Introduction
Basal cell carcinoma (BCC) is the most common skin
malignancy. Cancer registries do not collect data on this skin
cancer, so its prevalence and incidence are difficult to estimate.
According to the American Cancer Society, in 2006, >2 million
people were treated for cases of non-melanoma skin cancer (NMSC),
the majority of which were BCC (1).
The lifetime risk of developing skin cancer is estimated to be 1 in
5, and >97% of patients who develop skin cancer will have NMSC
(2). BCC accounts for 75% of all
skin cancers and is the most common skin malignancy. The incidence
of BCC is rising by 3–8% each year (3); thus, the average lifetime risk that a
Caucasian individual will develop BCC is 30% (4–6). BCC
rarely metastasizes (7) but may
cause extensive local tissue destruction when left untreated
(8,9). Hence, BCC is becoming a serious health
problem. Simple and cost-efficient medical treatments are clearly
required. A thorough understanding of BCC pathobiology is required
to develop such treatments. Considerable progress has been made
with respect to the understanding of BCC pathobiology during the
past few years.
BCC is characterized by abnormalities in the
Hedgehog (Hh) signalling pathway that result in constitutively
active Hh signalling. BCC tumours typically harbour an inactivating
mutation of the tumour suppressor patch (Ptch) gene or an
activating mutation in the smoothened (Smo) gene (10). Molecular studies have revealed that
the Ptch gene is a human homologue of the Drosophila patched gene
(11,12). Now known as PTCH, the gene encodes a
receptor for the Sonic hedgehog (Shh) pathway, which is important
for patterning and growth during vertebrate development (13). The Shh ligand binds to and inhibits
the PTCH receptor to allow signalling through the Shh pathway. As
an inhibitory protein, PTCH allows overactivation of the Shh
pathway in cases in which it has inactivating mutations. Recent
animal studies have revealed that mice overexpressing Shh in the
context of normal PTCH expression and activity develop multiple
BCCs and features of NBCCs (14,15).
Activating somatic mutations in SMO, a seven-transmembrane protein
immediately downstream of PTCH, were found in a selection of
sporadic BCCs, and transgenic mice overexpressing mutant SMO have
been revealed to develop skin abnormalities similar to those of
BCCs (16). These findings indicate
that SMO serves as a proto-oncogene. Overexpression of GLI
proteins, transcription factors activated by SMO, in mouse models
has been revealed to induce BCCs (17,18).
Furthermore, continued Shh signalling has been revealed to be
required for BCC carcinogenesis. A previous study demonstrated that
mice engineered to conditionally express GLI-2 exhibited BCC
regression when GLI-2 expression was inactivated (19). All of these studies support the
concept that Shh signalling overactivation is necessary and perhaps
sufficient for BCC development.
CD44 is a transmembrane cell-adhesion glycoprotein
that participates in and regulates many cellular processes,
including cellular growth, survival, differentiation, lymphocyte
homing, and motility (20,21). CD44 affects a variety of cellular
processes, most likely since multiple isoforms of the protein are
produced by alternative splicing (22–24).
CD44s, the smallest (standard) form of CD44 (CD44s), weighs
approximately 80–95 kDa and lacks all variable CD44 exons. In
breast cancer, cells undergoing EMT exhibit increased CD44
expression and TISC characteristics (25–27).
CD44 expression has been described within TISC populations;
however, the isoform responsible for its TISC characteristics
remains unknown (21). CD44s, which
is ubiquitously expressed in epithelial tissues, is the predominant
CD44 variant and has recently been proposed to be essential for
epithelial-to-mesenchymal transition (EMT) (28). Recent studies have demonstrated that
the RNA-binding protein IMP3 stabilizes CD44 mRNA to facilitate
cell migration and, more importantly, that CD44s combined with IMP3
can serve as a predictive biomarker for HCC (29). Collectively, the findings of these
studies indicate that CD44s plays an important role in HCC
progression.
To date, there is no evidence revealing that a
relationship exists between CD44s expression and vismodegib
resistance in BCC. In the present study, a CD44-harbouring
lentiviral vector was used to express CD44s in a BCC cell line and
then vismodegib cytotoxicity was evaluated in the cell line.
Additionally, an in vivo model was used to evaluate the
efficacy of vismodegib in mice implanted with tumours.
Materials and methods
Cell lines
The BCC cells, used in the present study, were
isolated from BCC tumors that arose in irradiated Patched 1
(Ptch1)+/− mice, according to the protocol
developed by So et al (30).
The BCC cell lines were cultured in Gibco; Thermo Fisher
Scientific, Inc., M154F media supplemented with 2% chelexed
heat-inactivated foetal bovine serum, 1% penicillin-streptomycin,
and 0.05 mM calcium chloride. Silibinin, carboxymethylcellulose
(CMC), Harris haematoxylin, dimethyl sulfoxide (DMSO), and trypan
blue were obtained from Sigma Aldrich; The human embryonic kidney
cell line 293FT [American Type Culture Collection (ATCC),
Rockville, MD, USA] was grown in DMEM supplemented with 10% fetal
calf serum (HyClone; GE Healthcare Life Sciences). All cell lines
were cultured at 37°C in a humidified atmosphere with 5%
CO2.
Construction of the recombinant
lentiviral vector encoding the CD44s gene
The sequences of the primers for the whole-length
human CD44s cDNA sequence were as follows: sense,
5′-GCGTCGACATGGACAAGTTTTGGTGGCACGCAGCCTG-3′ and antisense,
5′-CGGGATCCTTACACCCCAATCTTCATGTCCAC-3′. Briefly, the CD44s gene was
amplified by PrimeSTAR® GXL DNA Polymerase (Takara Bio,
Inc.) using BCC cell cDNA as a template, and then the CD44s gene
and pLenti vector were digested by the enzymes SalI and
BamHI. Following recycling electrophoresis, the CD44s gene
was subcloned into the pLenti plasmid, and the resulting
recombinant plasmid, pLenti-CD44s, was identified by sequencing
demonstrating its successful construction. Primer synthesis and DNA
sequencing were performed by Shanghai Shangon Co., Ltd.. The viral
particles were generated by the co-transfection of 293FT cells
(American Type Culture Collection) with pLenti-CD44s or pLenti-CON
and two packaging vectors via calcium phosphate-mediated
transfection. Three days after transfection, the cell culture
supernatants were harvested (2,000 × g for 5 min), filtered through
filters with 0.45-µm pores and concentrated 100-fold by
ultracentrifugation at 7,000 × g for 16 h. The viral particles were
then stored in small aliquots at −80°C.
Detection of CD44s mRNA levels in BCC
cells by quantitative RT-PCR
CD44s mRNA expression in BCC cells infected with
LV-CD44s was compared with that in BCC cells infected with LV-CON.
Total RNA was isolated by TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
CD44s mRNA expression levels were detected by quantitative RT-PCR
(qRT-PCR) using a LightCycler 480 Instrument (Roche Diagnostics)
and a SYBR® Premix Ex Taq™ kit (Takara Bio, Inc.), and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as a
normalizing control. The specific sequences of these PCR primers,
which were designed by Primer premier 5.0 software (Premier Biosoft
International), were as follows: CD44s forward,
5′-GGAGCAGCACTTCAGGAGGTTAC-3′ and reverse,
5′-GGAATGTGTCTTGGTCTCTGGTAGC-3′; and GAPDH forward,
5′-TCATGGGTGTGAACCATGAGAA-3′ and reverse,
5′-GGCATGGACTGTGGTCATGAG-3′. Cycling was performed under the
following conditions: 95°C for 10 min (to activate DNA polymerase),
followed by 40 cycles of 95°C for 15 sec, 55°C for 20 sec, and 72°C
for 10 sec. The specificity of the amplification products was
confirmed by melting curve analysis. Independent experiments were
performed in triplicate. The relative mRNA expression levels of the
samples were normalized against the mRNA expression level of GAPDH.
The cycle threshold (Cq) value was the output from the instrument
software, and the relative expression level was calculated using
2−ΔΔCq method according to the following formula: ΔΔCq
(target gene)=Cq (target gene)-Cq (control gene) (31).
Detection of target protein expression
by western blotting
Protein was extracted from the cultured cells with
RIPA lysis buffer (1% NP40, 0.1% sodium dodecyl sulfate (SDS), 100
µg/ml phenylmethylsulfonyl fluoride, and 0.5% sodiumdeoxycholate in
PBS) containing a proteinase inhibitor (Roche Diagnostics) on ice
for 30 min. The supernatants were collected by centrifugation at
12,000 × g for 20 min at 4°C, and the protein concentrations were
determined using a BCA assay kit (Pierce Biotechnology; Thermo
Fisher Scientific, Inc.). Twenty micrograms of protein mixed with
2×SDS loading buffer [125 mM Tris-HCl, 4% SDS, 20% glycerol, 100 mM
dithiothreitol (DTT), and 0.2% bromophenol blue] was loaded into
each lane and separated by 10% sodium dodecylsulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) before being transferred onto
polyvinylidenedifluoride membranes (PVDF; EMD Millipore), which
were blocked with 5% non-fat dry milk in TBST (20 mM Tris-HCl, pH
7.5; 150 mM NaCl; 0.1% Tween-20) for 2 h at room temperature. The
membranes were then incubated with antibodies against Shh (dilution
1:500; cat. no. ab53281), Smo (dilution 1:1,000; cat. no. ab113438)
and Ptch1 (dilution 1:800; cat. no. ab53715; all from Abcam,
Cambridge, UK) or GAPDH (dilution 1:2,000; cat. no. 51332; Cell
Signaling Technology, Inc.) overnight at 4°C. Finally, the
membranes were washed and then incubated with horse radish
peroxidase (HRP)-conjugated goat anti-mouse IgG (dilution 1:5,000;
cat. no. 7076) or HRP-conjugated goat anti-rabbit IgG (dilution
1:5,000; cat. no. 7074; both from Cell Signaling Technology, Inc.)
for 2 h at room temperature. The resulting protein bands were
detected using enhanced chemiluminescence (ECL) luminol reagent
(EMD Millipore). GAPDH was used to detect target protein level
modulation, and the images were analysed by Image-Pro®
Plus software (Media Cybernetics).
Apoptosis assay
Approximately 1×106 cells were
centrifuged at 2500 × g for 5 min, washed with cold BioLegend Cell
Staining Buffer, and then resuspended in Annexin V Binding Buffer.
Approximately 100 µl of cell suspension was transferred into a 5-ml
test tube, to which 5 µl of FITC Annexin V and 5 µl of 7-ADD
Viability Staining Solution were subsequently added. The cells were
then gently vortexed and incubated in the dark for 15 min at room
temperature. The volume of the solution was increased to 500 µl
with Annexin V Buffer and analysed with a FACS Calibur Flow
Cytometer (BD Biosciences).
Transwell assay
A Transwell assay was performed with a pre-coated
cell invasion kit (pore size, 8.0 µm; Corning, Inc.). BCC cells
(5×104/well) were allowed to migrate from the upper
chamber to the lower chamber, which contained medium with 30% FBS.
Following 60 h of incubation, the cells that had migrated through
the membrane were stained with 0.1% crystal violet at room
temperature for 20 min and then counted under a light microscope (6
random fields/well).
MTT detection
BCC cells were seeded in 96-well plates at a density
of 5×103 cells/well. The cells were treated with various
agents at 24 h after seeding. Following 48 h of incubation under
normal culture conditions, the cells were treated with MTT at a
final concentration of 5 mg/ml. Four hours later, DMSO
(Sigma-Aldrich; Merck KGaA) was added to the wells to dissolve the
crystals. The wells were shaken horizontally for 10 min. The OD
value was assessed at 405 nm by a micro-plate reader (Bio-Rad
Laboratories, Inc.). The IC50 value was determined based
on the relative absorbance of MTT, which was determined by probit
regression analysis with SPSS18.0 statistical software (SPSS,
Inc.).
In vivo treatments
BCC cells were cultured in a 75-cm2
flask, after which cell suspensions containing 2×106
cells were subcutaneously transplanted into 6-week-old athymic
female BALB/c nude mice (~16 g per mouse). Each group was comprised
of five mice. All the mice were maintained in caged housing in a
specifically designed pathogen-free (SPF) isolation facility at
24°C with a 12-h light/dark cycle, and fed rodent chow and water
ad libitum. All the mice exhibited a 50-mm3
tumour mass on day 7 after transplantation. The mice were divided
into two groups, each of which was comprised of 5 animals. The
animals in group 1, or the LV-CON group, received LV-CON treatment,
and the animals in group 2, or the LV-CD44s group, received
LV-CD44s treatment twice a week. The tumour volume was evaluated at
day 6, 10, 12, 14, 16, 18, 20, 22 and 24 post-transplantation. Two
orthogonal diameters of each tumour were measured with Vernier
callipers. The tumour volume was calculated with the following
formula: tumor volume = (lengthxwidth2)/2. Tumours with
weights outside the range of 50–200 mg at the start of the
treatment period were excluded from the study. On day 24 after
transplantation, all the mice were euthanized by 20% of
CO2/min asphyxiation. The tumor masses were weighed
using electronic scales. The tumours were collected and fixed in
10% formalin. Immunohistochemistry (IHC) was used to detect CD31
expression in tumour tissues. Relative tumour size (RTS) was
calculated as the tumour volume at the time of measurement divided
by the tumour volume at the time of treatment. The mean RTS value
was plotted as a function of time for the different treatment
groups. All in vivo protocols were approved by the
Institutional Animal Care and Use Committee of The Second
Affiliated Hospital of Xi'an Jiaotong University (No. 2016061).
Statistical analysis
Statistical analyses were performed using SPSS
statistical software 18.0 (SPSS, Inc.). Normally distributed data
are presented as the mean ± SD of at least three independent
experiments. Student's t-tests or one-way analysis of variance
(ANOVA) followed by Tukey's post hoc test were performed to assess
statistical significance. The results were considered statistically
significant at P<0.05.
Results
Lentivirus-mediated CD44s expression
in BCC cells
CD44 was initially identified as a lymphocyte homing
receptor and transmembrane glycoprotein that is commonly expressed
in embryonic stem cells and haematopoietic and cancer stem cells
(32,33). However, little research on the
function of CD44s in BCC cells has been reported. It has been
demonstrated that lentiviral-mediated gene expression facilitates
effective, stable gene expression in multiple biological systems
and assists in the elucidation of gene functions in numerous cell
types. Lentiviruses containing CD44s constructs were used to
express CD44s in BCC cells. Lentiviruses containing blank
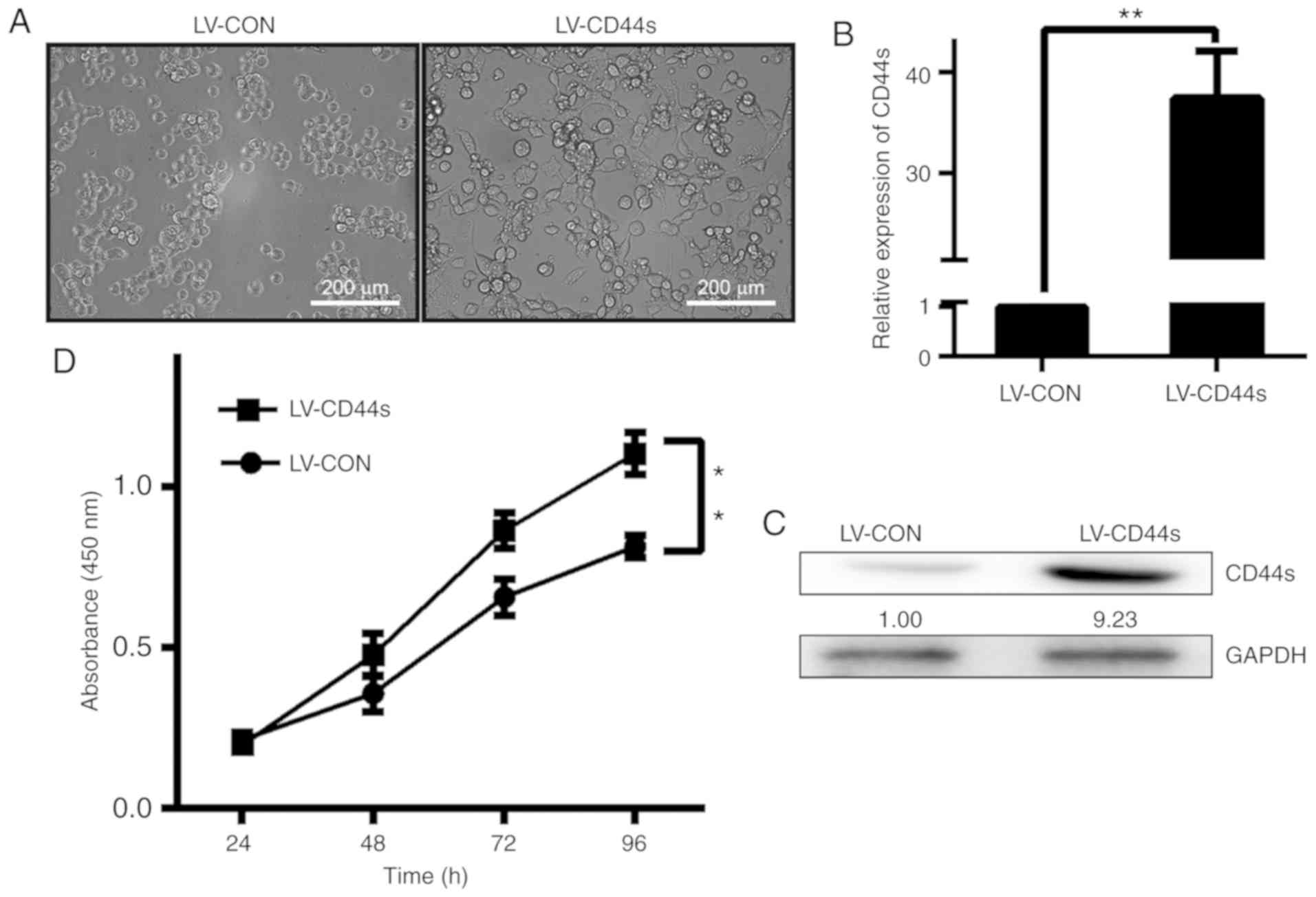
constructs (LV-CON)were used as controls (Fig. 1A). As revealed in Fig. 1A, compared with BCC control cells,
CD44s overexpression cells exhibited a more mesenchymal phenotype
with elongated shape and reduced cell-cell contact as observed by
inverted microscopy. CD44s mRNA expression in BCC cells was
compared with that in parental BCC cells and LV-CON cells by
qRT-PCR. To confirm the expression of CD44s in the aforementioned
cell clones, CD44s protein expression was detected by western
blotting. It was revealed that CD44s expression, as demonstrated by
qRT-PCR and western blotting, was higher in BCC cells infected with
LV-CD44 than in LV-CON cells (Fig. 1B
and C). After the CD44s construct (LV-CD44s) was transfected
into BCC cells, we selected the cell clones stably expressing CD44s
and cultured and analysed them separately. Cell proliferation was
monitored for 96 h after the BCC cells were infected with LV-CD44s
and LV-CON. The growth of the cells infected with LV-CD44s was
markedly increased compared with that of the cells infected with
LV-CON (Fig. 1D).
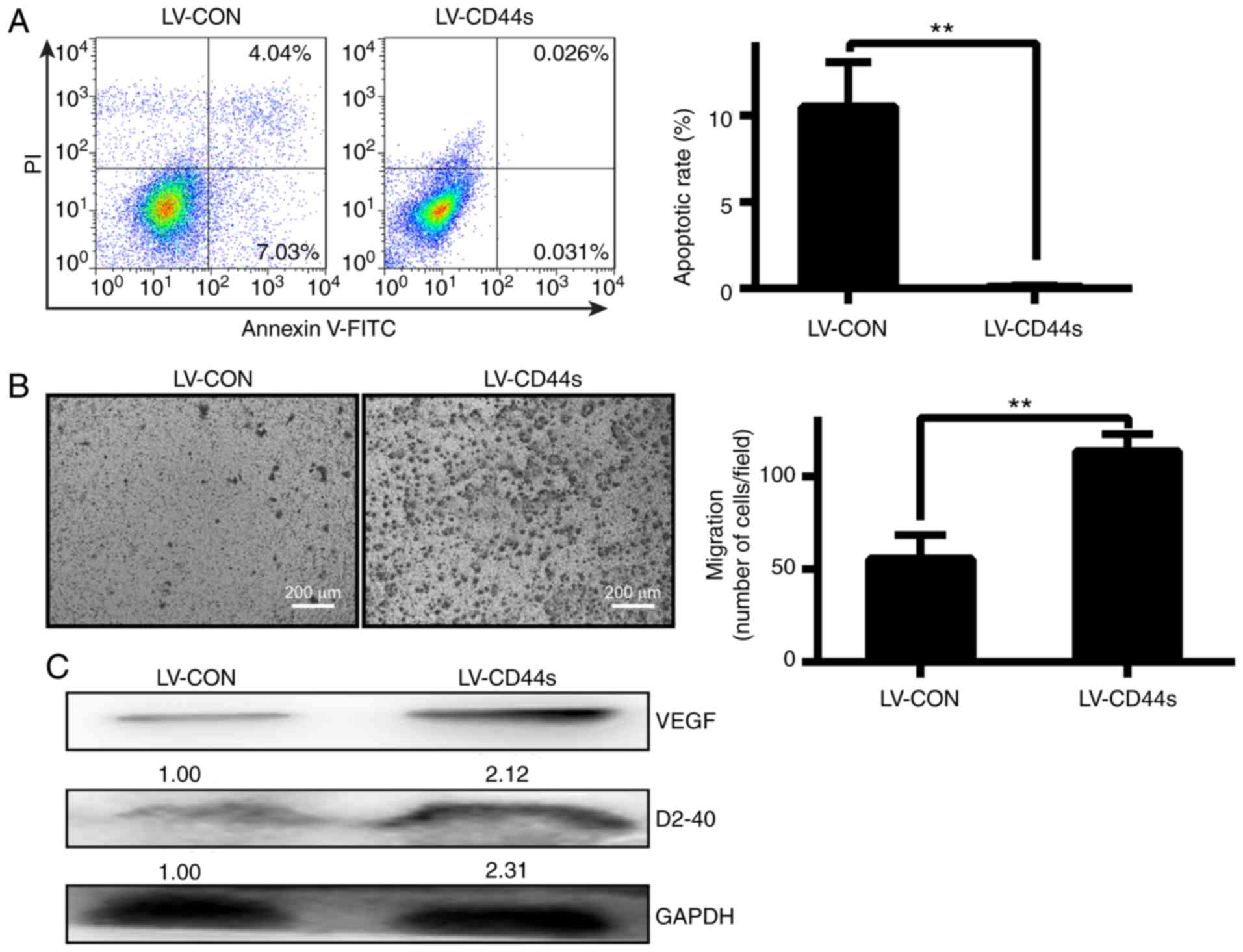
CD44s affects BCC cell apoptosis and
proliferation in vitro and in vivo
CD44 is a transmembrane glycoprotein and is
overexpressed in BCC, which suggests that it is required for cancer
proliferation. First, BCC cells were successfully infected with a
lentivirus (Fig. 1A). FACS assay
revealed that the number of viable cells in the LV-CD44s group was
significantly decreased compared with that in the LV-CON group
(**P<0.01, Fig. 2A). A Transwell
assay revealed that the migration potential of the cells was
significantly increased when CD44s was overexpressed by LV-CD44s in
BCC cells (**P<0.01, Fig. 2B).
Furthermore, VEGFA and D2-40 expression in the LV-CD44s group was
significantly increased compared with that in the LV-CON
group(Fig. 2C). Based on these
results, it was concluded that CD44s plays a critical role in BCC
cell proliferation and migration potential.
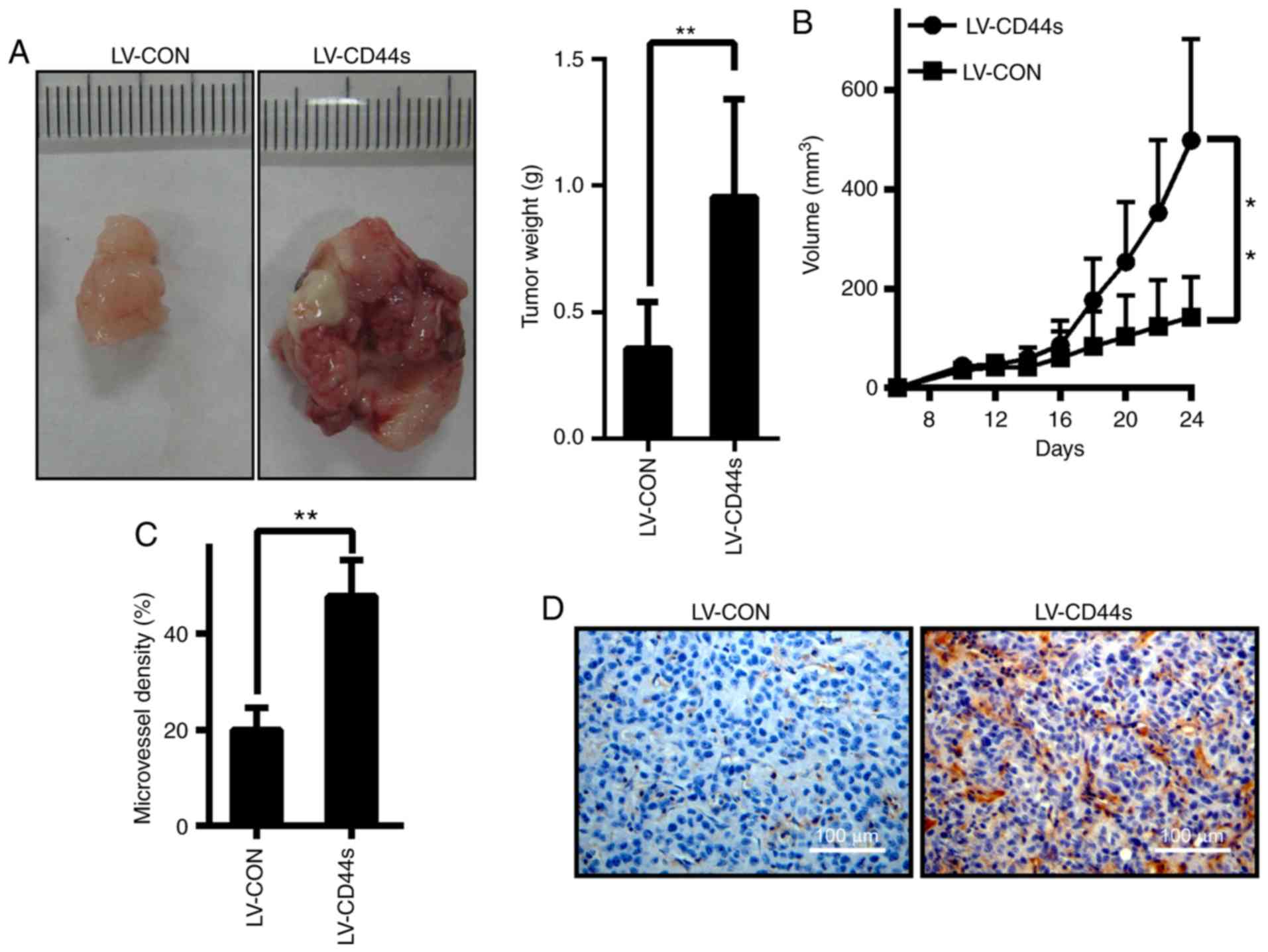
To examine the effects of CD44s on tumour growth
in vivo, BCC cells stably expressing control vectors or
CD44s were inoculated into the subcutaneous tissue of BalB/C nude
mice. All the animals developed tumours 7 days after inoculation.
The tumours in the LV-CD44s group were significantly larger than
those in the LV-CON group at 24 days after inoculation (Fig. 3A). The mean tumour volume in mice
inoculated with BCC cells expressing LV-CON was 143.20±81.41
mm3, while that in mice inoculated with BCC cells
expressing LV-CD44s was 498.23±204.65 mm3 (Fig. 3B). Given that angiogenesis is an
integral component of BCC, it was explored whether CD44s plays a
role in BCC angiogenesis. Immunohistochemical analysis revealed
that CD31 (a marker for microvessels denoting enhanced
angiogenesis) levels were significantly higher in tumours in the
CD44s group than in tumours in the LV-CON control group (Fig. 3C and D), suggesting that CD44s has
angiogenic effects in BCC. These data demonstrated that CD44s plays
an important role in promoting BCC cell growth in vivo.
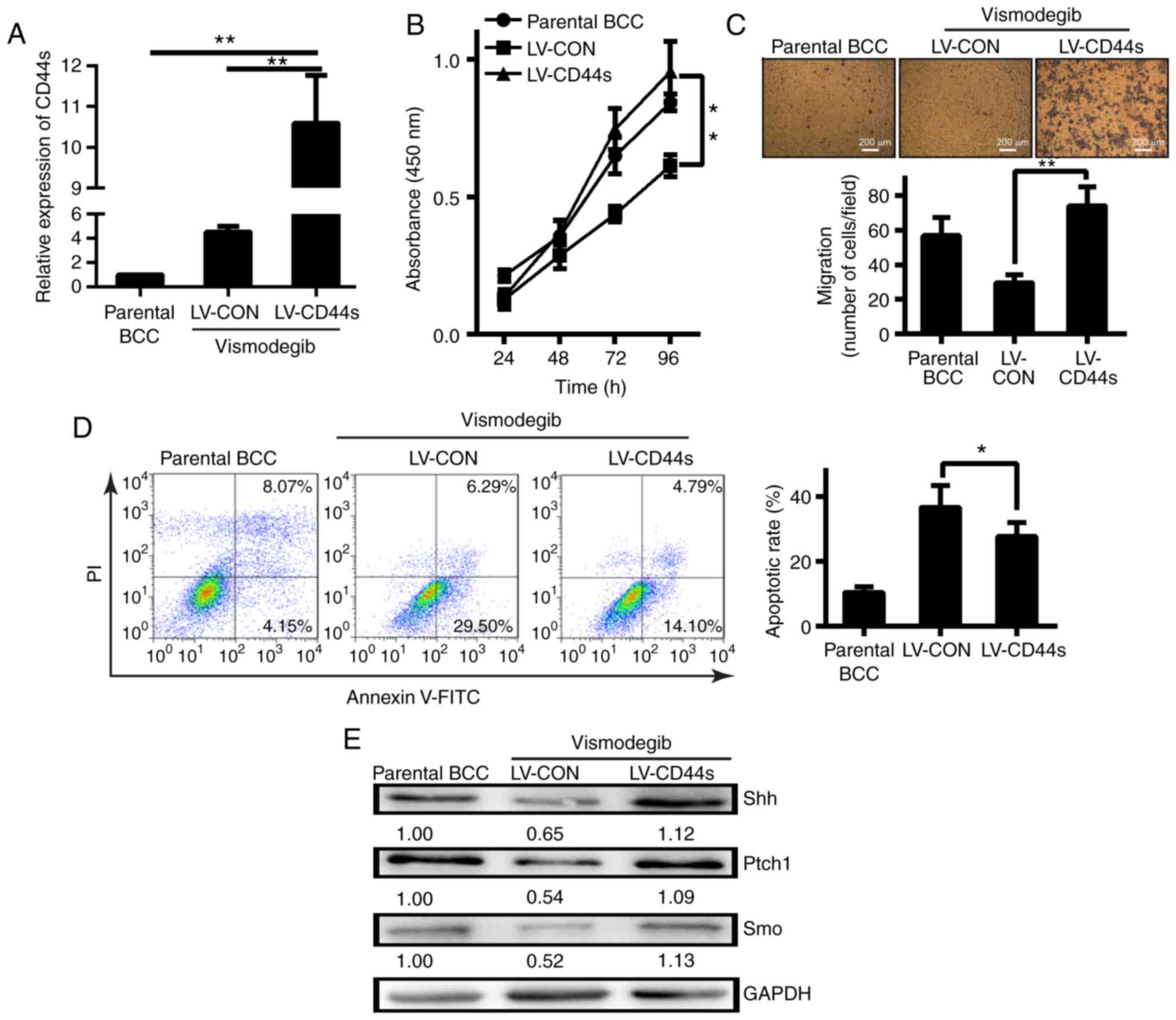
Effects of the Hh pathway on BCC cells
with CD44 expression
We next examined the effects of CD44 expression on
BCC characteristics, including CD44s expression, BCC proliferation,
migration and apoptosis, after vismodegib treatment. The BCC cell
line expressing CD44 demonstrated greater expression (Fig. 4A) and proliferation after vismodegib
treatment than the control or parental cell lines (Fig. 4B). Vismodegib treatment reduced BCC
cell migration, while CD44s expression reversed this effect
(Fig. 4C). BCC cell apoptosis was
also assessed and it was revealed that more LV-CON cells than
CD44-expressing BCC cells underwent apoptosis (Fig. 4D). CD44-positive cells revealed
upregulated expression of the Hh pathway proteins Shh, Ptch1, and
Smo compared with CD44-negative cells (Fig. 4E). Thus, CD44s-positive BCC cells
had increased proliferation and migration abilities and underwent
less apoptosis compared with CD44-negative cells (LV-CON). These
properties are dependent on Hh signalling. The levels of the
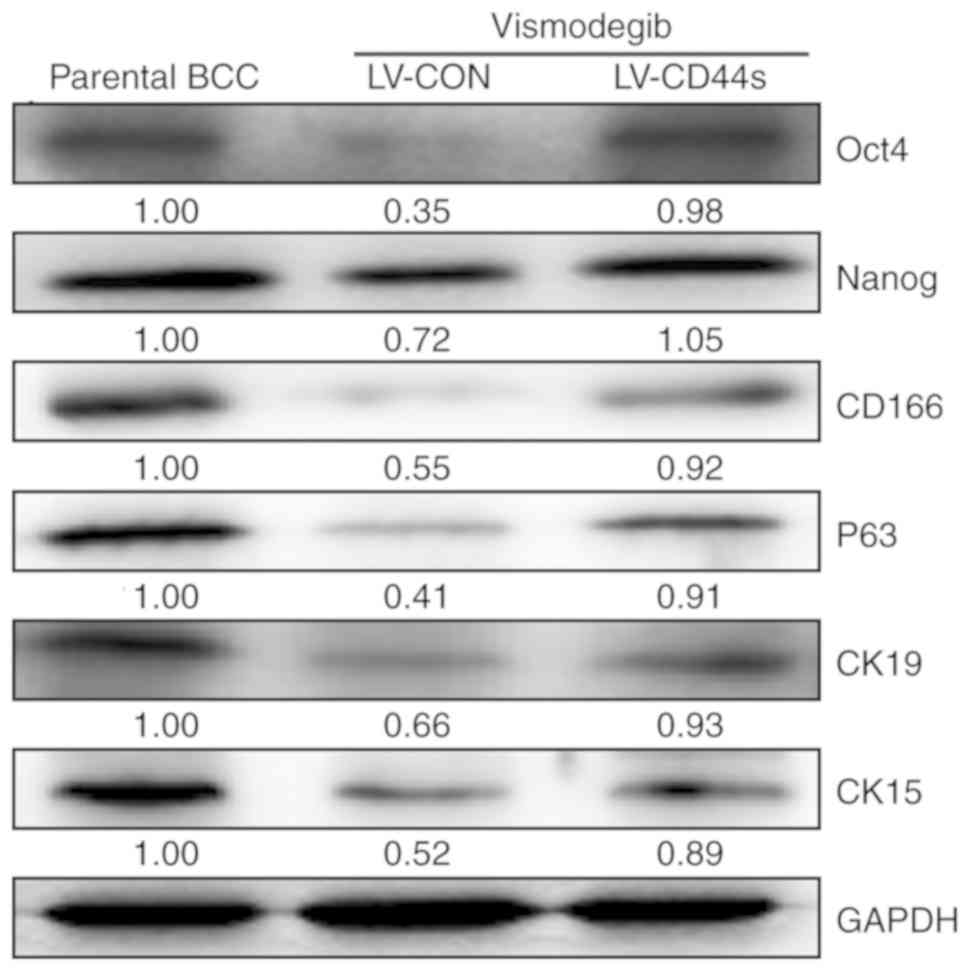
self-renewal proteins Oct2 and Nanog and other related stem cell
markers were decreased in BCC cells lacking CD44 (LV-CON) after
vismodegib treatment. However, the levels of these proteins
remained upregulated in BCC cells with CD44s overexpression
(Fig. 5). Thus, BCC cell growth was
associated with sustained increases in the levels of CD44, Hh
pathway proteins, and some self-renewal proteins.
Discussion
The present study is the first to demonstrate that
the Hh signalling pathway plays a vital role in the maintenance of
chemotherapy resistance in BCC cells with CD44 expression. BCC
cells were grown with CD44 overexpression and it was revealed that
this cell line displayed increased expression of Hh pathway
proteins and certain self-renewal proteins. Inhibition of Hh
signalling using vismodegib blocked BCC cell growth. BCC cells were
highly sensitive to vismodegib therapy in vitro, and this
therapy sensitivity was reversed by CD44 overexpression. Cells with
CD44 overexpression displayed increased proliferation and
migration, phenotypic features that were attenuated following Hh
pathway inhibition with vismodegib.
The pivotal molecular abnormality in BCC
carcinogenesis is aberrant Hh signalling pathway activation. The Hh
signalling pathway was first described in genetic studies of
embryonic segmentation and imaginal disk specification in
Drosophila. It is highly conserved from insects to vertebrates, and
vertebrates have multiple homologues of several components of the
pathway (34). The following three
homologues are found in mammals: Shh, Indian hedgehog (Ihh), and
Desert hedgehog (Dsh). Shh is the most commonly expressed and best
characterized homologue and is crucial for the nervous system,
axial skeleton, lung, skin, hair, and stem cell population
development and maintenance. Shh is synthesized as a 45-kDa
precursor protein that is auto-catalytically cleaved and covalently
modified by palmitate and cholesterol. Shh is secreted and binds to
its receptor, Ptch1. Ciliary ablation was revealed to strongly
inhibit the development of BCC and medulloblastoma when these
tumours were driven by an activated form of the transmembrane
protein SMO. Conversely, ciliary removal accelerated tumourigenesis
induced by constitutively active GLI-2 (35,36).
CD44 is a transmembrane glycoprotein and the
principal cell surface receptor for hyaluronic acid, a major
component of the ECM (37). CD44
plays an important role in communication in cell-matrix
interactions and also plays a role in cell motility, matrix
degradation, proliferation, and survival. The major form of CD44 on
epithelial cells is CD44s (standard), but some cells possess an
isoform of CD44 known as CD44v (variant). Several studies have
revealed that an association exists among CD44v6 expression,
gastric cancer lymph node metastasis, and prognosis (38,39).
In the present study, lentiviral vectors harbouring CD44s were used
to overexpress the protein to analyse its function. CD44 expression
was displayed by BCC cells with malignant transformation, a
property that may be associated with Hh pathway activation. The
prognostic value of the tumor microvascular density (MVD) in cancer
has been examined in several studies, with correlations with tumor
recurrence, disease-free or overall survival. Thus, the association
with angiogenesis was assessed by measuring MVD. There are a few
factors produced by MVD that are responsible for the induction of
angiogenesis, with VEGF and D2-40, is thought to have a key role in
it (40).
Vismodegib is the first oral medicine approved by
the US Food and Drug Administration for the treatment of adults
with advanced BCC (both locally advanced and distantly metastatic
BCC) that has recurred after surgery or cannot be resected or
irradiated. Vismodegib is a competitive antagonist of SMO, a
component of the Hh signalling pathway. Total SMO inhibition causes
the transcription factor GLI-1 to remain inactive, which in turn
suppresses the expression of genes regulated by the Hh signalling
pathway (41). However, vismodegib,
which targets the Hh signalling pathway, has not been demonstrated
to inhibit the growth of BCC cells with CD44s overexpression in
vitro. It is possible that Hh pathway inhibition synergizes
with CD44s expression. Vismodegib has not been demonstrated to
inhibit the growth of BCC cells with lentivirus-mediated CD44s
overexpression in nude mice in vivo. This will be addressed
in a future study.
In the present study, we defined a subgroup of BCC
cells comprised of CD44-positive cells that have properties that
are very different from those of unselected cancer cells, including
the property of chemotherapy resistance. The Hh signalling pathway
is important in the maintenance of these CD44-overexpressing cells,
and Hh inhibition acts to reverse therapy resistance in these
cells. Similar results have been obtained in research involving
other cancer cell lines. Our correlative scientific study revealed
that the combination of Hh inhibition and oncogene molecular
downregulation may be beneficial in only a minority of patients
with BCC, namely, patients whose tumours express CD44 at low
levels.
In conclusion, CD44s is highly expressed in BCC
cells, as demonstrated by qRT-PCR and western blotting, and
differences were observed between the proliferation rates of
LV-CD44s BCC cells and those of LV-CON BCC cells by growth curve
analysis. In vitro, vismodegib treatment reduced growth and
migration in LV-CON BCC cells; however, LV-CD44s expression
reversed these changes. In vivo, the growth of BCC tumours
stably infected with CD44s constructs was significantly increased
in the transplanted nude mouse model compared with that of BCC
tumours comprised of control cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by Shaanxi Municipal
Science and Technology Scientific and Technological project (no.
S2016YFJM0130).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JR, XM and CT contributed to the study conception
and design of the experiments. JR, XM, CT and ZL performed
experiments. CT acquired data. JR and XM contributed with the
statistical analysis of the data. CT and ZL participated in the
writing of the manuscript. JR, XM, CT and ZL drafted, edited,
critically revised and approved final version of manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All in vivo protocols were approved by the
Institutional Animal Care and Use Committee of The Second
Affiliated Hospital of Xi'an Jiaotong University (No. 2016061).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rigel DS, Friedman RJ and Kopf AW:
Lifetime risk for development of skin cancer in the U.S.
population: Current estimate is now 1 in 5. J Am Acad Dermatol.
35:1012–1013. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Szeimies RM and Karrer S: Towards a more
specific therapy: Targeting nonmelanoma skin cancer cells. Br J
Dermatol. 154 (Suppl 1):16–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holterhues C, Vries E, Louwman MW,
Koljenović S and Nijsten T: Incidence and trends of cutaneous
malignancies in the Netherlands, 1989–2005. J Invest Dermatol.
130:1807–1812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Senerchia AA, Ribeiro KB and
Rodriguez-Galindo C: Trends in incidence of primary cutaneous
malignancies in children, adolescents, and young adults: A
population-based study. Pediatr Blood Cancer. 61:211–216. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roewert-Huber J, Lange-Asschenfeldt B,
Stockfleth E and Kerl H: Epidemiology and aetiology of basal cell
carcinoma. Br J Dermatol. 157 (Suppl 2):47–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kurian RR, Di Palma S and Barrett AW:
Basal cell carcinoma metastatic to parotid gland. Head Neck Pathol.
8:349–353. 2014.PubMed/NCBI
|
|
8
|
Moser S, Borm J, Mihic-Probst D, Jacobsen
C and Kruse Gujer AL: Metastatic basal cell carcinoma: Report of a
case and review of the literature. Oral Surg Oral Med Oral Pathol
Oral Radiol. 117:e79–e82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sleightholm RL, Willcockson JR, Watley DC,
Durden FL and Foster JM: Bilateral lymphatic spread of metastatic
basal cell carcinoma. Plast Reconstr Surg Glob Open. 4:e11822016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Athar M, Tang X, Lee JL, Kopelovich L and
Kim AL: Hedgehog signalling in skin development and cancer. Exp
Dermatol. 15:667–677. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hahn H, Wicking C, Zaphiropoulous PG,
Gailani MR, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E,
Unden AB, Gillies S, et al: Mutations of the human homolog of
Drosophila patched in the nevoid basal cell carcinoma syndrome.
Cell. 85:841–851. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johnson RL, Rothman AL, Xie J, Goodrich
LV, Bare JW, Bonifas JM, Quinn AG, Myers RM, Cox DR, Epstein EH Jr
and Scott MP: Human homolog of patched, a candidate gene for the
basal cell nevus syndrome. Science. 272:1668–1671. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stone DM, Hynes M, Armanini M, Swanson TA,
Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, et
al: The tumour-suppressor gene patched encodes a candidate receptor
for sonic hedgehog. Nature. 384:129–134. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fan H, Oro AE, Scott MP and Khavari PA:
Induction of basal cell carcinoma features in transgenic human skin
expressing sonic hedgehog. Nat Med. 3:788–792. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oro AE, Higgins KM, Hu Z, Bonifas JM,
Epstein EH Jr and Scott MP: Basal cell carcinomas in mice
overexpressing sonic hedgehog. Science. 276:817–821. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie J, Murone M, Luoh SM, Ryan A, Gu Q,
Zhang C, Bonifas JM, Lam CW, Hynes M, Goddard A, et al: Activating
smoothened mutations in sporadic basal-cell carcinoma. Nature.
391:90–92. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki
H, Hui CC and Dlugosz AA: Basal cell carcinomas in mice
overexpressing Gli2 in skin. Nat Genet. 24:216–217. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nilsson M, Undèn AB, Krause D, Malmqwist
U, Raza K, Zaphiropoulos PG and Toftgård R: Induction of basal cell
carcinomas and trichoepitheliomas in mice overexpressing GLI-1.
Proc Natl Acad Sci USA. 97:3438–3443. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li C, Chi S and Xie J: Hedgehog signaling
in skin cancers. Cell Signal. 23:1235–1243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ponta H, Sherman L and Herrlich PA: CD44:
From adhesion molecules to signalling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zöller M: CD44: Can a cancer-initiating
cell profit from an abundantly expressed molecule? Nat Rev Cancer.
11:254–267. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng C and Sharp PA: Regulation of CD44
alternative splicing by SRm160 and its potential role in tumor cell
invasion. Mol Cell Biol. 26:362–370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Formby B and Stern R: Phosphorylation
stabilizes alternatively spliced CD44 mRNA transcripts in breast
cancer cells: Inhibition by antisense complementary to casein
kinase II mRNA. Mol Cell Biochem. 187:23–31. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weg-Remers S, Ponta H, Herrlich P and
König H: Regulation of alternative pre-mRNA splicing by the ERK
MAP-kinase pathway. EMBO J. 20:4194–4203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dang H, Ding W, Emerson D and Rountree CB:
Snail1 induces epithelial-to-mesenchymal transition and tumor
initiating stem cell characteristics. BMC Cancer. 11:3962011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shuang ZY, Wu WC, Xu J, Lin G, Liu YC, Lao
XM, Zheng L and Li S: Transforming growth factor-β1-induced
epithelial-mesenchymal transition generates ALDH-positive cells
with stem cell properties in cholangiocarcinoma. Cancer Lett.
354:320–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rudzki Z and Jothy S: CD44 and the
adhesion of neoplastic cells. Mol Pathol. 50:57–71. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu S, Wu X, Zhou B, Xu Z, Qin J, Lu H, Lv
L, Gao Y, Deng L, Yin J and Li G: IMP3 combined with CD44s, a novel
predictor for prognosis of patients with hepatocellular carcinoma.
J Cancer Res Clin Oncol. 140:883–893. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
So PL, Langston AW, Daniallinia N, Hebert
JL, Fujimoto MA, Khaimskiy Y, Aszterbaum M and Epstein EH Jr:
Long-term establishment, characterization and manipulation of cell
lines from mouse basal cell carcinoma tumors. Exp Dermatol.
15:742–750. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Patrawala L, Calhoun T,
Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra
D, Zhou J, Claypool K, et al: Highly purified CD44+ prostate cancer
cells from xenograft human tumors are enriched in tumorigenic and
metastatic progenitor cells. Oncogene. 25:1696–1708. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hooper JE and Scott MP: Communicating with
hedgehogs. Nat Rev Mol Cell Biol. 6:306–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wong SY, Seol AD, So PL, Ermilov AN,
Bichakjian CK, Epstein EH Jr, Dlugosz AA and Reiter JF: Primary
cilia can both mediate and suppress hedgehog pathway-dependent
tumorigenesis. Nat Med. 15:1055–1061. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han YG, Kim HJ, Dlugosz AA, Ellison DW,
Gilbertson RJ and Alvarez-Buylla A: Dual and opposing roles of
primary cilia in medulloblastoma development. Nat Med.
15:1062–1065. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jang BI, Li Y, Graham DY and Cen P: The
role of CD44 in the pathogenesis, diagnosis, and therapy of gastric
cancer. Gut Liver. 5:397–405. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Okayama H, Kumamoto K, Saitou K, Hayase S,
Kofunato Y, Sato Y, Miyamoto K, Nakamura I, Ohki S, Sekikawa K and
Takenoshita S: CD44v6, MMP-7 and nuclear Cdx2 are significant
biomarkers for prediction of lymph node metastasis in primary
gastric cancer. Oncol Rep. 22:745–755. 2009.PubMed/NCBI
|
|
39
|
Tsuchida A, Nagakawa Y, Kasuya K, Matudo
T, Kyo B, Suzuki Y, Aoki T, Sato E, Nagao T and Itoi T:
Significance of CD44s and CD44v6 expression in pancreaticobiliary
maljunction. Hepatogastroenterology. 58:1877–1881. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Riabov V, Gudima A, Wang N, Mickley A,
Orekhov A and Kzhyshkowska J: Role of tumor associated macrophages
in tumor angiogenesis and lymphangiogenesis. Front Physiol.
5:752014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ingham PW and McMahon AP: Hedgehog
signaling in animal development: Paradigms and principles. Genes
Dev. 15:3059–3087. 2001. View Article : Google Scholar : PubMed/NCBI
|