Introduction
Pancreatic ductal adenocarcinoma (PDAC) is
exceptionally malignant, progressive and typically associated with
poor prognosis, with a 5-year survival rate of only 5–15% (1). In 2018, 55,000 patients with
pancreatic cancer were newly diagnosed in the United States, and
44,330 patients died. It is reasonable to estimate that, by 2030,
pancreatic cancer will be the second leading cause of
cancer-associated mortality (2–4).
Gemcitabine (GEM) is well established as a standard
drug for patients with PDAC, improving the survival rate of
patients and bringing marked therapeutic benefits since its
discovery several decades ago (5).
However, only 20–30% of patients with PDAC benefit from GEM
treatment, and its therapeutic effect is limited despite
co-administration with other chemotherapeutic agents. The
unfavorable outcome is largely attributed to the development of
tumor drug resistance (TDR) (6–8).
TDR is one of the bottlenecks in tumor therapy, and
various mechanisms involved in TDR have been described (9,10). One
of the well-established mechanisms involves the adenosine
triphosphate-binding cassette (ABC) transporters, which are
membrane transporter proteins that decrease the endocellular
accumulation of a drug using the energy produced from the
decomposition of adenosine triphosphate (11–14).
At present, 15 ABC transporters have been demonstrated to serve a
role as drug pumps in TDR. Specifically, P glycoprotein (P-gp), ABC
subfamily G member 2 (ABCG2) and multidrug resistance-associated
protein 1 (MRP1) are positively associated with TDR in PDAC
(15). Although numerous studies
have reported on inhibitors of ABC transporters to overcome TDR,
their side effects and suboptimal safety profile limit the
application of these agents (16–18).
Furthermore, previous studies have demonstrated that the activation
of the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)
signaling pathway is associated with a decreased drug effect in
human breast adenocarcinoma and hepatocellular carcinoma (19–21).
Therefore, inhibitors of PI3K or AKT in combination with
chemotherapeutic drugs have a synergistic effect in cancer therapy
(22,23). Nuclear factor (NF)-κB is vital for
the development of drug resistance by regulating the expression
levels of numerous genes (24). A
previous study revealed that 3,3′-diindolylmethane could inhibit
the activation of NF-κB, resulting in the chemosensitization of
tumors (25,26). However, inhibitors of PI3K/AKT/NF-κB
invariably lead to unacceptable side effects.
Low-intensity low-frequency ultrasound (LILFU) is a
physical stimulus that can open cell membranes, and increase the
transmission of molecules and genes via the sonoporation effect.
Recently, LILFU has been recognized as a safe and effective method
in tumor therapy, offering high penetrating ability and the
advantage of contact with deep organs compared with other
non-invasive techniques, such as light beam treatment. As physical
energy, LILFU can be applied to relatively limited areas.
Therefore, it has the advantage of accuracy, low systemic toxicity
and few side effects. Nevertheless, few studies have focused on the
efficacy and feasibility of this method to increase
chemosensitivity in vivo, and the underlying mechanism of
the enhancing effect of LILFU.
The present study aimed to demonstrate whether LILFU
could enhance the cytotoxicity and therapeutic effect of GEM in
GEM-resistant ASPC-1 cells both in vitro and in vivo,
and to illustrate the role of ABC transporters and the
PI3K/AKT/NF-κB signaling pathway in the LILFU-induced reversal of
TDR.
Materials and methods
Cell culture
ASPC-1/GEM cells (American Type Culture Collection)
were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium
(HyClone; Cytiva) supplemented with 10% fetal bovine serum
(Biological Industries), penicillin G (100 U/ml) and streptomycin
(100 g/ml; Sigma-Aldrich; Merck KGaA) in an incubator at 37°C with
5% carbon dioxide. The medium was supplemented with GEM (1 µg/ml;
Hanson Pharma) to maintain the drug-resistant phenotype. The cells
were cultured in drug-free medium for 1 week prior to the
experiments.
Exposure to LILFU
An ultrasound probe with an HPCTB-360 transducer
(China Shipbuilding Industry Corporation 715 Research Institute)
was used for ultrasound stimulation. The transducer used degassed
sterile water in the device, and was settled on steel supports to
maintain a distance of 20 mm between the transducer and the cells
in the 6-well plates. The parameters of the ultrasound were as
follows: 360 kHz, 50% duty cycle (on 3 sec, off 3 sec), 1 min.
Determination of the optimal LILFU
parameters and the half-maximal inhibitory concentration of
GEM
ASPC-1/GEM cells (1×106 cells/well) were
seeded in 6-well plates and exposed to LILFU with different
acoustic intensities (0, 0.06, 0.20, 0.40, 0.67 and 1.10
W/cm2) to determine the optimal acoustic intensity of
LILFU. Following incubation for 24 h, cell viability was detected
using a Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular
Technologies, Inc.). Furthermore, 1×106 cells/well were
seeded in 6-well plates and incubated with different concentrations
of GEM (0, 0.625, 1.25, 2.5, 5.0 and 10 mg/ml). After 24 h of
treatment, cell viability was detected using the CCK-8 assay.
Cell cytotoxicity assay
ASPC-1/GEM cells were treated with LILFU, GEM or
GEM+LILFU for 24 h and seeded into 96-well plates (1,000
cells/well). Furthermore, ASPC-1/GEM cells were treated with GEM,
GEM+A66 (Beyotime Institute of Biotechnology), GEM+TGX221 (Beyotime
Institute of Biotechnology) or GEM+BAY11-7082 (Beyotime Institute
of Biotechnology) for 24 h and seeded into 96-well plates (1,000
cells/well). A66, TGX221 and BAY11-7082 are specific inhibitors of
PI3K p110α, PI3K p110β and NF-κB, respectively, and the
concentrations used in the experiment were 32 nM, 5 nM and 10 µM,
respectively. A total of 10 µl CCK-8 solution was added to each
well. Subsequently, the cells were incubated for 2 h. A multimode
plate reader (BioTek ELx808; BioTek Instruments, Inc.) was used to
measure the absorbance at 450 nm.
Colony formation assay
ASPC-1/GEM cells were seeded into 6-well plates and
subjected to various treatments for 24 h. Subsequently, the cells
were trypsinized into single cells, seeded into 6-well plates
(1,000 cells/well) and incubated at 37°C with 5% carbon dioxide.
After 14 days, the colonies were washed with PBS (Beyotime
Institute of Biotechnology), fixed with 4% paraformaldehyde at room
temperature for 30 min and stained with 0.1% crystal violet
solution at room temperature for 10 min. The cells were washed
thrice with double-distilled water. Images were captured with a
digital camera after drying, and colonies containing >50 cells
were counted.
Apoptosis assay
The cells were incubated in 6-well plates and
subjected to various treatments. The cells were collected after 24
h, washed twice with cold PBS and resuspended in 500 µl cold
binding buffer. Subsequently, 5 µl Annexin V-fluorescein
isothiocyanate (BD Biosciences) was added. The mixture was
protected from light and incubated at room temperature for 15 min.
Finally, 5 µl propidium iodide was added 5 min prior to examination
using a flow cytometer (BD Canto II; BD Biosciences).
Western blotting
Total, nuclear and membrane proteins were extracted
separately using RIPA Lysis Buffer, the Nuclear and Cytoplasmic
Protein Extraction Kit and the Membrane and Cytosol Protein
Extraction Kit (Beyotime Institute of Biotechnology). The present
study detected the expression levels of NF-κB in the nuclear
protein samples, PI3K-p110α, PI3K-p110β and AKT in total protein
samples, and P-gp, ABCG2 and MRP1 in membrane protein samples
separately. Subsequently, the protein concentration was determined
using a Bicinchoninic Acid Protein Assay kit (Beyotime Institute of
Biotechnology). Protein was separated by 10 or 12% SDS-PAGE in four
lanes and transferred to polyvinylidene difluoride membranes (EMD
Millipore). The membranes were blocked with 5% skimmed milk at room
temperature for 1 h, and incubated with primary antibodies
overnight at 4°C. Furthermore, the membranes were incubated with an
appropriate horseradish peroxidase-conjugated secondary antibody
(cat. no. 7074; dilution 1:1,000; Cell Signaling Technology, Inc.)
at room temperature for 1 h. Finally, the target proteins were
detected using enhanced chemiluminescence reagent (EMD Millipore).
Enhanced chemiluminescence signals were examined and analyzed using
Image Lab version 4.1 software (Bio-Rad Laboratories, Inc.).
Antibodies against P-gp (cat. no. 13342; dilution 1:1,000), ABCG2
(cat. no. 42078; dilution 1:1,000), MRP1 (cat. no. 72202; dilution
1:1,000), PI3K-p110α (cat. no. 4249; dilution 1:1,000), PI3K-p110β
(cat. no. 3011; dilution 1:1,000), AKT (cat. no. 4685; dilution
1:1,000), NF-κB (cat. no. 8242; dilution 1:1,000) and β-actin (cat.
no. 4970; dilution 1:1,000) were purchased from Cell Signaling
Technology, Inc.
Xenograft tumor model
A total of 1×107 ASPC-1/GEM cells were
subcutaneously inoculated into the right oxter of 6-week-old female
BALB/c nude mice, weighing ~18–22 g (Shanghai SLAC Co.). A total of
20 mice were maintained. The environment of mice was at the SPF
level. A cycle of 12 h of light and 12 h of dark were maintained
each day. The temperature was ~25°C and the humidity was around
50%. Mice were provided sufficient water and food. The bedding was
changed twice a week. All feeding and operating procedures were
approved by the Animal Ethics Committee of the Second Affiliated
Hospital of Zhejiang University School of Medicine. Tumor volumes
were calculated using the following formula: Volume
(mm3)=A (mm) × B (mm)2/2, where A and B are
the longest and shortest diameters, respectively. When the tumors
grew to 50–100 mm3, the mice were randomly divided into
four groups: Control, LILFU, GEM and GEM+LILFU. The mice were
anesthetized with pentobarbital (80 mg/kg) via intraperitoneal
injection, and the experimental animals were fixed on the
workbench. GEM was injected into the tail vein at a dose of 50
mg/kg (27–29). The ultrasonic transducer was fixed
on the holder. The tumor surface was coated with medical ultrasonic
couplant, and was placed directly under the transducer (Fig. S1). The parameters of the ultrasound
were as follows: 360 kHz, 50% duty cycle (on 3 sec, off 3 sec), 5
min. Following treatment, whole blood samples were collected into
prechilled heparinized tubes at t=5, 15, 30, 60, 120, 360 and 720
min and plasma was separated from whole blood. The mice were
treated once every 3 days for a total of 15 days, and the
experiment ended on day 21. We considered the average diameter of
tumors to exceed 20 mm, tumor growth or metastasis rapidly to cause
infection or necrosis, rapid weight loss of more than 15–20% as
humane endpoints. Once the state of the mice reached the humane
endpoints, the mice were immediately anesthetized with
pentobarbital (80 mg/kg) and sacrificed by cervical dislocation.
Each mouse only had a single subcutaneous tumor nodule. The GEM
concentration in the plasma was measured by high-performance liquid
chromatography (HPLC, AB Sciex Company). Fifty microliters of mouse
plasma was mixed with 50 µl of methanol, and then GEM was extracted
from the plasma by the addition of 3.5 ml of isopropanol/ethyl
acetate (1:2.5, v/v). The samples were vortexed for 10 min and then
centrifuged at 4,800 × g for 10 min. The supernatants were
collected and evaporated to dryness under a gentle stream of
nitrogen at 60°C. The residue was dissolved in a 50 µl methanol and
then centrifuged at 8,000 × g for 10 min before use. The flow rate
of the mobile phase was 0.2 ml/min. Twenty microliters of sample
was used to measure drug absorption at 268 nm. Standard curves for
GEM were generated using commercially available compounds.
Immunohistochemical staining
Tumor specimens were fixed with 4% paraformaldehyde
and embedded in paraffin. The slices were dewaxed, rehydrated,
incubated with antigen retrieval solution (pH 6.0), processed with
30 ml/l hydrogen peroxide to block endogenous peroxidase activity
and blocked with 4% normal goat serum (Beyotime Institute of
Biotechnology). The slices were incubated with a mouse anti-human
antibody against Ki-67 (cat. no. 9449, dilution 1:400, Cell
Signaling Technology, Inc.) overnight at 4°C, followed by
incubation with a secondary anti-mouse biotin-conjugated antibody
(cat. no. ab6788, dilution 1:1,000, Abcam) for 30 min at 37°C.
Subsequently, the slices were washed with PBS, developed using
diaminobenzidine and counterstained with hematoxylin.
Statistical analysis
The normal distribution of experimental data was
verified using the Shapiro-Wilk test. All data are presented as the
mean ± standard deviation. Comparisons among multiple groups were
performed using one-way ANOVA, and a post hoc test was performed
using the Tukey's correction. P<0.05 was considered to indicate
a statistically significant difference. All analyses were performed
using SPSS Statistics version 20.0 software (IBM Corp.).
Results
Exposure to LILFU enhances GEM-induced
cytotoxicity in ASPC-1/GEM cells
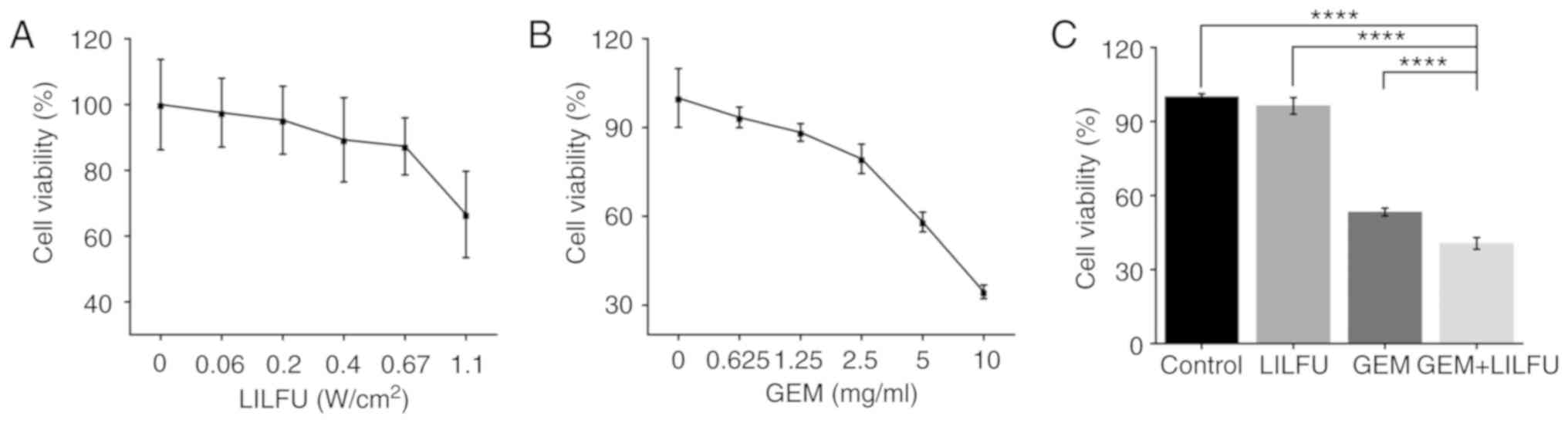
LILFU-irradiated cells were incubated for 24 h to
identify the appropriate acoustic parameters which did not inhibit
cell viability. Cell viability was >95% for an acoustic
intensity of ≤0.2 W/cm2 (Fig. 1A). Furthermore, the half-maximal
inhibitory concentration of GEM was 6.63 mg/ml (Fig. 1B). The acoustic parameter of 0.2
W/cm2 and the concentration of 6.63 mg/ml were used in
the subsequent experiments. Furthermore, the cells were treated
with GEM, LILFU or GEM+LILFU. The cell viability was 40.59% in the
GEM+LILFU group compared with 53.29% in the GEM group, and the
difference was statistically significant (Fig. 1C).
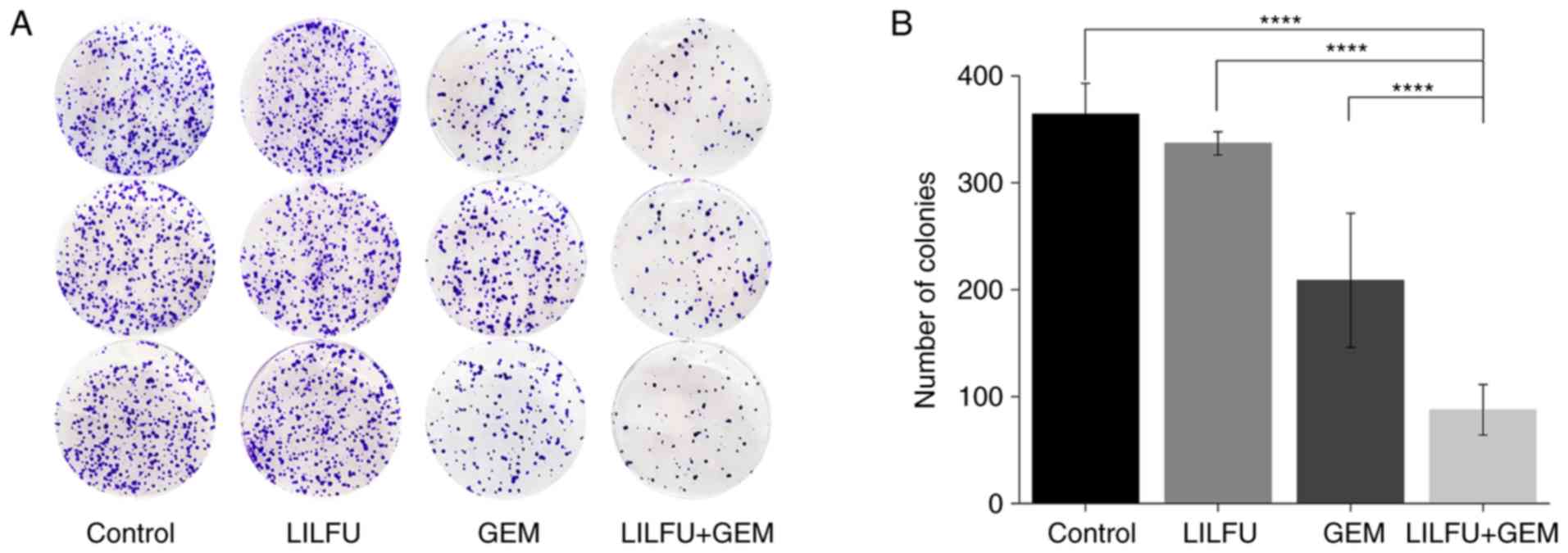
LILFU combined with GEM enhances the
inhibition of ASPC-1/GEM cell proliferation
There was no apparent difference observed between
the LILFU and control groups. The proliferative ability of the GEM
group was lower than that observed in the control group, as
demonstrated by the colony formation assay. The number of colonies
was significantly reduced in the GEM+LILFU group compared with in
the GEM group (88 vs. 209 colonies, respectively; Fig. 2A and B).
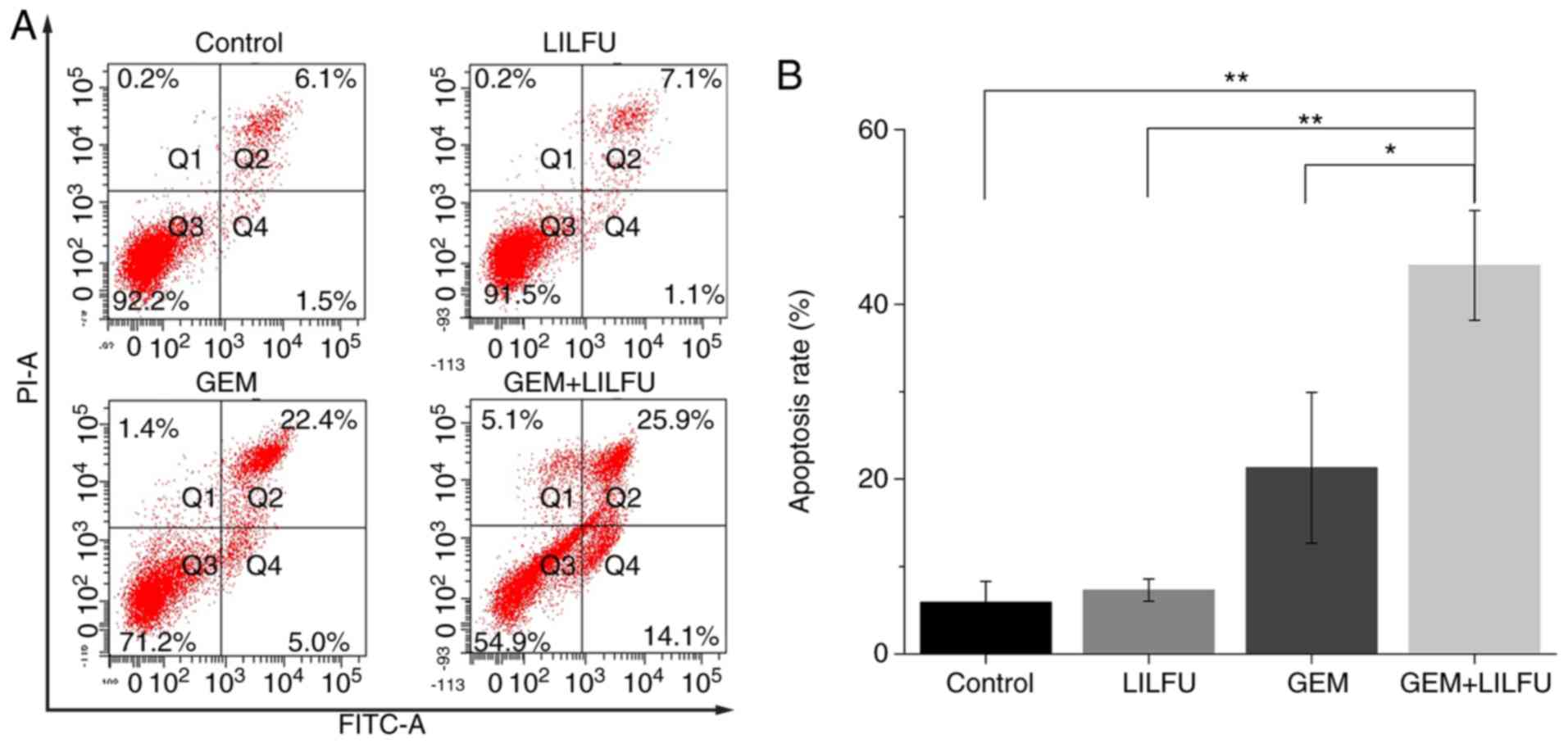
LILFU enhances GEM-induced apoptosis
in ASPC-1/GEM cells
Flow cytometry was used to determine the apoptotic
rates of the ASPC-1/GEM cells. The apoptotic rate of cells treated
with GEM was 27.4%, which was higher than that recorded in the
control group. Furthermore, the apoptotic rate of cells treated
with GEM+LILFU was 40%, which was significantly higher than that of
cells treated with GEM (Fig. 3A and
B).
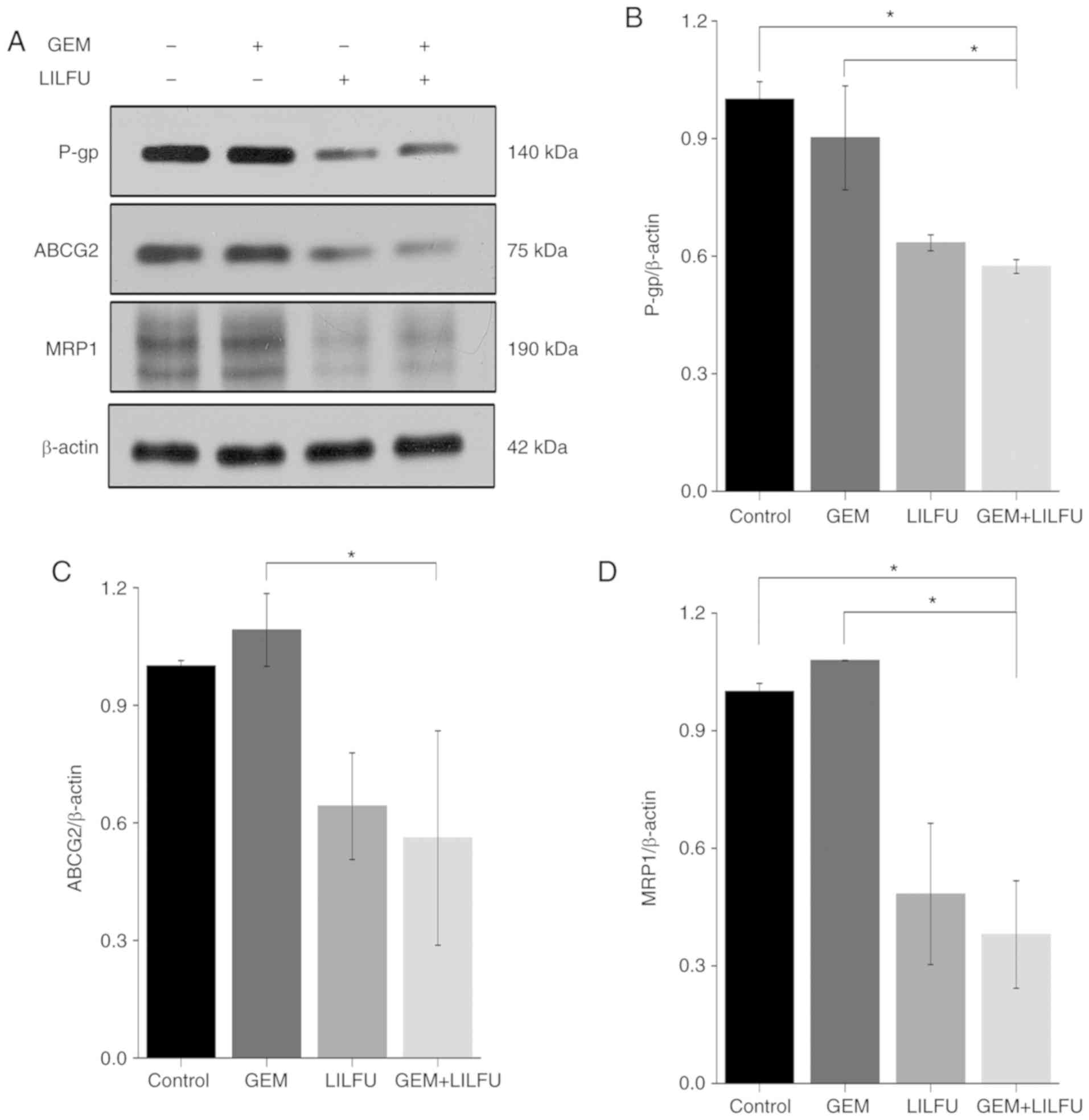
Exposure to LILFU induces the
reduction of ABC transporter expression
The present study revealed that the expression
levels of P-gp, ABCG2 and MRP-1 were reduced in LILFU group
compared to the Control group, whereas there was almost no change
observed in the GEM group. Furthermore, the expression levels of
P-gp, ABCG2 and MRP-1 were decreased more significantly in the
GEM+LILFU group (Fig. 4A-D). This
finding indicated that exposure to GEM+LILFU cause a reduction in
the expression levels of these proteins.
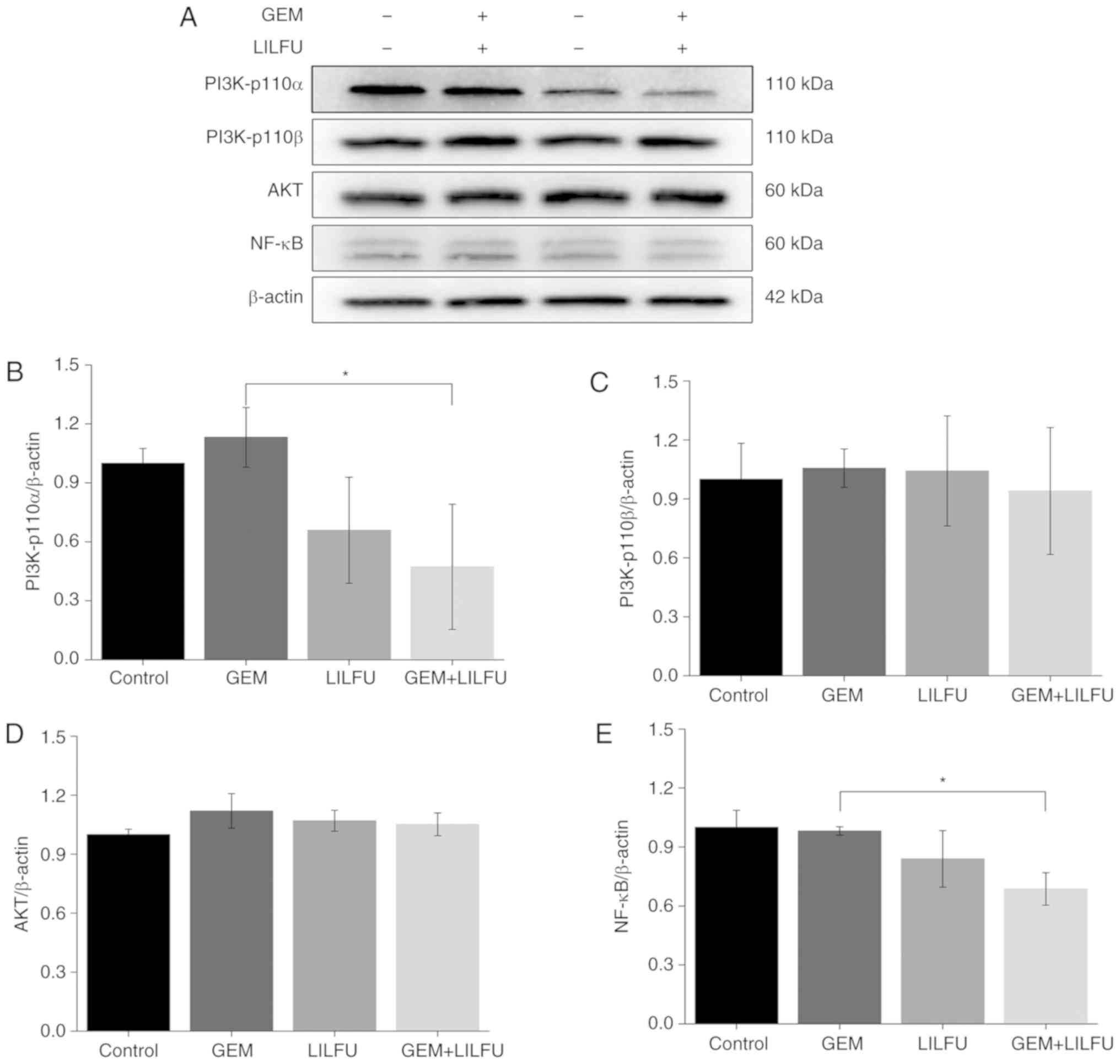
Exposure to LILFU induces the
reduction of PI3K/AKT/NF-κB pathway-associated protein
expression
The expression levels of PI3K-p110α, PI3K-p110β, AKT
and NF-κB were determined via western blotting to examine the
effect of LILFU on the PI3K/AKT/NF-κB signaling pathway. The
expression levels of PI3K-p110β and AKT were not markedly altered
in the group, LILFU group or GEM+LILFU group compared to the
control group (Fig. 5A, C and D).
However, the expression levels of PI3K-p110α and NF-κB were
decreased following treatment with GEM+LILFU compared with these
levels in the GEM group (Fig. 5A, B and
E).
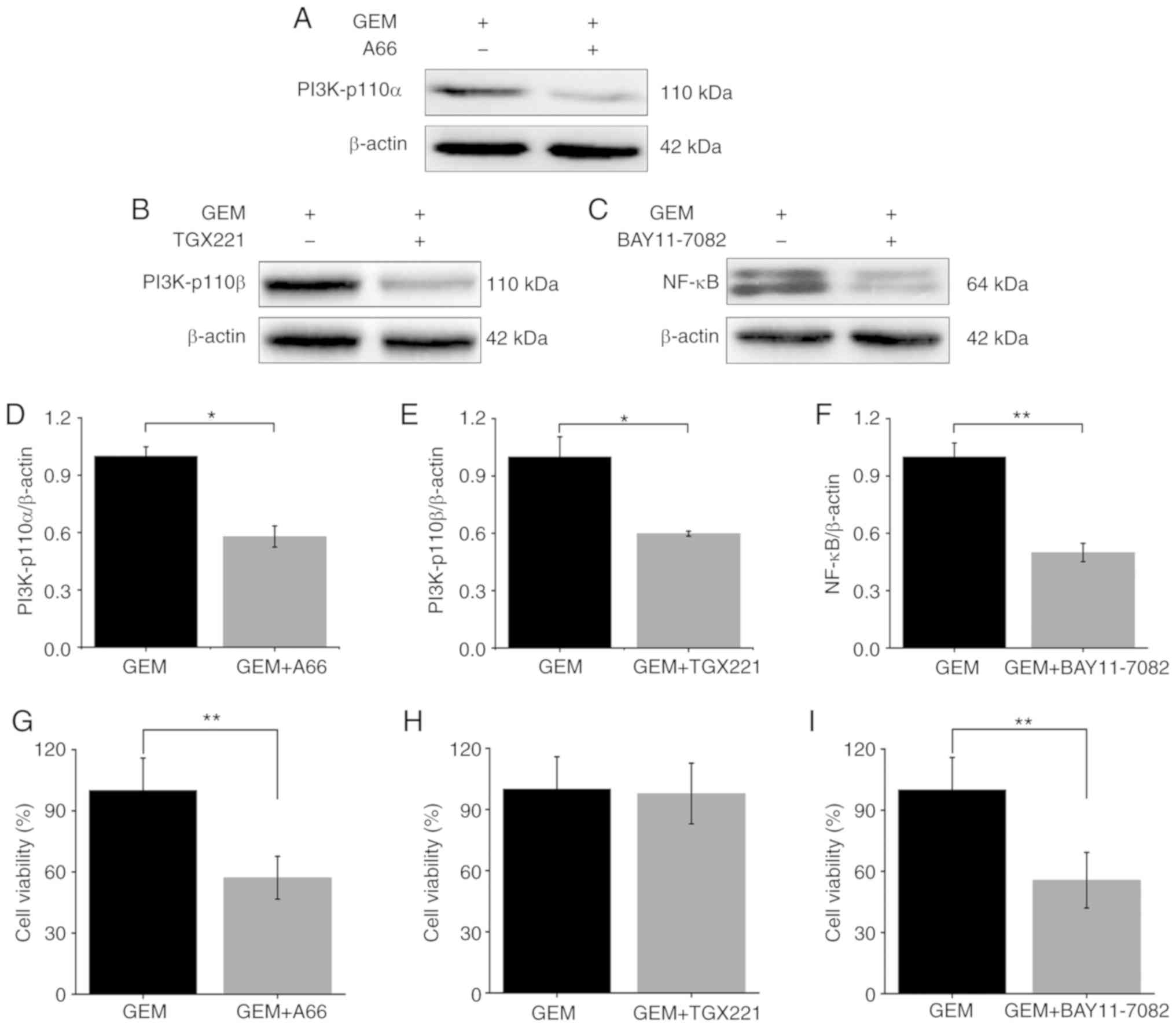
In addition, ASPC-1/GEM cells were treated with GEM
combined with A66 (PI3K-p110α inhibitor), TGX221 (PI3K-p110β
inhibitor) and BAY11-7082 (NF-κB inhibitor). Firstly, the
expression levels of PI3K-p110α, PI3K-p110β and NF-κB were
determined. Subsequently, cell viability was detected using a CCK-8
assay. Following the combined treatments, the expression levels of
PI3K-p110α, PI3K-p110β and NF-κB were reduced when compared to the
GEM treated alone group (Fig.
6A-F). Cell viability was decreased in the GEM+A66 and
GEM+BAY11-7082 groups when compared with the GEM alone treated
group (Fig. 6G and I). However, the
cell viability was not significantly decreased in the GEM+TGX221
group (Fig. 6H).
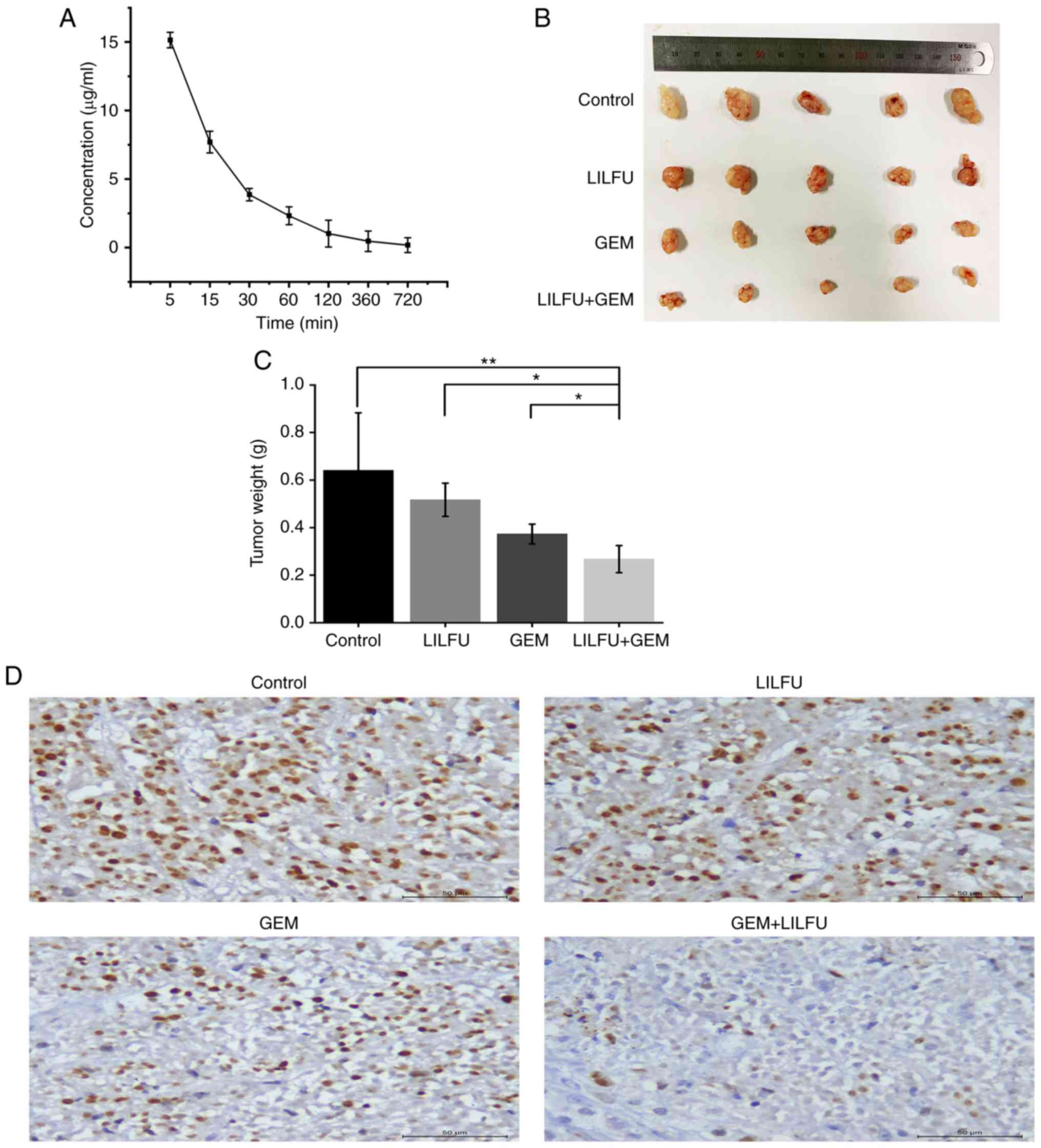
Exposure to LILFU enhances the effect
of GEM in vivo
ASPC-1/GEM cell xenograft models were established to
further identify the effect of LILFU in vivo. The blood
concentration of GEM was 14.08 µg/ml at 5 min and then gradually
decreased over time. (Fig. 7A).
Tumors observed in the LULFU+GEM group were markedly smaller than
those observed in the GEM group (Fig.
7B and C). Furthermore, immunohistochemical staining revealed
that the expression level of Ki-67 was decreased in the GEM+LILFU
group compared with that in the GEM group (Fig. 7D).
Discussion
Previous studies have identified various mechanisms
of drug resistance in pancreatic ductal adenocarcinoma (PDAC).
Firstly, ABC transporters are critical mediators of drug efflux,
which leads to decreased intracellular accumulation of drugs and
development of tumor drug resistance (TDR) (30,31).
Secondly, critical genetic mutations contribute to TDR. For
example, tumors with the BRCA mutation exhibit increased resistance
to carboplatin compared with tumors not harboring this mutation
(32). Furthermore, numerous
signaling pathways, including the PI3K/AKT/NF-κB and Notch
signaling pathways, are involved in drug resistance in PDAC.
Banerjee et al, demonstrated that activation of NF-κB is
mostly associated with resistance to chemotherapy in PDAC (25).
Due to their short wavelength and high frequency,
ultrasonic waves have strong directivity when propagating in the
medium and strong penetrating power. At present, low-frequency
ultrasound is used in various clinical fields, such as in
vivo thrombolysis, analgesia, desensitization and dental
surgery. Additionally, Yu et al reported that low-frequency
ultrasound has a synergistic antibacterial effect on bacteria and
chlamydia in combination with drugs or antibiotics (33). Therefore, ultrasound can serve a
biological role under the premise of ensuring safety and
feasibility within a certain frequency and intensity range.
Ultrasound can selectively increase the permeability
of the tumor cell membrane to accumulate higher intracellular
concentrations of drugs in the treatment of chronic myelogenous
leukemia and ovarian carcinoma (34,35).
Hassan et al (36) observed
higher sensitivity in drug-resistant uterine sarcoma cells
following exposure to ultrasound compared with in cells exhibiting
a normal response to treatment. Therefore, ultrasound may improve
the anticancer effect of doxorubicin in resistant cells (36). In addition, ultrasound-induced local
hyperthermia was found to increase the cellular uptake of drugs and
induce death of drug-resistant cells (37). Furthermore, Ning et al
(38) reported that high-intensity
focused ultrasound enhances the effect of bufalin by inducing
apoptosis in PDAC. Liu et al chose ultrasound parameters
with a frequency of 300 kHz, an average intensity of 1
W/cm2, a time of 6 min, and a duty cycle of 50% to treat
ovarian cancer xenografts in vivo (39). Huang et al chose ultrasound
parameters with a frequency of 1 MHz, an average intensity of 0.74
W/cm2, a time of 5 min, and a duty cycle of 20% both
in vitro and in vivo (40). Wu et al chose continuous
ultrasound parameters with a frequency of 1 MHz, an average
intensity of 1.2 W/cm2, and a time of 10 sec (41). Hassan et al chose ultrasound
parameters with a frequency of 1 MHz, an average intensity of 0.4
W/cm2, a time of 1 min, and a duty cycle of 10%
(36). Sun et al chose
continuous ultrasound parameters with a frequency of 300 KHz, an
average intensity of 1 W/cm2, and a time of 40 sec
(42). He et al chose
ultrasound parameters with a frequency of 300 KHz, an average
intensity of 2 W/cm2, a time of 10 min both in
vitro and in vivo (43).
Liu et al chose ultrasound parameters with a frequency of 1
MHz, an average intensity of 0.4 W/cm2, a time of 20 min
(37). Based on the above
references and our previous experiments, we chose ultrasound
parameters with a frequency of 360 KHz, an average intensity of 0.2
W/cm2, a time of 5 min, and a duty cycle of 50% in
vivo.
Based on the characteristics of ultrasound, the
present study aimed to use LILFU to reverse TDR in PDAC. The
present study demonstrated that LILFU enhanced the effect of GEM,
and suggested a possible underlying mechanism by which LILFU
reversed TDR. Overexpression of the ABC transporters, which pump
chemotherapeutic drugs out of the cytoplasm, invariably leads to
the development of TDR. The expression levels of P-gp are low prior
to treatment. However, the expression levels are upregulated after
chemotherapy which contributes to TDR (44). In the present study, inhibition of
ABC transporter expression by LILFU, accompanied by the
administration of intracellular chemotherapeutic drugs, markedly
increased the antitumor efficacy of the agents. Following exposure
to LILFU, the expression levels of P-gp, ABCG2 and MRP1 were
decreased, suggesting that LILFU reversed TDR via ABC transporters.
Furthermore, LILFU has some advantages over traditional inhibitors
of ABC transporters. Firstly, LILFU is more accurate than chemical
inhibitors and can target tumor lesions, potentially preventing the
development of systemic side effects caused by treatment. In
addition, similar to light beam treatment, LILFU is a type of
easily assessable physical energy, which can be applied in
vivo through a non-invasive approach without decreasing the
penetration ability.
Previous studies have demonstrated that the PI3K/AKT
signaling pathway is a mediator of chemoresistance in PDAC
(45,46). Zhang et al (47), demonstrated that overexpression of
galectin-1 activates the PI3K/AKT signaling pathway, and PI3K/AKT
cascade activation induces hepatocellular carcinoma resistance to
sorafenib and promotes the progression of liver cancer.
Furthermore, Liang et al (48) reported that STAT3 phosphorylation
activates the PI3K/AKT signaling pathway, which leads to increased
cisplatin resistance in ovarian cancer. To et al (49), demonstrated that CUDC-907 is a
PI3K inhibitor and exhibits a synergistic cytotoxic effect
on cisplatin-resistant cancer cells in combination therapy with
cisplatin. Furthermore, CUDC-907 was found to reverse cancer cell
resistance by inhibiting ABCC2 which is one of the ABC
transporters.
Examination of the ubiquitously expressed PI3K-p110
has revealed the distinct and various roles of each subunit in the
cell. However, in previous studies, it has been unclear whether
PI3K-p110α or PI3K-p110β is involved in the reversion of TDR.
Furthermore, previous studies have rarely investigated the effect
of ultrasound treatment on the PI3K/AKT/NF-κB signaling pathway. In
the present study, the expression levels of PI3K-p110α were
decreased following GEM+LILFU treatment, whereas the expression
levels of PI3K-p110β were not significantly altered. Therefore, it
was concluded that PI3K-p110α, rather than PI3K-p110β, may be
involved in TDR. In future studies, PI3K-p110α may be a target of
the PI3K signaling pathway and can be inhibited to reverse TDR. Ma
et al (50), treated
pancreatic cancer with triptolide and GEM, revealing that
triptolide enhances the sensitivity of pancreatic cancer cells to
GEM by inhibiting NF-κB signaling. Furthermore, the involvement of
NF-κB in the PI3K/AKT signaling pathway is commonly ignored.
Therefore, its role in TDR may have been overlooked. Additionally,
NF-κB expression is markedly reduced following GEM+LILFU treatment,
suggesting that it may also serve an essential role in
LILFU-induced reversion of TDR. As is known to us, NF-κB is bound
and inhibited by Inhibitor κB (IκB) proteins in cytoplasm and is
kept in an inactive state (51).
IKKα activated by the PI3K/AKT signaling pathway phosphorylates IκB
proteins and then IκB proteins are exposed to proteasomal
degradation, resulting in nuclear translocation and transcriptional
activation of NF-κB (52). We
propose the hypothesis that LILFU inhibited the PI3K-p110α/AKT
signaling pathway, causing IKKα to be inactive, thus preventing IκB
from being phosphorylated. Thus, IκB still bound to NF-κB and
inhibited NF-κB activation and nuclear translocation, which caused
the reduction of NF-κB in the nucleus. In addition, the present
study inhibited PI3K-p110β using TGX221, and the cell viability in
the TGX221 group was not significantly altered compared with GEM
group. However, when PI3K-p110α and NF-κB were inhibited, the cell
viability in the A66 and BAY11-7082 groups were markedly decreased.
Therefore, LILFU may enhance chemosensitivity via the
PI3K-p110α/AKT/NF-κB signaling pathway.
Hien et al (53) revealed that puerarin reduced the
expression levels of P-gp via the NF-κB signaling pathway in breast
cancer, which was consistent with the downregulation of NF-κB
observed in the present study. Previous studies have revealed that
the PI3K inhibitor CUDC-907 reverses the resistance of cancer cells
by inhibiting ABCC2 which is one of the ABC transporters (49). Therefore, it was concluded that
LILFU may downregulate the expression levels of ABC transporters
(P-gp, ABCG2 and MRP1) by inhibiting the PI3K-p110α/AKT/NF-κB
signaling pathway, thereby reversing the resistance of pancreatic
cancer.
LILFU has some obvious advantages over inhibitors of
ABC transporters and the PI3K/AKT/NF-κB signaling pathway. Firstly,
compared with chemical inhibitors, LILFU is more accurate and can
accumulate in tumor tissues without causing systemic side effects.
In addition, LILFU is more penetrating and can affect the deep
parts of organs or tissues. Therefore, LILFU can produce biological
effects in the body.
The present study used LILFU to reverse TDR without
apparent side effects. Combination treatment of chemotherapeutic
agents and LILFU may improve the chemosensitivity of cancer.
Therefore, LILFU may be a novel, non-invasive and promising
strategy for the reversion of TDR. Importantly, the combination
treatment considerably decreased the tumor volume in a xenograft
mouse model. This finding demonstrated that the LILFU-induced
increase in chemosensitivity in vivo is feasible, effective
and safe. In future studies, the LILFU intensity treatment window
or threshold should be explored to determine the treatment
parameters more precisely. Furthermore, the association between ABC
transporters and the PI3K/AKT/NF-κB signaling pathway in TDR should
be elucidated, with the aim to further reverse TDR.
In conclusion, LILFU improved the chemosensitivity
of ASPC-1/GEM cells, inhibited cell viability and proliferation,
and promoted cell apoptosis in the GEM+LILFU group. LILFU reversed
TDR by inhibiting the expression of ABC transporters, including
P-gp, ABCG2 and MRP-1. Furthermore, LILFU may reverse TDR by
inhibiting PI3K-p110α and NF-κB, instead of PI3K-p110β, in the
PI3K/AKT/NF-κB signaling pathway. Therefore, it was concluded that
LILFU may downregulate the expression levels of ABC transporters,
including P-gp, ABCG2 and MRP1, by inhibiting the
PI3K-p110α/AKT/NF-κB signaling pathway, thereby reversing
resistance in pancreatic cancer. Furthermore, treatment with
GEM+LILFU inhibited the growth of xenograft tumors in vivo
more effectively compared with GEM alone, and decreased the protein
expression levels of Ki-67. Moreover, several groups could be added
for mice inoculated with cells pretreated by GEM with or without
LILFU before surgery, which was a limitation of our study, to more
fully illustrate the effect of GEM+LILFU.
The results of the present study revealed that LILFU
is a promising treatment option for the reversal of TDR. Further
clinical studies are required to assess the feasibility and
efficacy of LILFU in reversing TDR.
Supplementary Material
Supporting Data
Acknowledgements
The GEM-resistant ASPC-1 (ASPC-1/GEM) cell line was
provided by Professor Min Li (University of Oklahoma, Norman, OK,
USA).
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81527803 and
81420108018), National Key R&D Program of China (grant no.
2018YFC0115900), Zhejiang Science and Technology Project (grant no.
2019C03077), Youth Natural Science Fund Project of Zhejiang
Province (grant no. LQ19H180004), and Natural Science Fund Project
of Ningbo (grant no. 2018A610380).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FQ performed the majority of the experiments and
wrote the article. JCh performed the majority of the analysis of
data and helped write the article. JCa and FD assisted with the
experiments. PH was in charge of project design and writing of the
article. All authors read and approved the manuscript and agree to
be accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The experimentation using nude mice was approved by
the Animal Ethics Committee of the Second Affiliated Hospital of
Zhejiang University School of Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saad AM, Turk T, Al-Husseini MJ and
Abdel-Rahman O: Trends in pancreatic adenocarcinoma incidence and
mortality in the United States in the last four decades; a
SEER-based study. BMC Cancer. 18:6882018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Sousa Cavalcante L and Monteiro G:
Gemcitabine: Metabolism and molecular mechanisms of action,
sensitivity and chemoresistance in pancreatic cancer. Eur J
Pharmacol. 741:8–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ina S, Hirono S, Noda T and Yamaue H:
Identifying molecular markers for chemosensitivity to gemcitabine
in pancreatic cancer: Increased expression of interferon-stimulated
gene 15 kd is associated with intrinsic chemoresistance. Pancreas.
39:473–485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo W, Yang G, Qiu J, Luan J, Zhang Y, You
L, Feng M, Zhao F, Liu Y, Cao Z, et al: Novel discoveries targeting
gemcitabine-based chemoresistance and new therapies in pancreatic
cancer: How far are we from the destination? Cancer Med.
8:6403–6413. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiorean EG, Cheung WY, Giordano G, Kim G
and Al-Batran SE: Real-world comparative effectiveness of
nab-paclitaxel plus gemcitabine versus FOLFIRINOX in
advanced pancreatic cancer: A systematic review. Ther Adv Med
Oncol. 11:17588359198503672019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vasan N, Baselga J and Hyman DM: A view on
drug resistance in cancer. Nature. 575:299–309. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aleksakhina SN, Kashyap A and Imyanitov
EN: Mechanisms of acquired tumor drug resistance. Biochim Biophys
Acta Rev Cancer. 1872:1883102019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mohammad IS, He W and Yin L: Understanding
of human ATP binding cassette superfamily and novel multidrug
resistance modulators to overcome MDR. Biomed Pharmacother.
100:335–348. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lowrence RC, Subramaniapillai SG,
Ulaganathan V and Nagarajan S: Tackling drug resistance with efflux
pump inhibitors: From bacteria to cancerous cells. Crit Rev
Microbiol. 45:334–353. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bugde P, Biswas R, Merien F, Lu J, Liu DX,
Chen M, Zhou S and Li Y: The therapeutic potential of targeting ABC
transporters to combat multi-drug resistance. Expert Opin Ther
Targets. 21:511–530. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fletcher JI, Williams RT, Henderson MJ,
Norris MD and Haber M: ABC transporters as mediators of drug
resistance and contributors to cancer cell biology. Drug Resist
Updat. 26:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Adamska A and Falasca M: ATP-binding
cassette transporters in progression and clinical outcome of
pancreatic cancer: What is the way forward? World J Gastroenterol.
24:3222–3238. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Falasca M and Linton KJ: Investigational
ABC transporter inhibitors. Expert Opin Investig Drugs. 21:657–666.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui H, Zhang AJ, Chen M and Liu JJ: ABC
Transporter inhibitors in reversing multidrug resistance to
chemotherapy. Curr Drug Targets. 16:1356–1371. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bates S, Kang M, Meadows B, Bakke S,
Choyke P, Merino M, Goldspiel B, Chico I, Smith T, Chen C, et al: A
Phase I study of infusional vinblastine in combination with the
P-glycoprotein antagonist PSC 833 (valspodar). Cancer.
92:1577–1590. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Knuefermann C, Lu Y, Liu B, Jin W, Liang
K, Wu L, Schmidt M, Mills GB, Mendelsohn J and Fan Z:
HER2/PI-3K/Akt activation leads to a multidrug resistance in human
breast adenocarcinoma cells. Oncogene. 22:3205–3212. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang W, Xiao Y, Li S, Zhu X, Meng L, Song
C, Yu C, Jiang N and Liu Y: Synergistic activity of magnolin
combined with B-RAF inhibitor SB590885 in hepatocellular carcinoma
cells via targeting PI3K-AKT/mTOR and ERK MAPK pathway. Am J Transl
Res. 11:3816–3824. 2019.PubMed/NCBI
|
|
21
|
Gao X, Qin T, Mao J, Zhang J, Fan S, Lu Y,
Sun Z, Zhang Q, Song B and Li L: PTENP1/miR-20a/PTEN axis
contributes to breast cancer progression by regulating PTEN via
PI3K/AKT pathway. J Exp Clin Cancer Res. 38:2562019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maira SM, Pecchi S, Huang A, Burger M,
Knapp M, Sterker D, Schnell C, Guthy D, Nagel T, Wiesmann M, et al:
Identification and characterization of NVP-BKM120, an orally
available pan-class I PI3-kinase inhibitor. Mol Cancer Ther.
11:317–328. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hirai H, Sootome H, Nakatsuru Y, Miyama K,
Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS and
Kotani H: MK-2206, an allosteric Akt inhibitor, enhances antitumor
efficacy by standard chemotherapeutic agents or molecular targeted
drugs in vitro and in vivo. Mol Cancer Ther. 9:1956–1967. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Banerjee S, Wang Z, Kong D and Sarkar FH:
3,3-Diindolylmethane enhances chemosensitivity of multiple
chemotherapeutic agents in pancreatic cancer. Cancer Res.
69:5592–5600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bharti AC and Aggarwal BB: Nuclear
factor-kappa B and cancer: Its role in prevention and therapy.
Biochem Pharmacol. 64:883–888. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ng SS, Tsao MS, Nicklee T and Hedley DW:
Wortmannin inhibits pkb/akt phosphorylation and promotes
gemcitabine antitumor activity in orthotopic human pancreatic
cancer xenografts in immunodeficient mice. Clin Cancer Res.
7:3269–3275. 2001.PubMed/NCBI
|
|
28
|
Gilles ME, Maione F, Cossutta M,
Carpentier G, Caruana L, Di Maria S, Houppe C, Destouches D,
Shchors K, Prochasson C, et al: Nucleolin targeting impairs the
progression of pancreatic cancer and promotes the normalization of
tumor vasculature. Cancer Res. 76:7181–7193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoon Y, Lim JW, Kim J, Kim Y and Chun KH:
Discovery of ursolic acid prodrug (NX-201): Pharmacokinetics and in
vivo antitumor effects in PANC-1 pancreatic cancer. Bioorg Med Chem
Lett. 26:5524–5527. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gottesman MM and Ambudkar SV: Overview:
ABC transporters and human disease. J Bioenerg Biomembr.
33:453–458. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lage H: An overview of cancer multidrug
resistance: A still unsolved problem. Cell Mol Life Sci.
65:3145–3167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tutt AN, Lord CJ, McCabe N, Farmer H,
Turner N, Martin NM, Jackson SP, Smith GC and Ashworth A:
Exploiting the DNA repair defect in BRCA mutant cells in the design
of new therapeutic strategies for cancer. Cold Spring Harb Symp
Quant Biol. 70:139–148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu H, Chen S and Cao P: Synergistic
bactericidal effects and mechanisms of low intensity ultrasound and
antibiotics against bacteria: A review. Ultrason Sonochem.
19:377–382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu T, Hu K, Bai J and Wang Z: Reversal of
adriamycin resistance in ovarian carcinoma cell line by combination
of verapamil and low-level ultrasound. Ultrason Sonochem. 10:37–40.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang S, Wang P, Wang X, Su X and Liu Q:
Activation of microbubbles by low-level therapeutic ultrasound
enhances the antitumor effects of doxorubicin. Eur Radiol.
24:2739–2753. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hassan MA, Furusawa Y, Minemura M,
Rapoport N, Sugiyama T and Kondo T: Ultrasound-induced new cellular
mechanism involved in drug resistance. PLoS One. 7:e482912012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Y, Cho CW, Yan X, Henthorn TK,
Lillehei KO, Cobb WN and Ng KY: Ultrasound-Induced hyperthermia
increases cellular uptake and cytotoxicity of P-glycoprotein
substrates in multi-drug resistant cells. Pharm Res. 18:1255–1261.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ning Z, Zhu Z, Wang H, Zhang C, Xu L,
Zhuang L, Yan X, Wang D, Wang P and Meng Z: High-intensity focused
ultrasound enhances the effect of bufalin by inducing apoptosis in
pancreatic cancer cells. OncoTargets Ther. 12:1161–1170. 2019.
View Article : Google Scholar
|
|
39
|
Liu L, Chang S, Sun J, Zhu S, Yin M, Zhu
Y, Wang Z and Xu RX: Ultrasound-mediated destruction of paclitaxel
and oxygen loaded lipid microbubbles for combination therapy in
ovarian cancer xenografts. Cancer Lett. 361:147–154. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang C, Huang S, Li H, Li X, Li B, Zhong
L, Wang J, Zou M, He X, Zheng H, et al: The effects of ultrasound
exposure on P-glycoprotein-mediated multidrug resistance in vitro
and in vivo. J Exp Clin Cancer Res. 37:2322018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu Y, Liu X, Qin Z, Hu L and Wang X:
Low-frequency ultrasound enhances chemotherapy sensitivity and
induces autophagy in PTX-resistant PC-3 cells via the endoplasmic
reticulum stress-mediated PI3K/Akt/mTOR signaling pathway.
OncoTargets Ther. 11:5621–5630. 2018. View Article : Google Scholar
|
|
42
|
Sun Y, Li Q, Xu Y, Pu C, Zhao L, Guo Z,
Ding X and Jin X: Study of the mechanisms underlying the reversal
of multidrug resistance of human neuroblastoma multidrug-resistant
cell line SK-N-SH/MDR1 by low-intensity pulsed ultrasound. Oncol
Rep. 29:1939–1945. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
He Y, Bi Y, Ji XJ and Wei G: Increased
efficiency of testicular tumor chemotherapy by ultrasound
microbubble-mediated targeted transfection of siMDR1. Oncol Rep.
34:2311–2318. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nieth C and Lage H: Induction of the
ABC-transporters Mdr1/P-gp (Abcb1), mrpl (Abcc1), and bcrp (Abcg2)
during establishment of multidrug resistance following exposure to
mitoxantrone. J Chemother. 17:215–223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim MP and Gallick GE: Gemcitabine
resistance in pancreatic cancer: Picking the key players. Clin
Cancer Res. 14:1284–1285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Andersson R, Aho U, Nilsson BI, Peters GJ,
Pastor-Anglada M, Rasch W and Sandvold ML: Gemcitabine
chemoresistance in pancreatic cancer: Molecular mechanisms and
potential solutions. Scand J Gastroenterol. 44:782–786. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang PF, Li KS, Shen YH, Gao PT, Dong ZR,
Cai JB, Zhang C, Huang XY, Tian MX, Hu ZQ, et al: Galectin-1
induces hepatocellular carcinoma EMT and sorafenib resistance by
activating FAK/PI3K/AKT signaling. Cell Death Dis. 7:e22012016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liang F, Ren C, Wang J, Wang S, Yang L,
Han X, Chen Y, Tong G and Yang G: The crosstalk between STAT3 and
p53/RAS signaling controls cancer cell metastasis and cisplatin
resistance via the Slug/MAPK/PI3K/AKT-mediated regulation of EMT
and autophagy. Oncogenesis. 8:592019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
To KK and Fu LW: CUDC-907, a dual HDAC and
PI3K inhibitor, reverses platinum drug resistance. Invest New
Drugs. 36:10–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ma JX, Sun YL, Yu Y, Zhang J, Wu HY and Yu
XF: Triptolide enhances the sensitivity of pancreatic cancer PANC-1
cells to gemcitabine by inhibiting TLR4/NF-κB signaling. Am J
Transl Res. 11:3750–3760. 2019.PubMed/NCBI
|
|
51
|
Mulero MC, Wang VY, Huxford T and Ghosh G:
Genome reading by the NF-κB transcription factors. Nucleic Acids
Res. 47:9967–9989. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Solt LA and May MJ: The IkappaB kinase
complex: Master regulator of NF-kappaB signaling. Immunol Res.
42:3–18. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hien TT, Kim HG, Han EH, Kang KW and Jeong
HG: Molecular mechanism of suppression of MDR1 by puerarin from
Pueraria lobata via NF-kappaB pathway and cAMP-responsive element
transcriptional activity-dependent up-regulation of AMP-activated
protein kinase in breast cancer MCF-7/adr cells. Mol Nutr Food Res.
54:918–928. 2010. View Article : Google Scholar : PubMed/NCBI
|