Introduction
Osteosarcoma (OS) is a common malignant bone tumor,
presenting especially in children and young adults, accounting for
approximately 19% of all cases of malignant bone cancers (1). Despite advancements in OS treatment,
including surgery, chemotherapy, and radiotherapy, the long-term
prognosis remains poor (2,3). Therefore, it is necessary to identify
reliable, noninvasive biologic markers to monitor OS and its
progression and to assess the response to therapy.
miRNAs are non-coding single-stranded RNAs of ~22
nucleotides in length. miRNAs downregulate approximately one-third
of mammalian protein-coding mRNAs through mRNA degradation and/or
translational suppression (4).
Increasing evidence indicates that numerous miRNAs play critical
roles in tumorigenesis and tumor progression (5,6).
Certain miRNAs are crucial oncogenes or tumor
suppressors in OS. miR-17, miR-214, and miR-18a-5p are upregulated
in OS and contribute to tumor growth (7–9), while
miR-423-5p, miR-491-5p, and miR-590-3p are downregulated and
function as tumor suppressors (10–12).
Differences in expression profiles between non-neoplastic and OS
tissues can partially elucidate the functions of miRNAs in
tumorigenesis. Previous studies have suggested that miR-95 has
distinct expression profiles in different types of cancers,
including hepatoma, lung cancer, and colorectal cancer (13–15).
However, the function of miR-95 in OS is unknown.
The present study aimed to investigate the role of
miR-95 in OS using in vitro and in vivo models and
publicly available expression data. Our results may help clarify
the mechanism underlying the miR-95-mediated effects on OS tumor
growth, thus potentially establishing it as a diagnostic
target.
Materials and methods
Cell culture and transfection
Human OS cell lines U2OS, MG-63, and Saos-2 were
obtained from the American Type Culture Collection (ATCC). Cells
were cultured in DMEM containing 10% FBS and 100 U/ml
penicillin/streptomycin (TransGen) at 37°C and 5% CO2.
The miR-95 inhibitor, miR-95 mimics, miR-95 antagomir, miR-499a-5p
inhibitor, and miRNA negative control (NC) were purchased from Ribo
Co. (Kunshan, China). The mimics, inhibitor, and negative control
were used to transfect OS cells with Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the
manufacturer's instructions. Following transfection at 48 h,
subsequent experimentation was performed.
Cell proliferation assay
OS cells transfected with the miR-95 inhibitor were
seeded at 3,000 cells/well in 96-well plates, and CCK-8 solution
(10 µl) was added to each well at 0, 24, 48, 72 and 96 h.
Absorbance was measured at 450 nm by GloMax (Promega) after
incubation for 2 h at 37°C.
RNA extraction and quantitative
RT-PCR
Normal bone tissues were surgically obtained from
patients at the Tianjin Union Medical Center. Informed consent was
provided by all subjects, and the Ethics Committee of Tianjin Union
Medical Center (Tianjin, China) approved the study protocol. The
periosteum and marrow of cortical bone were removed. The bone
tissues were ground and digested 3 times in 0.2% collagenase II and
0.25% pancreatin for 1 h on a stirrer to generate single-cell
suspensions. The cells and tissues were harvested in 1 ml
TRIzol.
Total RNA from cell lines and tissue samples were
extracted using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.)
reagent. The RNA concentration was detected using BioDrop
(BioDrop). Reverse transcription was carried out using the
EasyScript First-Stand cDNA Synthesis Kit (TransGen) in accordance
with the manufacturer's instructions. PCR was performed in
accordance with the instructions provided with the SYBR-Green Kit
(TransGen). The thermocycling program was as follows: 95°C for 5
min; (95°C for 15 sec; 60°C for 30 sec; 72°C for 20 sec) ×40
cycles; 72°C for 2 min; 4°C, for the remaining period. The
2−ΔΔCq method was used to calculate the relative
expression of the target gene (16). The forward and reverse primers for
miR-95 were as follows: miR-95-F, 5′-TGCGGTTCAACGGGTATTTATTG-3′ and
miR-95-R, 5′-CCAGTGCAGGGTCCGAGGT-3′. The forward and reverse
primers for U6, used as a reference, were as follows: U6 F,
5′-TGCGGGTGCTCGCTTCGGCAGC-3′ and U6 R,
5′-CCAGTGCAGGGTCCGAGGT-3′. The forward and reverse primers for
SCNN1A were as follows: SCNN1A-F, GCGGTGAGGGAGTGGTA and
SCNN1A-R, GGCGAAGATGAAGTTGC. The forward and reverse primers
for GAPDH, used as a reference, were as follows: GAPDH-F,
5′-CAAGCTCATTTCCTGGTATGAC-3′ and GAPDH-R,
5′-CAGTGAGGGTCTCTCTCTTCCT-3′.
Cell cycle assay
For the propidium iodide (PI) staining assay,
3×105 OS cells were cultured in each well of 6-well
plates. After transfection for 24 h, cells were harvested and fixed
overnight at 4°C with 80% ethanol. Thereafter, the cells were
incubated with PI (Sigma-Aldrich; Merck KGaA) for 20 min at 37°C.
Subsequently, flow cytometry was performed using the FACSCalibur
(BD Biosciences).
For the EdU assay, OS cells were seeded at
7×104 cells on each cell chamber slides and placed in
24-well plates. After transfection, the cells were evaluated in
accordance with the manufacturer's protocol (cat. 10310, Ribo
Co.).
Cell apoptosis assay
For PI/Annexin V staining, the cells were seeded at
3×105 per well and cultured in 6-well plates. At 48 h
after transfection with miR-95 mimics or miR-NC, cells were
harvested and detected using the Annexin V-Apoptosis Kit (BD
Biosciences) in accordance with the manufacturer's
instructions.
For TUNEL staining, tumor tissues were frozen and
sectioned. Thereafter, the sections were fixed, penetrated, and
stained using the TUNEL Assay for In Situ Apoptosis Detection Kit
(Invitrogen; Thermo Fisher Scientific, Inc.) prior to
4′,6-diamidino-2-phenylindole (DAPI) nuclear staining.
Western blot analysis
Cellular and tissue proteins were extracted using
radioimmunoprecipitation (RIPA) (ComWin, Changzhou, China),
determined by BCA protein assay and denatured in loading buffer
(ComWin) at 100°C. Proteins (30 µg) were resolved via SDS-PAGE
(10%) and transferred onto PVDF membranes. Thereafter, the
membranes were blocked with 5% fat-free milk for 1 h at room
temperature and were blotted overnight at 4°C with the primary
antibody against SCNN1A (dilution 1:1,000, cat. no. 10924;
Proteintech, Wuhan, China) and β-actin (dilution 1:1,000, cat. no.
20536; Proteintech). Following washing with TBS + Tween-20 (0.1%),
the membranes were incubated with HRP-labeled goat anti-rabbit IgG
(dilution 1:5,000, cat. no. 00001; Proteintech) for 1 h at room
temperature. Following washing again, the proteins in the blot were
visualized using ECL (Millipore), and images were captured using an
automatic chemiluminescence image analysis system (Tanon 5200;
Tanon Science and Technology Co. Ltd.).
Dual-luciferase reporter assay
For the SCNN1A 3′-UTR luciferase assay, U2OS
cells were transiently cotransfected with the SCNN1A 3′-UTR
luciferase reporter plasmid or the corresponding plasmid with
mutations in the miR-95 binding site, pmirGLO, and the pRL-TK
plasmid. After 30 h, firefly luciferase activity was quantified
using the luciferase reporter assay system (Promega).
Tumor xenografts
The male mice were maintained in a
temperature-controlled room (20±2°C) at a relative humidity of
40–70% with a 12 h light/dark cycle. All animal experiments were
approved by the Ethics Committee of Tianjin Union Medical Center. A
total of 10 NOD/SCID mice (20±2 g) aged 5 weeks were subcutaneously
injected with 2×106 U2OS cells. Three weeks later, the
mice were randomly divided into NC and Antagomir-treated groups
(five mice per group). The mice were peritumorally injected with
the miR-95 antagomir and miR-NC at a concentration of 5 nM every 3
days. The health status and behavior of the mice were monitored
daily. Tumors were measured every 4 days, and tumor volumes were
calculated. After 18 days, the mice were anesthetized by
intraperitoneal injection with 10% chloral hydrate (300 mg/kg), and
then euthanized by cervical dislocation. The mice did not exhibit
any signs of peritonitis before they were sacrificed. Following the
confirmation of death, the tumor tissues were removed from the
mice.
Immunohistochemistry and
immunofluorescence staining
Tissue samples were embedded and sectioned. The
sections were deparaffinized, subjected to antigen retrieval, and
treated with H2O2. Subsequently, the sections
were probed with anti-Ki67 antibody in an immunohistochemistry
assay or analyzed using the TUNEL In Situ Apoptosis Detection Kit
(Invitrogen; Thermo Fisher Scientific, Inc.) via immunofluorescence
before microscopy.
Statistical analysis
All data are expressed as the mean ± SD. The
experiments were performed in triplicate. Significant differences
were analyzed by the Student's t-test or one-way analysis of
variance (ANOVA) followed by Tukey's post-hoc test. Spearman's
correlation analysis was also performed. A P-value <0.05 was
considered as indicative of a statistically significant
difference.
Results
miR-95 is upregulated in OS
We obtained miRNA expression profiles for 20 OS
samples and 15 healthy controls from the GEO database (GSE65071)
(17). To identify candidate
noninvasive biomarkers for OS, we selected the top 10
differentially expressed miRNAs for further analysis (Table I). We first evaluated the expression
levels of 10 miRNAs reported in many tumor types. In particular,
miR-95 and miR-499a-5p are stably upregulated in other tumors,
including hepatocellular carcinoma and breast cancer (13,18,19).
However, the functions of these miRNAs in OS are unclear.
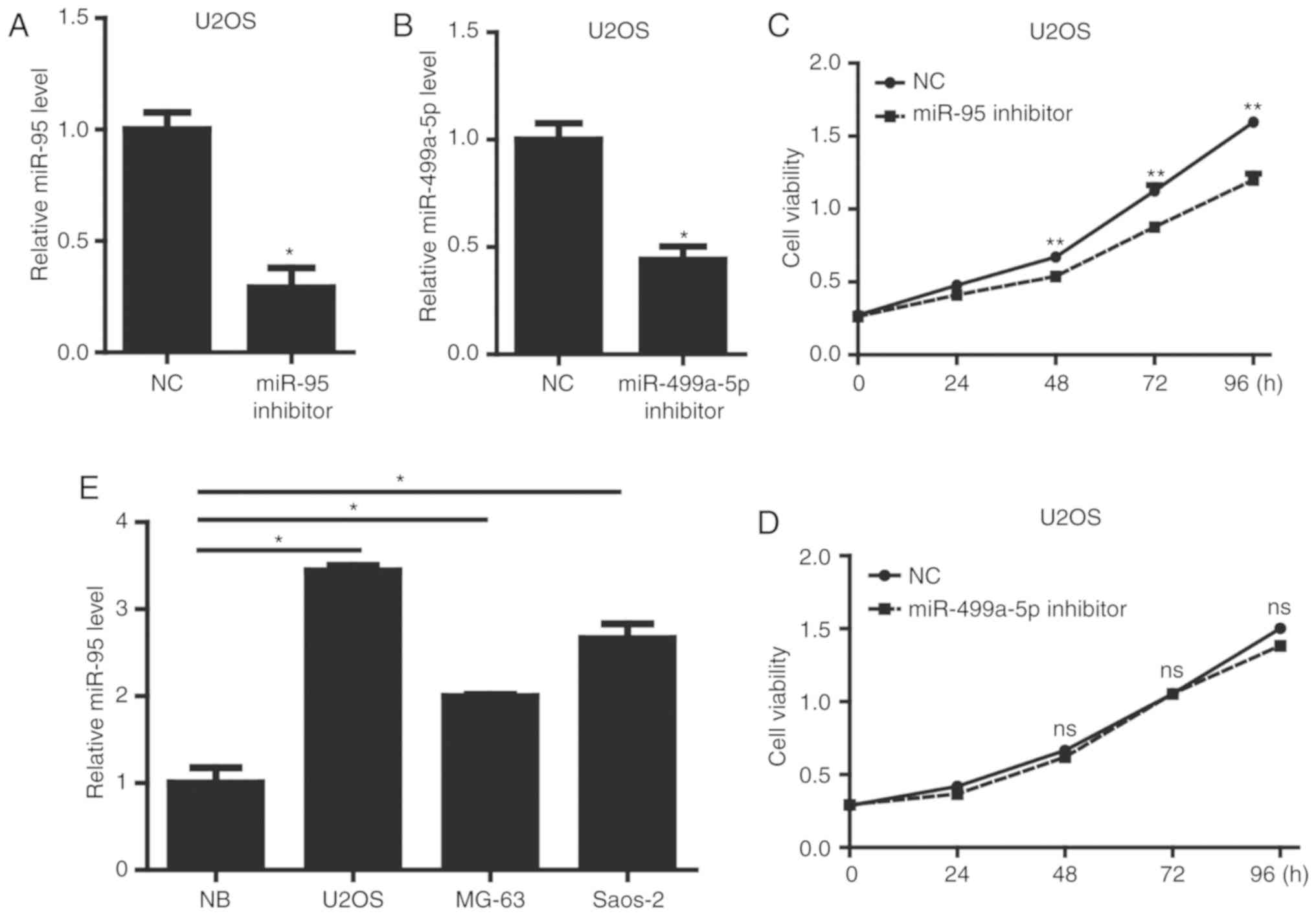
Accordingly, we performed gain-of-function studies by transfecting
an miR-95 or miR-499a-5p inhibitor into U2OS cells. As shown in
Fig. 1A and B, the miR-95 inhibitor
and miR-499a-5p inhibitor significantly downregulated the
expression level of miR-95 or miR-499a-5p, respectively, in U2OS
cells. We then used CCK-8 assays to examine alterations in the
proliferation after miR-95 or miR-499a-5p knockdown in U2OS cells.
Only miR-95 inhibition significantly reduced U2OS cell viability
(Fig. 1C and D). Moreover, we
evaluated the expression level of miR-95 in a panel of 3 OS cell
lines, including U2OS, MG-63 and Saos-2. Compared with the
expression in normal bone (NB) tissues, OS cell lines showed a
significant increase in the levels of miR-95 (Fig. 1E). These results showed that miR-95
is upregulated in OS cells and regulates OS cell viability.
 | Table I.Top 10 differentially expressed
miRNAs in GSE65071 (17). |
Table I.
Top 10 differentially expressed
miRNAs in GSE65071 (17).
| GEO database | miRNA_ID | t | β | logFC | P-value |
|---|
| GSE65071 | miR-663a | 22.36861 | 43.86 | 5.91579 | 1.20E-23 |
|
| miR-31-5p | 22.80988 | 44.554 | 5.74147 | 5.97E-24 |
|
| miR-203a | 17.22939 | 34.737 | 5.51882 | 1.12E-19 |
|
| miR-671-5p | 25.19218 | 48.097 | 5.47697 | 1.65E-25 |
|
| miR-499a-5p | 24.71057 | 47.407 | 5.4298 | 3.33E-25 |
|
| miR-346 | 21.7052 | 42.793 | 5.23055 | 3.52E-23 |
|
| miR-520h | 20.95204 | 41.545 | 5.19294 | 1.23E-22 |
|
| miR-95 | 24.75789 | 47.476 | 5.16319 | 3.11E-25 |
|
| cel-miR-39-3p | 16.99083 | 34.26 | 5.14319 | 1.80E-19 |
|
|
| 13.44421 | 26.486 | 5.13991 | 4.11E-16 |
miR-95 promotes cell cycle progression
and reduces apoptosis in OS cells
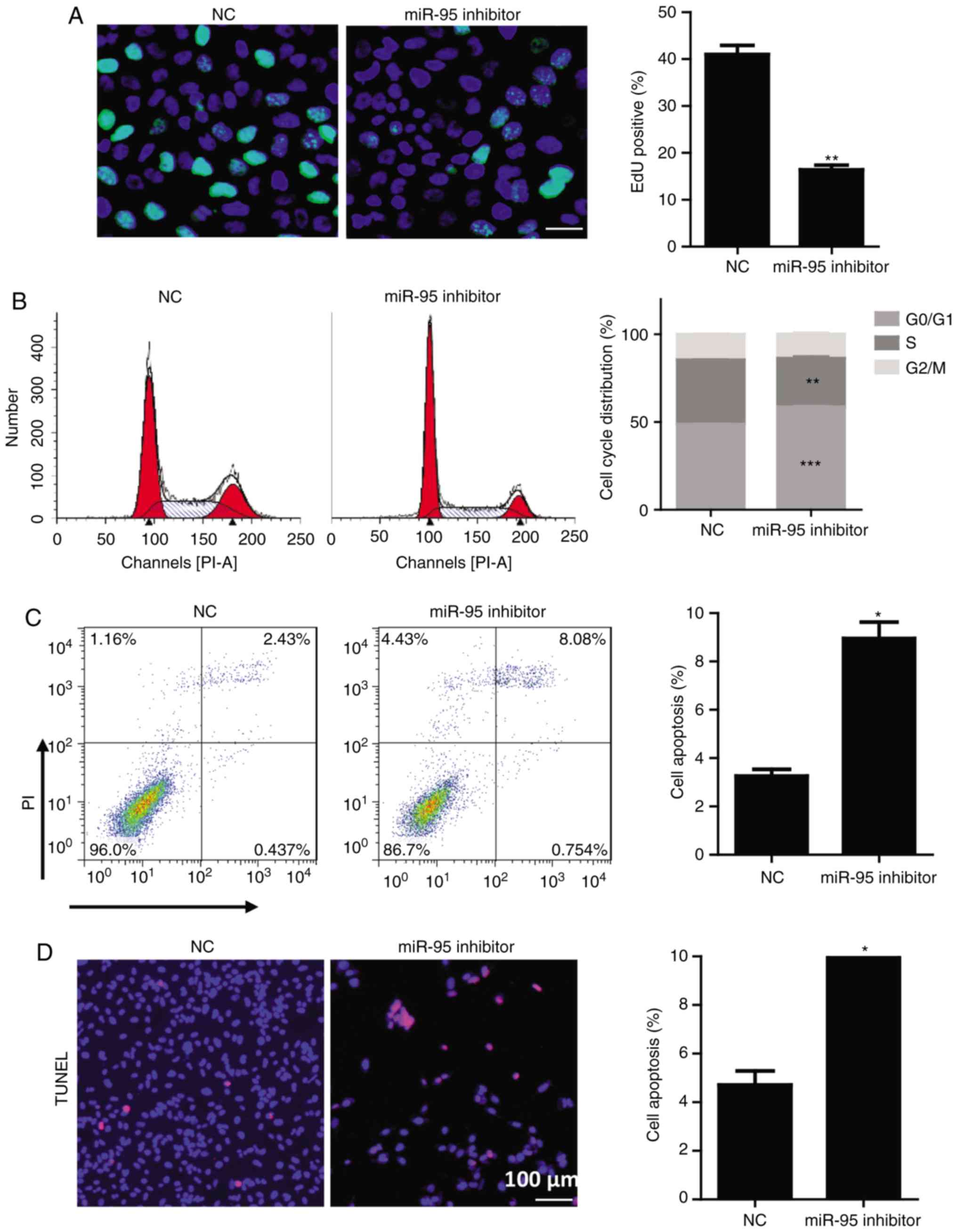
To further investigate the function of miR-95 in
cell cycle progression in OS cells, we performed propidium iodide
(PI) staining and 5-ethynyl-2′-deoxyuridine (EdU) assays. In these
analyses, the miR-95 inhibitor prevented U2OS and Saos-2 cell cycle
progression, as evidenced by a significant reduction in the
percentage of EdU-positive cells and the reduction in the ratio of
cells in the S phase (Figs. 2A and
B and S1A and B) and an
increase in the G0/G1 phase cells (Figs. 2B and S1B). A FACS analysis after PI/Annexin V
staining and TUNEL assays revealed that the miR-95 inhibitor
significantly induced U2OS and Saos-2 cell apoptosis (Figs. 2C and D and S1C and D). These results demonstrated
that miR-95 promotes tumor growth in OS by regulating cell cycle
progression and apoptosis.
SCNN1A is a target of miR-95
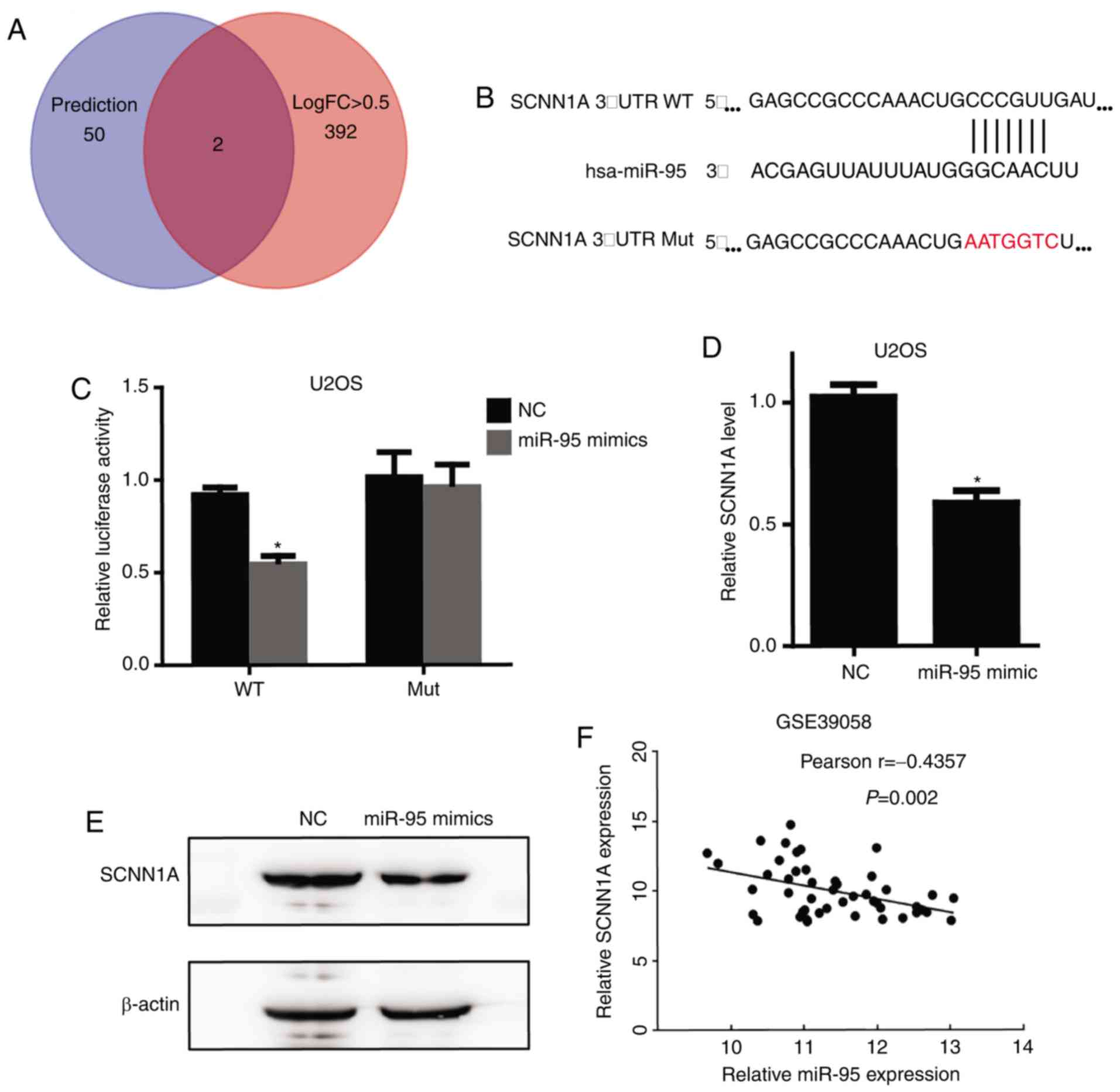
To identify the target genes of miR-95, we initially
used the publicly available miRNA target prediction website
TargetScan (www.targetscan.org), which predicted 52 potential
target genes (Table SI).
Furthermore, we determined the 394 downregulated genes with an
absolute value of LogFC >0.5 in OS from another GEO osteosarcoma
dataset (GSE39058) (Table SII)
(20). Both SCNN1A and
SNX1 were detected in these two datasets (Fig. 3A). However, the function of
SCNN1A, an miR-95 target, in regulating tumorigenesis is
largely unknown. Fig. 3B shows the
SCNN1A binding site for miR-95 and the sequence of mutant
SCNN1A 3′-UTR that we established. Furthermore,
overexpression of miR-95 mimics significantly repressed a
luciferase reporter containing the 3′-UTR of SCNN1A, and
this effect was completely abolished using a mutant SCNN1A
3′-UTR (Fig. 3C). Furthermore, we
performed RT-qPCR and Western blotting to confirm that
SCNN1A is a downstream target of miR-95 (Fig. 3D and E). In accordance with the GEO
database (GSE39058), SCNN1A mRNA expression level was
negatively correlated with the miR-95 expression level in OS
tissues (Fig. 3F).
SCNN1A is required for the biological
functions of miR-95 in OS cells
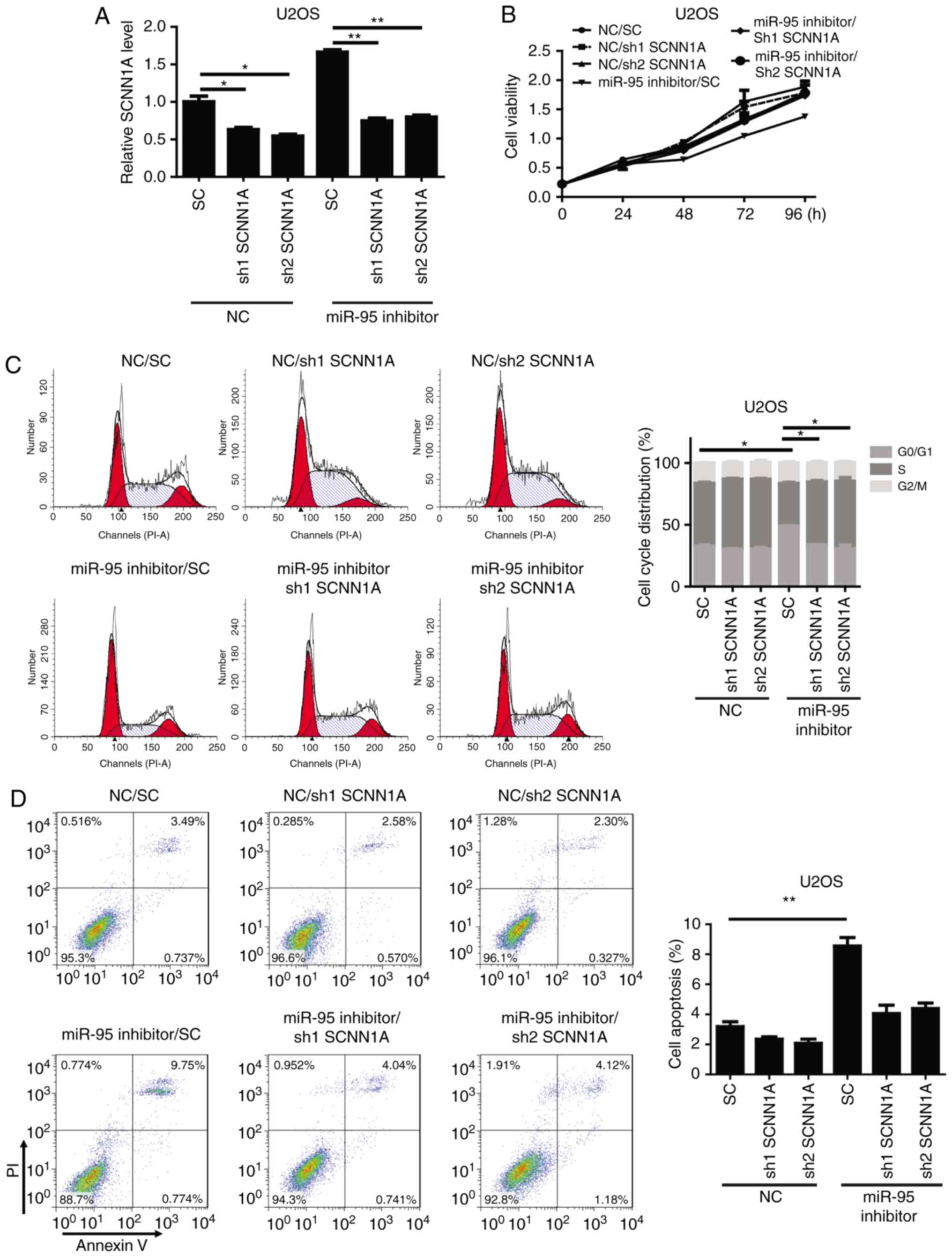
To confirm whether SCNN1A, an miR-95 target,
is critical for the regulation of OS cell cycle progression and
apoptosis, SCNN1A was knocked down by a specific shRNA in
miR-95-silenced U2OS and Saos-2 cells. We observed that shSCNN1A
significantly downregulated SCNN1A in the miR-95-silenced
U2OS and Saos-2 cells (Figs. 4A and
S2A). A functional assay revealed
that the knockdown of SCNN1A significantly reversed the effects of
the miR-95 inhibitor in U2OS cells, resulting in increased cell
viability, enhanced cell cycle progression, and decreased apoptosis
(Figs. 4B-D and S2B-D). These results indicate that SCNN1A
is a critical target of miR-95 during cell cycle regulation and
apoptosis in OS.
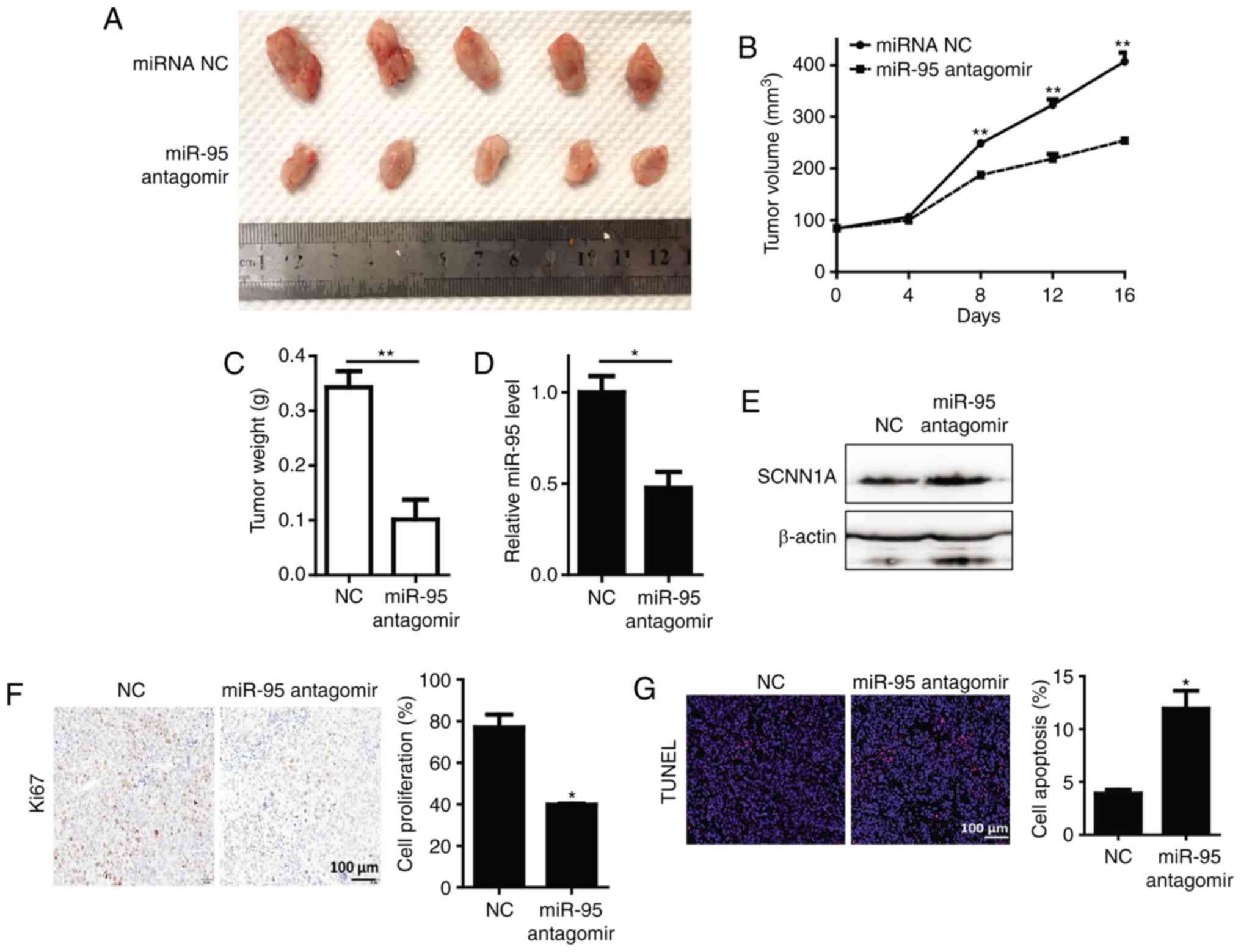
miR-95 antagomir suppresses the growth
of OS in vivo
To determine the effect of miR-95 in vivo, we
generated a subcutaneous nude mouse model. We found that
supplementation with miR-95 antagomir significantly inhibited tumor
growth, and resulted in a reduction in tumor weight and volume in
comparison with the control group (Fig.
5A-C). As expected, expression of miR-95 was also significantly
lower than that of the control group (Fig. 5D). In contrast, expression of SCNN1A
was upregulated in the control group (Fig. 5E). Immunohistochemical staining
revealed that the proliferation of U2OS tumor cells was
significantly decreased in the miR-95-antagomir group (Fig. 5F). Additionally, an increase in cell
apoptosis was shown using an immunofluorescence assay in the
miR-95-antagomir group (Fig.
5G).
Discussion
Osteosarcoma (OS), the most common type of pediatric
bone malignancy, commonly occurs in teenagers or children under 20
years of age (9). Despite marked
advancements, the treatment of metastatic OS remains a challenge
and there is a significant risk of relapse (21). Thus, the identification of novel
molecular diagnostics and therapeutic markers is necessary to
improve the survival rate of OS patients. Emerging evidence has
highlighted the role of miRNAs in human tumorigenesis, including OS
(22,23).
Among numerous miRNAs, miR-95 has been confirmed as
a critical oncogenic factor in human cancers, including hepatoma,
lung cancer, and colorectal cancer (13–15).
Our data indicated that miR-95 levels were markedly higher in OS
cell lines than those noted in normal bone tissues, which are in
contrast to the results reported by Niu et al, who found
that serum miR-95 levels were significantly decreased (24). Additional patient samples,
especially serum samples, are needed to confirm our conclusion that
miR-95 is an oncogenic miRNA in OS. We also evaluated the function
of miR-95 in vitro and in vivo. Previous studies have
reported that miR-95 promotes tumor progression in prostatic
cancer, hepatocellular carcinoma, and triple-negative breast cancer
(13,25,26).
After the loss of miR-95, we observed reduced proliferation, cell
cycle arrest at the G0/G1 phase, and increased apoptosis in U2OS
cells. An miR-95 antagomir suppressed the growth of OS in
vivo.
The epithelial sodium channel (ENaC) is a channel
composed of the following three subunits: SCNN1A, SCNN1B, and
SCNN1G. Of these proteins, SCNN1A expression levels are the highest
(27). Previous studies have
reported that SCNN1A is significantly associated with neuroblastoma
and breast cancer (27,28). Qian et al reported that
SCNN1B functions as a tumor-suppressor that is involved in
triggering the unfolded protein response in gastric cancer cells
(29). In the present study, we
confirmed that miR-95 directly interacts with the 3′-UTR of
SCNN1A, thus regulating OS. Future studies will focus on the
association between SCNN1A and the unfolded protein response.
In summary, our functional assays determined that
miR-95 plays an important role in OS by targeting SCNN1A.
Additionally, miR-95 promotes cell proliferation and inhibits cell
apoptosis in vitro and in vivo. Taken together, these
data support the use of miR-95 as a diagnostic marker and promote
miR-95 as a promising treatment strategy for OS.
Supplementary Material
Supporting Data
Acknowledgements
We thank Dr Jianjun Li of Nankai University for the
invaluable contributions to the discussion and technical
support.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contribution
RT, HL and GX designed the research. YG and SZ
performed the experiments. YJ and YG wrote the manuscript and
assisted in the experimental processes. ZF and QZ contributed
materials and performed the data analysis. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Tianjin Union Medical Center (approval no. 2019-B01).
Both the human tissue and animal experiments were approved by the
Ethics Committee of Tianjin Union Medical Center (Tianjin,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ottaviani G and Jaffe N: The epidemiology
of osteosarcoma. Cancer Treat Res. 152:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Laux CJ, Berzaczy G, Weber M, Lang S,
Dominkus M, Windhager R, Nöbauer-Huhmann IM and Funovics PT: Tumour
response of osteosarcoma to neoadjuvant chemotherapy evaluated by
magnetic resonance imaging as prognostic factor for outcome. Int
Orthop. 39:97–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bentwich L, Avniel A, Karov Y, Aharonov R,
Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, et al:
Identification of hundreds of conserved and nonconserved human
microRNAs. Nat Genet. 37:766–70. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prasad R and Katiyar SK: Down-regulation
of miRNA-106b inhibits growth of melanoma cells by promoting
G1-phase cell cycle arrest and reactivation of p21/WAF1/Cip1
protein. Oncotarget. 5:10636–10649. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hayashita Y, Osada H, Tatematsu Y, Yamada
H, Yanagisawa K, Tomida S, Yatabe Y, Kawahara K, Sekido Y and
Takahashi T: A polycistronic microRNA cluster, miR-17-92, is
overexpressed in human lung cancers and enhances cell
proliferation. Cancer Res. 65:9628–9632. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu C, Peng K, Guo H, Ren X, Hu S, Cai Y,
Han Y, Ma L and Xu P: miR-18a-5p promotes cell invasion and
migration of osteosarcoma by directly targeting IRF2. Oncol Lett.
16:3150–3156. 2018.PubMed/NCBI
|
|
8
|
Wu D, Zhang H, Ji F and Ding W:
MicroRNA-17 promotes osteosarcoma cells proliferation and migration
and inhibits apoptosis by regulating SASH1 expression. Pathol Res
Pract. 215:115–120. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rehei AL, Zhang L, Fu YX, Mu WB, Yang DS,
Liu Y, Zhou SJ and Younusi A: MicroRNA-214 functions as an oncogene
in human osteosarcoma by targeting TRAF3. Eur Rev Med Pharmacol
Sci. 22:5156–5164. 2018.PubMed/NCBI
|
|
10
|
Wang X, Peng L, Gong X, Zhang X, Sun R and
Du J: miR-423-5p inhibits osteosarcoma proliferation and invasion
through directly targeting STMN1. Cell Physiol Biochem.
50:2249–2259. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen T, Li Y, Cao W and Liu Y: miR-491-5p
inhibits osteosarcoma cell proliferation by targeting PKM2. Oncol
Lett. 16:6472–6478. 2018.PubMed/NCBI
|
|
12
|
Wang WT, Qi Q, Zhao P, Li CY, Yin XY and
Yan RB: miR-590-3p is a novel microRNA which suppresses
osteosarcoma progression by targeting SOX9. Biomed Pharmacother.
107:1763–1769. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye J, Yao Y, Song Q, Li S, Hu Z, Yu Y, Hu
C, Da X, Li H, Chen Q and Wang QK: Up-regulation of miR-95-3p in
hepatocellular carcinoma promotes tumorigenesis by targeting p21
expression. Sci Rep. 6:340342016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Zhang C, Hu L, He Y, Shi Z, Tang
S and Chen Y: Abnormal expression of miR-21 and miR-95 in cancer
stem-like cells is associated with radioresistance of lung cancer.
Cancer Invest. 33:165–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qin J, Teng JA, Zhu Z, Chen JX and Wu YY:
Glargine promotes human colorectal cancer cell proliferation via
upregulation of miR-95. Horm Metab Res. 47:861–865. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allen-Rhoades W, Kurenbekova L,
Satterfield L, Parikh N, Fuja D, Shuck RL, Rainusso N, Trucco M,
Barkauskas DA, Jo E, et al: Cross-species identification of a
plasma microRNA signature for detection, therapeutic monitoring,
and prognosis in osteosarcoma. Cancer Med. 4:977–988. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang X, Taeb S, Jahangiri S, Emmenegger
U, Tran E, Bruce J, Mesci A, Korpela E, Vesprini D, Wong CS, et al:
miRNA-95 mediates radioresistance in tumors by targeting the
sphingolipid phosphatase SGPP1. Cancer Res. 73:6972–6986. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qiu D, Han F and Zhuang H: MiR-499
rs3746444 polymorphism and hepatocellular carcinoma risk: A
meta-analysis. J Cancer Res Ther. 14 (Suppl):S490–S493. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kelly AD, Haibe-Kains B, Janeway KA, Hill
KE, Howe E, Goldsmith J, Kurek K, Perez-Atayde AR, Francoeur N, Fan
JB, et al: miRNA paraffin-based studies in osteosarcoma reveal
reproducible independent prognostic profiles at 14q32. Genome Med.
5:22013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heare T, Hensley MA and Dell'Orfano S:
Bone tumors: Osteosarcoma and Ewing's sarcoma. Curr Opin Pediatr.
21:365–372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Braconi C, Henry JC, Kogure T, Schmittgen
T and Patel T: The role of microRNAs in human liver cancers. Semin
Oncol. 38:752–763. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Niu J, Sun Y, Guo Q, Niu D and Liu B:
Serum miR-95-3p is a diagnostic and prognostic marker for
osteosarcoma. Springerplus. 5:19472016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xi M, Cheng L, Hua W, Zhou YL, Gao QL,
Yang JX and Qi SY: MicroRNA-95-3p promoted the development of
prostatic cancer via regulating DKK3 and activating Wnt/β-catenin
pathway. Eur Rev Med Pharmacol Sci. 23:1002–1011. 2019.PubMed/NCBI
|
|
26
|
Turashvili G, Lightbody ED, Tyryshkin K,
SenGupta SK, Elliott BE, Madarnas Y, Ghaffari A, Day A and Nicol
CJB: Novel prognostic and predictive microRNA targets for
triple-negative breast cancer. FASEB J. May 29–2018.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
27
|
You H, Ge Y, Zhang J, Cao Y, Xing J, Su D,
Huang Y, Li M, Qu S, Sun F and Liang X: Derlin-1 promotes
ubiquitylation and degradation of the epithelial Na+
channel, ENaC. J Cell Sci. 130:1027–1036. 2017.PubMed/NCBI
|
|
28
|
Varley KE, Gertz J, Roberts BS, Davis NS,
Bowling KM, Kirby MK, Nesmith AS, Oliver PG, Grizzle WE, Forero A,
et al: Recurrent read-through fusion transcripts in breast cancer.
Breast Cancer Res Treat. 146:287–297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qian Y, Wong CC, Xu J, Chen H, Zhang Y,
Kang W, Wang H, Zhang L, Li W, Chu ESH, et al: Sodium channel
subunit SCNN1AB suppresses gastric cancer growth and metastasis via
GRP78 degradation. Cancer Res. 77:1968–1982. 2017. View Article : Google Scholar : PubMed/NCBI
|