRNA-708-5p mimic (708–5p mimic) and scramble NC
mimic (NC mimic) were transfected into OS MG63 and SaOS-2 cells and
the transfection efficiency was verified (Fig. 1F and G). MG63 and SaOS-2 cells
transfected with 708-5p mimic showed significantly increased
miR-708-5p mRNA expression. To investigate whether miR-708-5p
affects the migration and invasion of OS, wound healing and
Transwell assays were conducted. The wound healing rate of MG63 (24
h) and SaOS-2 (48 h) was significantly decreased in the 708-5p
mimic group compared with the NC mimic group (Fig. 2A and B). Concordant with the wound
healing assay, the numbers of transmembrane migratory and invasive
cells were significantly decreased in the 708-5p mimic group
compared with the NC mimic group in the MG63 and SaOS-2 cells
(Fig. 2C and D).
Given that matrix metalloproteinases (MMPs) play a
crucial role during tumor metastasis, we also assessed the mRNA
(Fig. 3A and B) and protein levels
of MMP2, MMP7, and MMP9 (Fig. 3C-E)
in the MG63 and SaOS-2 cells. The mRNA and protein levels of MMP2,
MMP7, and MMP9 were significantly decreased after miR-708-5p was
overexpressed in MG63 cells (Fig.
3D). Likewise, the mRNA and protein levels of MMP2 and MMP9
were significantly decreased after miR-708-5p was overexpressed in
the SaOS-2 (Fig. 3E).
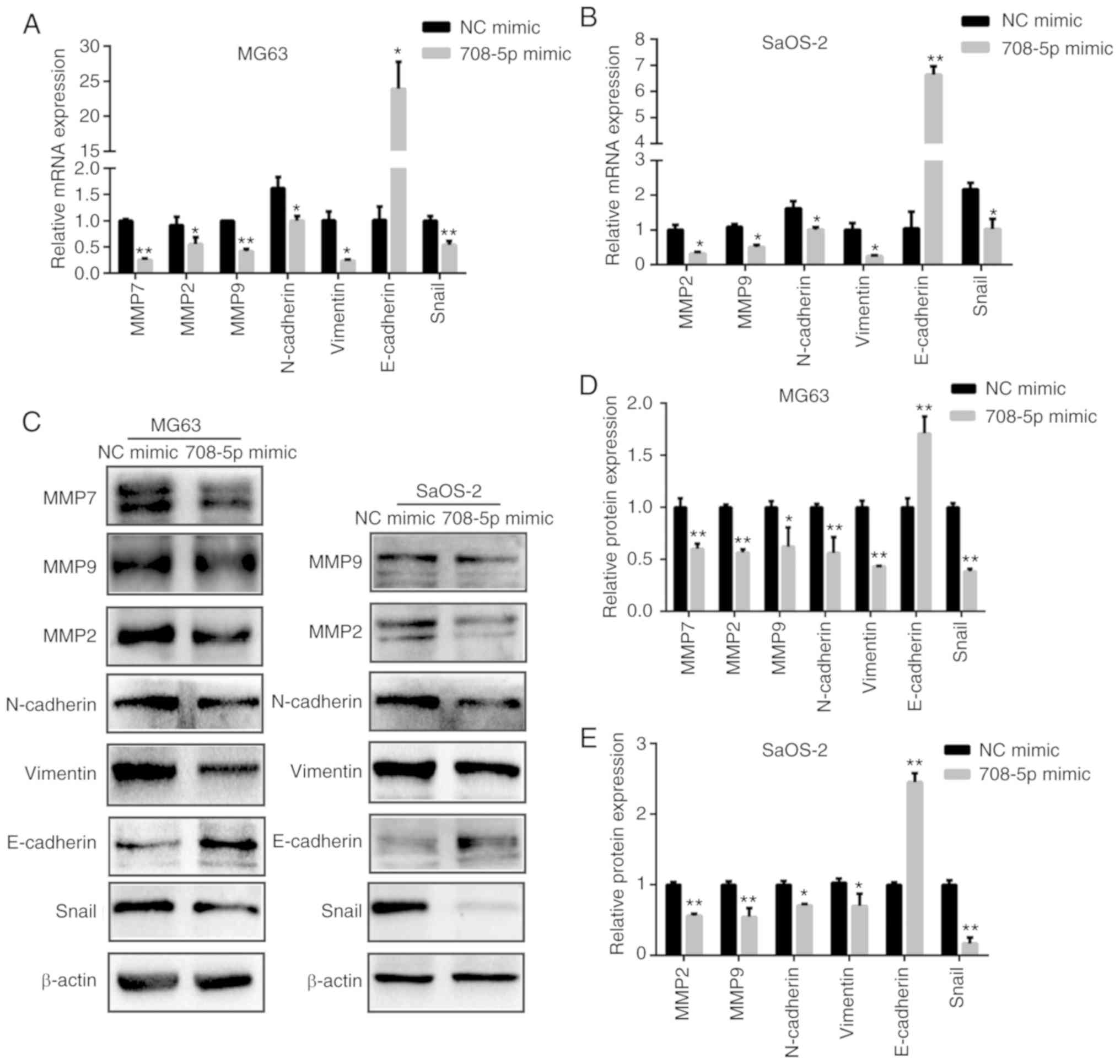 | Figure 3.miR-708-5p suppresses
epithelial-to-mesenchymal transition (EMT) of OS cells. (A) mRNA
and (C, left column) protein levels of MMP2, MMP7, MMP9,
N-cadherin, vimentin, E-cadherin and Snail after miR-708-5p
overexpression (708-5p mimics) compared to the scramble negative
control (NC mimic) in MG63 cells. (B) mRNA and (C, right column)
protein levels of MMP2, MMP9, N-cadherin, vimentin, E-cadherin and
Snail after miR-708-5p overexpression (708-5p mimics) compared to
the scramble negative control (NC mimic) in SaOS-2 cells. Relative
protein expression levels in (D) MG63 and (E) SaOS-2 cells. All
data are presented as mean ± SD from at least three independent
experiments. *P<0.05, **P<0.01, NC mimic vs. 708-5p mimic.
OS, osteosarcoma; MMP, matrix metalloproteinase. |
miR-708-5p suppresses
epithelial-to-mesenchymal transition (EMT) of OS cells
To further investigate whether miR-708-5p affects
EMT in OS, we detected the mRNA and protein levels of EMT-related
biomarkers. As shown in Fig. 3,
miR-708-5p overexpression significantly induced the expression of
epithelial biomarker E-cadherin while significantly repressing
mesenchymal biomarkers N-cadherin, vimentin, and EMT-related
transcription factor Snail. These results indicate that miR-708-5p
impairs the EMT of OS cells.
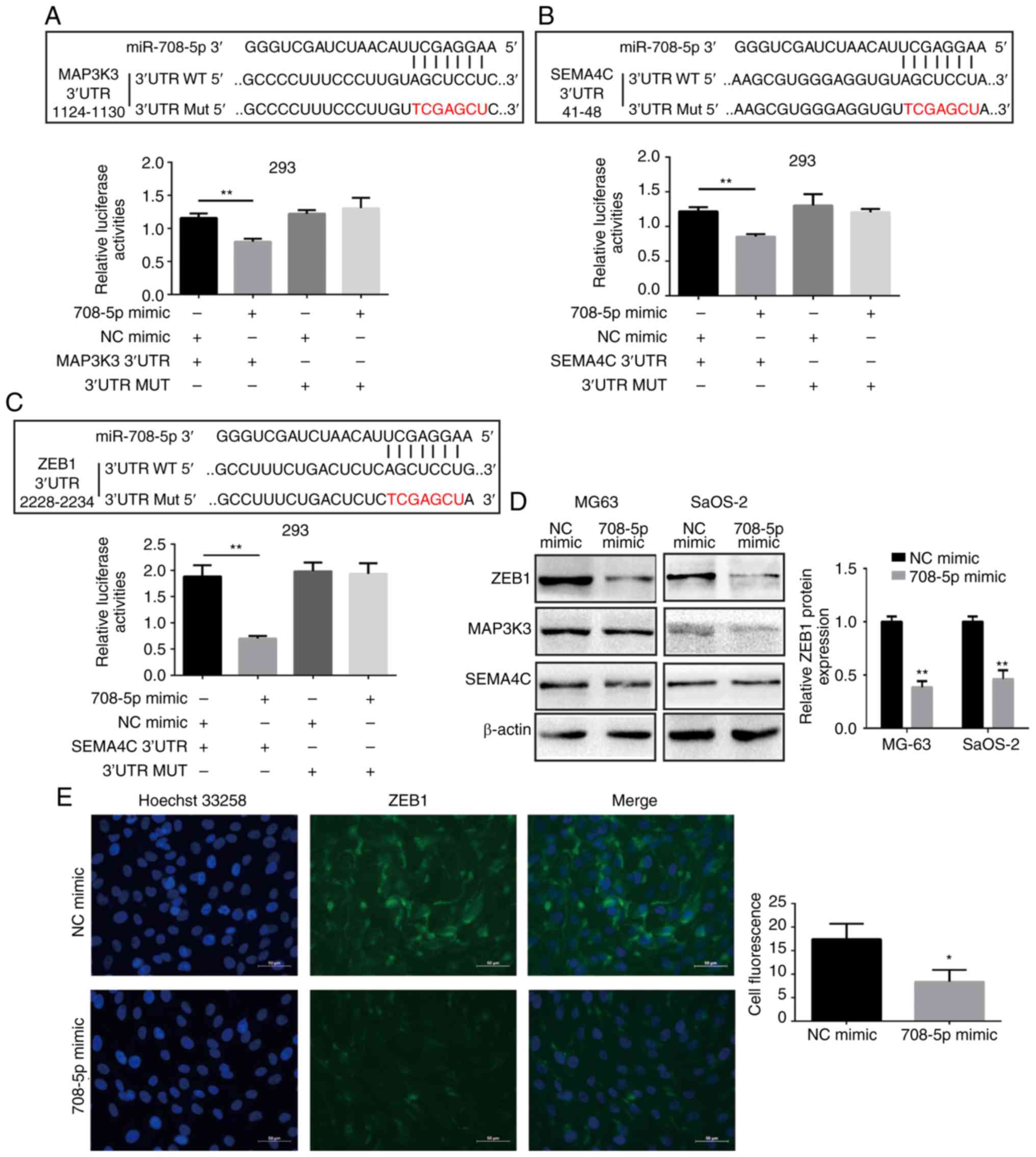
Zinc finger E-box-binding homeobox 1
(ZEB1) is a direct target gene of miR-708-5p
To explore the miR-708-5p-mediated downstream
regulator of OS cells, TargetScan7.2 (http://www.targetscan.org/vert_72/) was applied to
predict the potential target genes of miR-708-5p. Semaphorin 4C
(SEMA4C), mitogen-activated protein kinase kinase kinase 3
(MAP3K3), and ZEB1 are types of tumor
metastasis-associated genes and were thus screened as our candidate
target genes of miR-708-5p. To validate whether miR-708-5p binds to
the 3′UTR (3′ untranslated region) of these candidate genes, we
cloned a part of 3′UTR (containing the binding sites) of candidate
genes into pLG6-miR to construct a dual-luciferase reporter system.
3′UTR luciferase reporter plasmid (wild-type; WT) or 3′UTR mutated
(MUT) luciferase reporter plasmid (mutant type) with microRNA mimic
or NC mimic were co-transfected into 293T cells. miR-708-5p
overexpression significantly decreased the luciferase activities in
the wild-type 3′UTR of SEMA4C, MAP3K3, and ZEB1, whereas the mutant
type exhibited no significant changes (Fig. 4A-C). Nonetheless, only the protein
level of ZEB1 was decreased after miR-708-5p overexpression in MG63
and SaOS-2 cells (Fig. 4D). This
finding indicates that ZEB1 was directly targeted by miR-708-5p in
OS. Moreover, immunofluorescence results showed that miR-708-5p
overexpression could inhibit the fluorescence intensity of ZEB1 in
MG63 cells (Fig. 4E).
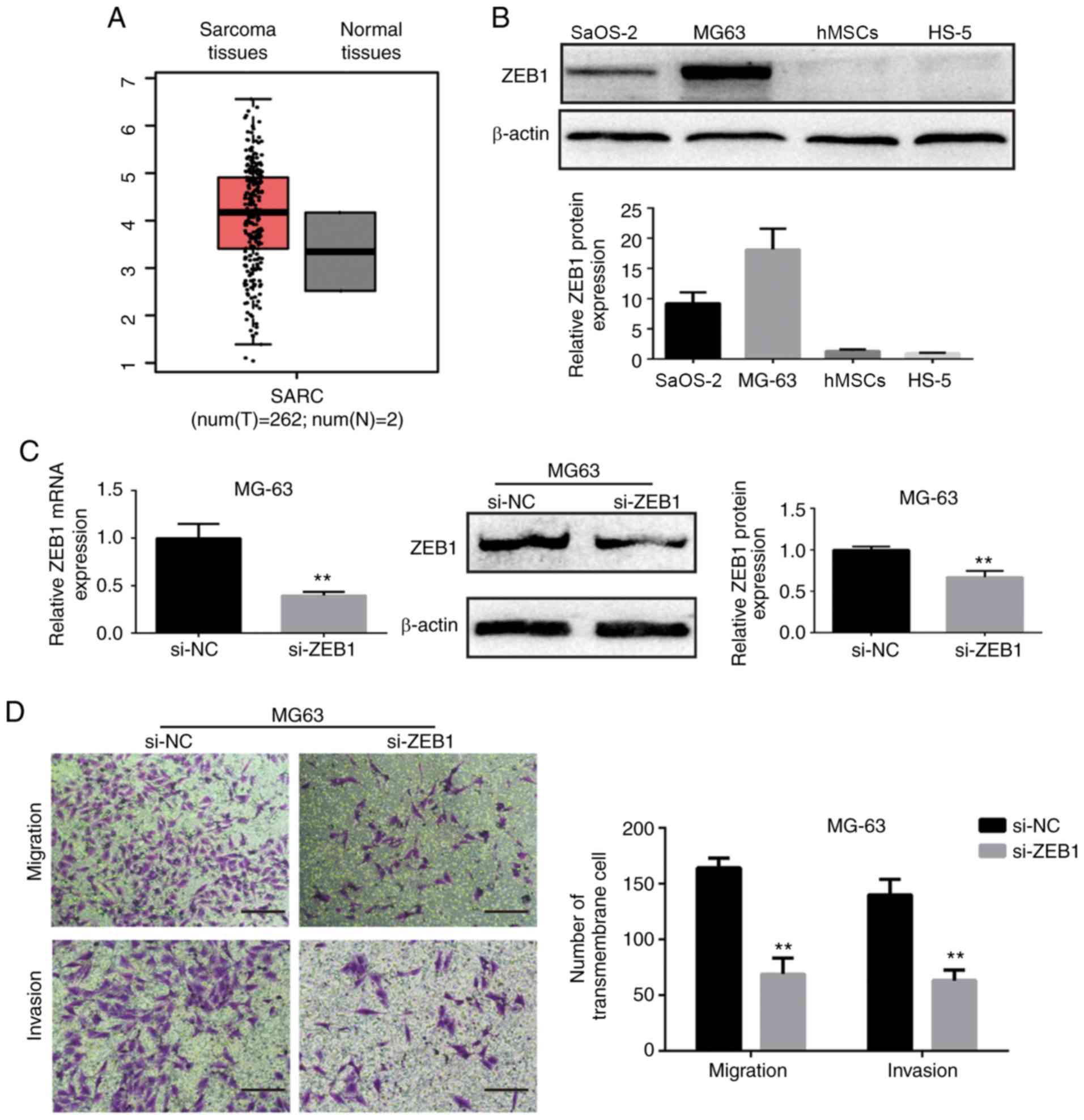
ZEB1 is upregulated in OS and
suppression of ZEB1 inhibits OS cell migration and invasion
We downloaded the expression box plot of ZEB1 in
sarcoma tissues and normal tissues from the Gene Expression
Profiling Interactive Analysis database (GEPIA), a web server for
cancer and normal gene expression profiling and interactive
analyses. As shown in Fig. 5A, ZEB1
was upregulated in sarcoma tissues when compared to that in normal
tissues. Hence, we further assessed the protein levels of ZEB1 in
OS MG63 and SaOS-2 cells. We found that ZEB1 was upregulated in
MG63 and SaOS-2 cells compared with that noted in the hMSCs and
HS-5 (Fig. 5B). These findings
suggest that ZEB1 may act as an oncogene in OS. To better elucidate
the role of ZEB1 in the metastasis of OS, ZEB1 was inhibited
through small-interference RNA (si-ZEB1). ZEB1 mRNA and protein
expression levels were significantly decreased after transfection
with siRNA, as determined by RT-qPCR and Western blot analysis
(Fig. 5C and E). Moreover, the
numbers of invasive and migratory (transmembrane) cells were
decreased after ZEB1 was effectively knocked down (Fig. 5D and F).
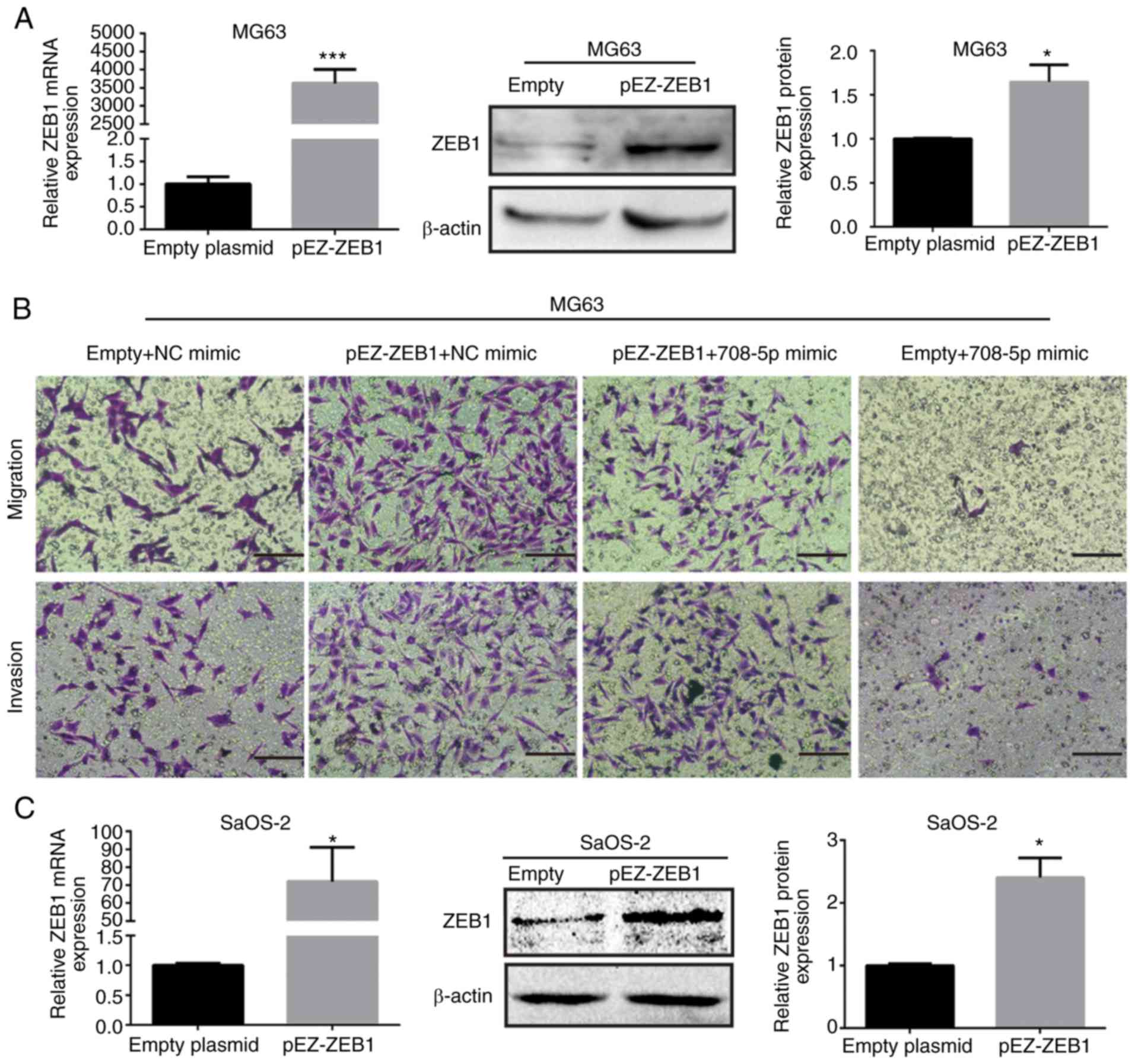
ZEB1 reverses the miR-708-5p-mediated
suppression of cell metastasis
To further confirm whether ZEB1 plays a vital role
in the miR-708-5p-mediated suppression of cell migration and
invasion, rescue experiments were conducted. As shown in Fig. 6A and C, the mRNA and protein
expression levels of ZEB1 were increased after transfected with
pEZ-ZEB1 in MG63 and SaOS-2 cell lines. Subsequently, the numbers
of invasive and migratory (transmembrane) cells were increased
after ZEB1 was reintroduced (Fig. 6B, D
and E). In brief, ZEB1 abrogated the miR-708-5p-mediated
inhibition of metastasis of OS cells.
Discussion
Currently, miRNAs are considered to regulate more
than 60% of human protein-coding genes (25). In addition, miRNAs generally bind to
the 3′-untranslated regions (3′UTR) of target mRNA genes
functioning in two patterns: Endonucleolytic cleavage and
translational repression or deadenylation (26). Except for the 3′UTR of mRNAs,
5′-UTRs or coding regions are factitiously the binding sites of
miRNAs, by which miRNAs could also develop an inhibitory effect on
target genes (27). Our colleagues
previously illuminated the miRNA-mRNA networks through biological
analysis (28), which elucidated
osteosarcomagenesis-associated miRNAs and provided a theoretical
basis for the discovery of the molecular mechanisms of osteosarcoma
(OS) carcinogenesis.
miR-708-5p is one of the key miRNAs in numerous
tumors and acts either as a tumor suppressor or promoter.
miR-708-5p was recently reported to be induced by glucocorticoid
receptor agonists in breast cancer cells. This characteristic of
miR-708-5p was found to inhibit cell proliferation, colony
formation, and cell-cycle arrest (29). Not alone, miR-708-5p was suggested
to be induced by glucocorticoids to impair metastasis through
targeting Rap1B in ovarian cancer (30). Furthermore, suppression of
miR-708-5p by polycomb group was found to increase metastasis by
calcium-induced cell migration (31). Restoration of miR-708-5p expression
in renal cancer cells was found to inhibit cell growth,
clonability, invasion, and migration, and induce apoptosis
(32). In contrast, miR-708-5p has
been found to be involved in the carcinogenesis in several types of
tumors. Silencing of miR-708-5p was found to promote apoptosis and
inhibit growth of bladder carcinoma cells through direct repression
of caspase-2 (33). In colorectal
cancer, miR-708-5p was reported to be significantly upregulated
when compared to normal tissues (34,35).
The proliferation and invasion of colorectal cancer cells were
inhibited. Thus, apoptosis was found to be promoted after silencing
of miR-708 (34).
In summary, miR-708-5p was found to have numerous
functions in multiple types of cancers. Sui et al (36) found that miR-708-5p inhibited the
growth and invasion of OS cells via regulating the URGCP/NF-κB
signaling pathway. However, more specific effects and mechanisms
that miR-708-5p may produce in OS, especially
epithelial-to-mesenchymal transition (EMT), are still ambiguous. To
fill this gap, we investigated differentially expressed microRNAs
(DEmiRNAs) in OS cells and marrow-derived mesenchymal stem cells
(hMSCs). The results showed that miR-708-5p was downregulated in
five OS cell lines (MG63, HOS, NY, SaOS-2, and Hu09) compared with
hMSCs. Tumor-initiating cells of OS may be derived from mesenchymal
stem cells in given bone circumstances (37). The differential expression of
miR-708-5p in OS cells and hMSCs prompted us to consider whether it
is involved in the occurrence and development of OS. Hence, we
further explored the function of miR-708-5p in MG63 and SaOS-2
cells, which are two representative OS cell lines. First, we
validated the downregulation of the expression of miR-708-5p in
MG63 and SaOS-2 cells in comparison with two types of normal cells,
namely, hMSCs and HS-5. Then, we transfected 20 nM of miR-708-5p
mimics into OS cells, and miR-708-5p was successfully
overexpressed. Restoration of miR-708-5p impaired the migration and
invasion abilities of the MG63 and SaOS-2 cells. MMP2, MMP7, and
MMP9 are three members of the matrix metallopeptidase gene family,
which are zinc-dependent enzymes and can cleave the components of
the extracellular matrix and molecules involved in signal
transduction. MMPs are thought to promote cancer cell metastasis
via disrupting the basement membrane and other components of ECM
(38). We further discovered that
the mRNA and protein levels of MMP2, MMP7, and MMP9 were
significantly decreased after miR-708-5p overexpression in MG63
cells. Likewise, MMP2 and MMP9 were significantly decreased after
miR-708-5p overexpression in the SaOS-2 cells.
miR-708-5p has been reported to suppress EMT in
renal cancer and melanoma cells (32,39).
After miR-708-5p transfection, epithelial marker E-cadherin was
significantly increased, whereas mesenchymal markers N-cadherin and
Vimentin were significantly decreased. Likewise, the mRNA and
protein level of Snail, one of EMT-TFs, was decreased after
miR-708-5p transfection. These results indicate that miR-708-5p
inhibited the migration and invasion of OS through impairing
EMT.
Searching and validating one or more direct target
genes are essential in clarifying the specific mechanism of certain
miRNAs in a given disease. We employed bioinformatics software to
predict the target genes of miR-708-5p. Three tumor
metastasis-associated genes, namely, MAP3K3, SEMA4C, and
ZEB1 were chosen, as our candidate genes. Although
miR-708-5p was found to bind to 3′UTR of the predicted target genes
by using dual-luciferase report assay in 293T cells, at the protein
level, only ZEB1 was significantly decreased after
miR-708-5p overexpression in OS MG63 and SaOS-2 cells. Combining
the results of the dual-luciferase report assay and Western blot
analysis, we concluded that ZEB1 was the direct target gene of
miR-708-5p in OS. To determine whether ZEB1 is involved in
miR-708-5p-mediated suppression of OS cell migration and invasion,
we conducted rescue experiments. We discovered that the
re-expression of ZEB1 reversed the inhibition of migration and
invasion mediated by miR-708-5p. This finding suggests that ZEB1
takes part in the miR-708-5p-mediated suppress of the migration and
invasion of OS cells. ZEB1 is highly expressed in OS tissues when
compared to normal tissue (18,40).
Moreover, the protein level of ZEB1 was found to be upregulated in
OS MG63 and SaOS-2 cells unlike that in the hMSCs and HS-5 cells.
Silencing of ZEB1 was found to inhibit the migration and invasion
of MG63 and SaOS-2 cells. Thus, a miR-708-5p/ZEB1/EMT axis that
mediates the metastasis of OS is established. In summary,
miR-708-5p is downregulated in OS, and reinduction of the
overexpression of miR-708-5p could suppress the migration and
invasion abilities of OS cells by directly targeting ZEB1. Thus,
miR-708-5p facilitates inhibition of EMT in OS cells. As a tumor
suppressor in OS, miR-708-5p may serve as a novel diagnostic and
therapeutic biomarker for OS.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81102035).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TF, ZZ and GZ conceived and designed the
experiments. TF performed the experiments. TF, ZZ and GZ collected,
analyzed and interpreted major of the data and wrote the manuscript
and provide final approval of the version to be published. YJ, HW,
XM, DL, YL and LL collected, analyzed and interpreted minor of the
data and revised the manuscript critically for important
intellectual content. All authors read and approved the final
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Human bone marrow were obtained from subjects at the
Children's Hospital of Chongqing Medical University (Chongqing,
China) as approved by the Ethics Committee. All healthy donors
provided informed consents. The study protocol was approved by the
Ethics Committee of Children's Hospital of Chongqing Medical
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ritter J and Bielack SS: Osteosarcoma. Ann
Oncol. 21 (Suppl 7):vii320–vii325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smeland S, Bielack SS, Whelan J, Bernstein
M, Hogendoorn P, Krailo MD, Gorlick R, Janeway KA, Ingleby FC,
Anninga J, et al: Survival and prognosis with osteosarcoma:
Outcomes in more than 2000 patients in the EURAMOS-1 (European and
American Osteosarcoma Study) cohort. Eur J Cancer. 109:36–50. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Botter SM, Neri D and Fuchs B: Recent
advances in osteosarcoma. Curr Opin Pharmacol. 16:15–23. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meazza C and Scanagatta P: Metastatic
osteosarcoma: A challenging multidisciplinary treatment. Expert Rev
Anticancer Ther. 16:543–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: Metazoan MicroRNAs. Cell.
173:20–51. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jones KB, Salah Z, Del Mare S, Galasso M,
Gaudio E, Nuovo GJ, Lovat F, LeBlanc K, Palatini J, Randall RL, et
al: miRNA signatures associate with pathogenesis and progression of
osteosarcoma. Cancer Res. 72:1865–1877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Delsin LEA, Roberto GM, Fedatto PF, Engel
EE, Scrideli CA, Tone LG and Brassesco MS: Downregulated
adhesion-associated microRNAs as prognostic predictors in childhood
osteosarcoma. Pathol Oncol Res. 25:11–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial- mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guarino M: Epithelial-mesenchymal
transition and tumour invasion. Int J Biochem Cell Biol.
39:2153–2160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao TT and Yang MH: Revisiting
epithelial-mesenchymal transition in cancer metastasis: The
connection between epithelial plasticity and stemness. Mol Oncol.
11:792–804. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yeung KT and Yang J:
Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol.
11:28–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vandewalle C, Van Roy F and Berx G: The
role of the ZEB family of transcription factors in development and
disease. Cell Mol Life Sci. 66:773–787. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Xu L, Li A and Han X: The roles
of ZEB1 in tumorigenic progression and epigenetic modifications.
Biomed Pharmacother. 110:400–408. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen A, Zhang Y, Yang H, Xu R and Huang G:
Overexpression of ZEB1 relates to metastasis and invasion in
osteosarcoma. J Surg Oncol. 105:830–834. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang R, Zhang C, Liu G, Gu R and Wu H:
MicroRNA-126 inhibits proliferation, migration, invasion and EMT in
osteosarcoma by targeting ZEB1. J Cell Biochem. 118:3765–3774.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yi L, Liu M and Tang Z: MicroRNA-130a
inhibits growth and metastasis of osteosarcoma cells by directly
targeting ZEB1. Mol Med Rep. 16:3606–3612. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng Y, Luan F, Zeng L, Zhang Y and Ma K:
MiR-429 suppresses the progression and metastasis of osteosarcoma
by targeting ZEB1. EXCLI J. 16:618–627. 2017.PubMed/NCBI
|
|
22
|
Strong AL, Jiang Q, Zhang Q, Zheng S, Boue
SM, Elliott S, Burow ME, Bunnell BA and Wang G: Design, synthesis,
and osteogenic activity of daidzein analogs on human mesenchymal
stem cells. ACS Med Chem Lett. 5:143–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou Y, Jiang R, An L, Wang H, Cheng S,
Qiong S and Weng Y: Benzo[a]pyrene impedes self-renewal and
differentiation of mesenchymal stem cells and influences fracture
healing. Sci Total Environ. 587-588:305–315. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-(-Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lytle JR, Yario TA and Steitz JA: Target
mRNAs are repressed as efficiently by microRNA-binding sites in the
5′ UTR as in the 3′ UTR. Proc Natl Acad Sci USA. 104:9667–9672.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Tang M, Ou L, Hou M, Feng T, Huang
YE, Jin Y, Zhang H and Zuo G: Biological analysis of cancer
specific microRNAs on function modeling in osteosarcoma. Sci Rep.
7:53822017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Senthil Kumar KJ, Gokila Vani M, Hsieh HW,
Lin CC, Liao JW, Chueh PJ and Wang SY: MicroRNA-708 activation by
glucocorticoid receptor agonists regulate breast cancer
tumorigenesis and metastasis via downregulation of NF-κB signaling.
Carcinogenesis. 40:335–348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin KT, Yeh YM, Chuang CM, Yang SY, Chang
JW, Sun SP, Wang YS, Chao KC and Wang LH: Glucocorticoids mediate
induction of microRNA-708 to suppress ovarian cancer metastasis
through targeting Rap1B. Nat Commun. 6:59172015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ryu S, McDonnell K, Choi H, Gao D, Hahn M,
Joshi N, Park SM, Catena R, Do Y, Brazin J, et al: Suppression of
miRNA-708 by polycomb group promotes metastases by calcium-induced
cell migration. Cancer Cell. 23:63–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saini S, Yamamura S, Majid S, Shahryari V,
Hirata H, Tanaka Y and Dahiya R: MicroRNA-708 induces apoptosis and
suppresses tumorigenicity in renal cancer cells. Cancer Res.
71:6208–6219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song T, Zhang X, Zhang L, Dong J, Cai W,
Gao J and Hong B: miR-708 promotes the development of bladder
carcinoma via direct repression of Caspase-2. J Cancer Res Clin
Oncol. 139:1189–1198. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lei SL, Zhao H, Yao HL, Chen Y, Lei ZD,
Liu KJ and Yang Q: Regulatory roles of microRNA-708 and microRNA-31
in proliferation, apoptosis and invasion of colorectal cancer
cells. Oncol Lett. 8:1768–1774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Piepoli A, Tavano F, Copetti M, Mazza T,
Palumbo O, Panza A, di Mola FF, Pazienza V, Mazzoccoli G, Biscaglia
G, et al: MiRNA expression profiles identify drivers in colorectal
and pancreatic cancers. PLoS One. 7:e336632012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sui C, Liu D, Hu Y and Zhang L:
MicroRNA-708-5p affects proliferation and invasion of osteosarcoma
cells by targeting URGCP. Exp Ther Med. 17:2235–2241.
2019.PubMed/NCBI
|
|
37
|
Gambera S, Abarrategi A, Rodríguez-Milla
MA, Mulero F, Menéndez ST, Rodriguez R, Navarro S and García-Castro
J: Role of activator protein-1 complex on the phenotype of human
osteosarcomas generated from mesenchymal stem cells. Stem Cells.
36:1487–1500. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoon SO, Park SJ, Yun CH and Chung AS:
Roles of matrix metalloproteinases in tumor metastasis and
angiogenesis. J Biochem Mol Biol. 36:128–137. 2003.PubMed/NCBI
|
|
39
|
Song XF, Wang QH and Huo R: Effects of
microRNA-708 on epithelial-mesenchymal transition, cell
proliferation and apoptosis in melanoma cells by targeting LEF1
through the Wnt signaling pathway. Pathol Oncol Res. 25:377–389.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yan H, Zhang B, Fang C and Chen L: miR-340
alleviates chemoresistance of osteosarcoma cells by targeting ZEB1.
Anticancer Drugs. 29:440–448. 2018. View Article : Google Scholar : PubMed/NCBI
|