Introduction
Worldwide, hepatocellular carcinoma (HCC) is
considered as the most common type of hepatic malignancy,
accounting for approximately 90% of all primary liver cancers
(1). According to research data,
HCC ranks fifth in incidence among the most common cancers in men
and seventh in women (2).
Unfortunately, the majority of patients are diagnosed at an
advanced stage of HCC with metastasis and are not indicated for
curative therapy, such as resection, transplantation or ablation
(3). Moreover, HCC is also
associated with the characteristics of rapid progression and a high
rate of lethality. Therefore, to enhance the survival and improve
the quality of life of HCC patients, research concerning the
identification of novel targets and the development of new
therapeutic strategies against HCC progression is urgently needed,
especially concerning the metastasis of HCC (4).
Recent studies have demonstrated that HCC is a
typical hypervascular tumor, with tissue composed of tumor cells
and blood vessels (5,6). Evidence has confirmed that
angiogenesis is a key process closely related to tumor initiation
and malignant evolution (7,8). In addition, Sun and Liao also
validated that physiological or pathological neovascularization is
a necessary process in the initiation of tumor tissue ischemia,
growth or metastasis (9). Hence,
anti-angiogenic treatment has received much attention. Moreover,
researchers have verified that tilting the balance toward
stimulatory angiogenic factors is a crucial mechanism used by tumor
cells to drive vascular growth and to obtain blood supply by
attracting and activating cells from the tumor microenvironment
(10). Furthermore, the endothelium
consists of a typical and important type of stromal cells present
in the tumor microenvironment. Studies have demonstrated that
endothelial cells are recruited by cancer cells by secreting
angiogenic factors to sustain tumor vascular networks (11). Moreover, tumor size and tumor
angiogenesis could be reduced when this crosstalk is interrupted
(12). Therefore, endothelial cells
are not only a critical part of the tumor microenvironment of HCC;
their association with cancer cells participates in the entire
process of tumor angiogenesis (4).
From this point of view, the dysfunction of endothelial cells in
the tumor microenvironment has important effects on the progression
and development of HCC.
Recently, suggested as a subclass of endogenous
non-coding RNAs existing in mammalian cells, circular RNAs
(circRNAs) have emerged as new ‘stars’ of the non-coding RNA world.
By virtue of the development of next-generation sequencing and
bioinformatics technology, the increasing abundance of expression
and biological function of circRNAs have been recognized (13). Additionally, circRNAs are considered
to feature high cell-type/tissue/developmental specific expression
and stable structure, and they mostly consist of exonic transcripts
which are generated during the process of back splicing (14,15).
Researchers have found that circRNAs possess numerous properties
conferred by their structure-a covalently closed continuous loop
with the 3′ and 5′ends joined together (16). Recently, we have found that circRNAs
are widely expressed and act as vital regulators in the process of
transcriptional and post-transcriptional gene expression in human
cells (17). Moreover, the
relevance of circRNAs in multiple human cancers has been
discovered, HCC included (18). The
dominant role played by pathophysiological regulation of circRNAs
has been verified by in-depth studies of circRNAs in the tumor
field (19). In addition, recent
studies have documented that circRNAs are involved in modulating
the functions of stimulated endothelial cells (20–22).
However, the expression and function of circRNAs in endothelial
cells exposed to an HCC microenvironment remain unclear.
In the present study, we constructed a co-culture
model of human primary hepatoma cells and umbilical vein
endothelial cells. Based on this model, we determined the
expression profile of circRNAs in endothelial cells under an HCC
microenvironment by high throughput sequencing analysis. At the
same time, a functional analysis and screening of differentially
expressed circRNAs with significance were carried out. Moreover, we
validated the effects of target circRNAs on endothelial cell
function through overexpression experiments.
Materials and methods
Cell culture
The human umbilical vein and HCC tissues were
obtained from the Northwest Women's and Children's Hospital
(Shaanxi, China) from patients suffered who underwent surgery from
March 2014 to February 2018 at the hospital. the average age of the
patients was 57±8 with the sex ratio at 3.83:1 (male vs. female).
The primary human umbilical vein endothelial cells (HUVECs) and
primary hepatoma cells (HPHCs) were extracted from these tissues.
After tissue fragmentation, separation and purification, HUVECs
were maintained in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich; Merck KGaA) supplemented with 10% growth factors
(ATCC); HPHCs were cultured in DMEM, supplemented with 10% fetal
bovine serum (FBS) (Thermo Fisher Scientific, Inc.). Cells that
were passaged to the second or third generation were used for
co-culture experiment. The Ethics Committee of Northwest Women and
Children's Hospital provided approval for all procedures in the
present study. Written informed consent was obtained from the
patients and their families.
For the establishment of an indirect co-culture
system, Transwell culture inserts in 6-well plates (0.4 µm
pore-size polyester membrane; Corning) were employed. Briefly,
after trypsinization and re-suspension with complete DMEM at a
density of 5×104 cells/ml, HPHCs were seeded in
Transwell inserts at 5×104 cells/ml, while HUVECs were
plated on Bioflex plates. Subsequently, cells were independently
incubated for 72 h to reach confluence, and then an additional 48 h
indirect co-culture was performed by using shared culture media.
Finally, HUVECs were cultured with the HPHC-conditioned media for
another 48 h.
RNA sequencing
HUVECs cultured under HCC microenvironment and
normal conditions were both divided into three groups of sequencing
samples. The whole sequencing process was completed in cooperation
with Lianchuan Biotech. Briefly, the RiboMinus Eukaryote kit
(Qiagen, Inc.) was used to remove rRNA from total RNA samples (3
mg) ahead of constructing the RNA-seq libraries. In accordance with
the manufacturer's protocol, we prepared the strand-specific
RNA-seq libraries using NEBNext Ultra Directional RNA Library Prep
kit for Illumina (NEB; New England BioLabs, Inc.). After
fragmentation of ribosome-depleted RNA samples (50 ng), random
hexamer primers were used for the synthesis of first- and
second-strand complementary DNA (cDNA). To remove the RNA template
strand and synthesize second-strand cDNA, dUTP mix was used.
Subsequently, to repair the cDNA fragment ends, we treated samples
with the End-It DNA End Repair kit (Epicentre®), and
then added an A at the 3′ ends of the DNA fragments by using Klenow
followed by adaptor ligation. Next, cDNA products were purified
with uracil DNA glycosylase (to remove the second-strand cDNA), and
13–16 cycles of PCR amplification were executed. Library quality
control was monitored with a Bioanalyzer 2100 (Agilent). Finally,
the sequencing process was completed using a HiSeq 2000 (Illumina)
on a 100-bp paired-end run. We deposited the RNA-sequencing data in
Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/) (accession code:
GSE77661).
Gene Ontology (GO) categories and
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway
analyses
Based on the Wallenius non-central hyper-geometric
distribution, we performed GO enrichment analysis on the circRNAs
with the GO seq R packages (23).
To detect the statistical enrichment of the circRNAs, KEGG pathway
enrichment analysis was executed using KOBAS software (http://www.genome.jp/kegg/).
Overexpression and transfection
The expression vector used in this study was pCDS-At
which was purchased from BioVector NTCC Inc. The sequences of
circ_4911 and circ_4302 were synthesized by TsingKe Biological
Technology. The circRNAs were cloned into the pCDS-At vector to
construct overexpression vectors. Transfection was achieved using
Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Briefly, the
HCC-conditioned HUVECs were seeded at a concentration of
1×105 cells/well in 6-well culture plates. After plating
for 24 h, transfection was performed with pCDS-circ_4911 and
pCDS-circ_4302 at concentrations of 1, 2 and 4 µg/ml for 24 h,
respectively. After transfection, the cells were washed twice with
PBS, cultured under regular conditions and used for experiments at
24 h.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Following transfection, HUVECs overexpressing the
target gene were collected for RT-qPCR. First, total RNAs from the
cultured cells were extracted with Trizol reagent (Invitrogen;
Thermo Fisher Scientific, Inc.). After RNA purification, the
PrimeScript 1st strand cDNA Synthesis Kit (Takara) was used for
first-strand cDNA generation with use of the ProFlex™ 3 × 32-well
PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Subsequently, the StepOne Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and the SYBR Green PCR kit (Takara)
were used simultaneously to measure target gene expression. GAPDH
was employed as the internal control. The qPCR procedure was as
follow: 1 min at 95°C, 20 sec at 95°C and 10 sec at 56°C and 15 sec
at 72°C for 35 cycles, and finally held at 4°C. The fold change of
target gene expression was assessed according to the
2−ΔΔCq method and normalized to that of GAPDH (24).
Cell proliferation assay
The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was performed to detect cell proliferation. The
HCC-conditioned HUVECs were seeded in 96-well culture plates
(Thermo Fisher Scientific, Inc.) at a concentration of
1×105 cells/well for a 24 h incubation. Then, to each
well 5 µl of 10 mg/ml MTT was added, and the plates were incubated
for a further 4 h at 37°C followed by another incubation for at
least 12 h with 1 M NaOH solution supplemented with 1% SDS. The
final absorbance at 490 nm was measured with a microplate
spectrophotometer (Bio-Tek Instruments, Inc.).
Transwell migration assay
For the detection of cell migration, a Transwell
24-well chamber (8.0 µm pore membranes; Corning) was used according
to the manufacturer's instructions. Briefly, the upper chamber
containing 100 µl of serum-free medium was seeded with
1×105 cells per well, and the lower chamber contained
600 µl of complete medium as the chemoattractant. Then, cells were
incubated at 37°C for 24 h. After that, cotton swabs were used to
remove the cells which remained on the upper surface of the
membrane, leaving the migrated cells on the lower surface. Next,
the cells were fixed and stained with 4% paraformaldehyde and 0.1%
crystal violet solution, respectively. Finally, the cells that
passed through the filter were photographed with an inverted
fluorescence microscope (magnification, ×20).
Cell cycle distribution
To analyze cell cycle distribution, flow cytometry
was utilized. Briefly, conditioned HUVECs at a concentration of
3×105 cells/well were plated in 6-well plates after
transfection with the overexpression vector. Next, we digested the
cells with 0.25% trypsin. After centrifugation, the cells were
collected and resuspended with PBS solution. Then, the cells were
fixed in pre-ice-cold 70% ethanol for 4 h at 4°C, washed with PBS
solution at least three times and digested for 30 min with RNase A
(KeyGen Biotech Corp., Ltd.) at 37°C. The cells were stained with
50 µg/ml propidium iodide (PI) (KeyGen Biotech Corp., Ltd.) for 30
min in the dark, and the stained cells were evaluated by flow
cytometry using a FACSAria II flow cytometer (Becton Dickinson).
The data are expressed as a proportion of the total and were
analyzed by Flow Jo 7.6.2 (Tree Star, Inc.).
Western blot analysis
The primary antibodies (anti-EGFR (cat. no. 4267S),
anti-p-EGFR (cat. no. 3777S), anti-p38 (cat. no. 8690S, anti-p-p38
(cat. no. 4511S), anti-cyclin D1 (cat. no. 55506S), anti-E-cadherin
(cat. no. 3195S) and anti-GAPDH (cat. no. 5174S) were purchased
from Cell Signaling Technology Inc. and diluted at 1:1,000, while
the species-specific horseradish peroxidase-conjugated secondary
antibody (cat. no. 7054S; Cell Signaling Technology) was diluted at
1:5,000. According to the manufacturer's instructions, the cells
were washed with pre-ice-cold PBS solution (P1022-500; Beijing
Solarbio Science & Technology Co., Ltd.) and protein extraction
was completed using RIPA lysis buffer (CW2334; CW Biotech). Next,
the proteins were isolated by centrifugation at 15,000 × g and 4°C
for 15 min. Then, the concentration of protein was determined using
the Pierce™ BCA Protein Assay kit (Thermo Fisher Scientific, Inc.).
Proteins were separated by electrophoresis through 10% SDS-PAGE
gels (Life Technologies; Thermo Fisher Scientific, Inc.), and the
protein lysates (30 µg) were transferred onto PVDF membranes
(Millipore). The membranes were blocked in 5% non-fat dry milk and
incubated at 4°C overnight with primary antibodies. Finally, the
protein bands were visualized and quantified using Image Lab
software (v3.0; Bio-Rad Laboratories, Inc.) and ImageJ software
(v1.46; National Institutes of Health).
Statistical analysis
Each sequencing sample was divided into three
groups, and all cellular experiments were repeated at least three
times. Tukey's test was performed for multiple comparisons between
groups and ANOVA analysis was conducted for multiple comparisons
between individual groups. All the analyses were performed using
SPSS v22.0 (IBM Inc.). GraphPad Prism 6 v6.06 (GraphPad Software)
was used as the image processing software in this study. P<0.05
was considered to indicate a statistically significant
difference.
Results
Profiling of circRNAs in the
individual-cultured HUVECs and HUVECs co-cultured with HPHCs
To investigate the circRNA expression profiles in
HUVECs under HCC microenvironment, we divided the HPHC-co-cultured
HUVECs (H) and naive HUVECs (CO) into three groups. Next, the
circRNA transcripts were characterized by using RNA-sequencing
analyses. Every sample group was sequenced on an Illumina HiSeq
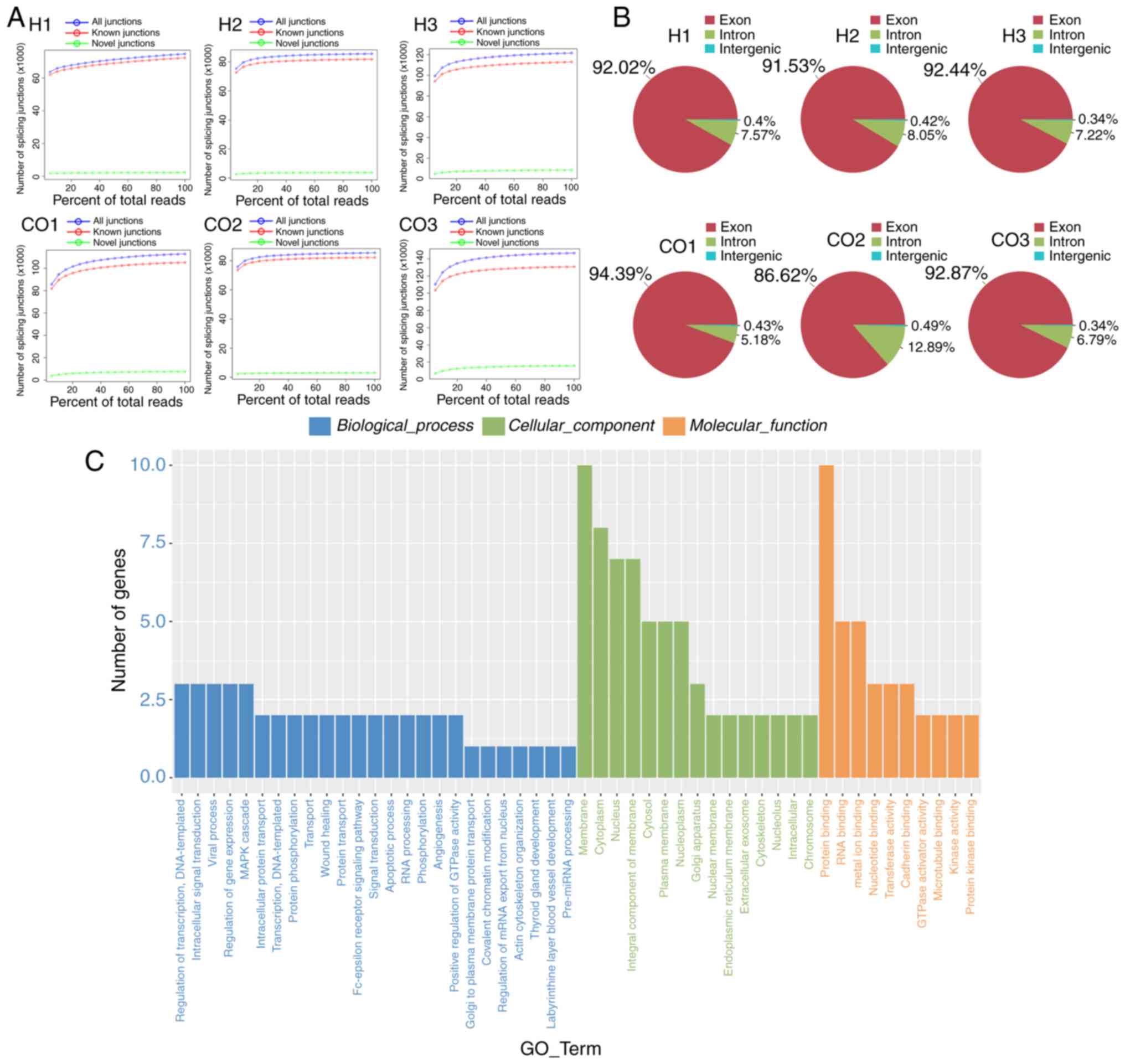
sequencer. The analysis results showed that the numbers of novel
splicing junctions between the H and CO groups were consistent
(Fig. 1A). Through transcriptional
analysis, we found that the transcriptional sources of circRNAs in
cells of the treatment and control groups were basically the same:
most of them were derived from exons; ~6–8% of circRNAs were
transcribed from introns; the intergenic circRNAs occupied <0.5%
of the transcript (Fig. 1B).
Afterwards, we analyzed the GO enrichment of these circRNAs to
further explore their potential functions. For biological
processes, circRNAs were mainly enriched in five processes:
‘regulation of transcription (DNA-templated)’, ‘intracellular
protein transduction’, ‘viral processes’, ‘regulation of gene
expression’ and the ‘MAPK cascade’. Concerning the cellular
components, the enriched GO terms included ‘membrane’ and
‘cytoplasm’. In addition, the circRNAs were predominately involved
in the molecular function of ‘protein binding’ (Fig. 1C).
Identification and validation of
differentially expressed circRNAs
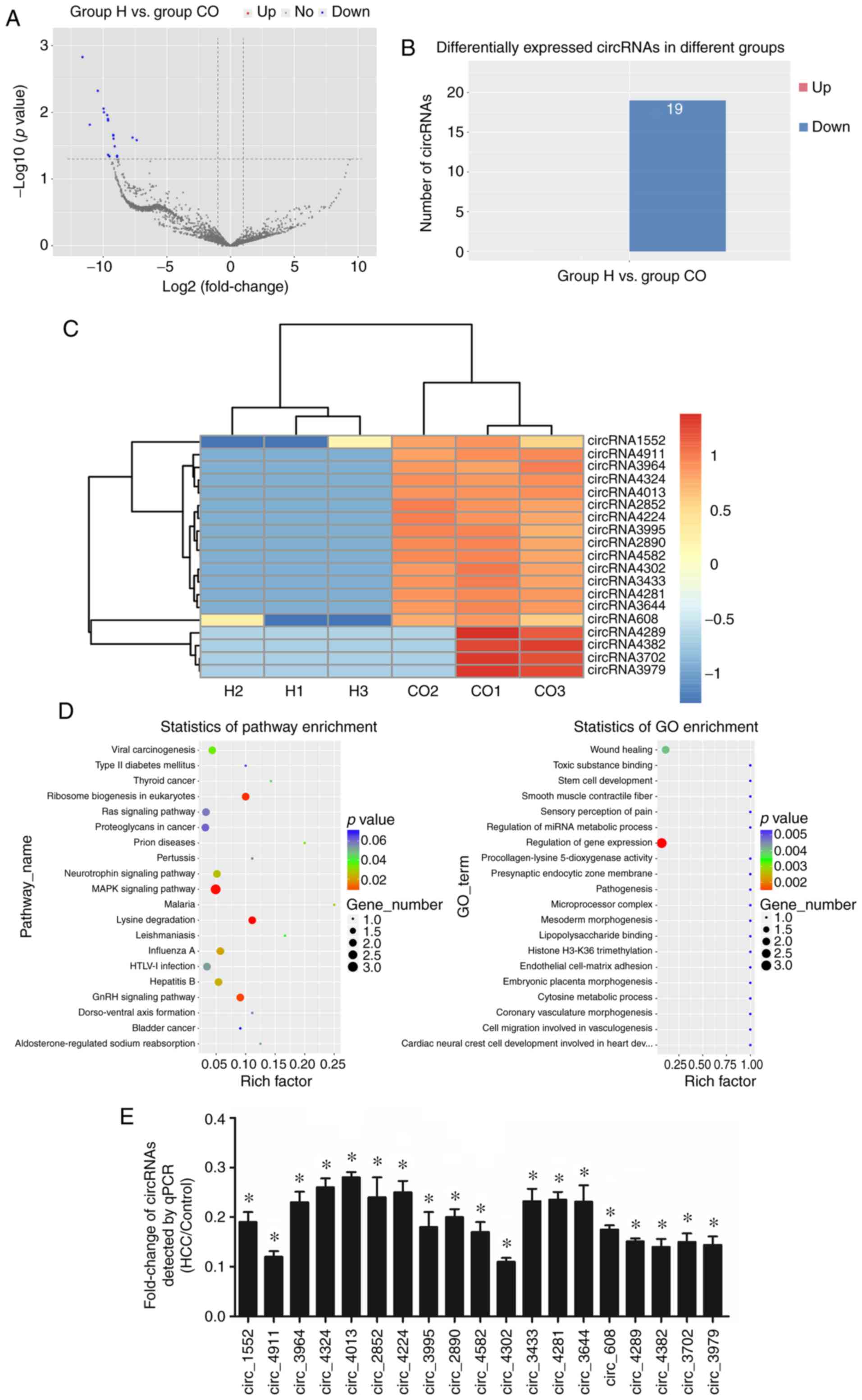
Next, to identify differentially expressed circRNAs
in HUVECs under HCC microenvironment, we profiled further analysis
on profiling circRNA expression in H and CO groups. According to
the volcano plot and differential circRNA expression enrichment
plot, nineteen significant differentially expressed circRNAs were
identified between the two groups (Fig.
2A and B). The heatmap displays the expression levels of these
19 circRNAs, and the results indicated that the expressions of
circRNAs were significantly downregulated in the treatment group
compared with the control, except circRNA-4289, circRNA-4382,
circRNA-3702 and circRNA-3979 (Fig.
2C). Subsequently, GO categorization and KEGG pathway analyses
were conducted to explore the putative function of these
differentially expressed circRNAs. As shown in Fig. 2D, the most circRNA-related GO term
was ‘regulation of gene expression’. For the pathway enrichment,
circRNAs were mainly involved in the ‘MAPK signaling pathway’,
‘ribosome biogenesis in eukaryotes’ and ‘lysine degradation’
(Fig. 2D). Consistent with the
sequencing results, the expression levels of all the circRNAs in
HUVECs under the HCC microenvironment were significantly
downregulated. To note, two most obviously down-modulated circRNAs
in the H group vs. the CO group were circ_4911 and circ_4302
(Fig. 2E).
Circ_4911 affects cellular function
and the expression of related proteins in HUVECs
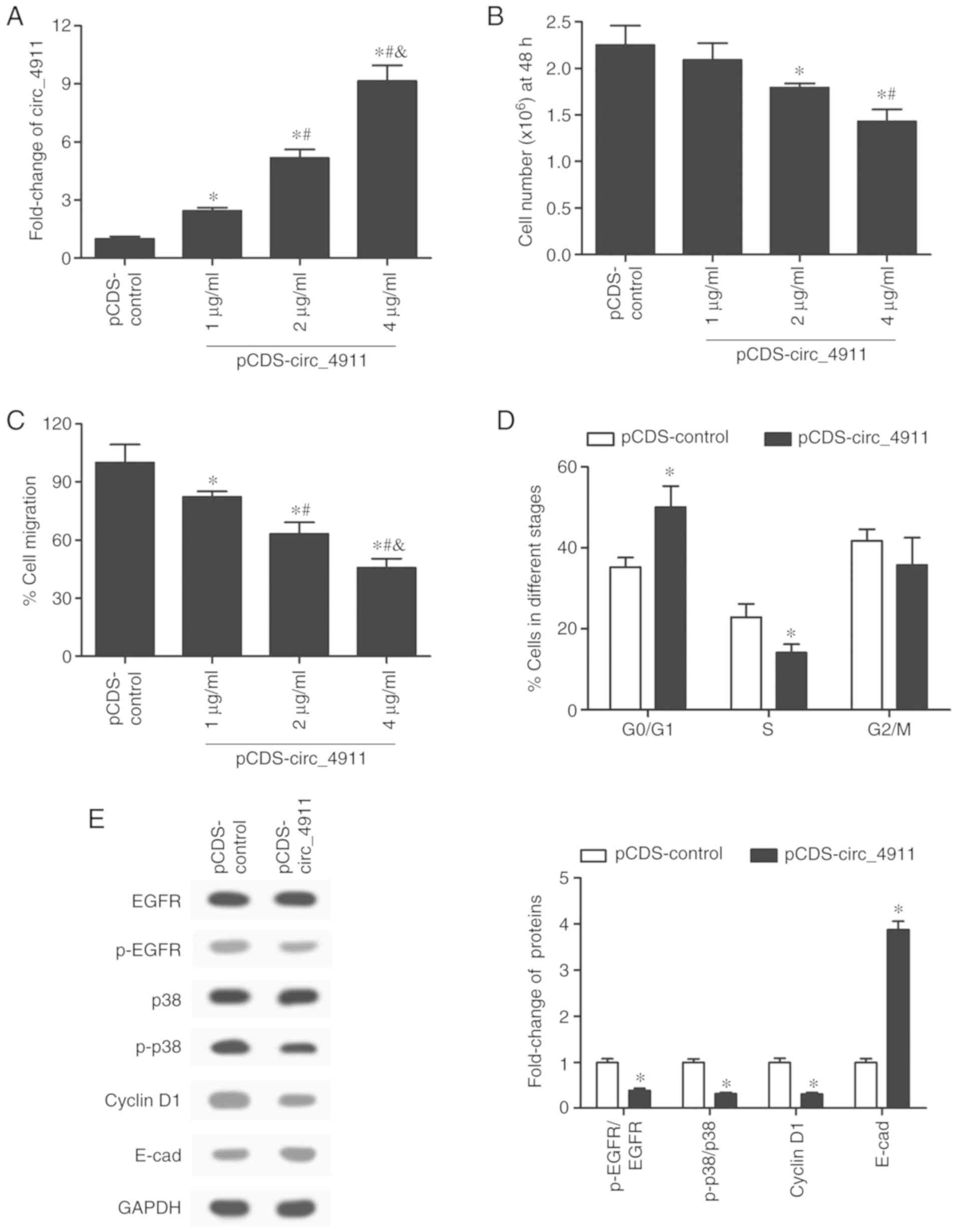
Once circ_4911 was identified as a significantly
downregulated gene in HUVECs under the action of HPHCs, our next
step was to explore the effect of circ_4911 on endothelial cell
function. To overexpress circ_4911, we used pCDS-At as the
expression vector, and then we transfected the cultured HUVECs with
pCDS-circ_4911 at concentrations of 1, 2 and 4 µg/ml. The
expression of circ_4911 was significantly and dose-dependently
increased in the circ_4911-overexpressing HUVECs (Fig. 3A). Subsequently, the results of the
cell proliferation assay showed that transfection with 4 µg/ml
pCDS-circ_4911 significantly inhibited the proliferation activity
of the HUVECs (Fig. 3B). Likewise,
overexpression of circ_4911 significantly decreased the migration
rate of HUVECs in a dose-dependent manner (Fig. 3C). In addition, circ_4911 also
modulated the cellular cycle of HUVECs; there was a significant
elevation in the proportion of cells in the G0/G1 stages when
circ_4911 was overexpressed, while the proportion of cells in the S
stage was declined; the ratio of cells in the G2/M stage was not
significantly affected by circ_4911 overexpression (Fig. 3D). To further validate the effects
of circ_4911 overexpression on cell function, we detected the
expression levels of several key proteins related to the cell
cycle, proliferation, migration and adhesion. The visualized and
quantified results of the western blot analysis indicated that
circ_4911 overexpression significantly suppressed the activation of
epidermal growth factor receptor (EGFR) and p38 and the expression
of cyclin D1, while it greatly increased the expression of
E-cadherin (Fig. 3E). In general,
the expression of circ_4911 was found to play an inhibitory role in
the proliferation and migration of HUVECs.
Circ_4302 plays a regulatory role in
cellular function and related protein expression of HUVECs
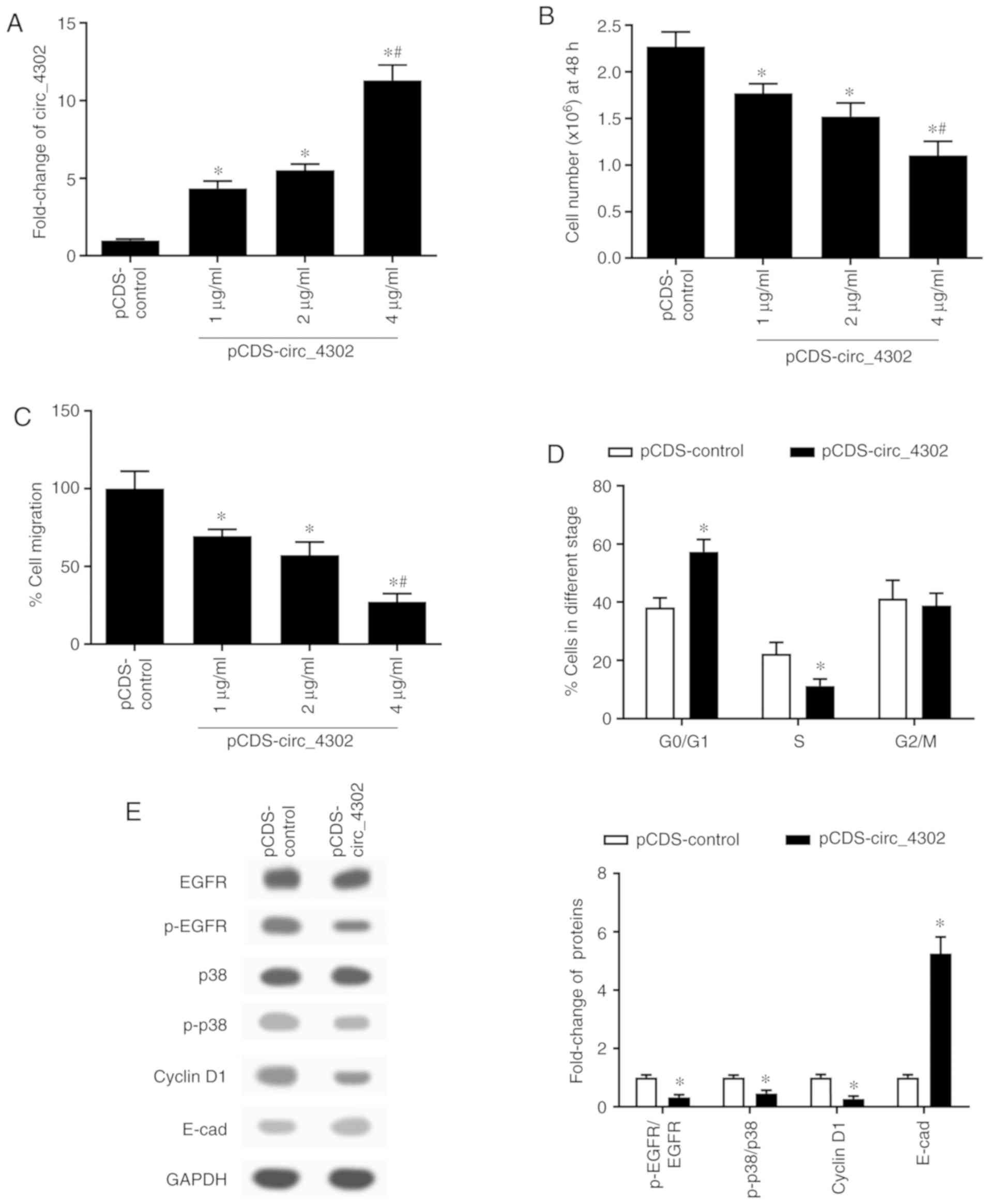
Circ_4302 was another circRNA selected as being
markedly downregulated in the HUVECs. To verify the function
exerted by circ_4302 on cellular events in HUVECs, we carried out
overexpression experiments as for circ_4911. First, the results of
RT-q PCR confirmed the effect of transfection with the
pCDS-circ_4302 vector (Fig. 4A).
Then, compared with the control group, the cell viability of the
HUVECs significantly declined after the transfection with
pCDS-circ_4302 (Fig. 4B).
Additionally, the cell migration rate of HUVECs was restrained by
circ_4302 overexpression vector in a dose-dependent manner
(Fig. 4C). According to the
experiments above, we found that the transfection effect at the
concentration of 4 µg/ml was the most obvious. Thus, we used 4
µg/ml pCDS-circ_4302 in subsequent experiments. Consistent with the
effect exerted by circ_4911 overexpression on the cell cycle,
overexpressing of circ_4302 similarly arrested HUVECs at the G0/G1
stage (Fig. 4D). Moreover, the
expression of p-EGFR, p-p38 and cyclin D1 was obviously decreased
in the HUVECs overexpressing circ_4302, while that of E-cadherin
was significantly enhanced (Fig.
4E). Taking together, circ_4302 had the same regulatory effects
as circ_4911 in regards to proliferation, migration and adhesion of
HUVECs.
Discussion
In recent years, sequencing and biotechnology have
become important technologies with great promise in biological
research. RNA-sequencing, also called transcriptome sequencing
technology, is an emerging technology based on high-throughput
sequencing and reflects the expression levels of RNAs (small RNAs
and non-coding RNAs included) in conditioned samples. In the
present study, we analyzed and predicted the distribution,
expression and function of circRNAs in human umbilical vein
endothelial cells (HUVECs) under a HCC microenvironment through the
use of RNA-sequencing.
CircRNAs, as a special type of non-coding RNA
molecule, have emerged as one of the latest research ‘hotspots’ in
the field of RNA. In the early years, RNA splicing errors,
transcriptional noise and RT-PCR artefacts were once the labels of
circRNAs (25). However,
researchers have since discovered that circRNAs have widely
enriched expression, as well as transcriptional and
post-transcriptional regulatory functions in human cells (17). Furthermore, studies have
demonstrated that circRNAs are expressed in a tissue- and
cell-specific manner (15). In this
study, we analyzed the circRNA expression profile in human
endothelial cells under an HCC microenvironment by utilizing
circRNA microarray analysis. The results indicated abundant
expression of circRNAs in HUVECs, and the majority were derived
from exon circularization. Moreover, these circRNAs were predicted
to be associated with a variety of cellular components, biological
processes and molecular functions.
In-depth studies provided evidence that circRNAs are
expressed at significantly different levels in stimulated HUVECs
and normal HUVECs, suggesting that circRNAs play a specific role in
endothelial cells. Analysis of the expression profiles of circRNAs
in HUVECs stimulated by ox-LDL revealed 943 circRNAs that were
differentially expressed with two-fold changes; among them,
hsa_circ_0003575 was upregulated dramatically in ox-LDL-induced
HUVECs (21). The expression of
cZNF292 was found to be significantly upregulated among the
circRNAs screened from hypoxia-induced HUVECs with differential
expression (20). Similarly,
researchers found a variety of circRNAs that were differentially
expressed in HUVECs exposed to hyperglycaemia (16). In the present study, by analyzing
the expression of circRNAs in human primary hepatoma cells (HPHCs)
co-cultured with HUVECs, we found 19 circRNAs that displayed
significant downregulation compared with the control group. In
addition, using RT-qPCR analysis, we verified the dysregulation of
these circRNAs, and screened out two circRNAs with the most
significant differential expression: circ_4911 and circ_4302.
To further explore the roles that circ_4911 and
circ_4302 play in the functions of HUVECs, we overexpressed cir4911
and circ_4302 in HUVECs. The results led us to the following
discovery. Overexpression of circ_4911 and circ_4302 significantly
inhibited the proliferation and migration of HUVECs, and arrested
cells at the GO/G1 stage thus affecting cell replication. The
expression levels of key proteins related to these cell events were
also repressed. In addition, the upregulation of E-cadherin
expression demonstrated that circ_4911 and circ_4302 could promote
cell adhesion of HUVECs. In previous studies, circRNAs was proven
as crucial molecular regulators of cell function in endothelial
cells. Dang and colleagues reported that knockdown of circRNA
hsa_circ_0010729 enhanced apoptosis, and could reverse
hypoxia-induced inhibition of proliferation and migration in HUVECs
(19). Under ox-LDL stimulation,
hsa_circ_0003575 silencing was demonstrated to promote
proliferation, increase the migration distance and enhance
angiogenesis in HUVECs, while apoptosis was decreased (21). Hsa_circRNA-0054633 was validated to
have protective effects against endothelial cell dysfunction
stimulated by high glucose (26).
Previously, endothelial cells were considered as one
of the key components in the tumor microenvironment, and they were
found to play an irreplaceable role in the development and
progression of tumors (27). More
importantly, the proliferation and migration of endothelial cells
are involved in the angiogenesis of tumors (11). It has been found that tumor cells
recruit endothelial cells and stimulate cell proliferation and
migration of endothelial cells by secreting signal factors, while
reducing cell adhesion, so as to maintain the vascular network of
tumors and ensure the growth of tumors (4,28,29).
In this study, differentially expressed circ_4911 and circ_4302
were selected from HUVECs in an HCC microenvironment, and both were
proven to play key roles in the cell cycle, proliferation,
migration and adhesion of endothelial cells. We proposed that
circ_4911 and circ_4302 are crucial molecules in the development of
HCC that may provide new targets for HCC therapy.
In conclusion, our study analyzed the expression
profile of circRNAs in HUVECs in an HCC microenvironment and
identified two significantly downregulated circRNAs: cir4911 and
circ_4302. Moreover, through overexpression experiments, we
demonstrated that circ_4911 and circ_4302 regulate the
proliferation, migration and adhesion of endothelial cells.
Therefore, we propose that circ_4911 and circ_4302 may play
important roles in the progression and development of HCC. However,
there are also limitations in the present study. The abnormal
expression of circ_4911 and circ_4302 should be identified in
tissues, and also the correlation between circRNAs and the clinical
characteristics of HCC patients should be analyzed. Moreover,
further research will aim to illustrate the potential molecular
mechanism of circ_4911 and circ_4302 in regulating the processes of
endothelial cells under an HCC microenvironment, based on classical
signaling pathways. These findings may provide novel targets for
HCC diagnosis and therapy.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Science and
Technology Research and Development Program of Shaanxi Province
(2011K13-03-11), the Science and Technology Project of Xi'an
[SF1203 (3)], and the State
Scholarship Fund of China (No. 01806285146).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KY and WC made substantial contributions to the
design of the present study. Data acquisition and interpretation
were conducted by KY, WC, XX, GC and YL. KY and ZJ wrote the
manuscript. WC and ZJ critically revised the manuscript for
important intellectual content. All authors approved the final
version of the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The experimental protocol of this study was approved
by the Committee on the Second Affiliated Hospital of Xi'an
Jiaotong University (Shaanxi, China). Written informed consent was
obtained from each participant prior to tissue collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun HZ, Song YL and Wang XY: Effects of
different anesthetic methods on cellular immune and neuroendocrine
functions in patients with hepatocellular carcinoma before and
after surgery. J Clin Lab Anal. 30:1175–1182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feng T, Yu H, Xia Q, Ma Y, Yin H, Shen Y
and Liu X: Cross-Talk mechanism between endothelial cells and
hepatocellular carcinoma cells via growth factors and integrin
pathway promotes tumor angiogenesis and cell migration. Oncotarget.
8:69577–69593. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tomizawa M, Kondo F and Kondo Y: Growth
patterns and interstitial invasion of small hepatocellular
carcinoma. Pathol Int. 45:352–358. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yukawa H, Suzuki K, Aoki K, Arimoto T,
Yasui T, Kaji N, Ishikawa T, Ochiya T and Baba Y: Imaging of
angiogenesis of human umbilical vein endothelial cells by uptake of
exosomes secreted from hepatocellular carcinoma cells. Sci Rep.
8:67652018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park JH, Kim SH, Choi MC, Lee J, Oh DY, Im
SA, Bang YJ and Kim TY: Class II histone deacetylases play pivotal
roles in heat shock protein 90-mediated proteasomal degradation of
vascular endothelial growth factor receptors. Biochem Biophy Res
Commun. 368:318–322. 2008. View Article : Google Scholar
|
|
8
|
Zhao Y and Adjei AA: Targeting
angiogenesis in cancer therapy: Moving beyond vascular endothelial
growth factor. Oncologist. 20:660–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun J and Liao JK: Induction of
angiogenesis by heat shock protein 90 mediated by protein kinase
akt and endothelial nitric oxide synthase. Arterioscler Thromb Vasc
Biol. 24:2238–2244. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weis SM and Cheresh DA: Tumor
angiogenesis: Molecular pathways and therapeutic targets. Nat Med.
17:1359–1370. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Manzi M, Bacigalupo ML, Carabias P, Elola
MT, Wolfenstein-Todel C, Rabinovich GA, Espelt MV and Troncoso MF:
Galectin-1 controls the proliferation and migration of liver
sinusoidal endothelial cells and their interaction with
hepatocarcinoma cells. J Cell Physiol. 231:1522–1533. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vasudev NS and Reynolds AR: Erratum to:
Anti-Angiogenic therapy for cancer: Current progress, unresolved
questions and future directions. Angiogenesis. 17:495–497. 2014.
View Article : Google Scholar
|
|
13
|
Zheng XB, Zhang M and Xu MQ: Detection and
characterization of ciRS-7: A potential promoter of the development
of cancer. Neoplasma. 64:321–328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szabo L and Salzman J: Detecting circular
RNAs: Bioinformatic and experimental challenges. Nat Rev Genet.
17:679–692. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao T, Chen Q, Fu L and Guo J: Circular
RNAs: Biogenesis, properties, roles, and their relationships with
liver diseases. Hepatol Res. 47:497–504. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shang FF, Luo S, Liang X and Xia Y:
Alterations of circular RNAs in hyperglycemic human endothelial
cells. Biochem Biophys Res Commun. 499:551–555. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hou LD and Zhang J: Circular RNAs: An
emerging type of RNA in cancer. Int J Immunopathol Pharmacol.
30:1–6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu L, Yao T, Chen Q, Mo X, Hu Y and Guo J:
Screening differential circular RNA expression profiles reveals
hsa_circ_0004018 is associated with hepatocellular carcinoma.
Oncotarget. 8:58405–58416. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dang RY, Liu FL and Li Y: Circular RNA
hsa_circ_0010729 regulates vascular endothelial cell proliferation
and apoptosis by targeting the miR-186/HIF-1α axis. Biochem Biophys
Res Commun. 490:104–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jes-Niels B, Nicolas J, Heumüller AW, Chen
W, Boon RA, Stellos K, Zeiher AM, John D, Uchida S and Dimmeler S:
Identification and characterization of hypoxia-regulated
endothelial circular RNA. Circ Res. 117:884–890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li CY, Ma L and Yu B: Circular RNA
hsa_circ_0003575 regulates oxLDL induced vascular endothelial cells
proliferation and angiogenesis. Biomed Pharmacother. 95:1514–1519.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Li Z, Jiang P, Peng M, Zhang X, Chen
K, Liu H, Bi H, Liu X and Li X: Circular RNA IARS (circ-IARS)
secreted by pancreatic cancer cells and located within exosomes
regulates endothelial monolayer permeability to promote tumor
metastasis. J Exp Clin Cancer Res. 37:1772018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Young MD, Wakefield MJ, Smyth GK and
Oshlack A: Gene ontology analysis for RNA-seq: Accounting for
selection bias. Genome Biol. 11:R142010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao W, Cheng Y, Zhang C, You Q, Shen X,
Guo W and Jiao Y: Genome-Wide identification and characterization
of circular RNAs by high throughput sequencing in soybean. Sci Rep.
7:56362017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan L, Lian W, Zhang X, Han S, Cao C, Li X
and Li M: Human circular RNA-0054633 regulates high glucose-induced
vascular endothelial cell dysfunction through the
microRNA-218/roundabout 1 and microRNA-218/heme oxygenase-1 axes.
Int J Mol Med. 42:597–606. 2018.PubMed/NCBI
|
|
27
|
Brenner W, Beitz S, Schneider E, Benzing
F, Unger RE, Roos FC, Thüroff JW and Hampel C: Adhesion of renal
carcinoma cells to endothelial cells depends on PKCmu. BMC Cancer.
10:1832010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meng J, Liu Y, Han J, Tan Q, Chen S, Qiao
K, Zhou H, Sun T and Yang C: Hsp90β promoted endothelial
cell-dependent tumor angiogenesis in hepatocellular carcinoma. Mol
Cancer. 16:722017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choi H and Moon A: Crosstalk between
cancer cells and endothelial cells: Implications for tumor
progression and intervention. Arch Pharm Res. 41:711–724. 2018.
View Article : Google Scholar : PubMed/NCBI
|