Introduction
Signal transducer and activator of transcription 3
(STAT3) is the vital member of the STAT family, which participates
in various cellular processes such as proliferation, apoptosis,
migration and differentiation (1).
In response to multiple extracellular stimuli that include diverse
growth factors as well as cytokines, cytoplasmic STAT3 becomes
phosphorylated at the residue Tyr705 by Janus-associated kinase
(JAK), forms homo- or heterodimers and translocates to the nucleus.
In the nucleus, STAT3 activates transcription of target genes by
binding to their promoters (2). Due
to its importance in cellular processes, STAT3 phosphorylation is
tightly regulated. Phosphorylated STAT3 can be dephosphorylated by
multiple protein tyrosine phosphatases (PTPs), including PTR
receptor type D (PTPRD), PTR receptor type T (PTPRT), Src homology
region 2 domain-containing phosphatase-1 (SHP1), Src homology
region 2 domain-containing phosphatase-2 (SHP2) or maternally
expressed gene 2 (MEG2), resulting in its inactivation
(3).
Cervical cancer is the second most commonly
diagnosed malignant tumor in women worldwide and the third-leading
cause of cancer-related deaths among women in less-developed
countries (4). Despite therapeutic
advances in recent years, the mortality and relapse rates of
cervical cancer remain high. Therefore, it is urgent to develop
novel effective therapeutic approaches to improve the outcome of
cervical cancer treatment. STAT3 has been reported to be abnormally
activated in cervical cancer, and a high level of STAT3
phosphorylation predicts poor clinical prognosis (5–7).
Furthermore, aberrant activation of STAT3 has been shown to promote
the growth of cervical cancer cells (8). All these findings suggest that STAT3
is a promising molecular target for the treatment of cervical
cancer.
Ropivacaine, a voltage-gated sodium channel
inhibitor, is widely used as a local anesthetic to relieve pain in
clinical practice (9). Apart from
the anesthetic advantages, increasing clinical evidence
demonstrates that the use of local anesthetics during cancer
surgery may decrease the risk of recurrence and metastasis
(10,11). In addition, recent studies show that
ropivacaine exhibits anticancer properties in multiple types of
cancers, including leukemia (12),
hepatocellular carcinoma (13),
colon cancer (14), pancreatic
cancer (14), gastric cancer
(15), esophageal cancer (16) and lung cancer (17) by diverse molecular mechanisms.
However, whether and how ropivacaine suppresses cervical cancer
cell growth remains unknown. In the present study, ropivacaine was
found to exert an inhibitory effect on the viability of cervical
cancer cells by suppressing cell cycle progression and promoting
cell apoptosis. Mechanistically, ropivacaine inhibited cervical
cancer cell growth by targeting the microRNA-96
(miR-96)/MEG2/pSTAT3 axis.
Materials and methods
Cell lines and cell culture
The human cervical cancer cell lines Siha and Caski
were obtained from Fengh Bio. Inc. (Changsha, China) and
EK-Bioscience, Inc. (Shanghai, China), respectively. The cells were
maintained in Dulbecco's modified Eagle's medium (DMEM; HyClone,
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (FBS) (Gibco, Life Technologies Inc., Thermo Fisher
Scientific, Inc.) and 1% penicillin/streptomycin (Sigma-Aldrich;
Merck KGaA) in a humidified incubator with 5% CO2 at
37°C.
For stable overexpression of STAT-3C, SiHa cells
were transfected with the pCMV-2B-STAT-3C vector using
Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's protocol. SiHa cells transfected with
the pCMV-2B backbone were used as control cells. Stable
transformants were selected with 800 µg/ml G418 (Calbiochem) for
two weeks before subsequent experimentation.
Antibodies and reagents
STAT3 (cat. no. #9139, dilution 1:2,000),
phospho-STAT3-Tyr705 (cat. no. #4093, dilution 1:500), cyclin D1
(cat. no. #2922, dilution 1:2,000), survivin (cat. no. #2802,
dilution 1:1,000), JAK2 (cat. no. #3230, dilution 1:500) and
phospho-JAK2-Tyr1007 (cat. no. #4406, dilution 1:500) antibodies
were purchased from Cell Signaling Technology. PTPRT (cat. no.
ab115848, dilution 1:1,000), SHP-1 (cat. no. ab124942, dilution
1:1,000) and SHP-2 (cat. no. ab131541, dilution 1:1,000) antibodies
were purchased from Abcam. PTPRD (cat. no. LS-C153706, dilution
1:1,000) antibody was purchased from Life Span BioSciences.
γ-tubulin (cat. no. sc-7396, dilution 1:500) antibody was obtained
from Santa Cruz Biotechnology. MEG2 (cat. no. MAB2668, dilution
1:1,000) was purchased from R&D Systems, Inc. Ropivacaine was
obtained from Selleck Chemicals. Pervanadate were purchased from
Sigma Aldrich; Merck KGaA.
Western blot analysis
Protein was harvested from cells and the protein
concentration was measured using the BCA method (Pierce Chemical;
Thermo Fisher Scientific, Inc.). Protein (50 µg) was resolved by
10% sodium dodecyl sulphate-polyacrylamide (SDS-PAGE) gel
electrophoresis and transferred to a nitrocellulose membrane. After
being blocked with 5% nonfat milk in TBST for 2 h at room
temperature, the membrane was incubated with the primary antibody
at 4°C overnight, followed by incubation with the corresponding
HRP-conjugated secondary antibody for 2 h at room temperature.
After being incubated with freshly prepared chemiluminescence
solution for 1–5 min, the membrane was observed using enhanced
chemiluminescence (ECL) detection reagent (Thermo Fisher
Scientific, Inc.) in an ImageQuant LAS4000 chemiluminescence imager
(GE Healthcare Life Sciences). Image Lab version 3.0 software
(Bio-Lab) was used to perform the densitometric analysis of blots.
Tubulin was used as the loading control.
Nuclear fraction isolation
The nuclear proteins of SiHa or Caski cells were
extracted using the NE-PER Nuclear and Cytoplasmic Extraction
Reagents (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Briefly, after being washed with
ice-cold PBS and centrifuged at 500 × g at 4°C for 3 min, the cells
was treated with CER I, shaken vigorously for 15–30 sec and
incubated at 4°C for 10 min. Then, CER II was added into the lysis,
followed by vigorous vortexing for 10 sec and incubation on ice for
1 min. After centrifugation at 16,000 × g at 4°C for 5 min, nuclear
extraction reagent was added to the pellet followed by incubation
at 4°C for 40 min with occasional vortexing. The nuclear fraction
was finally harvested following centrifugation at 16,000 × g at 4°C
for 10 min.
Luciferase assay
SiHa and Caski cells were seeded in 24-well plates
at a density of 2×104 cells/per well. On the second day,
the cells was co-transfected with 200 ng of pAPRE-luc reporter
plasmid and 20 ng pRL-TK plasmid as an internal control. After 24
h, the transferred cells were treated with different concentrations
(0, 0.25, 0.5, 1 mM) of ropivacaine for 72 h. Then, the cells were
collected for luciferase activity measurement with the
Dual-Luciferase Reporter Assay system (Promega) according to the
manufacturer's instructions.
CCK-8 assay
SiHa or Caski cells were plated into 96-well plates
at a density of 2,000 cells per well. On the following day, the
cells were treated with different concentrations (0, 0.25, 0.5, 1
mM) of ropivacaine for 72 h. Then, the viability of the cells was
detected by measurement of absorbance at 450 nm with the Cell
Counting Kit-8 (CCK-8) (Dojindo Laboratories) assay following the
manufacturer's instructions. The experimental optical density (OD)
value was normalized to the control OD value. Assays were performed
in triplicate, and the results are presented as means ± standard
deviation (SD).
BrdU incorporation assay
SiHa or Caski cells (2,000 cells/well) were plated
into 96-well plates. On the second day, the cells were treated with
the indicated concentrations (0, 0.25, 0.5, 1 mM) of ropivacaine
for 72 h. Then, the cells were further incubated in culture medium
with 10 µM BrdU for an additional 10 h. Subsequently, the cells
were fixed, and BrdU incorporation was determined using a BrdU Cell
Proliferation ELISA kit (cat. no. 11647229001, Roche Applied
Science) following the manufacturer's protocol. The BrdU density
was analyzed by measurement of absorbance at 450 nm. The
experiments were performed in triplicate and the results are
presented as means ± standard deviation (SD).
Colony formation assay
SiHa and Caski cells were seeded into 6-well plates
at a density of 2,000 cells per well. Cells were allowed to grow in
DMEM/10% FBS medium with different concentrations (0, 0.25, 0.5, 1
mM) of ropivacaine for 72 h for 2 weeks. After being fixed with
ice-cold methanol and stained with 0.5% crystal violet solution for
20 min at room temperature, the colony numbers were calculated
using ImageJ software, version 1.49 [National Institutes of Health
(NIH), Bethesda, MD, USA]. Assays were performed in triplicate, and
the results are presented as means ± standard deviation (SD).
Cell cycle analysis
SiHa and Caski cells (1×105 cells/well)
were seeded into a 6-well plate. The following day, the cells were
treated with different concentrations (0, 0.25, 0.5, 1 mM) of
ropivacaine for 72 h and then collected by trypsinization. After
being washed twice with ice-cold phosphate-buffered saline (PBS),
the cells were fixed with 70% EtOH overnight. After incubation with
200 µg/ml DNase-free RNase A for 30 min at 37°C, the cells were
stained with 0.05 mg/ml PI for 15 min in the dark at room
temperature, followed by flow cytometric analysis.
Cell apoptosis analysis
SiHa and Caski cells (1×105 cells/well)
were seeded into a 6-well plate. After 24 h, the cells were treated
with different concentrations (0, 0.25, 0.5, 1 mM) of ropivacaine
for 72 h and then harvested by trypsinization. The cells were
washed twice with ice-cold PBS, and then stained with Annexin V and
propidium iodide (PI) using an Annexin V-FITC Apoptosis Detection
Kit (BD Biosciences). Apoptosis was measured by flow cytometric
analysis according to the manufacturer's protocol. Data were
analyzed using FlowJo software version 10 (Tree Star, Inc.).
siRNA transfections
siRNA for MEG2 was purchased from Invitrogen;
Thermo Fisher Scientific, Inc. SiHa cells were transfected with
MEG2 siRNA or NC siRNA with Lipofectamine RNAiMAX
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions.
Transient transfection of miR-96
mimics
miR-96 mimics (Ribo Company) were transfected into
SiHa cells using Lipofectamine RNAiMAX Transfection Reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. At 18 h post-transfection, the cells
were used for subsequent experiments.
Quantitative real-time PCR
miRNA was isolated with a mirVana miRNA Isolation
Kit (Ambion) according to the manufacturer's protocols. Expression
of miRNAs was measured with the PrimeScript miRNA RT-PCR kit
(Takara) following the manufacturer's instructions. U6 was used as
an endogenous control. The forward primer for miR-24 was
5′-TGGCTCAGTTCAGCAGGAACAG-3′. The forward primer for miR-96 was
5′-TTTGGCACTAGCACATTTTTGCT-3′. The forward primer for miR-126 was
5′-TCGTACCGTGAGTAATAATGCG-3′. The forward primer for miR-181a-5p
was 5′-AACATTCAACGCTGTCGGTGAGT-3′. The forward primer for miR-613
was 5′-AGGAATGTTCCTTCTTTGCC-3′ and the reverse primer for miRNAs
was the UnimiRqPCR Primer (Takara). The forward primer for U6 was
5′-ATTGGAACGATACAGAGAAGATT-3′ and the reverse primer for U6 was
5′-GGAACGCTTCACGAATTTG-3′.
Statistical analysis
All data were analyzed using the SPSS 17.0 software
(SPSS, Inc.) and are shown as mean ± standard deviation (SD). The
unpaired Student's t-test and one-way ANOVA followed by Dunnett's
test were used to determine the statistical significance for
comparing two groups and more than two groups, respectively.
P-values <0.05 were considered statistically significant.
Results
Ropivacaine inhibits the growth of
cervical cancer cells
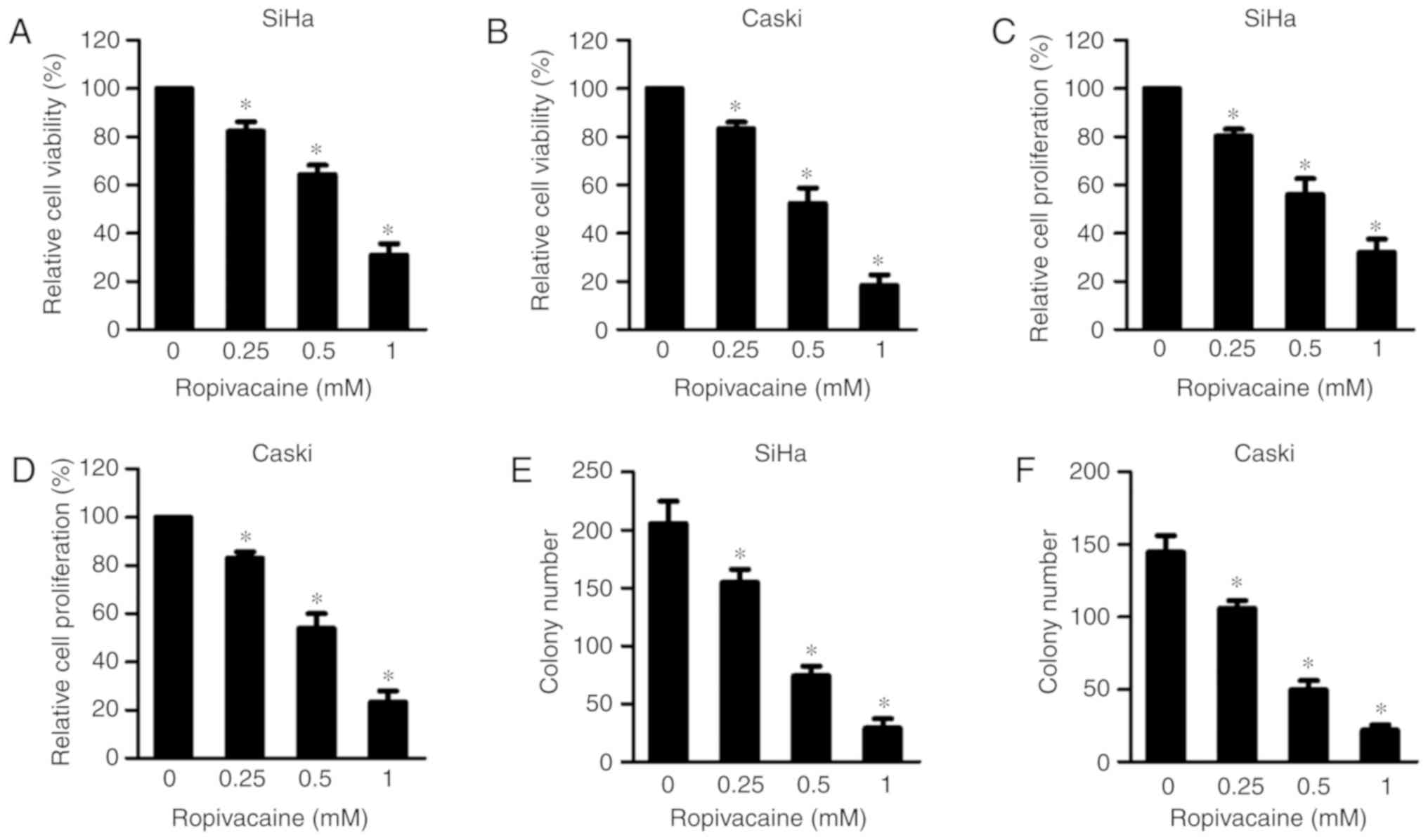
To explore the effects of ropivacaine on the
viability of cervical cancer cells, we treated two cervical cancer
cell lines, SiHa and Caski, with ropivacaine at different
concentrations (0, 0.25, 0.5 and 1 mM) for 72 h, and then measured
cell growth using a Cell Counting Kit-8 (CCK-8) assay. The results
showed that ropivacaine significantly inhibited the growth of both
SiHa and Caski cell lines in a dose-dependent manner (Fig. 1A and B). Accordingly, it also
significantly suppressed incorporation of 5-bromo-2′-deoxyuridine
(BrdU) into both SiHa and Caski cell lines (Fig. 1C and D), confirming its inhibitory
effect on the proliferation of cervical cancer cells. Furthermore,
the results of colony forming assays indicated that ropivacaine
significantly attenuated the survival ability of both SiHa and
Caski cell lines (Fig. 1E and F).
Together, these data indicated that ropivacaine exhibited a
significant inhibitory effect on cervical cancer cell growth.
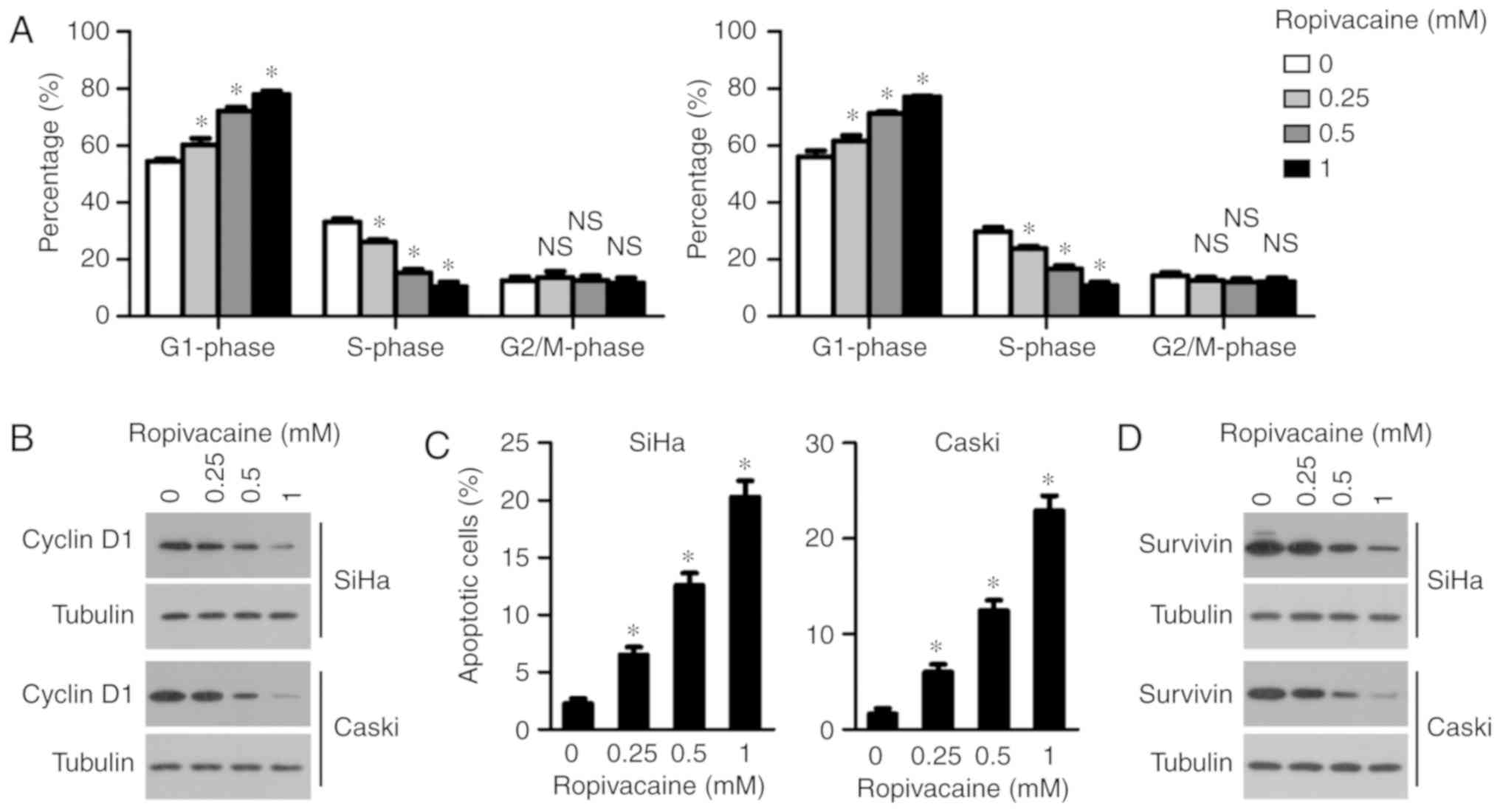
Ropivacaine suppresses the cell cycle
and promotes the apoptosis of cervical cancer cells
Both cell cycle suppression and an increase in cell
apoptosis lead to reduced viability in cancer cells. Therefore, we
next examined the effect of ropivacaine on the cell cycle and
apoptosis of cervical cancer cells. As shown in Fig. 2A, ropivacaine significantly
decreased the percentage of cells in the S-phase in a
dose-dependent manner. Consistent with these results, cyclin D1
expression was also suppressed by ropivacaine treatment, confirming
the drug's inhibitory effect on the cell cycle of cervical cancer
cells (Fig. 2B). We next determined
the effect of ropivacaine on the apoptosis of cervical cancer cells
via an Annexin V apoptosis detection assay. As shown in Fig. 2C, ropivacaine significantly induced
accumulation of apoptotic cells in both the SiHa and Caski cell
lines in a dose-dependent manner. Accordingly, expression of
survivin, a vital anti-apoptosis regulator, was suppressed in a
dose-dependent manner as well (Fig.
2D). Taken together, these results indicated that ropivacaine's
inhibitory effect on cervical cancer cell growth was mediated by
the decrease in cell cycle progression and an increase in
apoptosis.
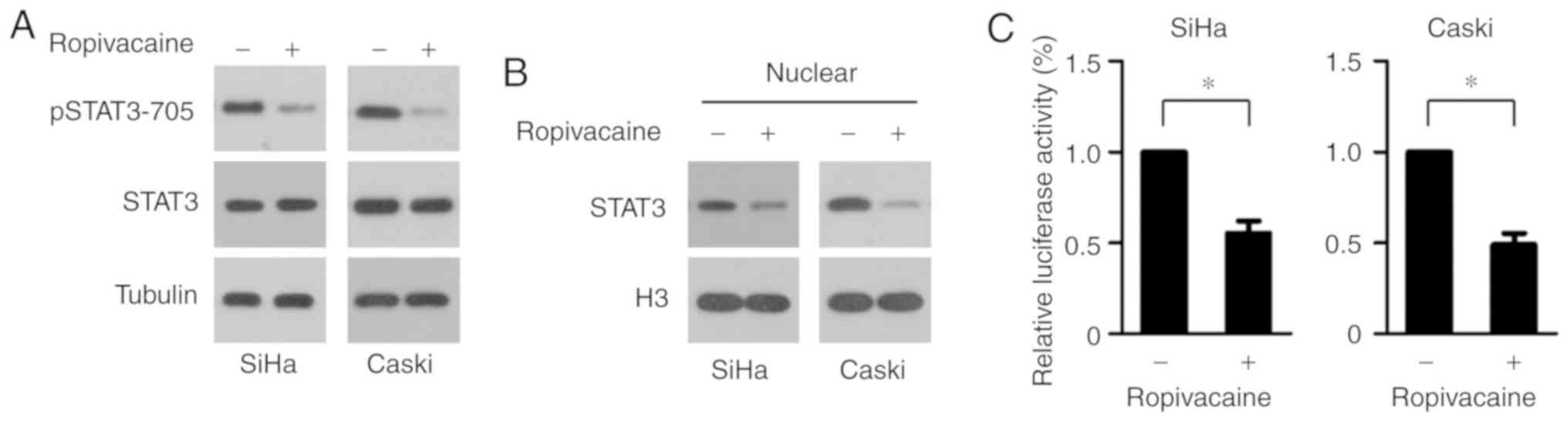
Ropivacaine suppresses phosphorylation
and transcriptional activation of STAT3
We next investigated the relevant signaling pathways
that mediated ropivacaine's inhibition of cervical cancer cell
viability. STAT3 is reported to be abnormally activated in cervical
cancers and to contribute to the initiation and development of such
cancers by controlling the expression of cell cycle and
anti-apoptosis regulators (7,8). Thus,
we hypothesized that ropivacaine might attenuate the viability of
cervical cancer cells by suppressing STAT3 activation. To test this
hypothesis, we first detected the effect of ropivacaine on
phosphorylation of STAT3 Tyr705 (pSTAT3-705), which is essential
for STAT3 activation. As shown in Fig.
3A, ropivacaine decreased the phosphorylation of STAT3. Since
the translocation of STAT3 from the cytoplasm to the nucleus is
modulated by its phosphorylation, we next examined whether
ropivacaine affected the expression of nuclear STAT3. Consistent
with the decrease in STAT3 phosphorylation, nuclear STAT3
expression was markedly inhibited by ropivacaine treatment
(Fig. 3B), while the expression of
total STAT3 protein was unchanged (Fig.
3A). To further investigate whether the reduction in nuclear
STAT3 affected its transcriptional activity, we used an acute-phase
response element (APRE) luciferase reporter, which responds to
STAT3 activation. The results showed that ropivacaine led to a
significant decrease in luciferase activity in both SiHa and Caski
cell lines (Fig. 3C), indicating
that the drug suppressed phosphorylation and transcriptional
activation of STAT3.
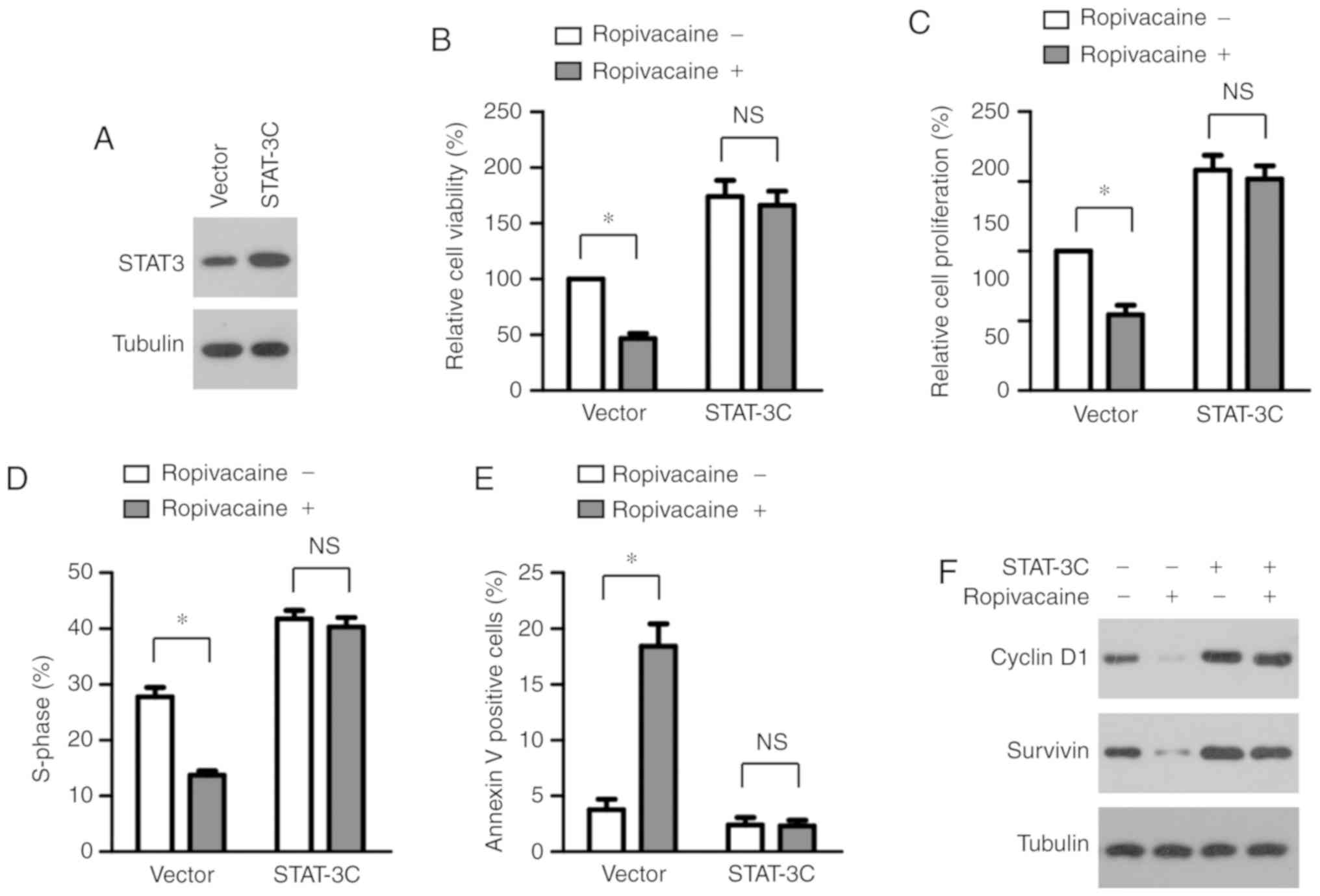
Inhibition of cervical cancer cell
growth mediated by ropivacaine is dependent on suppression of STAT3
activation
To test whether the decreased transcriptional
activation of STAT3 led to the inhibitory effects mediated by
ropivacaine on cervical-cancer cell viability, we established SiHa
cells stably expressing STAT-3C (STAT-3C), a constitutive active
form of STAT3 (Fig. 4A). We treated
both SiHa STAT-3C and Vector (control cells) transfected cells with
0.5 mM ropivacaine for 72 h and then performed a CCK-8 assay.
Consistent with a previous report (8), STAT-3C overexpression promoted the
viability of cervical cancer cells. Interestingly, it also markedly
reversed ropivacaine's inhibition of cell viability (Fig. 4B). Additionally, the forced
expression of STAT-3C effectively abrogated ropivacaine-mediated
suppression of cell proliferation and cell cycle progression, as
determined by BrdU incorporation assay and cell cycle analysis
(Fig. 4C and D). Furthermore, the
increased apoptosis of SiHa cells induced by ropivacaine was
rescued by STAT-3C overexpression (Fig.
4E). Accordingly, although we observed reduced expression of
cyclin D1 and survivin in the Vector cells in the presence of
ropivacaine, we found no significant change in the SiHa STAT-3C
transfected cells (Fig. 4F).
Together, these results indicated that ropivacaine attenuated the
growth of cervical cancer cells by suppressing STAT3
activation.
MEG2 mediates the inhibition of STAT3
phosphorylation by ropivacaine
To explore the molecular mechanism by which
ropivacaine inhibits STAT3 phosphorylation, we first examined its
effect on the activity of JAK2, a key upstream protein kinase of
STAT3 (2). As shown in Fig. 5A, ropivacaine had little effect on
JAK2 phosphorylation, suggesting that the drug may instead modulate
that of STAT3 via its negative regulators. Protein tyrosine
phosphatases (PTPs) regulate the transcriptional activity of STAT3
by dephosphorylating it (18). To
study whether PTPs participate in the reduction of STAT3
phosphorylation by ropivacaine, we treated SiHa cells with 0.5 mM
ropivacaine, with or without the PTP inhibitor pervanadate, for 72
h and then performed western blot analysis. As shown in Fig. 5B, ropivacaine significantly
decreased STAT3 phosphorylation, a process that pervanadate
completely reversed. Various PTPs, including PTPRD, PTPRT, SHP1,
SHP2 and MEG2, have been reported to regulate STAT3 phosphorylation
in multiple types of cancers and be involved in carcinogenesis
(2). To identify which PTP may
mediate the effect of ropivacaine, we first assessed the expression
of PTPRD, PTPRT, SHP1, SHP2 and MEG2 in SiHa cells after
ropivacaine treatment. The results showed that ropivacaine markedly
enhanced MEG2 expression, but we observed no significant changes in
the other PTPs (Fig. 5C),
suggesting that MEG2 mediates the ropivacaine-induced
decrease in STAT3 phosphorylation. To test this possibility, we
transiently transfected SiHa cells with MEG2 or
normal-control (NC) small interfering RNA (siRNA) and then treated
them with 0.5 mM ropivacaine for 72 h. Successful depletion of MEG2
in SiHa cells transfected with MEG2 siRNA (Si-MEG2) was
confirmed by real-time PCR analysis and western blot analysis
(Fig. 5D and E). MEG2
silencing almost completely rescued the reduction of phosphorylated
STAT3 induced by ropivacaine (Fig.
5F). Accordingly, MEG2 knockdown significantly
attenuated the suppression of STAT3 transcriptional activation and
cervical cancer cell viability mediated by ropivacaine (Fig. 5G). Taken together, these results
indicated that MEG2 mediated the inhibitory effect of
ropivacaine on phosphorylation of STAT3.
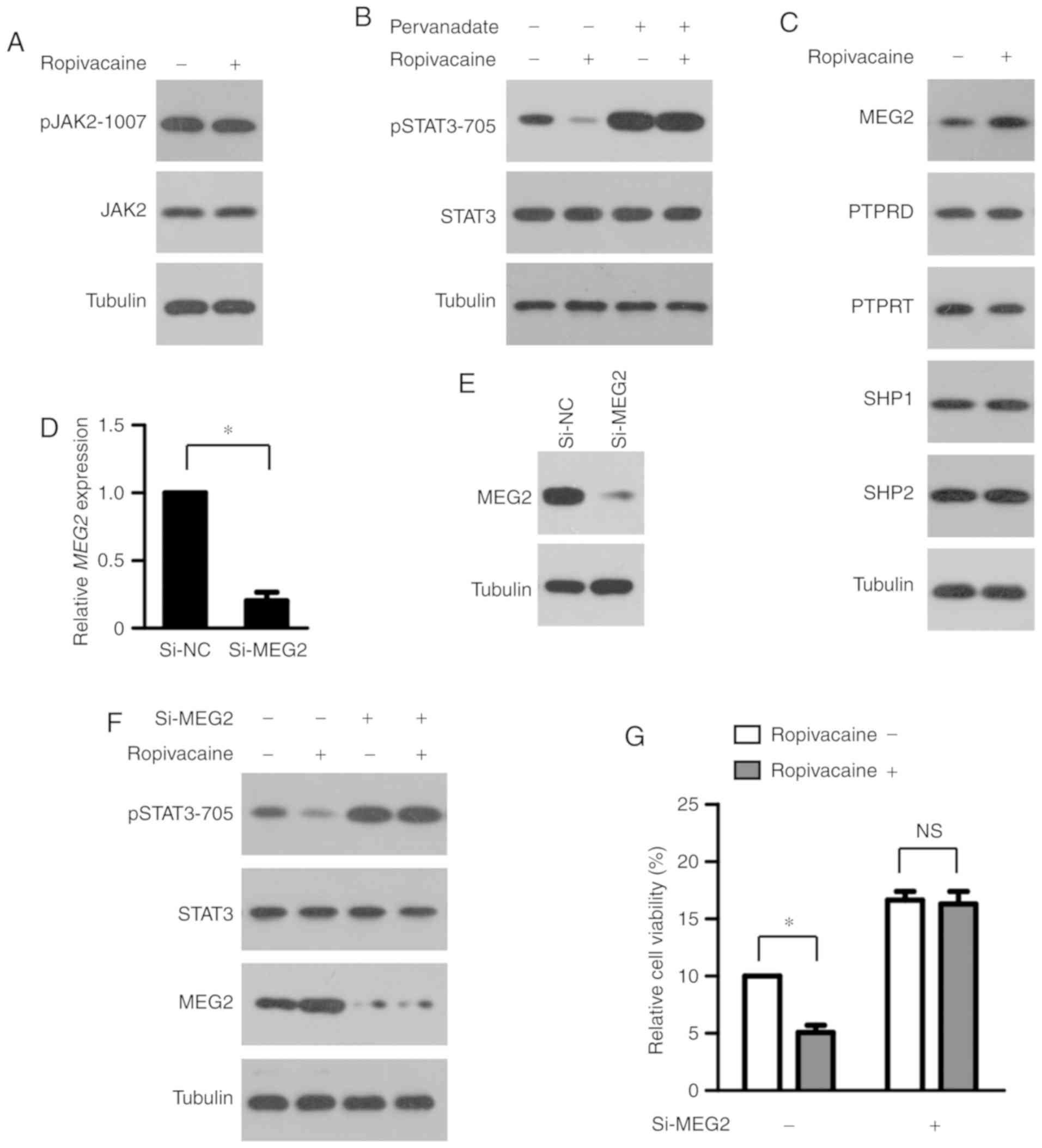 | Figure 5.Inhibitory effect of ropivacaine on
the phosphorylation of STAT3 is mediated by MEG2. (A)
Western blot analysis of pJAK2 levels in SiHa cells treated with
ropivacaine (0.5 mM) for 72 h. Tubulin was used as a loading
control. (B) SiHa cells were treated with ropivacaine (0.5 mM) and
pervanadate (50 µM), alone or in combination for 72 h, followed by
western blot analysis of pSTAT3 levels. Tubulin was used as a
loading control. (C) After 72 h of ropivacaine (0.5 mM) treatment,
the expression levels of indicated proteins in SiHa cells were
analyzed by western blot analysis. Tubulin was used as a loading
control. (D and E) Successful depletion of MEG2 in SiHa
cells transfected with MEG2 siRNA, as assessed by real-time
PCR analysis (D) and western blot analysis (E). (F and G) SiHa
cells transfected with NC siRNA or MEG2 siRNA were treated
with ropivacaine (0.5 mM) for 72 h, followed by western blot
analysis of pSTAT3 levels with tubulin as a loading control (F),
and CCK-8 assay (G). Results are shown as means ± SD of triplicate
measurements. The unpaired Student t-test was used to analyze the
data. *P<0.05. NS, not significant; pJAK2, phosphorylated Janus
associated kinase 2; pSTAT3, phosphorylated signal transducer and
activator of transcription 3; MEG2, maternally expressed
gene 2; PTPRD, PTR receptor type D; PTPRT, PTR receptor type T
(PTPRT); SHP1, Src homology region 2 domain-containing
phosphatase-1; SHP2, Src homology region 2 domain-containing
phosphatase-2. |
Ropivacaine upregulates MEG2 by
suppressing expression of miR-96
We next investigated how ropivacaine enhances
MEG2 expression. Recent studies have shown that MEG2
expression in cancers is mainly controlled by microRNAs, including
miR-24 (19), miR-96 (20), miR-126 (21), miR-181a-5p (22) and miR-613 (23). Therefore, we began by exploring the
effect of ropivacaine on expression of these microRNAs.
Interestingly, we found that the drug significantly increased the
expression of miR-96 alone, observing no significant changes in the
other microRNAs (Fig. 6A). This
indicated that miR-96 downregulation may lead to a
ropivacaine-induced increase in MEG2. To test this, we
transiently transfected miR-96 mimic into SiHa cells (Fig. 6B) and then measured the expression
of MEG2 after ropivacaine treatment. As shown in Fig. 6C, real-time PCR analysis revealed
the successful overexpression of miR-96 after ropivacaine
treatment. Furthermore, transfection of miR-96 mimic completely
reversed the ropivacaine-induced increase in MEG2 (Fig. 6D); accordingly, transfection also
completely abrogated the decrease in STAT3 phosphorylation caused
by ropivacaine. Furthermore, transfection of miR-96 mimic markedly
attenuated ropivacaine's inhibitory effect on STAT3 phosphorylation
(Fig. 6E) and significantly
abrogated its suppression of cervical cancer cell growth (Fig. 6F). Collectively, these results
indicated that ropivacaine upregulated MEG2 by suppressing
expression of miR-96.
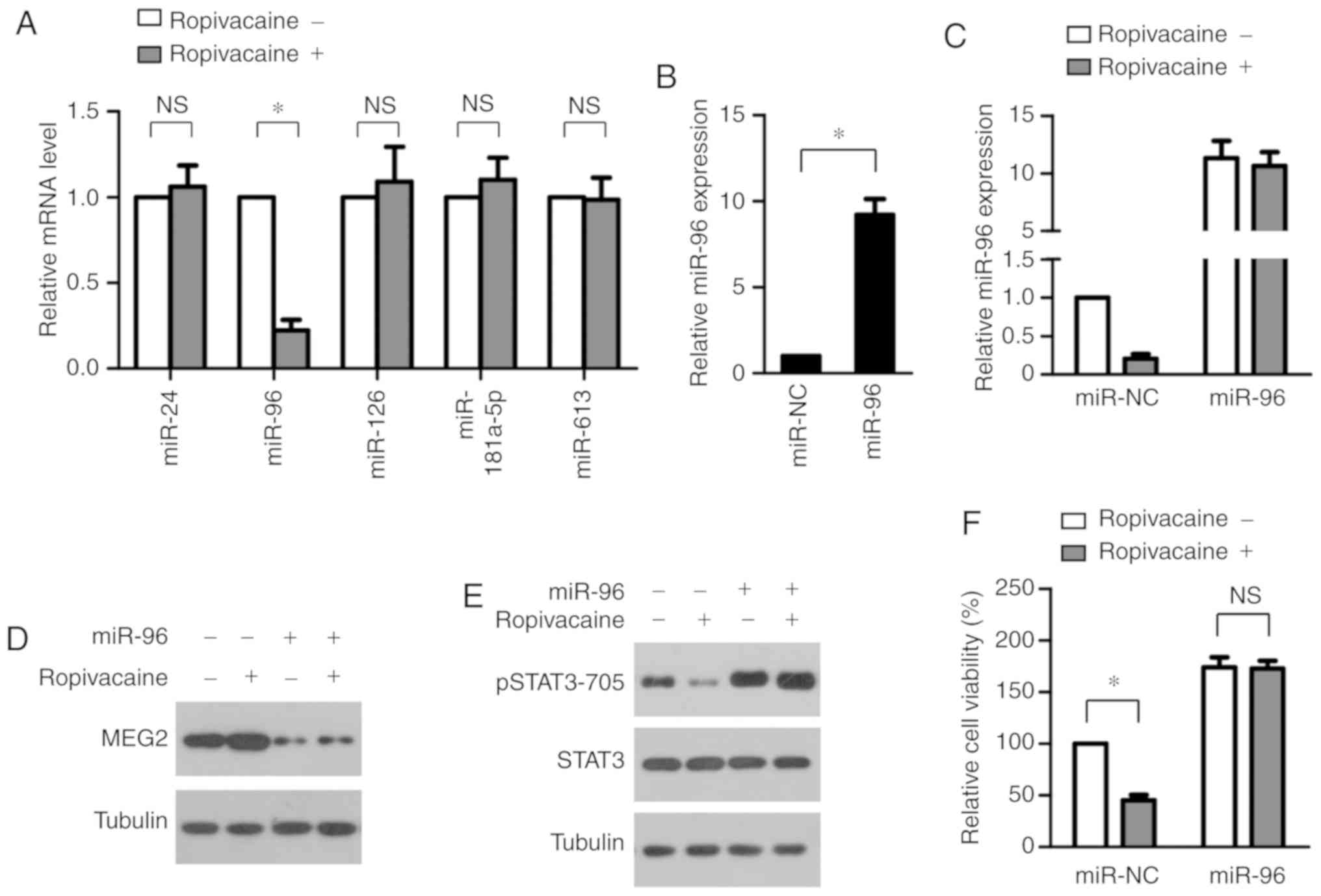 | Figure 6.Ropivacaine upregulates MEG2 via
suppressing the expression of miR-96. (A) SiHa cells were treated
with ropivacaine (0.5 mM) for 72 h, followed by real-time PCR
analysis of miR-24, miR-96, miR-126, miR-181a-5p and miR-613. (B)
Successful overexpression of miR-96 in SiHa cells transfected with
miR-96 mimics, as assessed by real-time PCR analysis. (C) After 72
h of ropivacaine (0.5 mM) treatment, the expression of miR-96 in
SiHa cells transfected with miR-NC or miR-96 mimics were analyzed
by real-time PCR analysis. (D) After 72 h of ropivacaine (0.5 mM)
treatment, the expression of MEG2 in SiHa cells transfected with
miR-NC or miR-96 mimics were analyzed by western blot analysis.
Tubulin was used as a loading control. (E and F) SiHa cells
transfected with miR-NC or miR-96 mimics were treated with
ropivacaine (0.5 mM) for 72 h, followed by western blot analysis of
pSTAT3 levels with tubulin as a loading control (E) and CCK-8 assay
(F). Results are shown as means ± SD of triplicate measurements.
The unpaired Student t-test was used to analyze the data.
*P<0.05. NS, not significant; pSTAT3, phosphorylated signal
transducer and activator of transcription 3; MEG2,
maternally expressed gene 2. |
Discussion
Accumulating evidence suggests that local
anesthetics play a beneficial role in reducing cancer recurrence
(24). Ropivacaine, a widely used
local anesthetic, has been shown to suppress the growth of a
variety of cancer cells via diverse molecular mechanisms. It can
inhibit the growth of breast cancer cells by disrupting
mitochondrial function (25), and
it has been reported to suppress that of chronic myeloid leukemia
by decreasing phosphatidylinositol-4,5-bisphosphate 3-kinase
(PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR)
signaling (12). Ropivacaine has
also been found to attenuate gastric cancer cell proliferation via
downregulation of extracellular signal-regulated kinase 1/2
(ERK1/2) signaling (15). Despite
these reports of the drug's antitumor benefits, its effect on
cervical cancer cell growth remains to be elucidated. In the
present study, we found that ropivacaine exerted an inhibitory
effect on cervical cancer cell growth. Our subsequent experiments
demonstrated that its inhibition of cell progression and promotion
of cell apoptosis led to suppression of such growth. In contrast
with the previously elucidated molecular mechanism underlying the
anticancer effect of ropivacaine, we found that ropivacaine
attenuated cervical cancer cell growth by suppressing
phosphorylation and transcriptional activation of signal transducer
and activator of transcription 3 (STAT3). Since elevated levels of
phosphorylated STAT3 are frequently found in cervical cancer tissue
and are positively correlated with poor prognosis (5,6), our
findings may provide novel insight into the treatment of cervical
cancer. In addition, as ropivacaine has been reported to suppress
migration and invasion of cancer cells in various types of cancers
(26–28), and STAT3 activation contributes to
migration and invasiveness of cervical cancer (29), it would be important to explore
whether ropivacaine affects cervical cancer cell migration or
invasion via suppression of STAT3 activation in future
investigations.
Phosphorylation and transcriptional activation of
STAT3 are precisely modulated by upstream protein kinase and
phosphatase (2). Our results
indicated that ropivacaine had little effect on the activation of
Janus associated kinase 2 (JAK2), the key protein kinase for STAT3,
suggesting that the drug may inhibit STAT3 phosphorylation by
affecting protein tyrosine phosphatases (PTPs). In accordance with
this hypothesis, we found that the suppression of STAT3
phosphorylation mediated by ropivacaine was reversed by the PTP
inhibitor pervanadate. Our further experiments demonstrated that
maternally expressed gene 2 (MEG2) mediated the suppression
of STAT3 phosphorylation induced by ropivacaine. Su et al
first showed that MEG2 could dephosphorylate STAT3 at Y705
via their direct interaction in breast cancer cells and that the
inactivation of STAT3 by MEG2 decreased the growth of breast
tumors (30). Subsequent studies
demonstrated that dephosphorylation of STAT3 by MEG2 plays
important roles in multiple physiological and pathological
processes. Physiologically, MEG2 was reported to regulate
erythroid-cell development by dephosphorylating STAT3 (31). Pathologically, MEG2-mediated
STAT3 dephosphorylation is involved in the development of breast
(30), prostate (32) and colorectal (33) cancers. However, whether the
molecular mechanism is the same in the progression of cervical
cancer remains unclear. We found that ropivacaine repressed STAT3
phosphorylation and cervical- ancer cell growth by increasing
expression of MEG2, which established a functional link
between MEG2 and STAT3 phosphorylation in cervical
cancer.
In a variety of cancer types, abnormal expression of
MEG2 is mainly caused by disordered miRNA expression. Liu
et al revealed that MEG2 is negatively regulated by
miR-181a-5p and is a tumor-suppressing gene in gastric cancer
(22). By targeting MEG2,
miR-24 and miR-96 suppress breast cancer cell growth and migration
(19,20). In cervical cancer, miR-613 has been
shown to contribute to tumor progression by repressing expression
of MEG2 (23). Therefore, we
speculated that ropivacaine may increase expression of MEG2
by regulating that of these miRNAs. Interestingly, ropivacaine
treatment markedly suppressed expression of miR-96 only, having
little effect on that of other miRNAs. Consistent with these
findings, miR-96 has been reported to be upregulated in human
cervical cancer tissues and to function as an oncogene to promote
carcinogenesis (34). Apart from
cervical cancer, miR-96 has been shown to be involved in various
other types of cancers (35,36).
Whether ropivacaine exerts a similar anticancer effect on these
cancer types deserves further investigation. In addition, the
molecular mechanism by which it represses miR-96 expression remains
to be clarified in further studies.
In conclusion, our results indicated that
ropivacaine inhibited the growth of cervical cancer cells by
suppressing the miR613/MEG2/pSTAT3 axis. These results
revealed the potent anti-growth effect of ropivacaine in cervical
cancer and provide novel insights into the molecular mechanism by
which this drug exerts its anticancer activity. Our findings
suggest that ropivacaine could have potential use as a novel agent
to treat cervical cancer patients.
Acknowledgements
Not applicable.
Funding
This research study was supported by the Science and
Technology Project of Guangdong Province (2015A020211001),
China.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
XC ad XS designed the experiments. XC, WL, XG and SH
performed the experiments and analyzed the data, XC and XS wrote
the manuscript. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
JAK
|
Janus associated kinase
|
|
PTPs
|
protein tyrosine phosphatases
|
|
MEG2
|
maternally expressed gene 2
|
References
|
1
|
Shi Y, Zhang Z, Qu X, Zhu X, Zhao L, Wei
R, Guo Q, Sun L, Yin X, Zhang Y and Li X: Roles of STAT3 in
leukemia (review). Int J Oncol. 53:7–20. 2018.PubMed/NCBI
|
|
2
|
Johnson DE, O'Keefe RA and Grandis JR:
Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev
Clin Oncol. 15:234–248. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Y, Shen Y, Wang S, Shen Q and Zhou X:
The role of STAT3 in leading the crosstalk between human cancers
and the immune system. Cancer Lett. 415:117–128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen CL, Hsieh FC, Lieblein JC, Brown J,
Chan C, Wallace JA, Cheng G, Hall BM and Lin J: Stat3 activation in
human endometrial and cervical cancers. Br J Cancer. 96:591–599.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takemoto S, Ushijima K, Kawano K,
Yamaguchi T, Terada A, Fujiyoshi N, Nishio S, Tsuda N, Ijichi M,
Kakuma T, et al: Expression of activated signal transducer and
activator of transcription-3 predicts poor prognosis in cervical
squamous-cell carcinoma. Br J Cancer. 101:967–972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shukla S, Shishodia G, Mahata S, Hedau S,
Pandey A, Bhambhani S, Batra S, Basir SF, Das BC and Bharti AC:
Aberrant expression and constitutive activation of STAT3 in
cervical carcinogenesis: Implications in high-risk human
papillomavirus infection. Mol Cancer. 9:2822010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shukla S, Mahata S, Shishodia G, Pandey A,
Tyagi A, Vishnoi K, Basir SF, Das BC and Bharti AC: Functional
regulatory role of STAT3 in HPV16-mediated cervical carcinogenesis.
PLoS One. 8:e678492013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yong L and Guang B: Intraperitoneal
ropivacaine instillation versus no intraperitoneal ropivacaine
instillation for laparoscopic cholecystectomy: A systematic review
and meta-analysis. Int J Surg. 44:229–243. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Oliveira GS Jr, Ahmad S, Schink JC,
Singh DK, Fitzgerald PC and McCarthy RJ: Intraoperative neuraxial
anesthesia but not postoperative neuraxial analgesia is associated
with increased relapse-free survival in ovarian cancer patients
after primary cytoreductive surgery. Reg Anesth Pain Med.
36:271–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Biki B, Mascha E, Moriarty DC, Fitzpatrick
JM, Sessler DI and Buggy DJ: Anesthetic technique for radical
prostatectomy surgery affects cancer recurrence: A retrospective
analysis. Anesthesiology. 109:180–187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng Q, Peng X and Yu H: Local anesthetic
drug inhibits growth and survival in chronic myeloid leukemia
through suppressing PI3K/Akt/mTOR. Am J Med Sci. 355:266–273. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Le Gac G, Angenard G, Clément B, Laviolle
B, Coulouarn C and Beloeil H: Local anesthetics inhibit the growth
of human hepatocellular carcinoma cells. Anesth Analg.
125:1600–1609. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bundscherer A, Malsy M, Gebhardt K,
Metterlein T, Plank C, Wiese CH, Gruber M and Graf BM: Effects of
ropivacaine, bupivacaine and sufentanil in colon and pancreatic
cancer cells in vitro. Pharmacol Res. 95-96:126–131. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang W, Cai J, Zhang H, Wang G and Jiang
W: Effects of lidocaine and ropivacaine on gastric cancer cells
through down-regulation of ERK1/2 phosphorylation in vitro.
Anticancer Res. 38:6729–6735. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Peng X and Zheng Q: Ropivacaine
inhibits the migration of esophageal cancer cells via
sodium-channel-independent but prenylation-dependent inhibition of
Rac1/JNK/paxillin/FAK. Biochem Biophys Res Commun. 501:1074–1079.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang HW, Wang LY, Jiang L, Tian SM, Zhong
TD and Fang XM: Amide-linked local anesthetics induce apoptosis in
human non-small cell lung cancer. J Thorac Dis. 8:2748–2757. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiong A, Yang Z, Shen Y, Zhou J and Shen
Q: Transcription factor STAT3 as a novel molecular target for
cancer prevention. Cancers (Basel). 6:926–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du WW, Fang L, Li M, Yang X, Liang Y, Peng
C, Qian W, O'Malley YQ, Askeland RW, Sugg SL, et al: MicroRNA
miR-24 enhances tumor invasion and metastasis by targeting PTPN9
and PTPRF to promote EGF signaling. J Cell Sci. 126:1440–1453.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong Y, Liang H, Uzair-Ur-Rehman, Wang Y,
Zhang W, Zhou Y, Chen S, Yu M, Cui S, Liu M, et al: miR-96 promotes
cell proliferation, migration and invasion by targeting PTPN9 in
breast cancer. Sci Rep. 6:374212016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu J, Li H, Ma J, Huang H, Qin J and Li
Y: PTPN9 promotes cell proliferation and invasion in Eca109 cells
and is negatively regulated by microRNA-126. Oncol Lett.
14:1419–1426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu Z, Sun F, Hong Y, Liu Y, Fen M, Yin K,
Ge X, Wang F, Chen X and Guan W: MEG2 is regulated by miR-181a-5p
and functions as a tumour suppressor gene to suppress the
proliferation and migration of gastric cancer cells. Mol Cancer.
16:1332017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li WT, Wang BL, Yang CS, Lang BC and Lin
YZ: MiR-613 promotes cell proliferation and invasion in cervical
cancer via targeting PTPN9. Eur Rev Med Pharmacol Sci.
22:4107–4114. 2018.PubMed/NCBI
|
|
24
|
Cassinello F, Prieto I, del Olmo M, Rivas
S and Strichartz GR: Cancer surgery: How may anesthesia influence
outcome? J Clin Anesth. 27:262–272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gong X, Dan J, Li F and Wang L:
Suppression of mitochondrial respiration with local anesthetic
ropivacaine targets breast cancer cells. J Thorac Dis.
10:2804–2812. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Piegeler T, Schläpfer M, Dull RO, Schwartz
DE, Borgeat A, Minshall RD and Beck-Schimmer B: Clinically relevant
concentrations of lidocaine and ropivacaine inhibit TNFα-induced
invasion of lung adenocarcinoma cells in vitro by blocking the
activation of Akt and focal adhesion kinase. Br J Anaesth.
115:784–791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu YJ, Li SY, Cheng Q, Chen WK, Wang SL,
Ren Y and Miao CH: Effects of anaesthesia on proliferation,
invasion and apoptosis of LoVo colon cancer cells in vitro.
Anaesthesia. 71:147–154. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li R, Xiao C, Liu H, Huang Y, Dilger JP
and Lin J: Effects of local anesthetics on breast cancer cell
viability and migration. BMC Cancer. 18:6662018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fan Z, Cui H, Xu X, Lin Z, Zhang X, Kang
L, Han B, Meng J, Yan Z, Yan X and Jiao S: MiR-125a suppresses
tumor growth, invasion and metastasis in cervical cancer by
targeting STAT3. Oncotarget. 6:25266–25280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Su F, Ren F, Rong Y, Wang Y, Geng Y, Wang
Y, Feng M, Ju Y, Li Y, Zhao ZJ, et al: Protein tyrosine phosphatase
Meg2 dephosphorylates signal transducer and activator of
transcription 3 and suppresses tumor growth in breast cancer.
Breast Cancer Res. 14:R382012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bu Y, Su F, Wang X, Gao H, Lei L, Chang N,
Wu Q, Hu K, Zhu X, Chang Z, et al: Protein tyrosine phosphatase
PTPN9 regulates erythroid cell development through STAT3
dephosphorylation in zebrafish. J Cell Sci. 127:2761–2770. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jung SN, Shin DS, Kim HN, Jeon YJ, Yun J,
Lee YJ, Kang JS, Han DC and Kwon BM: Sugiol inhibits STAT3 activity
via regulation of transketolase and ROS-mediated ERK activation in
DU145 prostate carcinoma cells. Biochem Pharmacol. 97:38–50. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang D, Cheng Z, Zhao M, Jiao C, Meng Q,
Pan H, Xie Y, Li L, Zhu Y, Wang W, et al: PTPN9 induces cell
apoptosis by mitigating the activation of Stat3 and acts as a tumor
suppressor in colorectal cancer. Cancer Manag Res. 11:1309–1319.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma X, Shi W, Peng L, Qin X and Hui Y:
MiR-96 enhances cellular proliferation and tumorigenicity of human
cervical carcinoma cells through PTPN9. Saudi J Biol Sci.
25:863–867. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lin H, Dai T, Xiong H, Zhao X, Chen X, Yu
C, Li J, Wang X and Song L: Unregulated miR-96 induces cell
proliferation in human breast cancer by downregulating
transcriptional factor FOXO3a. PLoS One. 5:e157972010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Haflidadóttir BS, Larne O, Martin M,
Persson M, Edsjö A, Bjartell A and Ceder Y: Upregulation of miR-96
enhances cellular proliferation of prostate cancer cells through
FOXO1. PLoS One. 8:e724002013. View Article : Google Scholar : PubMed/NCBI
|