Introduction
Although multimodal treatment, including surgical
resection, radiotherapy, and chemotherapy, prolongs survival,
glioblastoma (GBM) is still the most fatal primary intrinsic brain
cancer, with a median survival time of less than 2 years (1,2). GBMs
contain self-renewing, tumorigenic cancer stem cells that
contribute to tumor initiation and characteristic, malignant glioma
stem-like cells (GSLCs) (3,4). In addition, these GSLCs have been
reported to be closely associated with resistance to radiotherapy
and chemotherapy, although the underlying mechanism needs to be
further explored (5–7).
Conventional human glioma cell lines, such as U87,
U251, and T98G, may not represent the real GBMs in patients, for
several reasons. First, the serum-containing medium in which these
cell lines are cultured could potentially change the genomes of the
cells and their transcriptomes, giving rise to the depletion of
stem-like cells (8). Second, tumor
models constructed in mice by the injection of these cell lines
into the brain fail to develop the typical morphological features
of GBMs, such as diffuse infiltration into the surrounding tissue
and microvascular proliferations (8–10).
Therefore, it is unlikely that findings with these cell line models
can be correlated with the parameters of patients (10).
It is well known that activation of the canonical
Wnt/β-catenin signaling pathway inhibits the glycogen synthase
kinase 3 (GSK-3)-mediated degradation of β-catenin, resulting in
the accumulation of cytoplasmic β-catenin. Then, the β-catenin
translocates into the nucleus and interacts with transcriptional
complexes to regulate the downstream target genes, which can
facilitate cell proliferation and inhibit cell apoptosis (11,12).
CHIR99021 is a chemical compound that acts as an inhibitor of
GSK-3. It has been demonstrated to be useful for applications in
molecular biology for transforming the cell type into induced
pluripotent stem cells (iPSCs) and neurons (13,14).
However, there is no evidence that CHIR99021 can transform primary
low-grade glioma cells into GSLCs. In the present study, for the
first time to the best of our knowledge, the effect of CHIR99021 in
enriching cells with GSLC properties was explored, which would
enable the construction of a better model that is more similar to
clinical GBM.
Materials and methods
Preparation of the tumor
specimens
Glioma samples were obtained from the operation room
of the Neurosurgical Department of the Army General Hospital from
January 2017 to October 2017. All the patients, 8 females and 12
males aged from 18 to 65 years old, were informed and signed the
informed consent. A total of 20 glioma samples with low grades (WHO
I, 10; II, 10), as based on the WHO classification criteria, were
collected and used in the experiment. The study was approved by the
Ethics Committee of the Army General Hospital of Beijing (no.
2017-114).
Cell culture and experimental
groups
Glioma specimens were placed in a saline solution
after surgical extraction. Primary low-grade tumor cells were
obtained after mechanical dissociation and enzymatic digestion,
mainly based on a single method (15). Then, the cells were separated into
two groups. In the first group, the primary glioma-based cell
(PGBC) group, the cells were cultured in serum-free medium with
penicillin-streptomycin (1:100), B-27 (1:50), and N-2 supplement
(1:100; all from GIBCO; Thermo Fisher Scientific, Inc.),
recombinant human fibroblast growth factor-2 (FGF-2; 20 ng/ml),
epidermal growth factor (EGF; 20 ng/ml), and 5 µg/ml insulin
(Sigma-Aldrich; Merck KGaA). In the second group, the GSLC group,
the cells were cultured under the same conditions, except for the
addition of 100 nM CHIR99021 (Stemgent).
Flow cytometric analysis
Flow cytometry was used in order to find a suitable
concentration of CHIR99021 for cell survival and to detect the
expression level of stem cell markers in the two groups. In brief,
after being cultured in various concentrations of CHIR99021 (0,
10−3 M, 10−4 M, 10−5 M,
10−6 M, 10−7 M) for 48 h, the cells were
collected and washed with PBS. The apoptotic cells were detected
using an Annexin V-FITC apoptosis detection kit.
After culturing the two groups of cells for 48 h,
combinations of monoclonal antibodies against human CD133-FITC
(1:200 dilution; cat. no. 566597; BD Biosciences) and Nestin-PE
(1:400 dilution; cat. no. 561230; BD Biosciences) were added to the
cell suspensions at concentrations recommended by the manufacturer.
These cells were then analyzed by FACS (BD Accuri C6 flow
cytometry) after being incubated at 4°C in a dark place for 60 min.
This assay was performed in triplicate for the mixed cells derived
from grade I and II gliomas, and the results were presented as
average percentages.
Cell migration assay
Transwell chambers with an 8.0-µm pore (Costar;
Corning Incorporated) were used to detect cell migration abilities
after the cells were cultured at 37°C for 5 days. Briefly, the
cells were seeded into the upper chamber of the Transwell plates
(3.0×104 cells/well in 200 µl serum-free medium), while
the lower chamber was filled with 500 µl medium containing 10% FBS
as a chemoattractant. After incubation for 24 h, the cells
remaining on the upper surface of the filter were removed. The
cells that had migrated into the lower compartment were fixed with
methanol at room temperature (RT) for 20 min and stained with 0.5%
crystal violet (0.5 g crystal violet in 100 ml of 20% methanol;
cat. no. C0121; Beyotime Institue of Biotechnology) at RT for 15
min. The cells were counted visually in 5 random fields under a
light microscope (20X objective lens). In addition, the migrated
cells were dissociated, lysed, and quantified at 570 nm using a
spectrophotometer.
Transmission electron microscopy
After being cultured in a suitable medium for 7
days, the cells were fixed in 2.5% glutaraldehyde in a 100-mM
sodium cacodylate buffer (pH 7.4), dehydrated in a graded ethanol
series after OsO4 fixation, and embedded into Epon
(catalyst). Sections of 60 nm were imaged with a Tecnai Spirit
transmission electron microscope (FEI; Thermo Fisher Scientific,
Inc.).
Quantitative real-time PCR
To determine the role of CHIR99021 in the PI3K/AKT
signaling pathway in GSLCs, the gene expression levels of signal
transducer and activator of transcription 3 (STAT3), protein
kinase B (AKT), p85, p110, phosphoinositide 3-kinases
(PI3K), vascular endothelial growth factor (VEGF),
glycogen synthase kinase-3β (GSK-3β) and CD133 were
analyzed. Total RNA was extracted from the two groups on day 5
using TRIzol® Reagent. M-MLV reverse transcriptase was
used for cDNA synthesis. In brief, a mixture containing 2 µg of
total RNA, 0.75 µg oligo-dT primer (Tiangen Biotech Co., Ltd.), and
nuclease-free water in a total volume of 13.5 µl was heated at 70°C
for 5 min and then cooled on ice for another 5 min. The mixture was
supplemented with 4 µl M-MLV buffer, 1.25 µl dNTP, 0.5 µl RNasin,
and 0.75 µl M-MLV-RT up to a final volume of 20 µl, followed by
incubation at 42°C for 60 min.
The quantitative real-time PCR analysis was
performed using the SYBR Green Master Mix Kit (Takara Biotechnology
Co., Ltd.). Briefly, each PCR reaction mixture with a total volume
of 20 µl, including 10 µl 2X SYBR-Green Master Mix, 1 µl sense and
antisense primers (10 µmol/µl), and 1 µl cDNA, was run for 40
cycles, undergoing denaturation at 95°C for 15 sec, annealing at
60°C for 30 sec, and extension at 72°C for 30 sec. For relative
quantification, 2−ΔΔCq was calculated as an indication
of the relative gene expression levels of STAT3, AKT, p85, p110,
PI3K, VEGF, GSK-3β and CD133 (16). The primer sequences for the PCR
amplification of the genes are presented in Table I.
 | Table I.Primer sequences for qPCR. |
Table I.
Primer sequences for qPCR.
| STAT3 |
5′-TCTCCACCCAAGTGAAAGTGACGC-3′ |
5′-GGCAGTTCTCCTCCACCACCAAGC-3′ |
| AKT |
5′-TGTTGTAAAAAAACGCCG-3′ |
5′-TTTGTGACAGGAAAGCCC-3′ |
| p85 |
5′-CCCCAGGAACTCCACATA-3′ |
5′-TAACCATCCAGACCCCAC-3′ |
| p110 |
5′-TACTCAGTCCTGCGTGGG-3′ |
5′-TGGCTTTGAATCTTTGGC-3′ |
| PI3K |
5′-AGGTAGAGTGGTTGGGCG-3′ | 5′-
CTGGGATGAGTCTGGGGT-3′ |
| VEGF |
5′-CAGAAGTTGGACGAAAAGT-3′ |
5′-GCAGAAAGAGGAAAGAGGT-3′ |
| GSK-3 |
5′-ACTTTCTTGATGGCGACC-3′ |
5′-TTCTTTTTCTTCTGTGGG-3′ |
| CD133 |
5′-TTACGGCACTCTTCACCT-3′ |
5′-TATTCCACAAGCAGCAAA-3′ |
| β-actin |
5′-AGCGAGCATCCCCCAAAGTT-3′ |
5′-GGGCACGAAGGCTCATCATT-3′ |
Western blotting
The PGBCs and GSLCs were harvested by mechanical
dissociation after being cultured for 5 days. The cells were lysed
in ice-cold lysis buffer (RIPA) and phosphatase and protease
inhibitors for 30 min. The supernatants were collected after
centrifugation and quantified for protein content. BCA was used to
test the concentration of each sample, then equal amounts of
proteins (10 µg per lane) were electrophoretically fractionated in
8% sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred
to PVDF membranes. After the transfer, the membrane was blocked
with 5% skim milk and then incubated with the primary antibody
overnight at 4°C. The specific antibodies were against human STAT3
and nuclear factor kappa-light-chain-enhancer of activated B cells
(NF-κB) (1:1,000 dilution for both; product nos. 9139 and 8242,
respectively; Cell Signaling Technology, Inc.), GSK-3β, mTOR and
AKT (1:500 dilution for all of them; cat. nos. 615002, 610302 and
649002, respectively; BioLegend, Inc.), VEGF (1:1,000 dilution;
product code ab32152; Abcam), and β-actin (1:1,000 dilution; cat.
no. A5441; Sigma-Aldrich; Merck KGaA). Subsequently, the membrane
was incubated with HRP-conjugated goat anti-rabbit or
HRP-conjugated goat anti-mouse IgG (1:10,000 dilution for both;
product codes ab44171 and ab19195, respectively; Abcam) at RT for 2
h. Autoradiography of the membrane was performed using ECL western
blotting detection reagent (Thermo Fisher Scientific, Inc.) and
protein bands were quantified using ImageJ software (version 1.50i;
National Institutes of Health, Bethesda).
Immunological techniques
STAT3 serves as a hub for regulating key target
genes involved in tumor invasion and angiogenesis. Therefore, the
expression of STAT3 as well as related proteins was assessed.
Immunofluorescence (IF) was used in in vitro experiments. In
brief, after being cultured for 5 days, the GSLCs were fixed with
fresh cold 4% paraformaldehyde/PBS. The cells were blocked by 0.2%
Triton X-100/10% BSA for 1 h and then incubated overnight at 4°C.
After washing with PBS, appropriate secondary antibodies coupled
with fluorescent dyes (Alexa Fluor 594; dilution 1:1,000; cat. no.
R37117 and Alexa Fluor 488; 1:1,000; cat. no. A27034; Invitrogen;
Thermo Fisher Scientific, Inc.) were applied at RT for 1 h. The
following primary antibodies were used: anti-STAT3 (1:200 dilution;
product no. 9139; Cell Signaling Technology), anti-AKT (rabbit;
1:100; cat. no. 649002; BioLegend, Inc.), anti-VEGF (1:200
dilution; product code ab32152; Abcam), and anti-NF-κB (1:50
dilution; product no. 8242; Cell Signaling Technology). Nuclei were
labeled with 0.25 mg/ml DAPI (Sigma-Aldrich; Merck KGaA) for 15
min. Images were captured using a confocal laser scanning
microscope (CLSM Leica Microsystems).
Small interference RNA study
The functional analyses used small interference RNA
(siRNA) duplexes specific for STAT3 to knockdown STAT3 gene
expression. Transient transfections were performed using
Lipofectamine 2000 (Invitrogen Life Technologies; Thermo Fisher
Scientific, Inc.), according to the protocol of the manufacturer.
The GSLCs were transfected 24 h after being seeded into a 6-well
plate at a density of 2×105 cells/well. STAT3 siRNAs
(Santa Cruz Biotechnology, Inc.) were transfected with
Lipofectamine RNAimax transfection reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). Different concentrations of siRNA (200,
100 and 50 nM) were assessed. The knockdown effect was confirmed
using western blotting. In addition, the migration capacity of the
cells was detected as aforementioned.
Temozolomide (TMZ) treatment
The cytotoxicity of the two groups was determined by
culturing cells in various concentrations of TMZ (Sigma-Aldrich:
Merck KGaA), ranging from 0 to 1,600 µM. In order to determine the
half-maximal inhibitory concentration (IC50), an MTS
assay kit was performed according to the instructions after the
cells were cultured in different densities of TMZ (75, 150, and 300
µM) for 3 days. Furthermore, the apoptotic cells were detected
using an Annexin V-FITC apoptosis detection kit, in which vehicle
solvent dimethyl sulfoxide (DMSO) was used as a negative control.
Each assay was performed in triplicate, followed by an analysis of
the statistical significances.
In vivo study
Thirty female C57BL/6 mice (6–8 weeks old, supplied
by Beijing Vital River Laboratory Animal Technology Co., Ltd.) were
raised under sterile conditions. The use of animals was approved by
the Institutional Animal Care and Use Committee of the Army General
Hospital of Beijing (approval no. IACUC20170114-04). The mice were
randomly divided into 3 groups: The saline group, the PGBC group,
and the GSLC group. Then, the mice were sublethally irradiated with
2.5 Gy from a 137Cs source (2.115 Gy/min) before
transplantation. The PGBCs and GSLCs, suspended in a total volume
of 3 µl (approximately 1×105 cells), were
intracerebrally infused into the frontal lobe (coordinates: 1.0 mm
anterior, 2.5 mm ventral, and 1.8 mm lateral to the midline) using
a Hamilton syringe after exposing the bregma. After the surgery,
the animals were allowed to recover from the anesthesia and were
placed in the cages. The mice were sacrificed with an overdose of
pentobarbital (100 mg/kg) 14 days later. The weight of the mice and
the survival ratio over that period were recorded. Then, brain
tissue was cut into sections of 5 µm by using a cryostat (Leica CM
1850), and hematoxylin and eosin (H&E) staining was conducted
according to the instructions to reveal the cytomorphology of the
cells. In addition, IF staining as well as western blotting were
performed as in the in vitro procedure to reveal the
expression of related proteins.
Statistical analysis
Results were expressed as the mean ± standard
deviation, unless otherwise indicated. Statistically significant
differences between the two groups were determined by a two-tailed
Student's t-test or a one-way ANOVA followed by Tukey's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference. All tests were performed using SPSS 20.0
(IBM Corp.).
Results
CHIR99021 promotes the expression of
GSLC markers
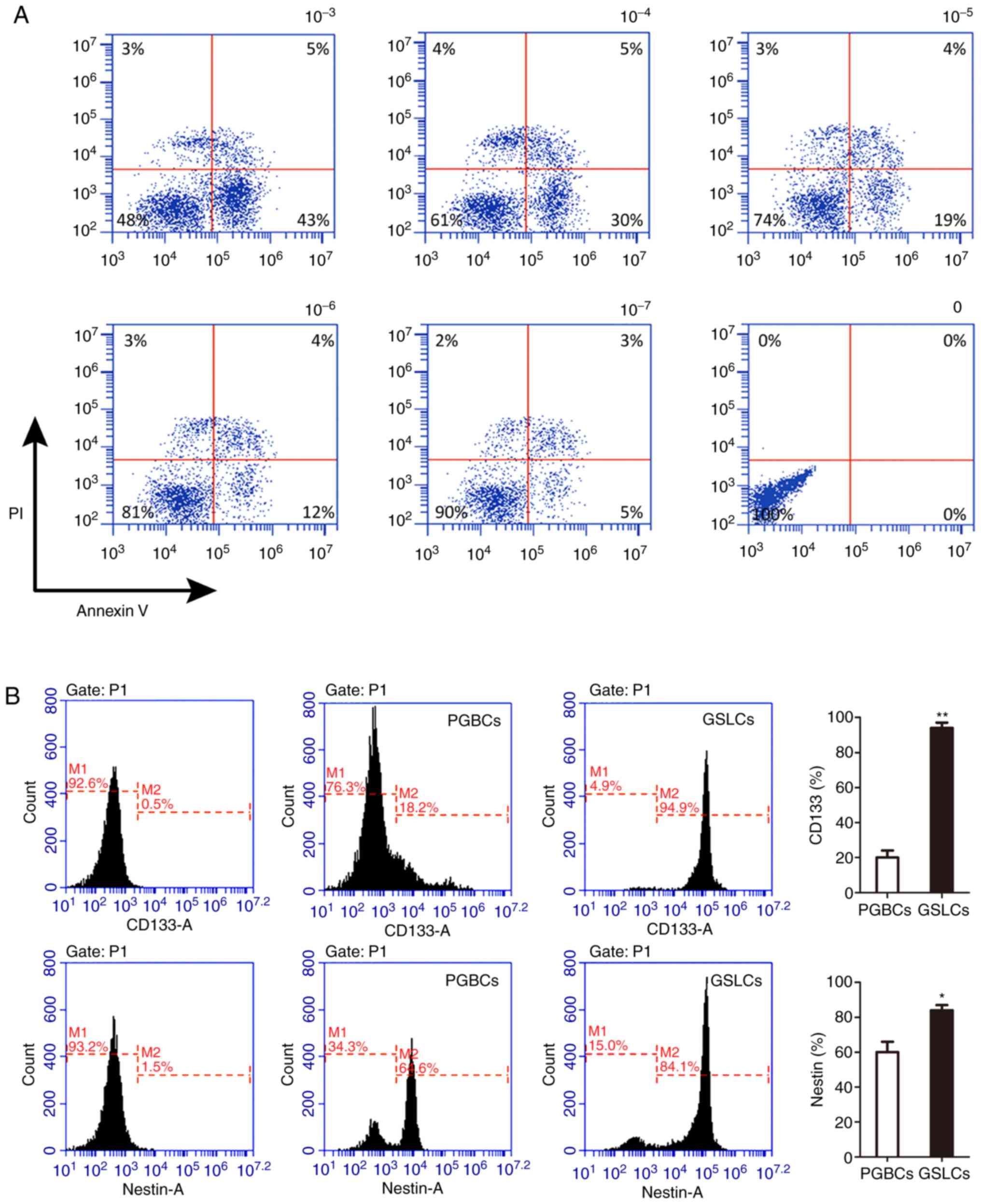
The suitable concentration of CHIR99021 was revealed
to be 100 nM after culturing the cells in different concentrations
of CHIR99021. With this concentration, the survival rate of the
primary low-grade glioma cells was ~90%. Moreover, the apoptotic
rate increased by ~10% when the concentration was increased ten
times (Fig. 1A). In the PGBCs, the
average percentages of CD133 and Nestin were 15.13±4.40% and
61.17±6.26%, respectively. These percentages were 93.83±2.20% and
83.93±1.98%, respectively, in cells cultured under 100 nM
CHIR99021, which were significantly higher percentages compared to
those in the PGBCs (P<0.01, Fig.
1B).
Proliferation and migration of the
GSLCs
Cellular cross-links and gliomasphere formation were
gradually formed by culturing in 100 nM CHIR99021 from day 3 to day
5 (Fig. 2A). In addition, the
migration ratios were confirmed by colorimetric assay and revealed
as the optical densities (OD) at 570 nm, which were ~15% and 38% in
the PGBCs and GSLCs groups, respectively (Fig. 2B). Ultrastructural observation of
cellular changes was carried out by using a transmission electron
microscopy (TEM). The GSLCs had more filopodia and lamellipodia and
a larger endoplasmic reticulum (ER), an organelle that is involved
in cell adhesion and migration (Fig.
2C).
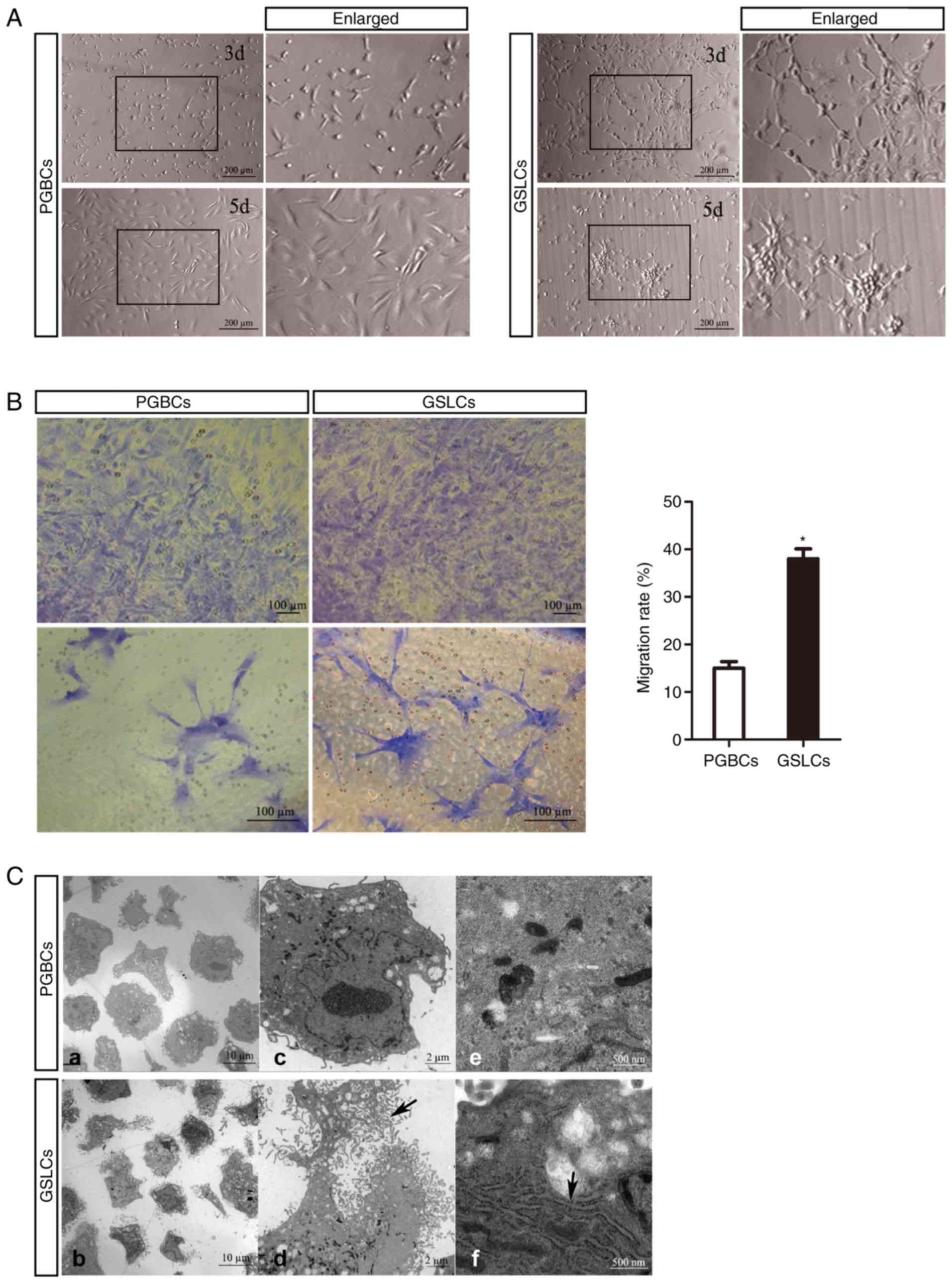 | Figure 2.Proliferation and migration of the
GSLCs and related ultra-structures. (A) Oval and fusiform
characteristics in PGBCs, and cross-links and sphere formation in
GSLCs. (B) Images of the migrated cells in Transwell assays used
for calculating of the migration rate (scale bar, 100 µm). (C)
Under TEM, more fiber-like structures around the GSLCs (a and b,
scale bar, 10 µm) were observed. At a higher magnification, these
structures were filopodia and lamellipodia (c, d and black arrow in
d, scale bar, 2 µm), in addition, more endoplasmic reticulum in the
GSLCs (e, f and black arrow in f, scale bar, 500 nm). *P<0.05.
GSLCs, glioma stem-like cells; PGBCs, primary glioma-based cells;
TEM, transmission electron microscopy. |
Effect of CHIR99021 on the PI3K/AKT
signaling pathway
The analysis revealed that GSK-3β was
expressed in the PGBCs but almost absent in the GSLCs. The
PI3K gene was significantly enhanced in the GSLCs compared
to the PGBCs (~288-fold). The heterodimers of PI3K, namely
p85 and p110, were respectively ~150-fold and 11-fold
higher in the GSLCs than in the PGBCs, which was in accordance with
the high PI3K activity in the GSLCs.
The AKT and mTOR genes, which are
well-characterized key downstream effectors of PI3K, were
highly expressed in the GSLCs (Fig.
3A). The expression levels of the related proteins (PI3K, AKT
and mTOR) were also enhanced in the GSLCs compared to the PGBCs
(Fig. 3B). The expression levels of
the STAT3, VEGF, and NF-κB genes, which are involved
in cell migration and invasion, were increased ~152-fold, 25-fold,
and 50-fold, respectively, in the GSLCs (Fig. 3A). Western blot analysis revealed
that the expression of STAT3, p-STAT3, VEGF, and NF-κB was
significantly increased in the GSLCs compared to the PGBCs
(Fig. 3B).
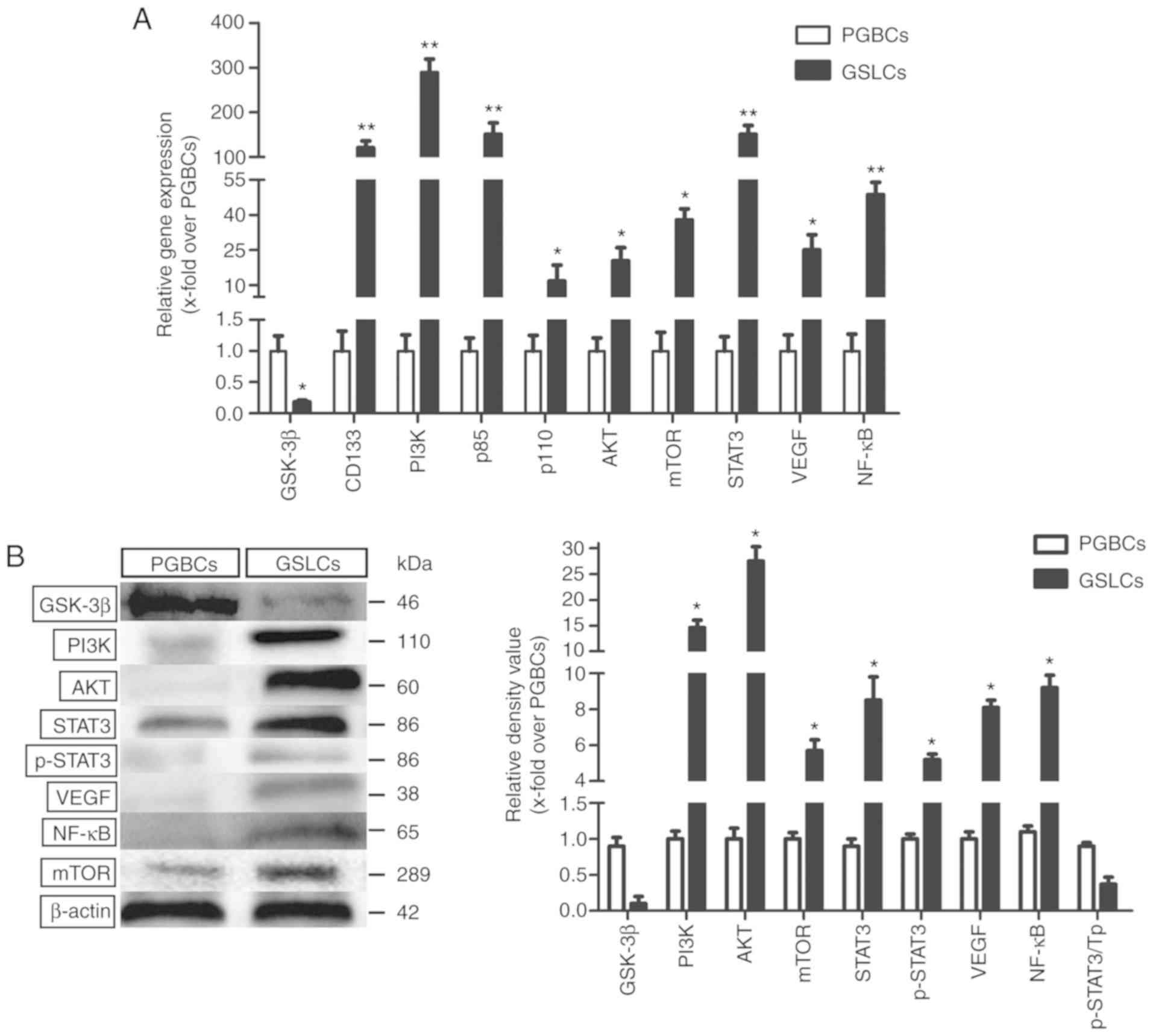 | Figure 3.Expression level of genes and
proteins relating to cell proliferation, migration and invasion.
(A) The gene expression levels detected by qRT-PCR. (B) The
expression levels of corresponding proteins detected by western
blotting. *P<0.05 and **P<0.01. AKT, protein kinase B;
GSK-3β, glycogen synthase kinase-3β; GSLCs, glioma stem-like cells;
mTOR, mammalian target of rapamycin; NF-κB, clear factor
kappa-light-chain-enhancer of activated B cells; PGBCs, primary
glioma-based cells; PI3K, phosphoinositide 3-kinase; p-STAT3,
phospho-STAT3; STAT3, signal transducer and activator of
transcription 3; Tp, total protein; VEGF, vascular endothelial
growth factor. |
Activation of STAT3
The IF staining of STAT3, AKT, VEGF and NF-κB was
positive in the GSLCs (Fig. 4A).
These results were in accordance with the changes in gene
expression. In addition, the knockdown effect of STAT3 in the GSLCs
was also detected by western blotting. In comparison to the control
group (untreated GSLCs) and the negative group (without siRNA
transfection), siRNA (200 and 100 nM) markedly inhibited the
expression of the STAT3 protein in the GSLCs. However, only 200 nM
siRNA could effectively decrease the p-STAT3 protein level
(Fig. 4B). Further study revealed
that 200 nM siRNA significantly decreased the expression of NF-κB
and VEGF (Fig. 4C). In the
Transwell experiment, 200 nM siRNA significantly inhibited the
migration of the GSLCs (Fig.
4D).
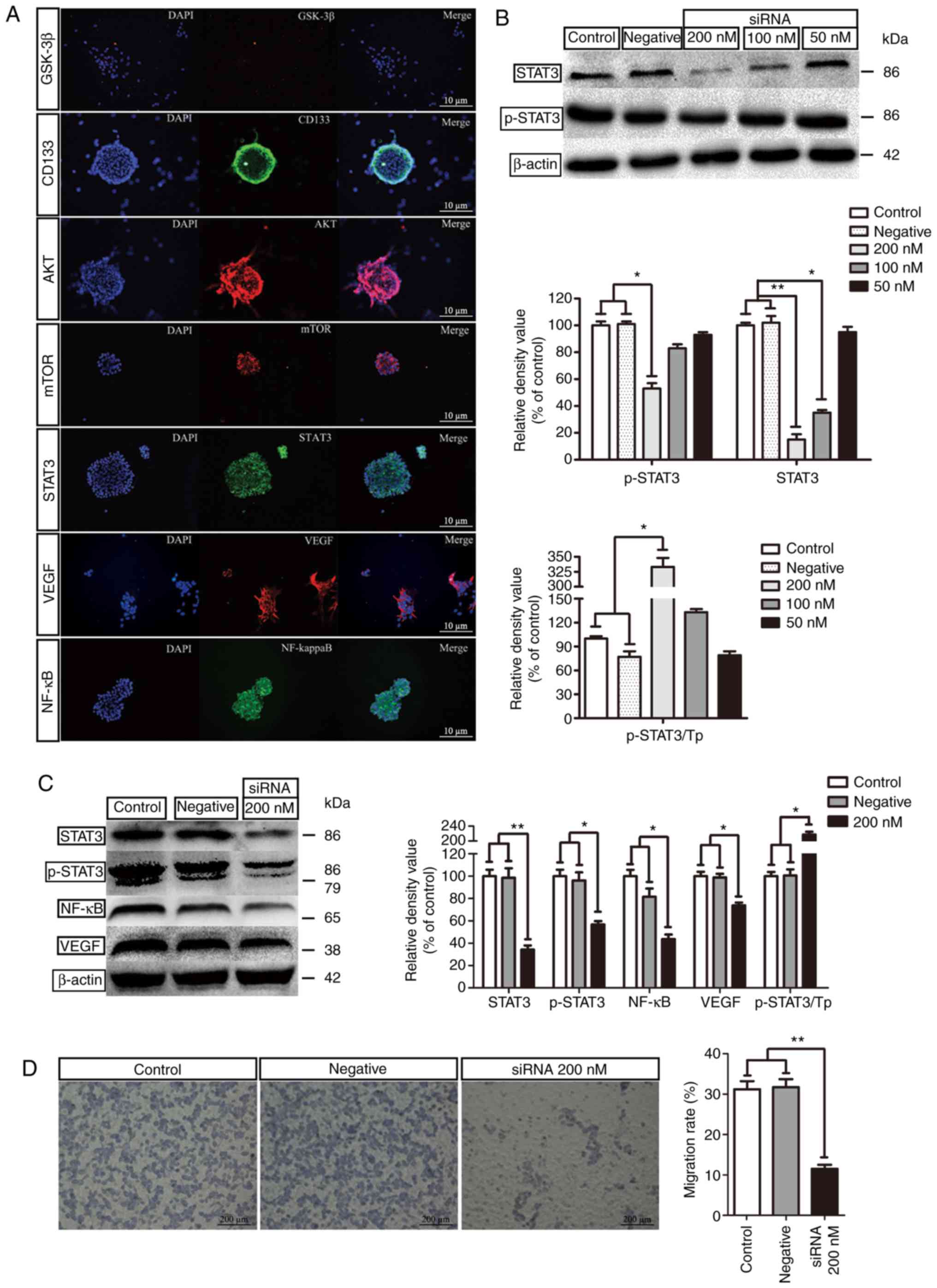 | Figure 4.Expression of STAT3 and related
proteins and siRNA study. (A) IF staining of proteins in GSLCs
(scale bar, 10 µm). (B) Knockdown effect of STAT3 in GSLCs by
siRNA. (C) The effect of 200 nM siRNA on the expression of STAT3,
p-STAT3, NF-κB and VEGF. (D) Transwell assay to detect the
migration rates after the siRNA study (scale bar: 200 µm).
*P<0.05 and **P<0.01. GSLCs, glioma stem-like cells; IF,
immunofluorescence; NF-κB, clear factor kappa-light-chain-enhancer
of activated B cells; p-STAT3, phospho-STAT3; siRNA, small
interference RNA; STAT3, signal transducer and activator of
transcription 3; Tp, total protein; VEGF, vascular endothelial
growth factor. |
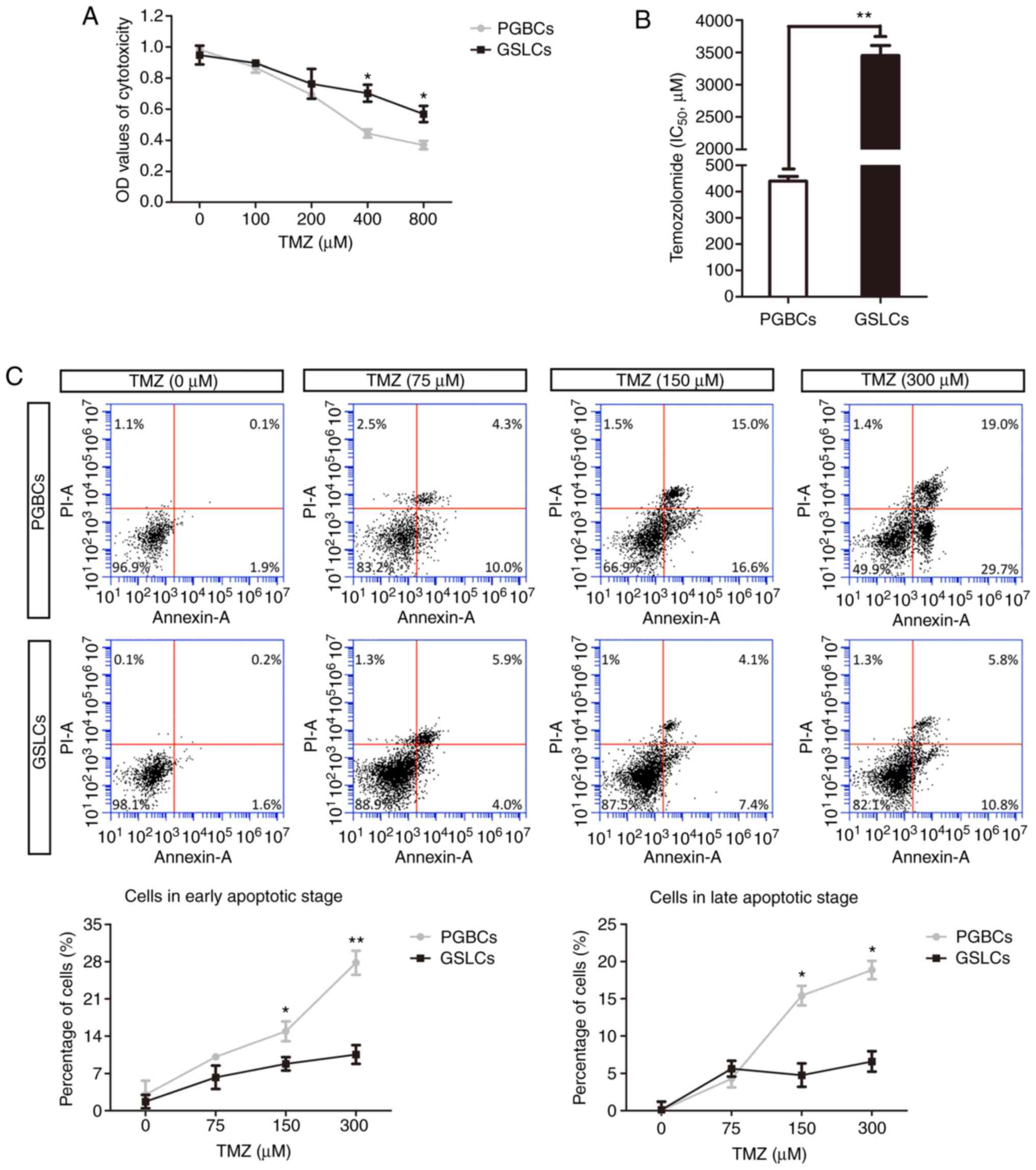
TMZ treatment
The cytotoxicity of mixed PGBCs and induced GSLCs
was determined by adding various concentrations of TMZ into the
medium. A significant difference of cytotoxicity between the PGBCs
and the GSLCs was observed when TMZ was added in a concentration of
400 µM (0.44±0.03 and 0.70±0.05, respectively, P<0.05; Fig. 5A). Further exploration revealed that
the IC50 values of the PGBCs and GSLCs to TMZ were
430±40.0 and 3450±350 µM, respectively (Fig. 5B).
Annexin V-FITC/PI analysis revealed that in the
DMSO-treated group (vehicle control), the cell apoptosis
percentages were ~2.00% and 1.80% for the PGBCs and GSLCs,
respectively. When the concentration of TMZ increased, the
proportion of apoptotic cells was increased in both groups,
however, it was more significant in the PGBCs (Fig. 5C). Additionally, early apoptosis
(Annexin+/PI−), as well as late apoptosis
(Annexin+/PI+), began to occur in both the
PGBC and GSLC groups after treatment with TMZ (75, 150 and 300 µM).
A significant difference between these two groups appeared at a
concentration of 150 µM for early apoptosis (14.90±1.87% vs.
8.80±1.25%, P<0.05) and for late apoptosis (15.43±1.31% vs.
4.77±1.58%, P<0.05; Fig.
5C).
In vivo validations in mice
The weight of the mice transplanted with the GSLCs
was significantly decreased than the weight of the mice injected
with the PGBCs and saline (Fig.
6A). In addition, no dead mice were found in the saline group,
1 death in the PGBC group, and 5 deaths in the GSLC group were
identified after the transplantation (Fig. 6B). H&E staining demonstrated
that there were numerous malignant cells with large nuclei and
active mitosis in the brain sections of the GSLC-transplanted mice
(Fig. 6C).
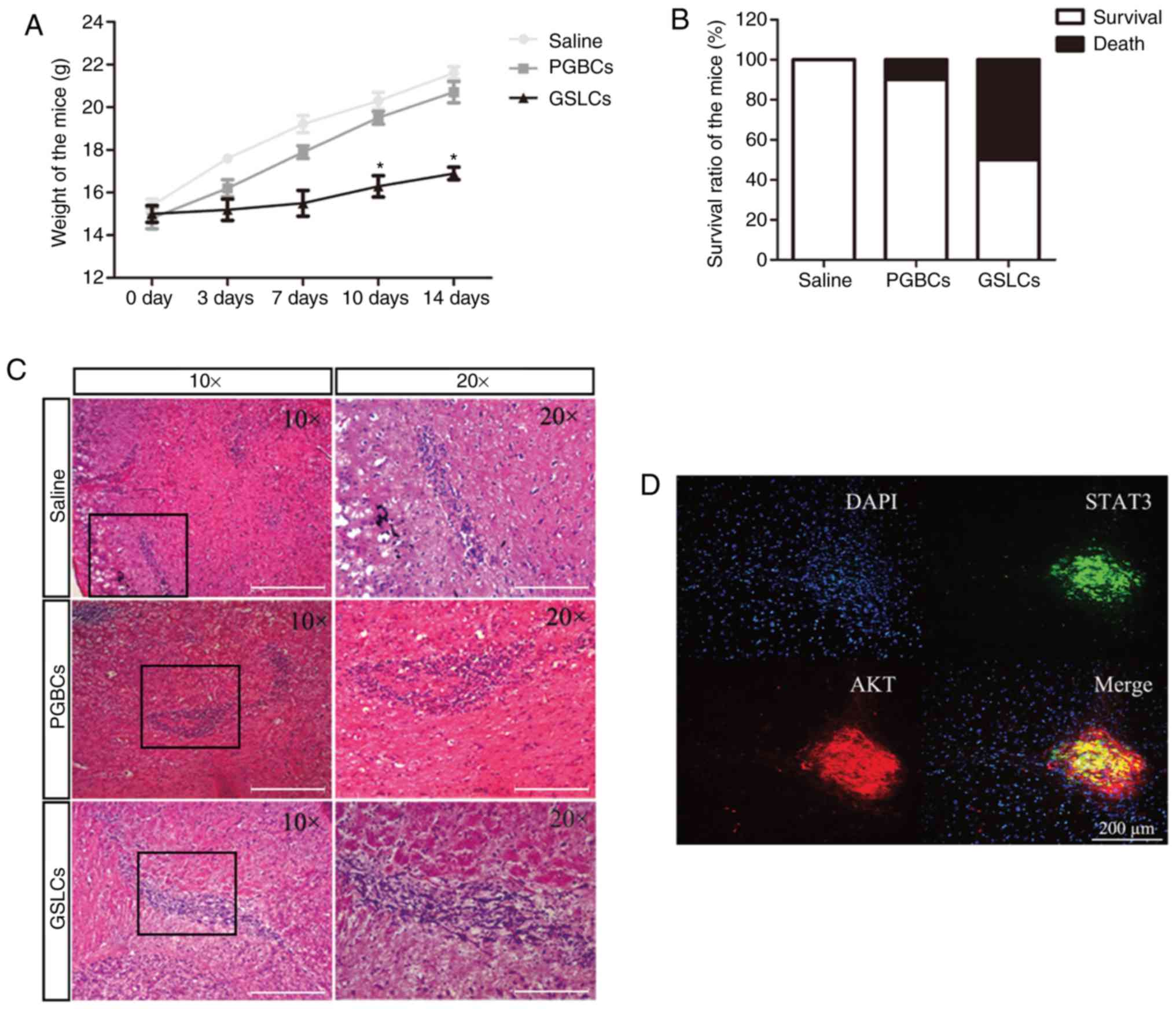 | Figure 6.In vivo experimental
validations in mice. (A) Weights of the mice injected with saline,
PGBCs and GSLCs. (B) Mortality rates of the mice transplanted with
saline, PGBCs and GSLCs. (C) Malignant cells in the brains of mice
revealed by H&E staining (scale bar, 200 µm). (D) Co-expression
of STAT3 and AKT in the GSLC-transplanted mice (scale bar, 200 µm).
(E and F) Expression levels of the STAT3, NF-κB, VEGF, and/or mTOR
proteins detected by IF and western blotting (scale bar, 250 µm).
*P<0.05 and **P<0.01. AKT, protein kinase B; GSLCs, glioma
stem-like cells; IF, immunofluorescence; mTOR, mammalian target of
rapamycin; NF-κB, clear factor kappa-light-chain-enhancer of
activated B cells; PGBCs, primary glioma-based cells; STAT3, signal
transducer and activator of transcription 3; VEGF, vascular
endothelial growth factor. |
IF revealed that STAT3 and AKT were co-expressed in
the brain sections of the GSLC-transplanted mice (Fig. 6D). Moreover, the NF-κB and VEGF
protein levels were significantly increased in the
GSLC-transplanted mice than in the PGBC- and saline-injected mice
(Fig. 6E). A western blot assay
revealed that the expression of the STAT3, NF-κB, VEGF, and mTOR
protein levels were significantly upregulated in the
GSLC-transplanted mice compared to the saline- and PGBC-injected
mice (Fig. 6F).
Discussion
Use of CHIR99021
Most glioma cell experiments are conducted using
cell lines such as U87 and U251. With these cell lines, however,
numerous clinical gliomas are not fully detected. Furthermore,
these cell lines may not accurately represent GBM characteristics.
The results of the present study revealed that CHIR99021, in a
concentration of 100 nM, could enrich primary low-grade glioma
cells with CD133-positive GSLC properties.
Based on some of the following existing studies, the
use of CHIR99021 was decided to establish a GSLC model of clinical
glioma tissue. A recent study demonstrated that cordycepin, derived
from cultured Cordyceps militaris, could induce a decrease
in cell viability, a downregulation of β-catenin, an increase in
apoptosis, and a reduction in TMZ resistance. However, all these
effects could be reversed with CHIR99021 (17). Another study reported that CHIR99021
activated the Wnt/β-catenin signaling pathway and then initiated
the differentiation of human embryonic stem cells (hESCs) by
inhibiting the degradation of β-catenin (18). Additionally, CHIR99021 was crucial
for maintaining the metabolic activity of differentiated rat
embryonic stem (ES) cells (19).
Furthermore, a tumor cell line was established by culturing cells
in a chemically defined N2B27 medium containing CHIR99021. The cell
lines revealed some indications of malignancy, such as Oct4
expression and a long-term expansion ability (20).
Proliferation and invasiveness of the
GSLCs
The present results revealed that the GSLCs had more
filopodia and lamellipodia and a larger endoplasmic reticulum.
These changes indicated that the proliferation and migration
abilities of the GSLCs were enhanced by CHIR99021, which was
consistent with the expression of related genes and proteins in the
PI3K/AKT/mTOR signaling pathway. Further exploration demonstrated
that the GSLCs significantly expressed invasion-related genes and
proteins, including STAT3, VEGF and NF-κB.
CHIR99021 specifically inhibits GSK-3, while
AKT inactivates GSK-3 through its Ser9
phosphorylation (21). In the
present study, qRT-PCR and western blot analysis were used to
demonstrate that AKT was activated by CHIR99021 in the
GSLCs. In addition, a similar genetic tendency in the classical
PI3K/AKT signaling pathway was revealed in the GSLCs, including a
heterodimer of PI3K, consisting of the regulatory subunit
p85 and the catalytic subunit p110. Furthermore, the
downstream key kinase mTOR in this pathway also exhibited
the same trend. The mTOR gene plays an essential role in the
activation of STAT3, which is followed by an upregulation of
the expression of angiogenesis-promoting factors (VEGF).
Both the activation and the upregulation contribute to cancer
stem-like cell survival/proliferation (22,23).
The NF-κB gene is another downstream transcription factor in
the classical PI3K/AKT signaling pathway. It has been implicated in
numerous hallmarks of cancer development, including
growth-factor-independent proliferation, apoptosis prevention,
unlimited replicative potential, and tissue invasion and metastasis
(24,25).
As a signal transducer and activator, STAT3
regulates the expression of target genes involved in the cell
cycle, apoptosis, cellular transformation, and tumor angiogenesis
(23). In the present study,
western blot analysis and IF staining revealed that CHIR99021
induced high expression of STAT3 and phosphorylated (p)-STAT3
proteins. Silencing STAT3 by siRNA also confirmed the effect
of CHIR99021, by downregulating related proteins (p-STAT3, VEGF,
and NF-κB). These findings indicated that STAT3 may function
as an integrator of these cellular signals, controlling the
migration and invasiveness of GSLCs, and thus could be a potential
therapeutic target in glioma treatment.
Resistance to TMZ and an animal
study
Existing publications have suggested that glioma
cancer stem cells are closely associated with resistance to
radiotherapy and chemotherapy. In addition, resistance to TMZ has
been revealed to be closely related to MGMT-mediated DNA repair in
glioma cancer stem cells (26–28),
however, the underlying mechanism remains to be elucidated. The
present research revealed that the high expression of STAT3 in the
GSLCs rendered the cells significantly resistant to TMZ, which was
not the case in the PGBCs. This may account for the recurrence of
gliomas. Whether TMZ resistance is mainly related to the increased
expression of STAT3 still requires further study. Irradiated mice
instead of nude mice were used in our animal studies in order to
mimic the clinical situation, in which the patient is often
radiated after surgery but still has clinical relapses. This
recurrence may be ascribed to the weak condition of the patient and
the high malignant properties of the cancer stem cells. The present
animal studies revealed that transplantation of the GSLCs had a
tumor-initiating capacity in the irradiated mice brain, and the
high expression of AKT, mTOR, STAT3, VEGF, and NF-κB in the brain
sections two weeks later also indicated migration and invasion of
the GSLCs in vivo.
Limitations
The present study focused only on the proliferation
and invasiveness of GSLCs, without further exploration of other
characteristics, such as tumor angiogenesis or inflammation.
Furthermore, although the present study provided evidence that
CHIR99021 could enhance the expression of malignancy-related
biological features, only the well-known PI3K/ATK pathway and STAT3
were explored. Finally, the mechanism of TMZ resistance requires
further investigation.
CHIR99021 has the potential to promote the migration
and invasiveness of induced GSLCs both in vitro and in
vivo, possibly by regulating the PI3K/AKT signaling pathway and
activating STAT3. In addition, the drug resistance capability of
the GSLCs was also enhanced. In conclusion, CHIR99021 may provide a
useful GSLC model, generated from patient-derived low-grade glioma
samples, for further study, helping to understand the pathogenesis
of therapeutic resistance and to screen for potential drug
candidates.
Acknowledgements
We would like to thank our colleague Dr CY Liang
from the Seventh Medical Center of PLA Army General Hospital for
providing insight and expertise that greatly assisted the
research.
Funding
The research was supported by the Beijing Municipal
Science & Technology Commission (Z17110000101). YY is supported
by the China Scholarship Council (201709110116).
Availability of data and materials
The datasets in the current study are available from
the corresponding author on reasonable request.
Authors' contributions
YY, OB and SSW designed this study. QQW and SSW
performed the cell experiments, and YY and YLS performed the animal
study and data collection. OB and SSW helped to perform data
analysis and interpretation. YY, QQW and YLS wrote the manuscript,
and OB and RXX critically revised manuscript for important
intellectual content. All authors read and approved the manuscript
and agree to be accountable for all aspects of the research in
ensuring that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patients, and ethical approval was obtained from the Ethics
Committee of the Army General Hospital of Beijing (No. 2017-114).
The use of animals was approved by the Institutional Animal Care
and Use Committee of the Army General Hospital of Beijing (approval
no. IACUC20170114-04).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sunayama J, Sato A, Matsuda K, Tachibana
K, Watanabe E, Seino S, Suzuki K, Narita Y, Shibui S, Sakurada K,
et al: FoxO3a functions as a key integrator of cellular signals
that control glioblastoma stem-like cell differentiation and
tumorigenicity. Stem Cells. 29:1327–1337. 2011.PubMed/NCBI
|
|
2
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lathia JD, Mack SC, Mulkearns-Hubert EE,
Valentim CL and Rich JN: Cancer stem cells in glioblastoma. Genes
Dev. 29:1203–1217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stopschinski BE, Beier CP and Beier D:
Glioblastoma cancer stem cells-from concept to clinical
application. Cancer Lett. 338:32–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hardee ME, Marciscano AE, Medina-Ramirez
CM, Zagzag D, Narayana A, Lonning SM and Barcellos-Hoff MH:
Resistance of glioblastoma-initiating cells to radiation mediated
by the tumor microenvironment can be abolished by inhibiting
transforming growth factor-β. Cancer Res. 72:4119–4129. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu G, Yuan X, Zeng Z, Tunici P, Ng H,
Abdulkadir IR, Lu L, Irvin D, Black KL and Yu JS: Analysis of gene
expression and chemoresistance of CD133+ cancer stem cells in
glioblastoma. Mol Cancer. 5:672006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee J, Kotliarova S, Kotliarov Y, Li A, Su
Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al:
Tumor stem cells derived from glioblastomas cultured in bFGF and
EGF more closely mirror the phenotype and genotype of primary
tumors than do serum-cultured cell lines. Cancer Cell. 9:391–403.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mahesparan R, Read TA, Lund-Johansen M,
Skaftnesmo KO, Bjerkvig R and Engebraaten O: Expression of
extracellular matrix components in a highly infiltrative in vivo
glioma model. Acta neuropathol. 105:49–57. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie Y, Bergstrom T, Jiang Y, Johansson P,
Marinescu VD, Lindberg N, Segerman A, Wicher G, Niklasson M,
Baskaran S, et al: The human glioblastoma cell culture resource:
Validated cell models representing all molecular subtypes.
EBioMedicine. 2:1351–1363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh VK, Kumar N, Kalsan M, Saini A and
Chandra R: Mechanism of induction: Induced pluripotent stem cells
(iPSCs). J Stem Cells. 10:43–62. 2015.PubMed/NCBI
|
|
14
|
Takeda Y, Harada Y, Yoshikawa T and Dai P:
Chemical compound-based direct reprogramming for future clinical
applications. Biosci Rep. 38(pii): BSR201716502018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim SS, Pirollo KF and Chang EH: Isolation
and culturing of glioma cancer stem cells. Curr Protoc Cell Biol.
67:1–23. 2015. View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bi Y, Li H, Yi D, Bai Y, Zhong S, Liu Q,
Chen Y and Zhao G: β-catenin contributes to cordycepin-induced MGMT
inhibition and reduction of temozolomide resistance in glioma cells
by increasing intracellular reactive oxygen species. Cancer Lett.
435:66–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng T, Zhai K, Chang Y, Yao G, He J,
Wang F, Kong H, Xin H, Wang H, Jin M, et al: CHIR99021 combined
with retinoic acid promotes the differentiation of primordial germ
cells from human embryonic stem cells. Oncotarget. 8:7814–7826.
2017.PubMed/NCBI
|
|
19
|
Petkov S, Hyttel P and Niemann H: The
small molecule inhibitors PD0325091 and CHIR99021 reduce expression
of pluripotency-related genes in putative porcine induced
pluripotent stem cells. Cell Reprogram. 16:235–240. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng X, Gao H, Wang Y, Yang B, Liu T, Sun
Y, Jin H, Jiang L, Li L, Wu M and Qian Q: Conversion of rat
embryonic stem cells into neural precursors in chemical-defined
medium. Biochem Biophys Res Commun. 431:783–787. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fan X, Xiong H, Wei J, Gao X, Feng Y, Liu
X, Zhang G, He QY, Xu J and Liu L: Cytoplasmic hnRNPK interacts
with GSK3β and is essential for the osteoclast differentiation. Sci
Rep. 5:177322015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang
Y, Deng J, Margolick JB, Liotta LA, Petricoin E III and Zhang Y:
Activation of the PTEN/mTOR/STAT3 pathway in breast cancer
stem-like cells is required for viability and maintenance. Proc
Natl Acad Sci USA. 104:16158–16163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan J, Zhang F and Niu R: Multiple
regulation pathways and pivotal biological functions of STAT3 in
cancer. Sci Rep. 5:176632015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kucuksayan HH and Akgun SS: Pl3K/Akt/NF-κB
Signalling Pathway on NSCLC Invasion. Med Chem. 6:234–238. 2016.
View Article : Google Scholar
|
|
25
|
Matsuoka T and Yashiro M: The Role of
PI3K/Akt/mTOR signaling in gastric carcinoma. Cancers. 6:1441–1463.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Happold C, Roth P, Silginer M, Florea AM,
Lamszus K, Frei K, Deenen R, Reifenberger G and Weller M:
Interferon-β induces loss of spherogenicity and overcomes therapy
resistance of glioblastoma stem cells. Mol Cancer Ther. 13:948–961.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qiu ZK, Shen D, Chen YS, Yang QY, Guo CC,
Feng BH and Chen ZP: Enhanced MGMT expression contributes to
temozolomide resistance in glioma stem-like cells. Chin J Cancer.
33:115–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lai IC, Shih PH, Yao CJ, Yeh CT, Wang-Peng
J, Lui TN, Chuang SE, Hu TS, Lai TY and Lai GM: Elimination of
cancer stem-like cells and potentiation of temozolomide sensitivity
by Honokiol in glioblastoma multiforme cells. PLoS One.
10:e01148302015. View Article : Google Scholar : PubMed/NCBI
|