Introduction
Glioblastoma multiforme (GBM) is the most common and
deadliest form of brain cancer (1–3).
Although current treatments are rigorous, including surgery and
chemotherapy, the prognosis for patients with GBM remains poor. The
median survival rate is 14.6 months, and the two-year survival rate
is 30% (1,4). This poor prognosis in GBM and other
solid tumors is often attributed to the presence of cancer stem
cells (CSCs) (5–8). This subpopulation of cells has the
unique abilities to self-renew, differentiate, and initiate
tumorigenesis. Due to this, CSCs play an important role in
recurrence and metastasis. Glioblastoma stem cells (GSCs) have also
been revealed to be resistant to conventional therapies (9,10),
underscoring the need to develop treatments that account for the
cellular heterogeneity found in GBM environments, specifically with
regard to GSCs.
While the properties of self-renewal,
differentiation, and tumorigenesis define GSCs, practically, it is
easier to use GSC markers for their identification. These markers
are proteins that are commonly expressed by GSCs and associated
with the stem cell phenotype (11).
A variety of GSC markers have been proposed and debate continues on
which markers are the most accurate (11). Three of the most commonly examined
GSC markers are CD133, SOX2 [SRY (sex determining region
Y)-box transcription factor 2], and Nestin. CD133 is
a cell surface glycoprotein of unknown function and was the first
identified GSC marker (6,8,11).
Cells that are CD133-positive have exhibited an increased
ability to form tumors in vivo. SOX2 is a marker of
embryonic stem cells used in somatic reprogramming. The knockdown
of SOX2 in GSCs has been revealed to reduce their
tumorigenesis and chemoresistance (12). Nestin is a class VI
intermediate filament also found in non-malignant neural stem
cells. Nestin-positive cells have demonstrated
radioresistance and increased tumor formation (13).
One drug that has shown promise in targeting CSCs is
salinomycin, a monocarboxylic polyether antibiotic isolated from
Streptomyces albus (14).
Salinomycin has been used as an anticoccidial in poultry since the
1970s, and it acts as an ionophore with a preference for
Na+ and K+ (15). A high-throughput study in 2009
identified the potential of salinomycin to kill stem cell-like
breast cancer cells and significantly reduce their tumor seeding
ability in mice (14). Several
other studies since then have revealed the effect of this drug on
other types of CSCs including acute myeloid leukemia, lung cancer,
colorectal cancer, and prostate cancer (16–18).
Salinomycin has been revealed to overcome ATP-binding cassette
transporters in acute myeloid leukemia, inhibit the Wnt signaling
in chronic lymphocytic leukemia, and induce apoptosis in both of
these cancers (19–21). The mechanism by which salinomycin
exerts these capabilities remains unknown.
In a previous study, we hypothesized that
salinomycin would specifically target the GSCs (22). Although several studies have
suggested that salinomycin is effective against GBM, the effect of
the drug on GSCs is understudied. Prior studies demonstrated that
salinomycin decreases the viability of GSC-enriched cultures more
than non-GSC enriched cultures, and that salinomycin inhibits
neurosphere formation, a functional marker for stemness (23–25).
In the present study, we corroborated these previous studies on the
susceptibility of GSC-enriched cultures and neurosphere formation.
Furthermore, novel evidence was provided of the ability of
salinomycin to decrease the expression of the GSC marker SOX2 at
both the transcriptional and translational level. Finally,
preliminary evidence was provided that salinomycin-induced death of
GSCs is achieved via the apoptotic pathway.
Materials and methods
Cell culture
Three human glioblastoma cell lines were used:
Established glioblastoma cell line of unknown origin U87-MG (ATCC
HTB-14) (26), primary glioblastoma
cell line SMC448 (kindly provided and STR-profiled by Dr Do-Hyun
Nam, Samsung Medical Center) (27),
and proneural patient-derived xenograft GBM line D456 (kindly
provided and STR-profiled by Dr G. Yancey Gillespie, Department of
Neurosurgery, University of Alabama at Birmingham) (28). A mouse neural stem cell line NE-4C
(ATCC CRL-2925) was also used. The cells were grown in either
serum-containing or serum-free culture media. Serum-free culture
cells were grown in neurobasal-A media supplemented with 1 mM
glutamine (Life Technologies; Thermo Fisher Scientific, Inc.), 8
µg/ml heparin (J.T. Baker Chemical Co.), 0.5X N-2 and 0.5X B-27
(both from Gibco; Thermo Fisher Scientific, Inc.), 1%
penicillin/streptomycin (Corning, Inc.), 20 ng/ml epidermal growth
factor and 10 ng/ml fibroblast growth factor (both from Shenandoah
Biotechnology, Inc.) (NBE media). Serum culture cells were grown in
Eagle's minimal essential medium supplemented with 10% fetal bovine
serum and 1% penicillin/streptomycin (Corning, Inc.) (EMEM
media).
Light microscopy
VistaVision Microscope (VWR #82026-630) at ×4
magnification was used to observe the morphology of cells in serum
and serum-free culture. The microscope at a magnification of ×10
was also used to observe sphere formation ability.
Salinomycin treatment
For all experiments, the same salinomycin treatment
procedure was followed. Cells were seeded in appropriate media (NBE
for serum-free culture, EMEM for serum culture) and incubated for
24 h at 37°C. After 24 h, salinomycin (MP Biomedicals, LLC)
reconstituted in dimethyl sulphoxide (DMSO) or mock control (DMSO
alone) was added to a final concentration of 0.1% v/v DMSO. Cells
were incubated for an additional 48 h at 37°C and then collected
for analysis.
Viability assay
For the viability assay, 1×104 cells in
100 µl of media per well were seeded in 96-well plates. Cell
Counting Kit-8 (CCK-8), a water-soluble tetrazolium salt-based
colorimetric assay, was used to determine the cell viability as per
the manufacturer's protocol (Sigma Aldrich-Merck KGaA). Briefly, 10
µl of CCK-8 solution per well was added to salinomycin-treated and
mock-treated cells, incubated in a humidified incubator at 37°C for
4 h, and the absorbance was measured at λ=450 nm.
Flow cytometry
For flow cytometry, 3×105 cells in 3 ml
of media per well were seeded in 6-well plates. Flow cytometry was
used to assess the expression of SOX2. NE-4C, U87-MG, D456, and
SMC448 were cultured in NBE (serum-free culture) or EMEM (serum
culture) for 48 h with 1 µM salinomycin or mock control (DMSO).
Cells were then pelleted, resuspended in 4% paraformaldehyde, then
in a permeabilization buffer (BD Cytofix/Cytoperm™; cat. no.
554722; BD Biosciences) on ice for 15 min, and stained with Alexa
Fluor 647 anti-SOX2 antibody (cat. no. 562139; dilution 1:10
dilution; BD Biosciences) on ice for 30 min. Flow cytometry was
then performed analyzing 20,000 events per sample using BD Accuri
C6 flow cytometer (BD Biosciences). Results were analyzed using
Accuri C6 software (v1.0; BD Biosciences).
Flow cytometry was also used to assess apoptosis.
U87-MG, D456, and SMC448 were cultured in NBE (serum-free culture)
or EMEM (serum culture) for 48 h with 1 µM salinomycin or mock
control. Cells were then pelleted, dissolved in Annexin V binding
buffer, and double-stained with Annexin V (Enzo Life Sciences) and
7-AAD (EMD Millipore). Flow cytometry was performed analyzing
20,000 events per sample using BD Accuri C6 flow cytometer.
Specific apoptosis was calculated as established previously using
the following equation: (Late apoptotic population with 1 µM
Sal-late apoptotic population with 0 µM Sal)/(100-late apoptotic
population with 0 µM Sal) ×100.
Finally, flow cytometry was used to assess caspase-3
cleavage. Caspase-3 cleavage was determined using
NucView® 488 Caspase-3 assay kit (Biotium; cat. no.
30029). The assay was conducted according to manufacturer's
protocol. Flow cytometry was performed analyzing 20,000 events per
sample using BD Accuri C6 flow cytometer.
Real-time quantitative PCR
(RT-qPCR)
For RT-qPCR, 3×105 cells in 3 ml of media
per well were seeded in 6-well plates. Primers were designed by
retrieving nucleotide sequences from the NCBI gene database for
SOX2 (NM_003106), Nestin (NM_006617),
Prominin1/CD133 (NM_001145847), and ACTB (NM_001101;
see Table I). ACTB was used
as a housekeeping gene control. Primers were synthesized by
Eurofins Genomics. RNA isolation was performed using PureLink RNA
Mini kit (Invitrogen, Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol for mammalian cultured cells. RNA
quantification was performed using NanoDrop (Thermo Fisher
Scientific, Inc.). Complementary DNA (cDNA) was synthesized using
qScript cDNA SuperMix (Quanta Biosciences) and Mastercycler nexus
gradient (Eppendorf) according to manufacturers' protocols.
Quantitative real-time PCR was performed using PerfeCTa SYBR Green
FastMix (Quanta Biosciences) using Eco Real-Time PCR system
(Illumina) according to manufacturers' protocols. The thermocycling
conditions were as follows: Forty cycles of 2-step cycling were
performed with denaturing at 95°C for 15 sec and annealing at 60°C
for 1 min. Relative quantification was determined using the
2−ΔΔCq method (29).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Human |
|---|
| ACTB |
| F |
CTCGTCGCCCACATAGGAA |
| R |
AGGGCTTCTTGTCCTTTCCTTC |
| CD133 |
|
| F |
GGTCCCTTCTGTGAACCAAC |
| R |
CAGATAAGTCAGCCAGGGAGC |
| Nestin |
| F |
GGTCCCTTCTGTGAACCAAC |
| R |
CAGATAAGTCAGCCAGGGAGC |
| SOX2 |
|
| F |
AAGCCCTGAAAGCGCAAGTCCTCAA |
| R |
GGCAGTGGTAGTGGTGGCATTAGCAG |
|
| Mouse |
|
| ACTB |
|
| F |
AAGAAGGCTATAGTCACCTCGG |
| R |
TGGTAATAATGCGGCCGGT |
| Nestin |
|
| F |
CTGGAAGAAGTTCCCAGGCTT |
| R |
GAAGATGTGGAAGGAGAGCGT |
| SOX2 |
|
| F |
ACAGCATGTCCTACTCGCAG |
| R |
CCTCGGACTTGACCACAGAG |
Sphere formation assay
A sphere formation assay was used to qualitatively
assess stemness. Dissociated single cells were seeded at either 10
or 100 cells per well in a 96-well plate with or without the
addition of 1 µM salinomycin (n=20). Cells were incubated at 37°C
for 7 days and then were evaluated for sphere formation using light
microscopy at a magnification of ×10.
Limiting dilution assay
A limiting dilution assay was used as a functional
assay for confirming stemness (2).
Dissociated single cells were plated into 96-well plates with
various seeding densities (1–10 cells/well with 20 wells per
condition) in NBE media with and without the addition of 1 µM
salinomycin (n=8). Sphere formation was visually confirmed within
each well. A diameter >50 µm was used as the criterion for
counting spheres.
Statistical analysis
All experiments were performed with at least
triplicates for each condition. Data were analyzed by a 2-tailed
t-test with equal or unequal variance and ANOVA with Dunnett's
multiple comparisons test. An f-test was used to determine variance
prior to the t-test or ANOVA. Data with P<0.05 were considered
to indicate a statistically significant difference.
Results
As demonstrated in Fig.
1A, cells grown with serum adhered to the bottom of the flask
and assumed an extended morphology. Alternatively, cells grown in
serum-free culture remained suspended in the media and adhered to
each other, forming spheres. Such a growth environment enriches the
culture for GSCs. To validate this model, GSC markers SOX2,
Nestin, and CD133 were analyzed by RT-qPCR. As revealed
in Fig. 1B-D cells grown in
serum-free culture had higher expression of these markers. For the
U87 cell line, the difference in GSC marker expression was
statistically significant for SOX2 and Nestin, but
not for CD133. SMC448 exhibited statistical significance for
all three GSC markers with P<0.01 for both SOX2 and
Nestin. D456 exhibited differences in GSC expression for all
markers, albeit not at statistically significant levels.
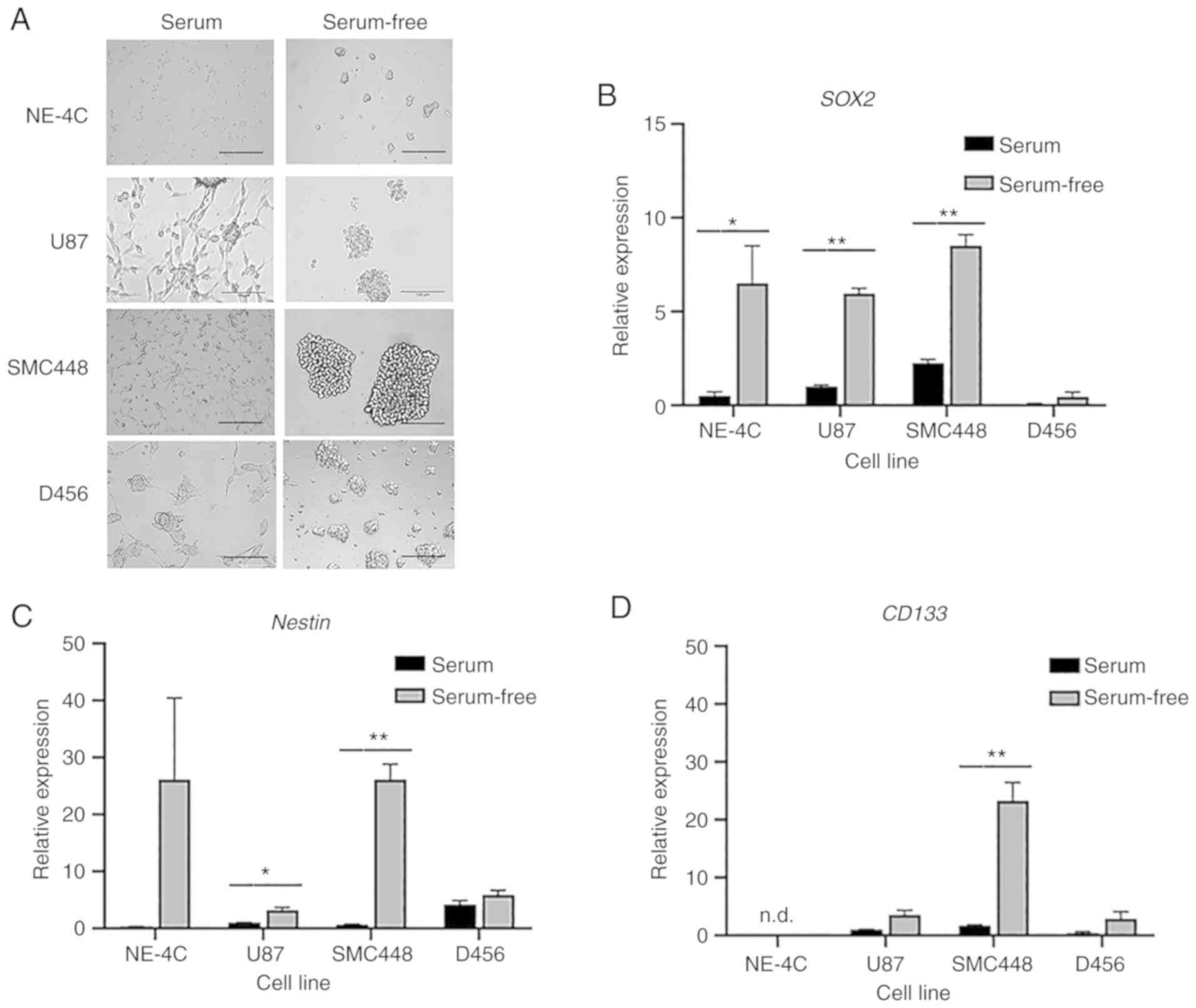 | Figure 1.Serum-free culture enriches GSCs. GBM
cells were grown in both serum and serum-free media and their
expression of GSC markers were evaluated. (A) Representative
bright-field images of NE-4C, U87, SMC448, and D456 cells after
four days of growth in serum-free and serum-containing media
exhibiting marked differences in cell morphology. Scale bar, 100
µm. (B-D) RT-qPCR analysis of the gene expression of (B)
SOX2, (C) Nestin, and (D) CD133 for NE-4C,
U87, SMC448, and D456 GBM cells. Error bars represent 95%
confidence intervals (n=3). *P<0.05, **P<0.01 for each gene
compared to the serum culture counterpart. These results indicated
increased stemness characteristics when GBM cells were grown in
serum-free media. GSCs, glioblastoma stem cells; GBM, glioblastoma;
SOX2, SRY (sex determining region Y)-box transcription
factor 2. |
Having established that serum-free culture enriched
GSC-like cells, this culture method was used to assess the
effectiveness of salinomycin against GSCs. Increasing doses of
salinomycin were added to cells grown in both serum and serum-free
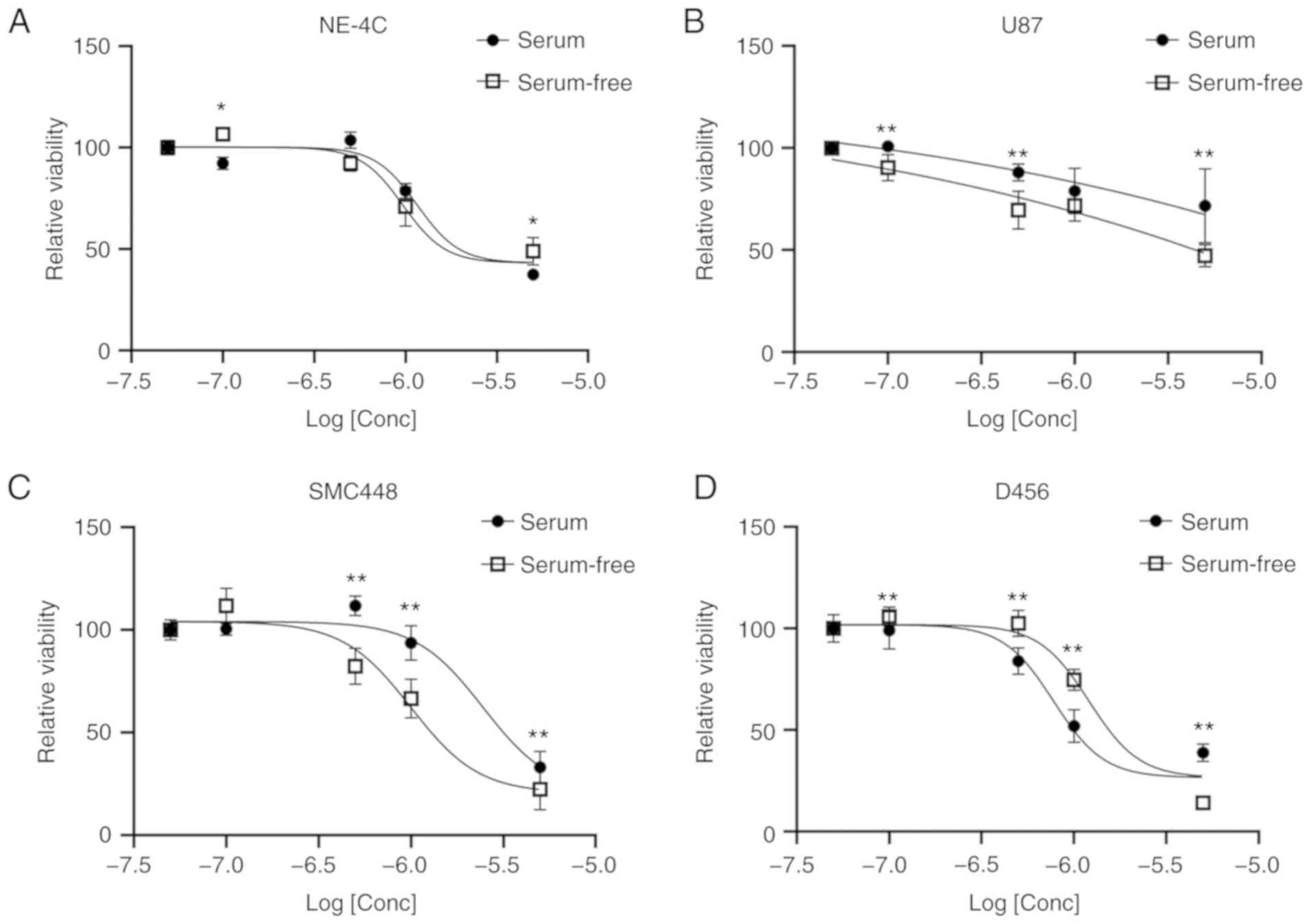
culture. All three GBM cell lines exhibited a decreasing viability
with increasing doses of salinomycin (Fig. 2). In both the U87 and SMC448 lines,
the cells grown in serum-free culture had lower viabilities,
suggesting the GSCs were more affected than the differentiated
cancer cells in the serum culture. However, lower viabilities were
observed in serum culture than in serum-free culture for the D456
cell line at all concentrations of salinomycin except at the
highest 5 µM. This result was unexpected and may indicate the
importance of the GBM subtype on the effectiveness of salinomycin.
It could also be explained by the lack of a statistically
significant difference in GSC markers between D456 cells grown in
serum vs. serum-free culture. The non-cancerous neural stem cell
line NE-4C was also investigated as a negative control (Fig. 2A). However, NE-4C cells exhibited
significant toxicity to salinomycin in both serum and serum-free
culture. This finding highlights the importance of understanding
the mechanism of action of salinomycin and its effect on the GSC
population.
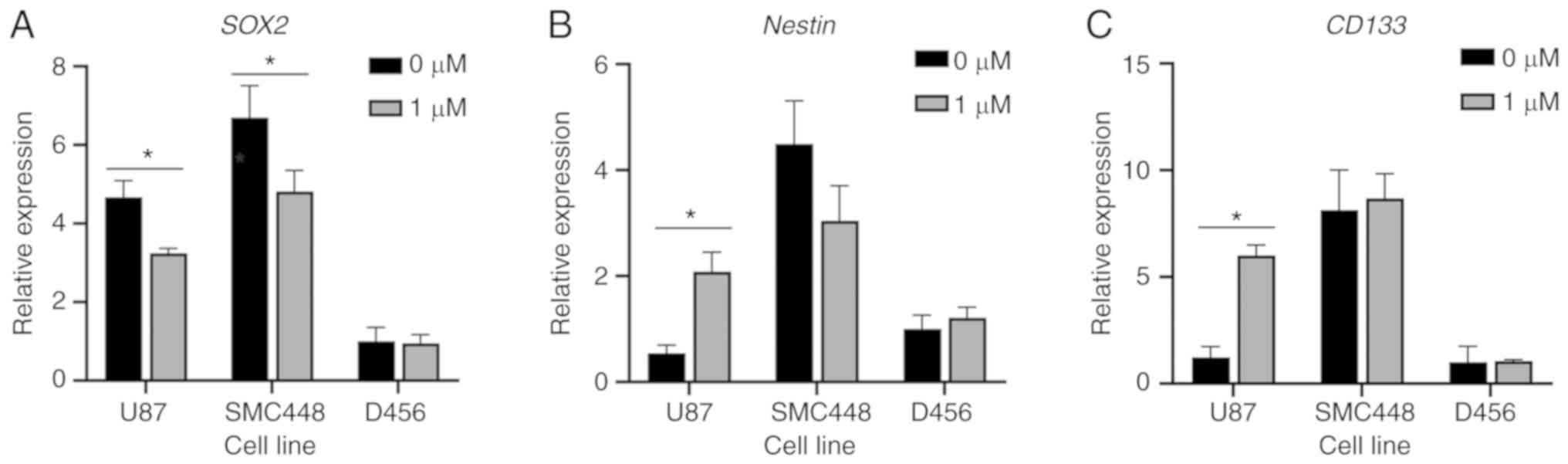
To more thoroughly assess the effect of salinomycin
on the GSC population, the mRNA expression of the GSC markers
SOX2, Nestin, and CD133 was evaluated. Cells grown in
sphere culture were treated for two days in triplicate with either
1 µM salinomycin or 0.1% DMSO. Salinomycin treatment significantly
decreased the SOX2 expression in U87 and SMC448 cells and
resulted in a non-statistically significant decrease in SOX2
expression in D456 cells (Fig. 3A).
However, the effect of salinomycin on Nestin and
CD133 was inconsistent (Fig. 3B
and C). Salinomycin increased the expression of Nestin
in U87 cells, decreased its expression in SMC448 cells, and had no
observable effect on its expression in D456 cells. None of these
results were statistically significant. For CD133, salinomycin
caused a statistically significant increase in U87 cells but
minimal changes in SMC448 and D456. The variance in marker
expression brings into question whether these markers are in fact
assessing the same types of cells.
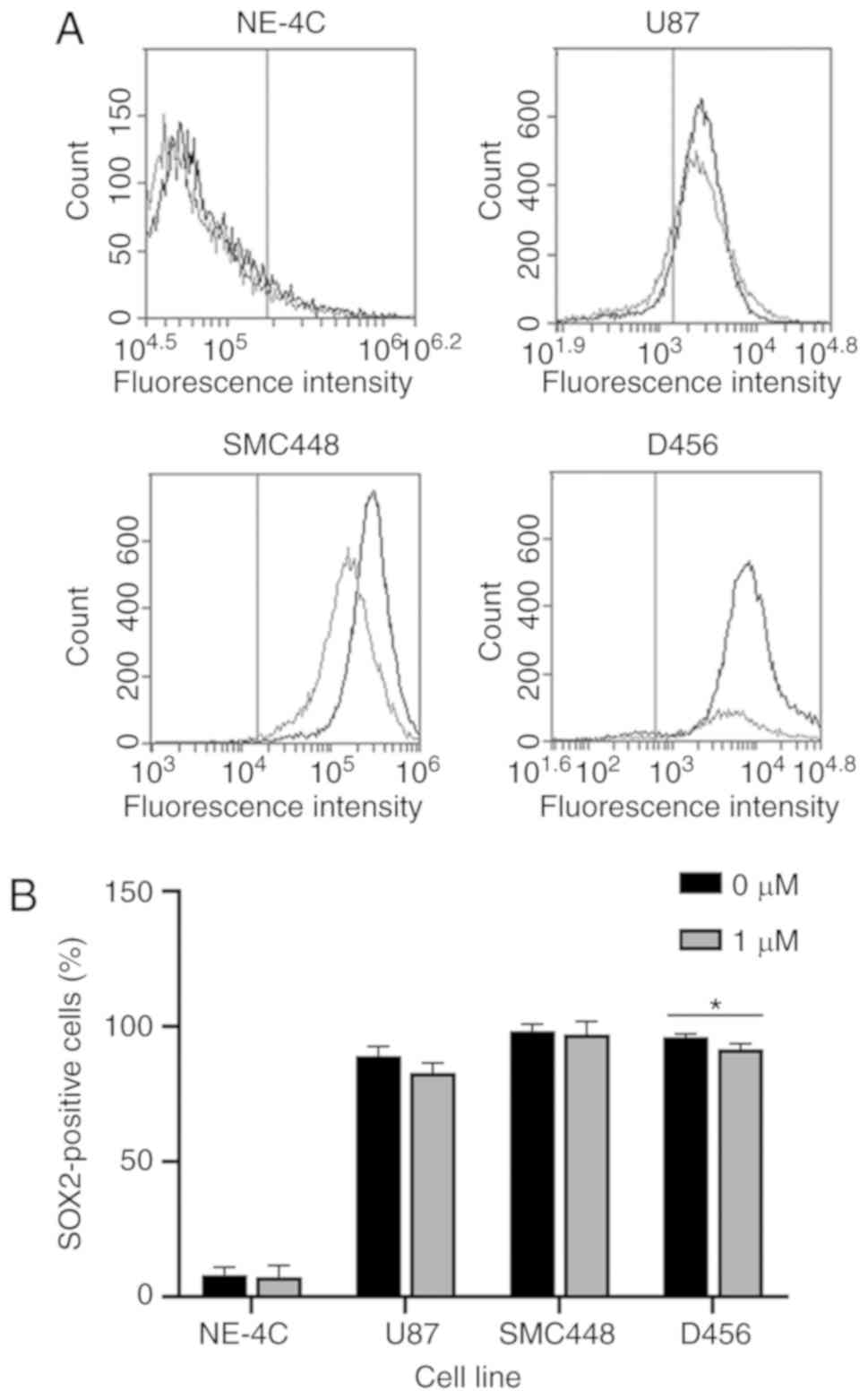
To further investigate the effect of salinomycin on
GSCs, the protein expression and GSC functionality were assessed.
Since salinomycin caused a decrease in SOX2 mRNA expression
for all three GBM cell lines, SOX2 protein expression was
analyzed using flow cytometry (Fig.
4). A statistically significant decrease in
SOX2-positive population in D456 expression, with a similar
decrease in U87 and SMC448 cells (albeit not at statistically
significant levels) was revealed. Placed in context with the
transcriptional results, these data indicated that while most of
the cells continue to express SOX2, salinomycin is reducing the
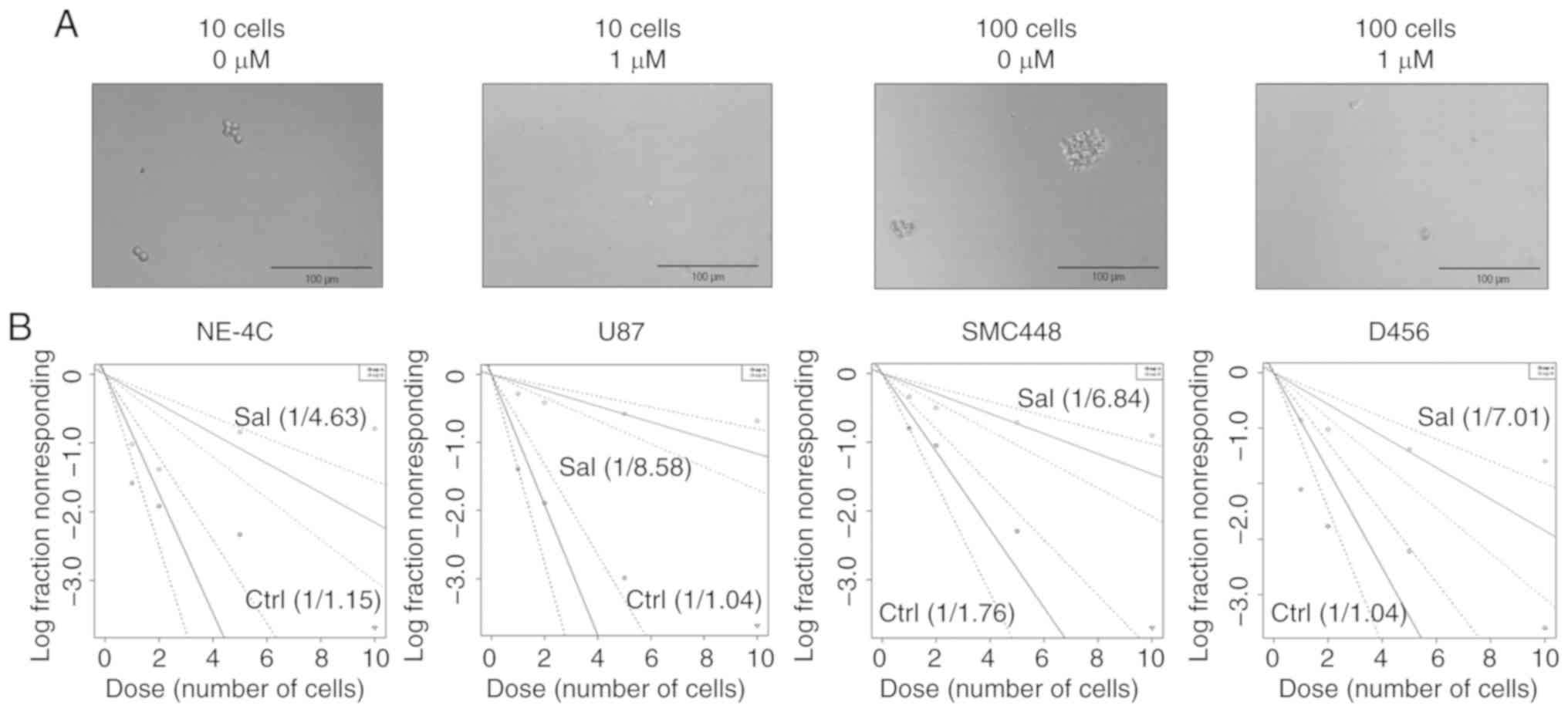
total amount of SOX2-positive population. The functional
impact of salinomycin on GSC clonogenicity was assessed via sphere
formation assay. Qualitatively, 1 µM of salinomycin was sufficient
to prevent sphere formation at both 10 and 100 cells/well seeding
densities, even though these densities were otherwise sufficient to
form spheres without salinomycin (Fig.
5A). Quantitatively using the extreme limiting dilution assay
(30), a significant reduction of
clonogenic population was also observed. Clonogenic potential of
U87 cells was altered from 1 of 1.04 to 1 of 8.58 cells after 1
week of 1 µM of salinomycin treatment (Fig. 5B). Similarly, the clonogenic
potential of SMC448 was altered from 1 of 1.76 to 1 of 6.84 and
D456 changed from 1 of 1.04 to 1 of 7.01. Notably, the clonogenic
potential of NE-4C mouse neural stem cells was also altered but to
a lower extent from 1 of 1.15 to 1 of 4.63 (comparison of slopes of
lines in Fig. 5B). Collectively,
these data indicated that salinomycin significantly and selectively
impacted GSC clonogenicity.
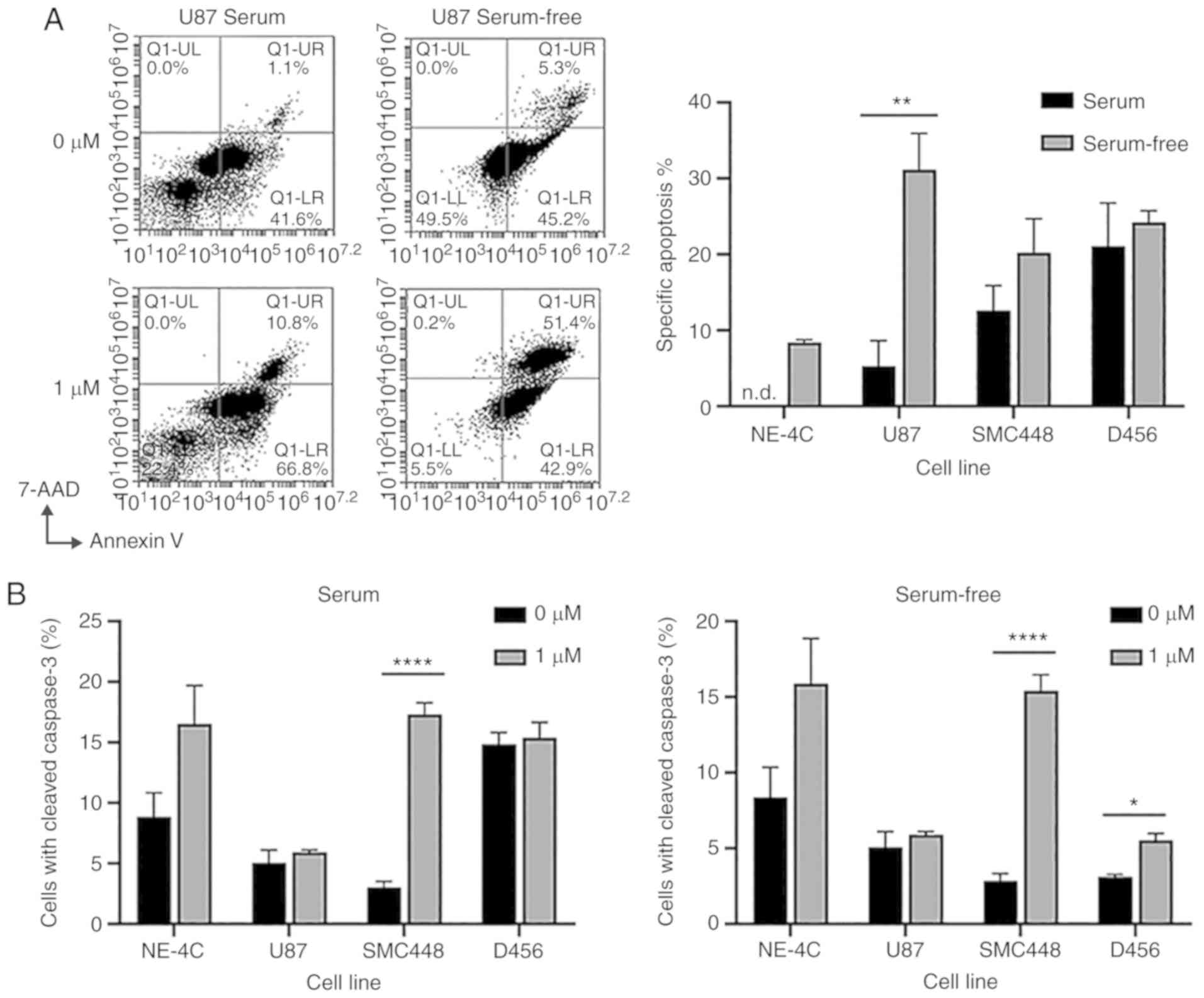
To investigate the mechanism of action of
salinomycin, an Annexin V/7-AAD assay was performed (Fig. 6A). This assay assesses the
percentage of cells that are healthy (lower left quadrant), early
apoptotic (lower right quadrant), late apoptotic (upper right
quadrant), and necrotic (upper left quadrant). For all cell lines,
salinomycin treatment led to an increased percentage of cells in
the early apoptotic and late apoptotic quadrants, but no increase
in the necrotic quadrant. This finding indicated that salinomycin
induced cell death via apoptosis rather than necrosis. The cells
grown in serum-free culture, which are GSC enriched, led to higher
rates of specific apoptosis than those grown with serum,
reinforcing the prior observation that salinomycin can to a certain
extent preferentially deplete GSCs. For U87, the difference in the
apoptotic rates between the serum-free culture and serum culture
was statistically significant. The GBM lines U87, SMC448, and D456
all exhibited higher rates of specific apoptosis than the
non-cancerous NE4C cell line, providing evidence that salinomycin
has greater toxicity for cancer cells than their non-cancerous
counterparts. To further investigate this potential mechanism, flow
cytometry was used to identify caspase-3 cleavage activity in
serum-free and serum culture cells with or without the addition of
salinomycin (Fig. 6B). Salinomycin
treatment led to statistically significant increases in caspase-3
cleavage for SMC448 cells in serum culture as well as for both
SMC448 and D456 cells in serum-free culture. The cells in serum and
serum-free culture exhibited similar trends in caspase-3 cleavage,
indicating that the increased apoptosis in U87 serum-free vs. serum
culture may be caused by a caspase-3-indepdendent pathway (31,32).
Discussion
We have previously postulated the potential of
salinomycin to selectively target GSCs (20). While salinomycin is yet to be the
subject of any full-scale clinical trials in cancer patients, its
emergence as a key anticancer agent has propelled it into the focus
of pre-clinical models using patient-derived cancer cells (33). The aim of the present study was to
investigate the impact of salinomycin on GSC-like cells in GBM to
assess the efficacy of the drug as a potential chemotherapy for GBM
patients. GSCs are a distinct subpopulation of cells within
glioblastomas which have the ability to initiate tumor growth and
self-renew (6,8,11).
Conventional therapies do not adequately target these cell
populations in glioblastoma, which may lead to inefficient tumor
elimination and cancer reemergence (11,34).
The antibiotic salinomycin had been first identified to target CSCs
nearly a decade ago, and subsequent studies on salinomycin have
been centered on its potential to treat similar stem cell-like
populations in various cancers (14). Additionally, drug delivery systems
have been implemented in vitro with salinomycin to
demonstrate the ability of the drug to deplete glioblastoma
populations while also crossing the blood-brain barrier (35).
In the present study, the potential of salinomycin
as a chemotherapeutic agent in the treatment of SOX2-positive GSCs
was demonstrated. GSC populations within the tumor microenvironment
must be targeted to adequately eliminate the tumor. One established
human glioblastoma cell line (U87-MG) and two patient-derived
glioblastoma cells lines (SMC448 and D456) were used in the present
study. U87-MG is a well-established and widely used glioblastoma
cell line of unknown origin that has been in culture since the
1960s (26). SMC448 is a
radioresistant xenograft cell line derived from a high-grade glioma
of unknown subtype (27). D456 is a
glioblastoma of the proneural subtype that was derived from a human
pediatric fronto-parietal GBM directly implanted into the flank of
immunocompromised mice (28).
Additionally, the mouse neural stem cell line NE-4C was used as a
non-cancerous control. The present results revealed that
salinomycin depleted the SOX2-positive GSC-enriched cell
populations. Notably, the GSC markers Nestin and
CD133 did not demonstrate the same consistent trend. This
finding could indicate that SOX2, Nestin, and CD133
are not measuring the same GSC population. This conclusion could be
a result of a heterogenous GSC population or one or more of these
markers not correctly identifying GSCs. An alternative conclusion
could be that protein expression or conformation but not mRNA
expression is indicative of the GSC population. Such a conclusion
has been previously suggested for the detection of CD133 in colon
cancer CSCs (36). Salinomycin was
also demonstrated to decrease neurosphere formation and decrease
the clonogenicity of GSC-like cells. Finally, it was demonstrated
that salinomycin induced apoptotic cell death in GBM cells.
Salinomycin has previously been linked to apoptotic
activity in leukemia, prostate, breast, and ovarian cancer
(16,17,37,38).
There is great potential for targeting CSCs, including manipulating
pro- and anti-apoptotic pathways in CSC populations (39). Salinomycin has additionally been
demonstrated to overcome anti-apoptotic cellular mechanisms in
human cancer cells (20). In the
case of ovarian cancer specifically, it is known that the
salinomycin-induced apoptotic pathway relies on the activation of
caspase-8 and death receptor 5 (40). Salinomycin has also been
demonstrated to enhance the apoptotic activity of TRAIL in
TRAIL-resistant GBM cells by modulating caspase-3 expression
(41). The apoptotic effects of
salinomycin have been shown to be retained even in biodegradable
drug-delivery systems, an indication that this pathway could serve
as a point of interest in more advanced pre-clinical trials
(42). Future study on salinomycin
as a GBM treatment option should regard the apoptotic pathway as a
factor that could help differentiate GSC death from normal neural
cell death. Future studies should assess the effect of salinomycin
on GBM in vivo to better understand its potential
therapeutic benefits before translation to clinical testing.
In the development of new drugs to treat
glioblastomas, it is important to consider the impact of these
novel treatments on the function of normal brain tissue. Cases of
neural toxicity induced by accidental salinomycin overdoses have
been documented in humans and animals previously (43–45).
Although our findings indicated that salinomycin decreased the
sphere formation potential for the non-cancerous stem cell line
NE-4C, they also revealed that salinomycin toxicity took effect at
a higher concentration for this neural stem cell line than the GBM
cell lines assessed. Additionally, the shifts in apoptotic activity
induced by salinomycin were not significant for NE-4C where large
shifts were observed in the glioblastoma lines. It has also been
observed that salinomycin triggers calcium-induced apoptosis in
Schwann cells and dorsal root ganglia, but this effect can be
inhibited by Na+/Ca2+ exchanger inhibitors
(46). A potential avenue that may
prove useful for subsequent salinomycin studies is the
implementation of synthetic structural analogs of the drug that
exhibit lower levels of toxicity towards healthy cells (47). Salinomycin has also been
demonstrated to have synergistic DNA damage effects when combined
with the common chemotherapy drug temozolomide, highlighting the
potential of salinomycin in combination therapies (48). As the drug is researched more
thoroughly within glioblastoma studies and other cancers, our
findings reaffirm that salinomycin toxicity towards healthy cells
must be a contributing factor towards the implementation of the
ionophore as a chemotherapeutic agent.
Acknowledgements
We gratefully acknowledge Dr Do-Hyun Nam (Samsung
Medical Center, Seoul, South Korea) and Dr G. Yancey Gillespie
(University of Alabama at Birmingham, Birmingham, AL) for kindly
providing SMC448 and D456 patient-derived GBM lines,
respectively.
Funding
The present study was supported by the National
Science Foundation under grant no. 1604677 (to YK), by The
University of Alabama Office for Research and Economic Development
(to YK), and by The University of Alabama Randall Research Scholars
Program (to JWM and WRR).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JWM and YK conceived and designed the study. JWM and
WRR conducted the experiments and acquired the data. JWM, WRR and
YK were involved in the experimental design, data analysis,
interpretation of data, and drafted and revised the manuscript. All
authors read the final manuscript and agree to be accountable for
all aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arvold ND and Reardon DA: Treatment
options and outcomes for glioblastoma in the elderly patient. Clin
Interv Aging. 9:357–367. 2014.PubMed/NCBI
|
|
2
|
Hanif F, Muzaffar K, Perveen K, Malhi SM
and Simjee ShU: Glioblastoma multiforme: A review of its
epidemiology and pathogenesis through clinical presentation and
treatment. Asian Pac J Cancer Prev. 18:3–9. 2017.PubMed/NCBI
|
|
3
|
Tamimi AF and Juweid M: Epidemiology and
outcome of glioblastoma. Chapter 8. In: Glioblastoma [Internet]. De
Vleeschouwer S (ed). Codon Publications. (Brisbane (AU)). 29251870.
2017.
|
|
4
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galli R, Binda E, Orfanelli U, Cipelletti
B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F and Vescovi
A: Isolation and characterization of tumorigenic, stem-like neural
precursors from human glioblastoma. Cancer Res. 64:7011–7021. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
9
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim Y, Joo KM, Jin J and Nam DH: Cancer
stem cells and their mechanism of chemo-radiation resistance. Int J
Stem Cells. 2:109–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lathia JD, Mack SC, Mulkearns-Hubert EE,
Valentim CL and Rich JN: Cancer stem cells in glioblastoma. Genes
Dev. 29:1203–1217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Song WS, Yang YP, Huang CS, Lu KH, Liu WH,
Wu WW, Lee YY, Lo WL, Lee SD, Chen YW, et al: Sox2, a stemness
gene, regulates tumor-initiating and drug-resistant properties in
CD133-positive glioblastoma stem cells. J Chin Med Assoc.
79:538–545. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neradil J and Veselska R: Nestin as a
marker of cancer stem cells. Cancer Sci. 106:803–811. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mitani M, Yamanishi T and Miyazaki Y:
Salinomycin: A new monovalent cation ionophore. Biochem Biophys Res
Commun. 66:1231–1236. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong TT, Zhou HM, Wang LL, Feng B, Lv B
and Zheng MH: Salinomycin selectively targets ‘CD133+’
cell subpopulations and decreases malignant traits in colorectal
cancer lines. Ann Surg Oncol. 18:1797–1804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y: Effects of salinomycin on cancer
stem cell in human lung adenocarcinoma A549 cells. Med Chem.
7:106–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim KY, Yu SN, Lee SY, Chun SS, Choi YL,
Park YM, Song CS, Chatterjee B and Ahn SC: Salinomycin-induced
apoptosis of human prostate cancer cells due to accumulated
reactive oxygen species and mitochondrial membrane depolarization.
Biochem Biophys Res Commun. 413:80–86. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fuchs D, Daniel V, Sadeghi M, Opelz G and
Naujokat C: Salinomycin overcomes ABC transporter-mediated
multidrug and apoptosis resistance in human leukemia stem cell-like
KG-1a cells. Biochem Biophys Res Commun. 394:1098–1104. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fuchs D, Heinold A, Opelz G, Daniel V and
Naujokat C: Salinomycin induces apoptosis and overcomes apoptosis
resistance in human cancer cells. Biochem Biophys Res Commun.
390:743–749. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu D, Choi MY, Yu J, Castro JE, Kipps TJ
and Carson DA: Salinomycin inhibits Wnt signaling and selectively
induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl
Acad Sci USA. 108:13253–13257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Magrath JW and Kim Y: Salinomycin's
potential to eliminate glioblastoma stem cells and treat
glioblastoma multiforme (Review). Int J Oncol. 51:753–759. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen T, Yi L, Li F, Hu R, Hu S, Yin Y, Lan
C, Li Z, Fu C, Cao L, et al: Salinomycin inhibits the tumor growth
of glioma stem cells by selectively suppressing glioma-initiating
cells. Mol Med Rep. 11:2407–2412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin LS, Jia PF, Zhang ZQ and Zhang SM:
ROS-p53-cyclophilin-D signaling mediates salinomycin-induced glioma
cell necrosis. J Exp Clin Cancer Res. 34:572015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xipell E, Gonzalez-Huarriz M, Martinez de
Irujo JJ, García-Garzón A, Lang FF, Jiang H, Fueyo J, Gomez-Manzano
C and Alonso MM: Salinomycin induced ROS results in abortive
autophagy and leads to regulated necrosis in glioblastoma.
Oncotarget. 7:30626–30641. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Allen M, Bjerke M, Edlund H, Nelander S
and Westermark B: Origin of the U87MG glioma cell line: Good news
and bad news. Sci Transl Med. 8:354re32016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Joo KM, Kim J, Jin J, Kim M, Seol HJ,
Muradov J, Yang H, Choi YL, Park WY, Kong DS, et al:
Patient-specific orthotopic glioblastoma xenograft models
recapitulate the histopathology and biology of human glioblastomas
in situ. Cell Rep. 3:260–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Friedman GK, Langford CP, Coleman JM,
Cassady KA, Parker JN, Markert JM and Yancey Gillespie G:
Engineered herpes simplex viruses efficiently infect and kill
CD133+ human glioma xenograft cells that express CD111.
J Neurooncol. 95:199–209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu Y and Smyth GK: ELDA: Extreme limiting
dilution analysis for comparing depleted and enriched populations
in stem cell and other assays. J Immunol Methods. 347:70–78. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bai X, Kinney WH, Su WL, Bai A, Ovrutsky
AR, Honda JR, Netea MG, Henao-Tamayo M, Ordway DJ, Dinarello CA and
Chan ED: Caspase-3-independent apoptotic pathways contribute to
interleukin-32γ-mediated control of mycobacterium tuberculosis
infection in THP-1 cells. BMC Microbiol. 15:392015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu G, Zou H, Luo T, Long M, Bian J, Liu
X, Gu J, Yuan Y, Song R, Wang Y, et al: Caspase-dependent and
caspase-independent pathways are involved in cadmium-induced
apoptosis in primary rat proximal tubular cell culture. PLoS One.
11:e01668232016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Klose J, Trefz S, Wagner T, Steffen L,
Preißendörfer Charrier A, Radhakrishnan P, Volz C, Schmidt T,
Ulrich A, Dieter SM, et al: Salinomycin: Anti-tumor activity in a
pre-clinical colorectal cancer model. PLoS One. 14:e02119162019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tigli Aydin RS, Kaynak G and
Gumusderelioglu M: Salinomycin encapsulated nanoparticles as a
targeting vehicle for glioblastoma cells. J Biomed Mater Res A.
104:455–464. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kemper K, Sprick MR, de Bree M, Scopelliti
A, Vermeulen L, Hoek M, Zeilstra J, Pals ST, Mehmet H, Stassi G and
Medema JP: The AC133 epitope, but not the CD133 protein, is lost
upon cancer stem cell differentiation. Cancer Res. 70:719–729.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Al Dhaheri Y, Attoub S, Arafat K, Abuqamar
S, Eid A, Al Faresi N and Iratni R: Salinomycin induces apoptosis
and senescence in breast cancer: Upregulation of p21,
downregulation of survivin and histone H3 and H4 hyperacetylation.
Biochim Biophys Acta. 1830:3121–3135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kaplan F and Teksen F: Apoptotic effects
of salinomycin on human ovarian cancer cell line (OVCAR-3). Tumour
Biol. 37:3897–3903. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He YC, Zhou FL, Shen Y, Liao DF and Cao D:
Apoptotic death of cancer stem cells for cancer therapy. Int J Mol
Sci. 15:8335–8351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Parajuli B, Shin SJ, Kwon SH, Cha SD,
Chung R, Park WJ, Lee HG and Cho CH: Salinomycin induces apoptosis
via death receptor-5 up-regulation in cisplatin-resistant ovarian
cancer cells. Anticancer Res. 33:1457–1462. 2013.PubMed/NCBI
|
|
41
|
Calzolari A, Saulle E, De Angelis ML,
Pasquini L, Boe A, Pelacchi F, Ricci-Vitiani L, Baiocchi M and
Testa U: Salinomycin potentiates the cytotoxic effects of TRAIL on
glioblastoma cell lines. PLoS One. 9:e944382014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Norouzi M, Abdali Z, Liu S and Miller DW:
Salinomycin-loaded nanofibers for glioblastoma therapy. Sci Rep.
8:93772018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rollinson J, Taylor FG and Chesney J:
Salinomycin poisoning in horses. Vet Rec. 121:126–128. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Story P and Doube A: A case of human
poisoning by salinomycin, an agricultural antibiotic. N Z Med J.
117:U7992004.PubMed/NCBI
|
|
45
|
van der Linde-Sipman JS, van den Ingh TS,
van nes JJ, Verhagen H, Kersten JG, Beynen AC and Plekkringa R:
Salinomycin-induced polyneuropathy in cats: Morphologic and
epidemiologic data. Vet Pathol. 36:152–156. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Boehmerle W and Endres M: Salinomycin
induces calpain and cytochrome C-mediated neuronal cell death. Cell
Death Dis. 2:e1682011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Borgstrom B, Huang X, Hegardt C, Oredsson
S and Strand D: Structure-activity relationships in salinomycin:
Cytotoxicity and phenotype selectivity of semi-synthetic
derivatives. Chemistry. 23:2077–2083. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xipell E, Aragón T, Martinez-Velez N, Vera
B, Idoate MA, Martínez-Irujo JJ, Garzón AG, Gonzalez-Huarriz M,
Acanda AM, Jones C, et al: Endoplasmic reticulum stress-inducing
drugs sensitize glioma cells to temozolomide through downregulation
of MGMT, MPG, and Rad51. Neuro Oncol. 18:1109–1119. 2016.
View Article : Google Scholar : PubMed/NCBI
|