Introduction
Primary tumor sidedness has prognostic and
predictive value in metastatic colorectal cancer (CRC), and has
thus emerged as a new biomarker (1,2).
Several analyses revealed that right-sided colorectal cancer (RCRC)
exhibited significantly worse prognosis than left-sided colorectal
cancer (LCRC) (3–5), and anti-EGFR therapy clearly
benefitted patients with LCRC, whereas patients with RCRC derived
limited benefit (6–10). However, the mechanism of the
differences between RCRC and LCRC has not been fully
elucidated.
RCRC and LCRC have different clinicopathological and
molecular characteristics. RCRC is generally characterized by being
more common in women, and associated with Lynch syndrome, sessile
serrated adenoma/polyp (SSA/P), mitogen-activated protein kinase
signaling, microsatellite instability-high (MSI-H), deficiency of
mismatch repair genes, CpG island methylation, and KRAS and
BRAF V600E mutations (11–15).
LCRC is more common in men, and associated with familial
adenomatous polyposis syndrome, traditional serrated adenoma (TSA),
chromosomal instability, ERBB1 and ERBB2
amplifications, and APC, p53, and NRAS mutations
(11–15). Based on these clinicopathological
and molecular differences, primary tumor sidedness is considered to
be associated with prognosis and efficacy of targeted therapy.
Mutations in RNF43 have been reported in
several solid tumors, such as colorectal (16–18),
gastric (19), pancreatic (20), ovarian (21), and endometrial (22) cancers. RNF43 encodes a
RING-type E3 ubiquitin ligase, and the protein is predicted to
contain a transmembrane domain, a protease-associated domain, an
ectodomain, and a cytoplasmic RING domain (23). Expression of RNF43 results in
increased ubiquitination of frizzled receptors, and an alteration
in their subcellular distribution, resulting in reduced surface
levels of these receptors. RNF43 is considered to negatively
regulate WNT signaling, and functions as a tumor suppressor. Loss
of RNF43 results in decrease or lack of degradation of frizzled
receptors, with an enhancement of WNT signaling. In cancer cells,
inactivation of RNF43 through RNF43 mutation is one of the
causes of permanent activation of the WNT signaling pathway
(23).
Serrated neoplasia, which is a precancerous lesion
of CRC, is associated with primary tumor sidedness: SSA/P is
associated with RCRC, while TSA is associated with LCRC (24). Recently, several studies revealed
the importance of RNF43 mutation in the serrated neoplasia
pathway, i.e., RNF43 mutation was associated with serrated
neoplasia pathway such as SSA/P (25) and TSA (26,27).
Moreover, it has been reported that RNF43 mutation in
serrated neoplasia is associated with BRAF V600E mutation
(17), which is recognized as one
of the characteristics of RCRC and a significant negative
prognostic factor in metastatic CRC (1,2).
Collectively, it was surmised that RNF43 mutation may play
different roles in RCRC and LCRC. Recently, it has been reported
that RNF43 mutations contribute to tumorigenesis in RCRC
(18). However, to date, clinical
significance of RNF43 mutation have not been fully
investigated according to primary tumor sidedness. It was
hypothesized that the clinical significance of RNF43
mutations differ between RCRC and LCRC. To test this hypothesis,
the clinicopathological characteristics and survival outcome of
patients with RNF43 mutation in RCRC and LCRC were
investigated.
Materials and methods
Patients
This retrospective study was approved by the Ethics
Committee of the Niigata University School of Medicine, and
performed in accordance with the Helsinki Declaration (G2015-0816).
All methods were performed in accordance with the relevant
guidelines and regulations, and written informed consent was
obtained from the patients. A total of 201 Japanese patients (117
male and 84 female patients; median age 65 years old; range, 30–94
years) with stage I–IV CRC according to AJCC, 7th edition (28) who underwent a primary tumor
resection between January 2009 and December 2015 at the Niigata
University Medical and Dental Hospital or Niigata Cancer Center
Hospital were included in this study. The median follow-up period
was 34 months (range, 1–92 months). Patients diagnosed with
adenocarcinoma were included. Patients under 18 years old were
excluded. Patients with synchronous double primary CRC or other
active concurrent malignant diseases, inflammatory bowel disease or
familial adenomatous polyposis were excluded. No patient had
received neoadjuvant chemoradiation. Typically, chemotherapy was
administered according to the Japanese Society for Cancer of the
Colon and Rectum (JSCCR) guidelines (29). Adjuvant chemotherapy, including
fluorouracil or its derivatives ± oxaliplatin, was usually
administered in stage III patients for six months. For patients
with unresectable metastatic diseases, molecular targeted therapy
was administered according to RAS mutational status.
In the present analysis, RNF43 mutational
prevalence, spectrum and frequency between our cohort and TCGA
samples were compared. The mutation information for the TCGA
CRC-sequenced samples (n=489) was obtained from the cBioPortal
(https://www.cbioportal.org/) (30) to assess mutation frequency.
Comprehensive genomic sequence
analysis of primary tumors
As previously described (15,31–34),
formalin-fixed, paraffin-embedded (FFPE) samples were used for
next-generation sequencing (NGS), and genetic alterations,
including RNF43, were evaluated. Briefly, hematoxylin and
eosin-stained sections were used to assess tumor content, to ensure
that >50% tumor content was present. Where applicable, unstained
sections were macro-dissected to enrich for tumor content. DNA was
extracted using a BioStic FFPE Tissue DNA Isolation Kit (Mo Bio
Laboratories, Inc.). All sample preparation, NGS, and
bioinformatics analysis were performed in a CLIA/CAP-accredited
laboratory (KEW, Inc.). DNA fragment libraries (50–150 ng) were
prepared and enriched for the 415-gene panel with CANCERPLEX
Version 3.0 (KEW, Inc.). An average 500X sequencing depth was
achieved using Illumina MiSeq or NextSeq platforms. A proprietary
bioinformatics platform and knowledge base were used to process
genomic data and to identify multiple genomic abnormalities,
including SNPs, small indels, copy number variation, and
translocations. An allelic fraction threshold of 10% was used for
SNPs and indels, and thresholds of >2.5-fold for gain, and
0.5-fold for loss, were used. Tumors were assessed for the presence
of MSI on the basis of an extended loci panel. In addition to the
Bethesda panel (35), a collection
of 950 regions consisting of tandem repeats of one, two or three
nucleotides with a minimum length of 10 bases was used (31). Tumor mutational burden was
calculated as the number of non-synonymous mutations per megabase
of sequence in the panel (panel size=1.3 Mb).
RNF43 status and clinicopathological
characteristics
The 201 patients were classified into RNF43
wild-type or RNF43 mutant-type; moreover, RNF43
mutant-type were subdivided into right-sided RNF43
mutant-type or left-sided RNF43 mutant-type according to
primary tumor sidedness. Primary tumor location was determined by
operative findings. Cancer in the cecum, ascending colon, hepatic
flexure, or transverse colon was classified as RCRC; while cancer
in the splenic flexure, descending colon, sigmoid colon,
rectosigmoid, or rectum was classified as LCRC (15).
Statistical analysis
Statistical analyses were performed with IBM SPSS
Statistics 22 (IBM Japan, Inc.). Fisher's exact test was used to
evaluate the associations between RNF43 status and
clinicopathological characteristics. To clarify clinicopathological
characteristics which were independently associated with
RNF43 mutation, factors with a P-value of <0.10 in
univariate analyses were entered into a multivariate analysis.
Logistic analysis was performed to identify factors that were
independently associated with RNF43 mutation. Five-year
overall survival (OS) rates were estimated using the Kaplan-Meier
method. The log-rank test was used to assess for significant
differences between subgroups. P-values <0.05 were considered to
indicate statistically significant differences.
Results
Alteration of RNF43 in Japanese
CRC
To date, there has been no studies regarding genetic
alterations of RNF43 among Japanese CRC patients; hence, the
genetic alterations of RNF43 were evaluated and compared
with The Cancer Genome Atlas (TCGA) data (https://www.cbioportal.org/). RNF43
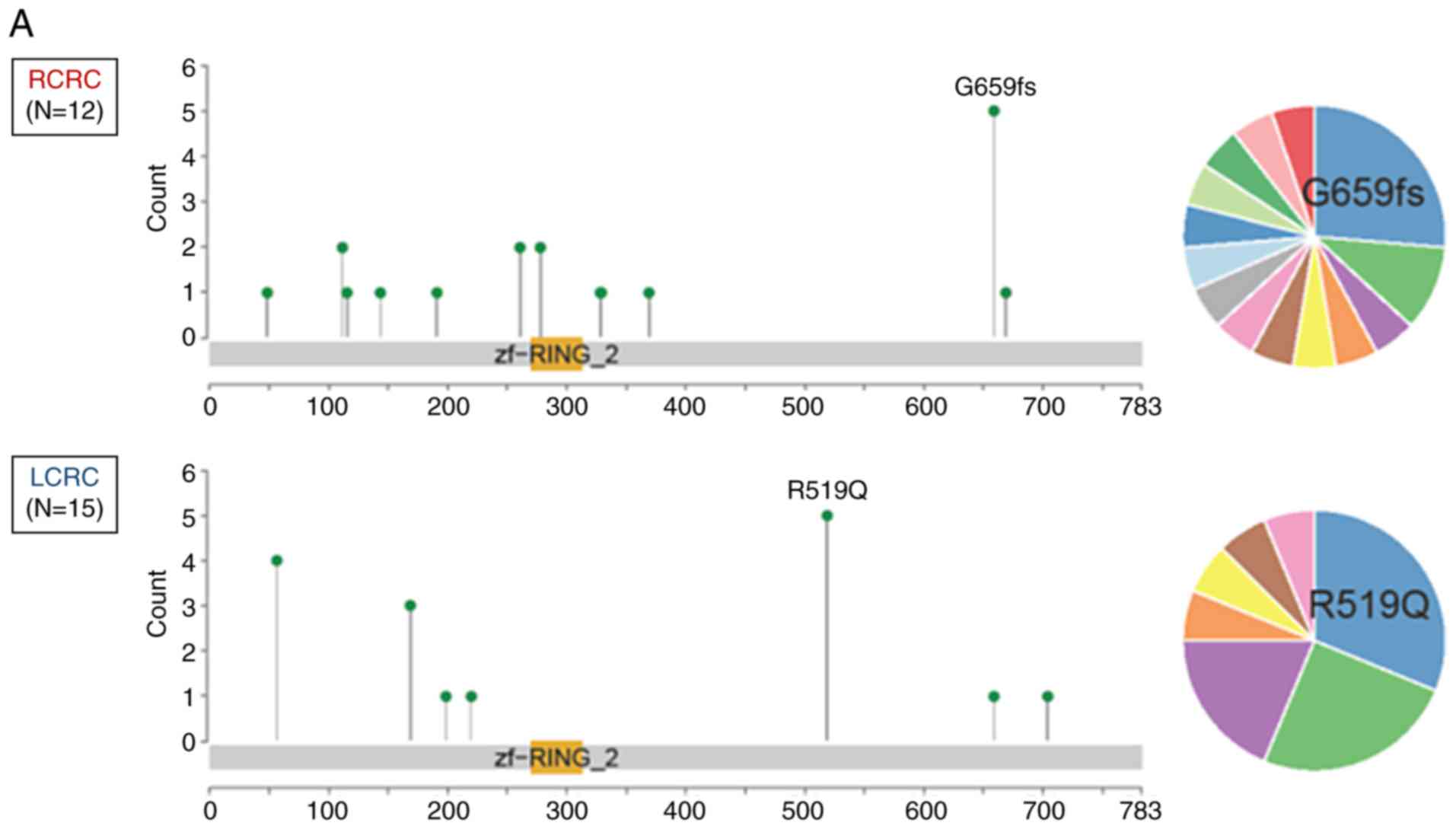
nonsense/frameshift mutation was more frequently observed in RCRC
compared with LCRC in both of the Japanese cohorts (P<0.001;
Figs. 1A and 2A) and TCGA samples (P=0.042; Figs. 1B and 2B).
Clinicopathological characteristics in
relation to RNF43 mutation status
The 415-gene panel assessment successfully detected
genetic alterations in all 201 patients. The 415-gene panel
assessment revealed that 174 (87%) patients were RNF43
wild-type and 27 (13%) patients were RNF43 mutant-type.
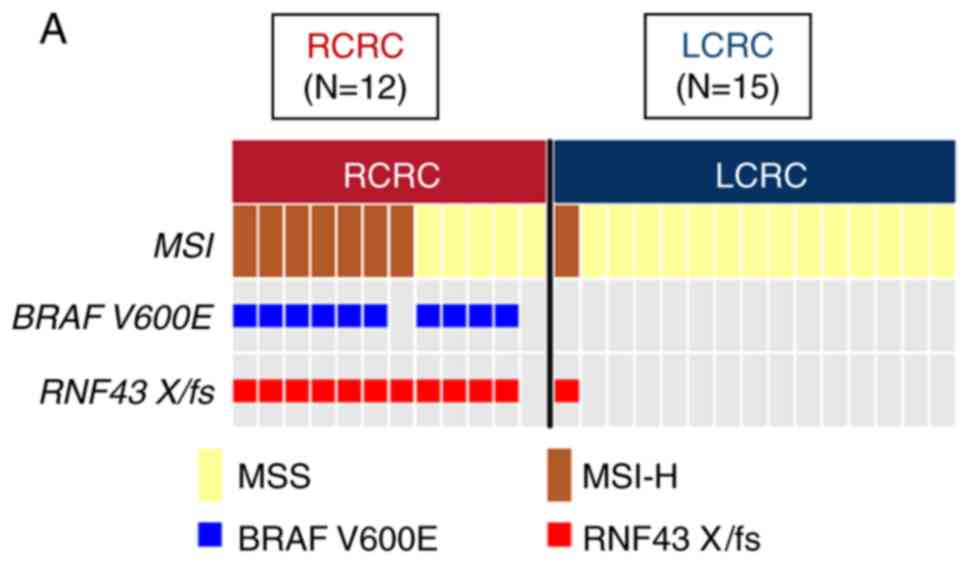
RNF43 mutant-type was significantly associated with age
(≥65; P=0.003), females (P=0.006), absence of venous invasion
(P=0.003), absence of distant metastasis (P=0.021), BRAF
V600E mutation (P<0.001), and MSI-H (P<0.001), and
multivariate analysis revealed that age (≥65), absence of venous
invasion, and BRAF V600E mutation were independently
associated with RNF43 mutation (Table I).
 | Table I.RNF43 gene status and other
clinicopathological characteristics in colorectal cancer. |
Table I.
RNF43 gene status and other
clinicopathological characteristics in colorectal cancer.
|
| RNF43 gene
status |
| Multivariate |
|---|
|
|
|
|
|
|---|
| Variables | Wild N (%) | Mutant N (%) | Univariate
P-value | Odds ratio (95%
CI) | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
<65 | 94 (46) | 6 (3) | 0.003 | 1 |
|
|
≥65 | 80 (40) | 21 (10) |
| 3.04
(1.03–8.90) | 0.042 |
| Sex |
|
|
|
|
|
|
Male | 108 (53) | 9 (4) | 0.006 |
|
|
|
Female | 66 (33) | 18 (9) |
|
|
|
| Location |
|
|
|
|
|
| Right
side | 44 (22) | 12 (6) | 0.062 |
|
|
| Left
side | 130 (65) | 15 (7) |
|
|
|
| Tumor size
(mm) |
|
|
|
|
|
|
<50 | 75 (37) | 13 (6) | 0.679 |
|
|
|
≥50 | 99 (49) | 14 (7) |
|
|
|
| pT category |
|
|
|
|
|
| T1,
2 | 20 (10) | 4 (2) | 0.539 |
|
|
| T3,
4 | 154 (76) | 23 (11) |
|
|
|
| Histopathological
grading |
|
|
|
|
|
| G1,
2 | 128 (63) | 19 (9) | 0.816 |
|
|
| G3 | 46 (23) | 8 (4) |
|
|
|
| Lymphatic
invasion |
|
|
|
|
|
|
Absence | 65 (32) | 14 (7) | 0.203 |
|
|
|
Presence | 109 (54) | 13 (6) |
|
|
|
| Venous
invasion |
|
|
|
|
|
|
Absence | 35 (17) | 13 (6) | 0.003 | 1 |
|
|
Presence | 139 (69) | 14 (7) |
| 0.18
(0.06–0.52) | 0.002 |
| pN category |
|
|
|
|
|
| N0 | 49 (24) | 10 (5) | 0.362 |
|
|
| N1,
2 | 125 (62) | 17 (8) |
|
|
|
| cM category |
|
|
|
|
|
| M0 | 72 (36) | 18 (9) | 0.021 |
|
|
| M1 | 102 (51) | 9 (4) |
|
|
|
| APC |
|
|
|
|
|
|
Wild-type | 29 (14) | 9 (4) | 0.061 |
|
|
|
Mutant | 145 (72) | 18 (9) |
|
|
|
| KRAS |
|
|
|
|
|
|
Wild-type | 105 (52) | 21 (10) | 0.091 |
|
|
|
Mutant | 69 (34) | 6 (3) |
|
|
|
| BRAF
V600E |
|
|
|
|
|
|
Wild-type | 171 (85) | 17 (9) |
<0.001 | 1 |
|
|
Mutant | 3 (1) | 10 (5) |
| 45.68
(9.76–213.81) |
<0.001 |
| MSI |
|
|
|
|
|
|
MSI-H | 7 (3) | 8 (4) |
<0.001 |
|
|
|
MSS | 167 (84) | 19 (9) |
|
|
|
Genetic alterations of the MAPK
pathway other than BRAF V600E mutation in RNF43 mutant-type
Seventeen of the 27 patients with RNF43
mutant-type had no BRAF V600E mutation. Nine of the 17
patients had mutations other than BRAF V600E in the MAPK
pathway: 6 patients had KRAS mutation, 3 patients had
BRAF non-V600E mutation; however, no patient had NRAS
mutation.
RNF43 mutant-type according to primary
tumor sidedness
Among the 27 patients with RNF43 mutation, 12
patients were right-sided RNF43 mutant-type and 15
left-sided RNF43 mutant-type. As revealed in Fig. 2A, 11 of the 12 right-sided
RNF43 mutant-type had nonsense/frameshift mutations, while
14 of 15 left-sided RNF43 mutant-type had missense
mutations. Right-sided RNF43 mutant-type was significantly
associated with histopathological grade 3 (P=0.008), lymphatic
invasion (P=0.021), APC wild (P=0.003), BRAF V600E
mutation (P<0.001), MSI-H (P=0.008), and RNF43
nonsense/frameshift mutation (P<0.001) compared with left-sided
RNF43 mutant-type (Table
II; Fig. 2A).
 | Table II.Clinicopathological characteristics
according to primary tumor sidedness in RNF43 mutant
colorectal cancer. |
Table II.
Clinicopathological characteristics
according to primary tumor sidedness in RNF43 mutant
colorectal cancer.
|
| Primary tumor
sidedness |
|
|---|
|
|
|
|
|---|
| Variables | Right-sided N
(%) | Left-sided N
(%) | P-value |
|---|
| Age (years) |
|
|
|
|
<65 | 1 (4) | 5 (18) | 0.182 |
|
≥65 | 11 (40) | 10 (37) |
|
| Sex |
|
|
|
|
Male | 3 (11) | 6 (22) | 0.683 |
|
Female | 9 (33) | 9 (33) |
|
| Tumor size
(mm) |
|
|
|
|
<50 | 5 (18) | 8 (29) | 0.704 |
|
≥50 | 7 (26) | 7 (26) |
|
| pT category |
|
|
|
| T1,
2 | 1 (4) | 3 (11) | 0.605 |
| T3,
4 | 11 (40) | 12 (44) |
|
| Histopathological
grading |
|
|
|
| G1,
2 | 5 (18) | 14 (52) | 0.008 |
| G3 | 7 (26) | 1 (4) |
|
| Lymphatic
invasion |
|
|
|
|
Absence | 3 (11) | 11 (40) | 0.021 |
|
Presence | 9 (33) | 4 (15) |
|
| Venous
invasion |
|
|
|
|
Absence | 4 (15) | 9 (33) | 0.252 |
|
Presence | 8 (30) | 6 (22) |
|
| pN category |
|
|
|
| N0 | 3 (11) | 7 (26) | 0.424 |
| N1,
2 | 9 (33) | 8 (30) |
|
| cM category |
|
|
|
| M0 | 8 (30) | 10 (37) | 0.999 |
| M1 | 4 (15) | 5 (18) |
|
| APC |
|
|
|
|
Wild-type | 8 (30) | 1 (4) | 0.003 |
|
Mutant | 4 (15) | 14 (52) |
|
| KRAS |
|
|
|
|
Wild-type | 11 (40) | 10 (37) | 0.182 |
|
Mutant | 1 (4) | 5 (18) |
|
| BRAF
V600E |
|
|
|
|
Wild-type | 2 (7) | 15 (55) |
<0.001 |
|
Mutant | 10 (37) | 0 (0) |
|
| MSI |
|
|
|
|
MSI-H | 7 (26) | 1 (4) | 0.008 |
|
MSS | 5 (18) | 14 (52) |
|
| Variants of
RNF43 |
|
|
|
|
Nonsense or frameshift | 11 (40) | 1 (4) |
<0.001 |
|
Missense | 1 (4) | 14 (52) |
|
Overall survival in relation to RNF43
status and primary tumor sidedness
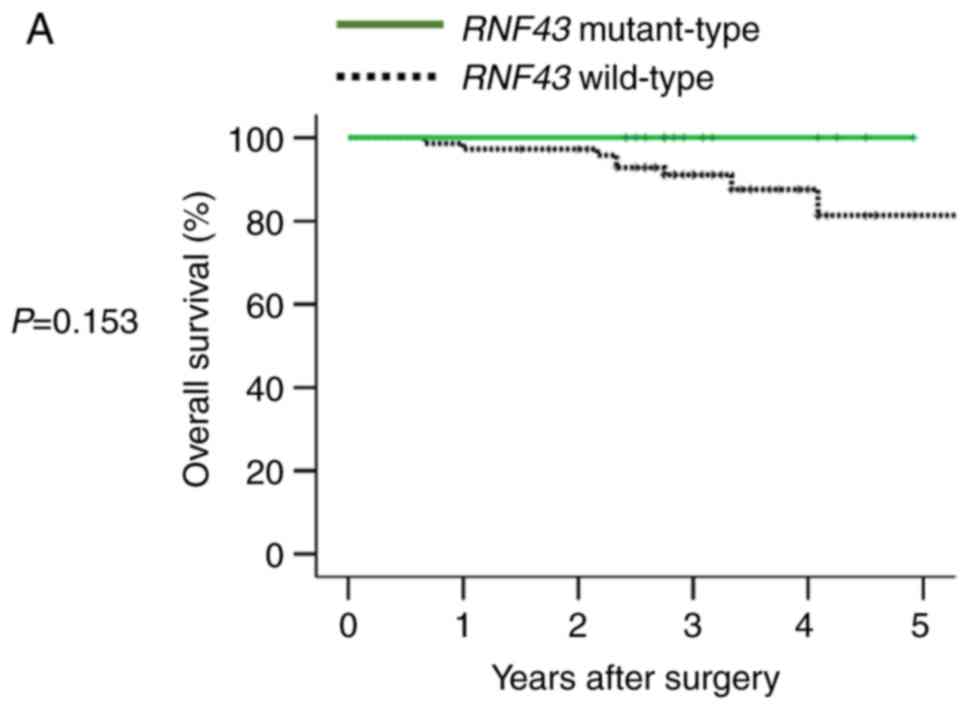
In 90 patients with stage I–III disease,
RNF43 mutation was not a significant prognostic factor for 5
year OS (Fig. 3A), and primary
tumor sidedness was not associated with RNF43
mutant-type.
In 111 patients with stage IV disease, RNF43
mutation was not a significant prognostic factor for OS (Fig. 3B). However, when RNF43
mutant-type was subdivided into right-sided RNF43
mutant-type or left-sided RNF43 mutant-type according to
primary tumor sidedness, right-sided RNF43 mutant-type
exhibited significantly worse overall survival than RNF43
wild-type and left-sided RNF43 mutant-type (P=0.001 and
P=0.023, respectively; Fig. 3C).
Regarding variants of RNF43 mutations, all right-sided
RNF43 mutant-type had nonsense mutation (R145X) or
frameshift mutation (P192fs, S262fs, G659fs), while all left-sided
RNF43 mutant-type had missense mutations (T58S, W200C,
R221W, R519Q; Table III). All the
four right-sided RNF43 mutant-type were older in age (≥65),
females, and BRAF V600E mutant-type. Three of four patients
with right-sided RNF43 mutation had two or more metastatic
sites; conversely, all five patients with left-sided RNF43
mutation had one metastatic site. While all patients with
right-sided RNF43 mutant-type succumbed to their cancer,
three of the five patients with left-sided RNF43 mutant-type
were alive at the final follow-up (Table III).
 | Table III.Clinical course of RNF43
mutant-type patients with Stage IV disease. |
Table III.
Clinical course of RNF43
mutant-type patients with Stage IV disease.
| Genetic
alteration | Age | Sex | Primary tumor
location | KRAS
status | BRAF
status | MSI status | Tumor mutational
burden | Initial metastatic
sites | Treatment | Survival status
(months after primary tumor resection) |
|---|
| S262fs | 71 | F | Right | Wild | V600E | MSS | 19 | Liver, Lung,
Spleen, Peritoneum | R2 resection
(Primary) → FOLFOX + Bmab → FOLFIRI | Dead (8
months) |
| R145X | 66 | F | Right | Wild | 26_34del,
V600E | MSS | 19 | Para-aortic lymph
node | R0 resection
(Primary and Para-aortic LN) → Lung, LN recurrence → FOLFOX +
Bmab | Dead (13
months) |
| P192fs | 80 | F | Right | Wild | V600E | MSS | 18 | Lung | R2 resection
(Primary) → XELOX + Bmab | Dead (20
months) |
| G659fs | 78 | F | Right | Wild | V600E | MSI-H | 48 | Liver,
Peritoneum | R2 resection
(Primary) → FOLFOX + Pmab | Dead (8
months) |
| R519Q | 35 | F | Left | Wild | Wild | MSS | 10 | Liver | R2 resection
(Primary) → FOLFOX + Bmab → R0 resection (Liver) → Liver and lung
recurrence → FOLFOX + Bmab → FOLFIRI + Pmab | Alive (44
months) |
| R519Q | 86 | M | Left | Wild | Wild | MSS | 11 | Lung | R2 resection
(Primary) → Xeloda → XELOX + Bmab → IRIS + Pmab | Alive (45
months) |
| W200C | 70 | F | Left | Wild | D594G | MSS | 19 | Liver | R2 resection
(Primary) → R0 resection (Liver) → Liver recurrence → R0 resection
(Liver) | Alive (40
months) |
| R221W | 77 | M | Left | Wild | Wild | MSS | 12 | Liver | R2 resection
(Primary) → XELOX → IRIS → Pmab | Dead (11
months) |
| T58S | 75 | F | Left | Wild | Wild | MSS | 11 | Liver | R2 resection
(Primary) → XELOX + Bmab → R0 resection (Liver) → Lung
recurrence | Dead (41
months) |
Discussion
This analysis has three main findings regarding
RNF43 mutations in CRC. Firstly, most of RNF43
mutations in RCRC were nonsense or frameshift mutations, while
those in LCRC were missense mutations. Secondly, right-sided
RNF43 mutant-type was significantly associated with
histopathological grade 3 and BRAF V600E mutation. Thirdly,
right-sided RNF43 mutant-type exhibited significantly worse
OS than left-sided RNF43 mutant-type. These results
indicated that right-sided RNF43 mutant-type is one of the
clinically important subtypes in CRC, and RNF43
nonsense/frameshift mutations, along with BRAF V600E
mutation, may be a possible cause of worse prognosis of RCRC.
In this analysis, it was revealed that 13% of the
Japanese CRC patients in this study had RNF43 mutations,
while 9% of patients in the TCGA cohort had RNF43 mutations
(36,37). Recently, several studies have
revealed the role of RNF43 mutations in the serrated
neoplastic pathway of CRC. Hashimoto et al reported that WNT
pathway gene mutations, including RNF43 mutation, were more
common in SSA/P with dysplasia than in SSA/P without dysplasia, and
suggested that WNT pathway gene mutations are involved in the
development of dysplasia in SSA/P (25). Tsai et al reported the
incidence of RNF43 mutation in SSA/P (10%) and TSA (28%),
and stated that RNF43 mutation is an early and specific
molecular aberration in the serrated neoplasia pathway (26). Yan et al reported
RNF43 germline and somatic mutation along with BRAF
V600E mutation in the serrated neoplasia pathway (16). However, the clinical significance of
RNF43 mutation has not been fully elucidated; hence, the
clinicopathological characteristics of RNF43 mutation were
investigated, with a focus on the association between RNF43
mutation and primary tumor sidedness.
To the best of our knowledge, this is the first
study which investigated the survival outcome of RNF43
mutant-type according to primary tumor sidedness in CRC. Previous
studies have reported that hotspot mutations, mainly frameshift
(R117fs and G659fs), are found in microsatellite-instable SSA/P and
CRC (23). In this analysis, 201
patients with stage I–IV CRC were investigated, and it was revealed
that 11 out of 12 right-sided RNF43 mutant-type had
nonsense/frameshift mutations, while 14 out of 15 left-sided
RNF43 mutant-type had missense mutations. Although RNF43
protein expression was not investigated, RNF43
nonsense/frameshift mutation may be a cause of loss of function of
RNF43 protein. It is speculated that RNF43
nonsense/frameshift mutation can become a cause of stimulation of
the WNT signaling pathway, and is associated with the aggressive
tumor biology of RCRC.
Approximately 5 to 9% patients with CRC have
BRAF V600E mutation, and BRAF V600E mutation is
recognized as a distinct molecular subtype of CRC (1,2).
Multiple studies have revealed that the BRAF V600E mutation
is associated with poor prognosis in metastatic CRC (38,39),
as well as poor response to anti-EGFR therapy in later lines of
therapy (40,41). In the present study, it was revealed
that 10 out of 12 right-sided RNF43 mutant-type had
BRAF V600E mutation. It is surmised that both RNF43
and BRAF V600E mutations are important for the tumor biology
of right-sided RNF43 mutant-type; i.e., RNF43
nonsense/frameshift mutation, along with BRAF V600E
mutation, induce enhancement of both the WNT and MAPK signaling
pathways, resulting in a worse prognosis in right-sided
RNF43 mutant-type.
In stage IV disease, it was revealed that
right-sided RNF43 mutant-type exhibited significantly worse
OS than left-sided RNF43 mutant-type, and all patients with
right-sided RNF43 mutant-type succumbed to their cancer.
These results suggest that right-sided RNF43 mutant-type is
a distinct subtype that has potentially worse prognosis. We
consider that RNF43 mutation should be treated differently
according to primary tumor sidedness, since the clinicopathological
characteristics and survival outcomes differ between right-sided
RNF43 mutant-type and left-side RNF43 mutant-type.
Thus, how should this dismal molecular subtype ‘right-sided
RNF43 mutant-type’ be treated? At present, right-sided
RNF43 mutant-type may be treated the same as BRAF
V600E mutant-type (1,2), since it was revealed that most
right-sided RNF43 mutant-type cases had BRAF V600E
mutation in this analysis. In the future, WNT signaling plus BRAF
inhibitors may be applied for right-sided RNF43 mutant-type
(ClinicalTrials.gov Identifier:
NCT02278133).
This analysis has some limitations. First, this
retrospective analysis was performed at two institutions. Second,
it included a small number of patients; specifically, the study had
only 90 patients with Stage I–III disease. Future analysis should
include a larger number of patients with CRC from large-scale
multi-institutional studies or a cancer registry. It is speculated
that the microbiome may be associated with tumor carcinogenesis and
phenotype, and certain bacteria may be associated with genetic
alterations in CRC. For example, it has been reported that
Fusobacterium nucleatum is enriched in tumor tissue of MSI-H
CRC (42,43). Although we do not have data linking
the microbiome to the results of our study at present, we plan to
investigate the relationship between genetic alteration of
right-sided CRC and the patient microbiome. Collectively, this
analysis is important for clarifying the clinicopathological
characteristics and prognosis of RNF43 mutant-type according
to primary tumor sidedness, and facilitating the research of future
treatment strategies.
In conclusion, clinicopathological characteristics
and survival outcome of patients with RNF43 mutation may
differ between RCRC and LCRC. In RCRC, RNF43 mutation may be
a small, but distinct molecular subtype that is associated with
aggressive tumor biology along with BRAF V600E mutation.
Future preclinical and clinical studies may have to focus on
RNF43 mutation to improve survival outcome in CRC.
Acknowledgements
Not applicable.
Funding
The present study was supported by Denka Co., Ltd.
Tokyo, Japan and, in part, by JSPS KAKENHI grant nos. JP18K08612
and JP17K10624.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
YS and AM provided substantial contributions to the
design and interpretation of data, and drafting of the article.
MaeN, HO, YoT, MasN, HK, YH, HI, MNag, HN, SM, YaT, and TW provided
substantial contributions to the acquisition of clinical data and
interpretation of data. YL and SO provided substantial
contributions to the statistical analysis of the data and creation
of the figures. TW critically revised the work and provided final
approval of article. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
This retrospective study was approved by the Ethics
Committee of the Niigata University School of Medicine, and
performed in accordance with the Helsinki Declaration (G2015-0816).
All methods were performed in accordance with the relevant
guidelines and regulations, and written informed consent was
obtained from the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors report no proprietary or commercial
interest in any product mentioned or concept discussed in this
article.
References
|
1
|
National Comprehensive Cancer Network, .
NCCN clinical practice guidelines in oncology-colon cancer (version
2, 2019). https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
|
|
2
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson
A, Bodoky G, et al: ESMO consensus guidelines for the management of
patients with metastatic colorectal cancer. Ann Oncol.
27:1386–1422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Loupakis F, Yang D, Yau L, Feng S,
Cremolini C, Zhang W, Maus MK, Antoniotti C, Langer C, Scherer SJ,
et al: Primary tumor location as a prognostic factor in metastatic
colorectal cancer. J Natl Cancer Inst. 107:dju4272015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weiss JM, Pfau PR, O'Connor ES, King J,
LoConte N, Kennedy G and Smith MA: Mortality by stage for right-
versus left-sided colon cancer: Analysis of surveillance,
epidemiology, and end results-Medicare data. J Clin Oncol.
29:4401–4409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishihara S, Murono K, Sasaki K, Yasuda K,
Otani K, Nishikawa T, Tanaka T, Kiyomatsu T, Kawai K, Hata K, et
al: Impact of primary tumor location on postoperative recurrence
and subsequent prognosis in nonmetastatic colon cancers: A
multicenter retrospective study using a propensity score analysis.
Ann Surg. 267:917–921. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Holch JW, Ricard I, Stintzing S, Modest DP
and Heinemann V: The relevance of primary tumour location in
patients with metastatic colorectal cancer: A meta-analysis of
first-line clinical trials. Eur J Cancer. 70:87–98. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Petrelli F, Tomasello G, Borgonovo K,
Ghidini M, Turati L, Dallera P, Passalacqua R, Sgroi G and Barni S:
Prognostic survival associated with left-sided vs. right-sided
colon cancer: A systematic review and meta-analysis. JAMA Oncol.
3:211–219. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tejpar S, Stintzing S, Ciardiello F,
Tabernero J, Van Cutsem E, Beier F, Esser R, Lenz HJ and Heinemann
V: Prognostic and predictive relevance of primary tumor location in
patients with RAS wild-type metastatic colorectal cancer:
Retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA
Oncol. 3:194–201. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arnold D, Lueza B, Douillard JY, Peeters
M, Lenz HJ, Venook A, Heinemann V, Van Cutsem E, Pignon JP,
Tabernero J, et al: Prognostic and predictive value of primary
tumour side in patients with RAS wild-type metastatic colorectal
cancer treated with chemotherapy and EGFR directed antibodies in
six randomized trials. Ann Oncol. 28:1713–1729. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boeckx N, Koukakis R, Op de Beeck K, Rolfo
C, Van Camp G, Siena S, Tabernero J, Douillard JY, André T and
Peeters M: Primary tumor sidedness has an impact on prognosis and
treatment outcome in metastatic colorectal cancer: Results from two
randomized first-line panitumumab studies. Ann Oncol. 28:1862–1868.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Breivik J, Lothe RA, Meling GI, Rognum TO,
Børresen-Dale AL and Gaudernack G: Different genetic pathways to
proximal and distal colorectal cancer influenced by sex-related
factors. Int J Cancer. 74:664–669. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iacopetta B: Are there two sides to
colorectal cancer? Int J Cancer. 101:403–408. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hutchins G, Southward K, Handley K, Magill
L, Beaumont C, Stahlschmidt J, Richman S, Chambers P, Seymour M,
Kerr D, et al: Value of mismatch repair, KRAS, and BRAF mutations
in predicting recurrence and benefits from chemotherapy in
colorectal cancer. J Clin Oncol. 29:1261–1270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen H, Yang J, Huang Q, Jiang MJ, Tan YN,
Fu JF, Zhu LZ, Fang XF and Yuan Y: Different treatment strategies
and molecular features between right-sided and left-sided colon
cancers. World J Gastroenterol. 21:6470–6478. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimada Y, Kameyama H, Nagahashi M,
Ichikawa H, Muneoka Y, Yagi R, Tajima Y, Okamura T, Nakano M,
Sakata J, et al: Comprehensive genomic sequencing detects important
genetic differences between right-sided and left-sided colorectal
cancer. Oncotarget. 8:93567–93579. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan HHN, Lai JCW, Ho SL, Leung WK, Law WL,
Lee JFY, Chan AKW, Tsui WY, Chan ASY, Lee BCH, et al: RNF43
germline and somatic mutation in serrated neoplasia pathway and its
association with BRAF mutation. Gut. 66:1645–1656. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eto T, Miyake K, Nosho K, Ohmuraya M,
Imamura Y, Arima K, Kanno S, Fu L, Kiyozumi Y, Izumi D, et al:
Impact of loss-of-function mutations at the RNF43 locus on
colorectal cancer development and progression. J Pathol.
245:445–455. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lai C, Sun W, Wang X, Xu X, Li M, Huang D,
Xu E, Lai M and Zhang H: RNF43 frameshift mutations contribute to
tumourigenesis in right-sided colon cancer. Pathol Res Pract.
215:1524532019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi
ST, Siu HC, Deng S, Chu KM, Law S, et al: Whole-genome sequencing
and comprehensive molecular profiling identify new driver mutations
in gastric cancer. Nat Genet. 46:573–582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang X, Hao HX, Growney JD, Woolfenden S,
Bottiglio C, Ng N, Lu B, Hsieh MH, Bagdasarian L, Meyer R, et al:
Inactivating mutations of RNF43 confer Wnt dependency in pancreatic
ductal adenocarcinoma. Proc Natl Acad Sci USA. 110:12649–12654.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ryland GL, Hunter SM, Doyle MA, Rowley SM,
Christie M, Allan PE, Bowtell DD; Australian Ovarian Cancer Study
Group, ; Gorringe KL and Campbell IG: RNF43 is a tumour suppressor
gene mutated in mucinous tumours of the ovary. J Pathol.
229:469–476. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Giannakis M, Hodis E, Jasmine Mu X,
Yamauchi M, Rosenbluh J, Cibulskis K, Saksena G, Lawrence MS, Qian
ZR, Nishihara R, et al: RNF43 is frequently mutated in colorectal
and endometrial cancers. Nat Genet. 46:1264–1266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Serra S and Chetty R: Rnf43. J Clin
Pathol. 71:1–6. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO classification of tumours of the digestive system.
4th. IARC; Lyon: 2010
|
|
25
|
Hashimoto T, Yamashita S, Yoshida H,
Taniguchi H, Ushijima T, Yamada T, Saito Y, Ochiai A, Sekine S and
Hiraoka N: WNT pathway gene mutations are associated with the
presence of dysplasia in colorectal sessile serrated
adenoma/polyps. Am J Surg Pathol. 41:1188–1197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsai JH, Liau JY, Yuan CT, Lin YL, Tseng
LH, Cheng ML and Jeng YM: RNF43 is an early and specific mutated
gene in the serrated pathway, with increased frequency in
traditional serrated adenoma and its associated malignancy. Am J
Surg Pathol. 40:1352–1359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sekine S, Yamashita S, Tanabe T, Hashimoto
T, Yoshida H, Taniguchi H, Kojima M, Shinmura K, Saito Y, Hiraoka
N, et al: Frequent PTPRK-RSPO3 fusions and RNF43 mutations in
colorectal traditional serrated adenoma. J Pathol. 239:133–138.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th. Springer;
New York: 2010
|
|
29
|
Watanabe T, Muro K, Ajioka Y, Hashiguchi
Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S,
et al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2016 for the treatment of colorectal cancer. Int J Clin
Oncol. 23:1–34. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nagahashi M, Wakai T, Shimada Y, Ichikawa
H, Kameyama H, Kobayashi T, Sakata J, Yagi R, Sato N, Kitagawa Y,
et al: Genomic landscape of colorectal cancer in Japan: Clinical
implications of comprehensive genomic sequencing for precision
medicine. Genome Med. 8:1362016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shimada Y, Yagi R, Kameyama H, Nagahashi
M, Ichikawa H, Tajima Y, Okamura T, Nakano M, Nakano M, Sato Y, et
al: Utility of comprehensive genomic sequencing for detecting
HER2-positive colorectal cancer. Hum Pathol. 66:1–9. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shimada Y, Tajima Y, Nagahashi M, Ichikawa
H, Oyanagi H, Okuda S, Takabe K and Wakai T: Clinical significance
of BRAF Non-V600E mutations in colorectal cancer: A retrospective
study of two institutions. J Surg Res. 232:72–81. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Oyanagi H, Shimada Y, Nagahashi M,
Ichikawa H, Tajima Y, Abe K, Nakano M, Kameyama H, Takii Y,
Kawasaki T, et al: SMAD4 alteration associates with invasive-front
pathological markers and poor prognosis in colorectal cancer.
Histopathology. 74:873–882. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Boland CR, Thibodeau SN, Hamilton SR,
Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA,
Fodde R, Ranzani GN and Srivastava S: A national cancer institute
workshop on microsatellite instability for cancer detection and
familial predisposition: Development of international criteria for
the determination of microsatellite instability in colorectal
cancer. Cancer Res. 58:5248–5257. 1998.PubMed/NCBI
|
|
36
|
Cancer Genome Atlas Network, .
Comprehensive molecular characterization of human colon and rectal
cancer. Nature. 487:330–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Colorectal Adenocarcinoma. TCGA, PanCancer
data. https://www.cell.com/pb-assets/consortium/pancanceratlas/pancani3/index.html
|
|
38
|
Tol J, Nagtegaal ID and Punt CJ: BRAF
mutation in metastatic colorectal cancer. N Engl J Med. 361:98–99.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Peeters M, Oliner KS, Parker A, Siena S,
Van Cutsem E, Huang J, Humblet Y, Van Laethem JL, André T, Wiezorek
J, et al: Massively parallel tumor multigene sequencing to evaluate
response to panitumumab in a randomized phase III study of
metastatic colorectal cancer. Clin Cancer Res. 19:1902–1912. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Seymour MT, Brown SR, Middleton G, Maughan
T, Richman S, Gwyther S, Lowe C, Seligmann JF, Wadsley J, Maisey N,
et al: Panitumumab and irinotecan versus irinotecan alone for
patients with KRAS wild-type, fluorouracil-resistant advanced
colorectal cancer (PICCOLO): A prospectively stratified randomised
trial. Lancet Oncol. 14:749–759. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee DW, Han SW, Kang JK, Bae JM, Kim HP,
Won JK, Jeong SY, Park KJ, Kang GH and Kim TY: Association between
fusobacterium nucleatum, pathway mutation, and patient
prognosis in colorectal cancer. Ann Surg Oncol. 25:3389–3395. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hamada T, Zhang X, Mima K, Bullman S,
Sukawa Y, Nowak JA, Kosumi K, Masugi Y, Twombly TS, Cao Y, et al:
Fusobacterium nucleatum in colorectal cancer relates to
immune response differentially by tumor microsatellite instability
status. Cancer Immunol Res. 6:1327–1336. 2018. View Article : Google Scholar : PubMed/NCBI
|