Introduction
Type VI collagen secreted from host cells has been
shown to influence the proliferation of cancer cells (1–3). High
collagen type VI alpha 1 chain (COL6A1) mRNA expression was
observed in astrocytoma using reverse transcription quantitative
PCR (RT-qPCR) analysis (4).
Overexpression of COL6A1 mRNA and the COL6A1 polypeptide was
detected in non-small cell lung cancer using RT-qPCR and
immunohistochemical (IHC) analyses (5). IHC analysis also indicated that COL6A1
expression is higher in castration-resistant prostate carcinoma
than in adjacent normal tissue (6).
In addition, gene expression profile analysis showed that
COL6A1 mRNA expression is a predictive marker of poor
prognosis in clear cell renal cell carcinoma (7). Proteomic analysis revealed that COL6A1
expression is associated with poor prognosis in patients with
glioblastoma (8). Similarly, IHC
analysis indicated that COL6A1 expression predicts the prognosis of
patients with cervical cancer (9).
Thus, previous reports suggest that type VI collagen, which
contains the COL6A1 polypeptide as one of the three α chains, is
important for cancer cell proliferation.
Zhu et al indicated that COL6A1 knockdown
(KD) inhibits the proliferation of prostate cancer LNCaP cells.
COL6A1 promotes proliferation through the JAK-STAT pathway
(6). Owusu-Ansah et al also
reported that COL6A1 is involved in the migration and invasion of
highly metastatic pancreatic cancer BxPC-M8 cells (10). Recently, we reported the existence
of a non-triple helical polypeptide encoded by COL6A1, NTH
α1(VI), in the conditioned media of cancer cell lines (11). NTH α1(VI), rather than the COL6A1
polypeptide of type VI collagen, may be directly involved in cancer
cell proliferation, migration, and invasion.
Extracellular matrix (ECM)-derived peptides, known
as matrikines or matricryptins (hereafter, matricryptins), are
defined as ‘enzymatic fragments of ECM containing exposed
matricryptic sites’, according to Davis et al (12). Arresten, canstatin, and tumstatin
are matricryptins derived from the NC1 domains of type IV collagen
α1, α2, and α3 chains, respectively; they all possess
anti-angiogenic and antitumor activities (13,14).
It has been reported that endotrophin, the C5 domain derived from
COL6A3, promotes malignant tumor progression (15). As described above, tumors express
COL6A1 (5,6), whose expression is associated with the
prognosis of patients with certain types of cancer (8,9).
Additionally, Willumsen et al observed COL6A1- and
COL6A3-derived peptides in some tumors (16); as indicated above, NTH α1(VI) is
thought to be involved in cancer cell proliferation, migration, and
invasion. Therefore, we evaluated whether NTH α1(VI) and/or derived
peptide(s) are involved in cancer cell proliferation using highly
metastatic human pancreatic cancer S2-VP10 cells, given that
S2-VP10 cells constitutively express many matrix metalloproteases
(17).
Materials and methods
Antibodies and type VI collagen
A mouse monoclonal antibody (#141) that recognizes
COL4A1, NTH α1(IV), COL6A1, and NTH α1(VI) was prepared in our
laboratory (Nippon Kayaku, Tokyo, Japan) and characterized as
previously described (11,18,19).
Rabbit anti-COL6A1 polyclonal antibody (cat. no. NBP1-59126) was
purchased from Novus Biologicals. Rabbit anti-COL6A1 (N-term)
polyclonal antibody (cat. no. AP6587a) and mouse anti-COL6A2
monoclonal antibody (cat. no. AT1585a) were purchased from Abgent
Inc. Mouse anti-HSP90 monoclonal antibody (cat. no. 610418) was
purchased from BD Biosciences. Horseradish peroxidase-labeled sheep
anti-mouse (cat. no. NA931-1ML) and donkey anti-rabbit (cat. no.
NA934-1ML) IgG antibodies were purchased from GE Healthcare. Human
type VI collagen was purchased from BD Biosciences.
Small interfering RNAs (siRNAs)
Control and COL6A1 siRNAs were purchased from Thermo
Fisher Scientific, Inc. The sequences of all siRNAs except for the
control siRNA are shown in Table
I.
 | Table I.siRNA sequences used in this
study. |
Table I.
siRNA sequences used in this
study.
| Name | Sequence |
|---|
| COL6A1#1 sense |
5′-CCUGUUCUUUGUGCUGGACACCUCU-3′ |
| COL6A1#1
antisense |
5′-AGAGGUGUCCAGCACAAAGAACAGG-3′ |
| COL6A1#2 sense |
5′-GCAUAGACAAGAAGUGUCCAGAUUA-3′ |
| COL6A1#2
antisense |
5′-UAAUCUGGACACUUCUUGUCUAUGC-3′ |
| COL6A1#3 sense |
5′-GCCGUCGAUGCCAUGGACUUUAUCA-3′ |
| COL6A1#3
antisense |
5′-UGAUAAAGUCCAUGGCAUCGACGGC-3′ |
TaqMan probes
Collagen type IV alpha 1 (COL4A1), COL6A1, and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH) TaqMan probes were
purchased from Thermo Fisher Scientific, Inc.
Cell lines and cell cultures
The human breast cancer cell line, MDA-MB-436
(20), was purchased from the
American Type Culture Collection and cultured in Roswell Park
Memorial Institute (RPMI)-1640 medium supplemented with 10% fetal
bovine serum (FBS; Tissue Culture Biologicals). The human
pancreatic cancer cell line, S2-VP10 (17), was purchased from the Cell Resource
Center for Biomedical Research, Institute of Development, Aging and
Cancer, Tohoku University (Miyagi, Japan), and cultured in
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS. Both cell lines were cultured at
37°C in a 5% CO2 atmosphere. Notably, we confirmed that
both cell lines were mycoplasma-negative.
Transfection of siRNAs and cell
proliferation assays
Cells (1×104/well) were plated into
12-well plates and cultured at 37°C in a 5% CO2
atmosphere. After overnight incubation, the cells were transfected
with the siRNAs indicated in the figure legends using Lipofectamine
RNAiMAX (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Briefly, 100 µl of OptiMEM (Thermo
Fisher Scientific, Inc.) containing 10 pmol of siRNA was mixed with
100 µl of OptiMEM containing 2.0 µl of Lipofectamine RNAiMAX. The
mixture was incubated at room temperature for 20 min. After
incubation, the mixture was added to the cells, which were
maintained at 37°C in a 5% CO2 atmosphere. Three hours
later, the culture medium was replaced with fresh medium. After
siRNA transfection, the indicated amount of type VI collagen, 1 ml
of conditioned medium, or 1 ml of DMEM supplemented with 10% FBS
was added to the cells. The cells were then cultured for 72 h at
37°C in a 5% CO2 atmosphere and subsequently subjected
to a cell proliferation assay and cell lysate preparation. In some
experiments, the siRNA-treated conditioned medium was collected at
the end of the culture period. The conditioned medium was
fractionated with Amicon Ultra-4 or Ultra-15 Centrifugal Filter
Units (molecular weight cutoff, 10 kDa; Merck KGaA), as indicated
in the section ‘Conditioned medium for the cell proliferation
assay’; the rescue activities of the fractions were assessed in
cell proliferation assays.
To evaluate cell proliferation, we calculated
proportional proliferation (% of control) assessed by methylene
blue staining as described in the section ‘Methylene blue
staining.’
Methylene blue staining
We assessed cell proliferation by methylene blue
staining as previously described with slight modifications
(21,22). Briefly, the medium was removed from
the wells and the plate was allowed to dry at room temperature.
Five hundred microliters of methanol was added to each well, and
the plate was kept at room temperature for 2 min to fix the cells.
Next, methanol was removed from the wells and the plate was allowed
to dry at room temperature. Thereafter, 500 µl of 0.05% methylene
blue was added to each well, and the plate was incubated at room
temperature for 30 min, after which the methylene blue solution was
removed. The plate was washed three times with distilled water; 1
ml of 3% HCl was added to each well to dissolve the methylene blue
stain, and the absorbance at 660 nm was measured using a SpectraMAX
M3 plate reader (Molecular Devices, LLC) with SoftMAX Pro Software
version 6.4.2 (Molecular Devices, LLC). All cell proliferation
assays were performed in triplicate and each siRNA, drug treatment,
and rescue experiment was repeated at least three times. Data are
expressed as the mean ± SD.
Conditioned medium for the cell
proliferation assay
A total of 2×105 S2-VP10 cells were
plated per well in a 6-well plate and cultured for two days at 37°C
in a 5% CO2 atmosphere. The conditioned medium obtained
after incubation was used to evaluate the ability to rescue the
repressed proliferation of KD cells. In some experiments, the
conditioned media were fractionated in size using Amicon Ultra-4 or
Ultra-15 Centrifugal Filter Units according to the manufacturer's
instructions. The conditioned medium was centrifuged at 5,000 × g
for 25 min at 4°C in the filter units, followed by filtration
through a 0.45-µm polyvinylidene difluoride membrane filter (Merck
KGaA); fractions containing molecules larger than 10 kDa (>10
kDa fraction) or smaller than 10 kDa (<10 kDa fraction) were
collected separately. Nine volumes of DMEM were added to one volume
of the >10 kDa fraction, whereas one volume of FBS was added to
nine volumes of the <10 kDa fraction. Each fraction was used in
the cell proliferation assay.
Concentration of <10 kDa fraction
by acetone precipitation
The proteins were concentrated by acetone
precipitation. Briefly, nine volumes of ice-cold acetone were added
to one volume of the <10 kDa fraction and the mixture was
incubated at −20°C for more than 2 h. This mixture was centrifuged
at 15,000 × g for 10 min at 4°C, and the pellet was subjected to
SDS-PAGE.
Preparation of cell lysates
The cells were washed three times with
phosphate-buffered saline (PBS, pH 7.4) and harvested. Lysis buffer
[50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.5% Triton X-100, and 10 mM
phenylmethylsulfonyl fluoride] was added to the cells, which were
then incubated on ice for 30 min. After cell lysis, the mixture was
centrifuged at 20,000 × g for 15 min at 4°C, and the supernatants
were used as cell lysates. Protein concentrations were determined
using a BCA Protein Assay Kit (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Ten micrograms of
cell lysate were subjected to western blotting.
Western blotting analysis
Western blotting was performed as previously
described (11,18). Briefly, an equal amount of 2X
concentrated sample buffer containing 2-mercaptoethanol (2-ME) [125
mM Tris-HCl (pH 6.8), 20% glycerol, 10% 2-ME, and 4% SDS] was added
to each cell lysate or supernatant and heated at 95°C for 5 min.
Under non-reducing conditions, a 2X concentrated sample buffer
without 2-ME was used. The mixture was loaded onto a 7.5% SDS-PAGE
gel, 5–20% SuperSep Ace precast gel (Wako Pure Chemical
Industries), or 15–20% SuperSep Ace precast gel (Wako Pure Chemical
Industries) and electrophoresed. Precision Plus Protein Dual Color
Standards (Bio-Rad Laboratories, Inc.) were used as molecular
weight markers. The gel was transferred to a polyvinylidene
difluoride membrane (PerkinElmer), which was blocked with
Tris-buffered saline-Tween 20 [TBS-T; 20 mM Tris-HCl (pH 7.5), 150
mM NaCl, and 0.05% Tween 20] containing 5% skim milk (BD
Biosciences). The membrane was incubated with predetermined
antibodies and washed with TBS-T. All primary antibodies, except
for the anti-COL6A1 (N-term) and anti-COL6A2 antibodies, were
diluted to 1 µg/ml with TBS-T containing 5% skim milk prior to use.
The anti-COL6A1 (N-term) and anti-COL6A2 antibodies were diluted to
1 µg/ml with Can Get Signal® (Toyobo). Bound antibodies
were detected with horseradish peroxidase-labeled anti-mouse or
anti-rabbit IgG secondary antibodies diluted to 0.25 µg/ml in TBS-T
containing 5% skim milk. The washed membrane was developed with ECL
Western Blotting Detection Reagent (GE Healthcare) and visualized
on Hyperfilm ECL (GE Healthcare) according to the manufacturer's
instructions. To reuse the membrane for some experiments, it was
incubated at 55°C for 30 min in stripping buffer [62.5 mM Tris-HCl
(pH 6.5), 100 mM 2-ME, and 2% SDS] and reprobed with another
antibody as previously described (23).
Coomassie Brilliant Blue (CBB)
staining
CBB staining was performed using a Rapid Stain CBB
Kit (Nacalai Tesque) according to the manufacturer's
instructions.
RNA extraction and cDNA synthesis
RNA was extracted and purified using an RNeasy Mini
Kit (Qiagen) according to the manufacturer's instructions.
cDNA was synthesized using purified RNA and
SuperScript III (Thermo Fisher Scientific, Inc.) as follows.
Briefly, 500 ng of RNA was mixed with 1 µl of 50 µM oligo
d(T)20 (Thermo Fisher Scientific, Inc.) and 1 µl of 10
mM dNTP Mix (Thermo Fisher Scientific, Inc.). The mixture was
adjusted to 13 µl with sterile distilled water, followed by heating
at 65°C for 5 min. After heat denaturation, the mixture was
incubated at 4°C for 1 min. Next, 4 µl of 5X First-Strand Buffer
(Thermo Fisher Scientific, Inc.), 1 µl of 0.1 M DTT (Thermo Fisher
Scientific, Inc.), 1 µl of RNaseOUT (Thermo Fisher Scientific,
Inc.), and 1 µl of SuperScript III were added to the mixture. For
cDNA synthesis, the mixture was incubated at 50°C for 30 min,
followed by denaturation at 75°C for 15 min. The cDNA was used as a
template in RT-qPCR.
Reverse transcription quantitative
PCR
The indicated mRNA expression was assessed by
RT-qPCR using Rotor Gene Q (Qiagen) according to the manufacturer's
instructions. Briefly, a reaction mixture containing 10 µl of 2X
TaqMan Gene Expression Master Mix (Thermo Fisher Scientific, Inc.),
1 µl TaqMan probe (Thermo Fisher Scientific, Inc.), 1 µl cDNA, and
8 µl dH2O was prepared and subjected to qPCR. Cycling
conditions were as follows: Pre-denaturation at 95°C for 10 min;
denaturation at 95°C for 15 sec; annealing and extension at 60°C
for 1 min; number of cycles, 40. After the reaction, the relative
ΔCq of the target gene (ΔΔCq) was calculated using GAPDH
mRNA as an internal standard (24).
The expression level of COL6A2 mRNA was compared to that of
COL6A1 mRNA. All RT-qPCR experiments were performed in
duplicate, and each RT-qPCR experiment was repeated at least three
times. Data are expressed as the mean ± SD.
Statistical analysis
All statistical analyses were carried out with Exsus
version 8.1.0 software (CAC Croit Corp.), and were based on at
least three independent experiments; the data are expressed as the
mean ± SD. Dunnett's multiple comparison test was used to determine
statistical differences among the four groups. Student's t-test was
used to determine statistically significant differences between two
groups. P<0.05 was considered as statistically significant.
Results
Expression of NTH α1(VI) by S2-VP10
cells: COL6A1 silencing reduces cell proliferation and NTH α1(VI)
expression
S2-VP10 cells expressed the NTH α1(VI)/COL6A1 chain
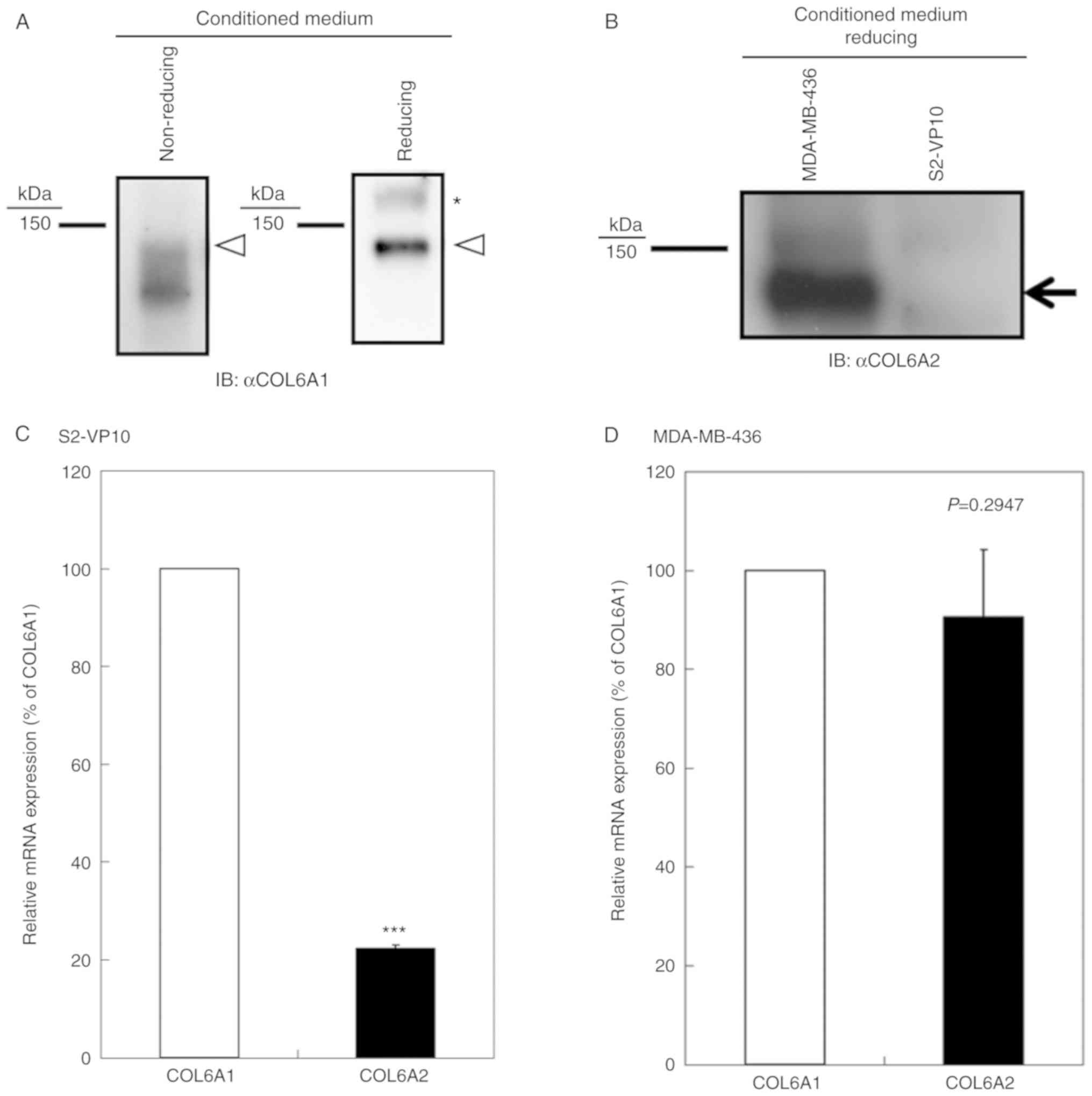
(Fig. 1A), whereas only faint
expression of the COL6A2 polypeptide was found (Fig. 1B). Notably, a smear band was
observed under non-reducing conditions (Fig. 1A). It is known that COL6A1 contains
cysteine residues (1). Therefore,
the smear band may have resulted from differences in intramolecular
disulfide bonds. In addition, the COL6A2 mRNA expression
level was 22.3±0.7% of the COL6A1 mRNA expression level
(Fig. 1C). We found that MDA-MB-436
cells expressed COL6A1 and COL6A2 during our preliminary
experiments (data not shown). Thus, we compared the COL6A1
mRNA/COL6A2 mRNA ratio between S2-VP10 and MDA-MB-436 cells.
The results revealed that the COL6A2 mRNA expression level
was comparable to that of COL6A1 mRNA expression (90.5±13.6%
of the COL6A1 mRNA expression level) in the MDA-MB-436 cells
(Fig. 1D), indicating that NTH
α1(VI) is a major form of the COL6A1 gene product in S2-VP10
cells, as type VI collagen is composed of α1, α2, and α3 chains
(1,25), requiring equal amounts of each
chain.
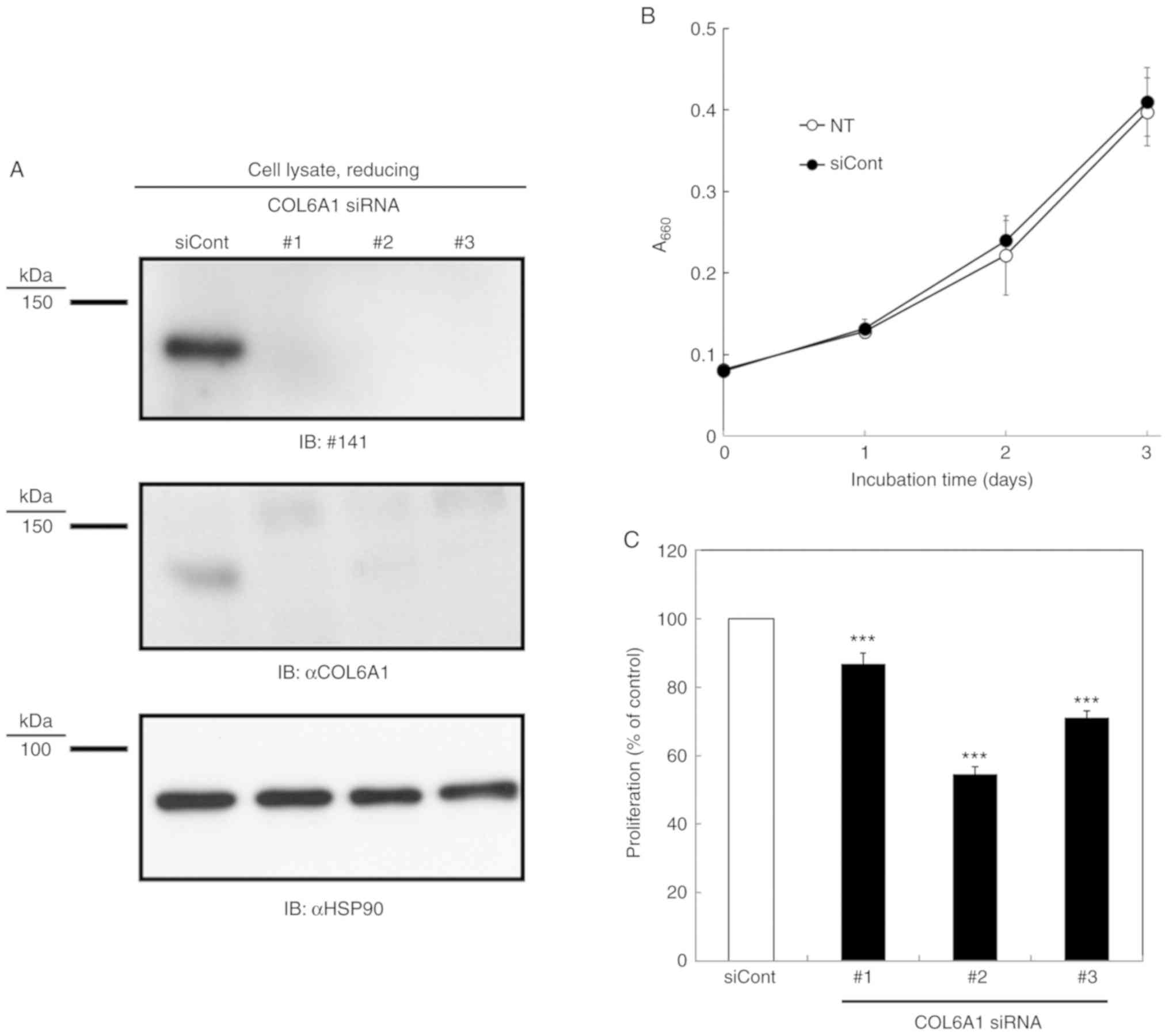
COL6A1 KD repressed COL6A1 expression (Fig. 2A). Next, we confirmed that COL6A1 KD
inhibited S2-VP10 cell proliferation as previously reported
(6). Representative proliferation
curves of S2-VP10 cells transfected with or without control siRNA
are shown in Fig. 2B, indicating
that the transfection of control siRNA does not affect the
proliferation of S2-VP10 cells. Moreover, Fig. 2C indicates that COL6A1 KD inhibited
the proliferation of S2-VP10 cells.
Requirement for fragmentation of NTH
α1(VI) for enhanced proliferation
Repressed proliferation of S2-VP10 cells by COL6A1
KD suggests that COL6A1 gene products including type VI
collagen, NTH α1(VI), and/or NTH α1(VI)-derived peptides are
involved in cell proliferation. The rescue activity was evaluated
by adding type VI collagen or the conditioned medium of S2-VP10
cells to COL6A1-silenced S2-VP10 cells; type VI collagen did not
show rescue activity (Fig. 3A),
whereas the conditioned medium showed rescue activity compared to
DMEM supplemented with 10% FBS (Fig. 3B
and C), indicating that NTH α1(VI) and/or derived peptides are
responsible for the rescue activity. To test whether NTH α1(VI) or
its fragments hold this activity, the conditioned medium was
fractionated by size; rescue activity with a strength similar to
that of non-fractionated conditioned medium was found in the <10
kDa fraction, whereas the >10 kDa fraction containing NTH α1(VI)
showed no rescue activity (Fig.
3C-E), indicating that enhanced proliferation requires NTH
α1(VI) fragmentation. To confirm that the active components in the
<10 kDa fraction were derived from NTH α1(VI), we assessed the
rescue activity of the <10 kDa fraction of COL6A1 KD cells. We
observed that the <10 kDa fraction of control siRNA-treated
cells was able to rescue cell proliferation (Fig. 4A and B), whereas the <10 kDa
fraction of COL6A1 KD cells appeared to exhibit lower rescue
activity (Fig. 4C). The slight
rescue activity of the <10 kDa fraction of COL6A1 KD cells
likely resulted from the small number of active fragments that may
have been produced from preexisting NTH α1(VI) prior to KD
treatment. Notably, we also confirmed that NTH α1(VI) with a size
of 140 kDa was present in the >10 kDa fraction (Fig. 4D) and that the <10 kDa fraction
contained only peptides less than 10 kDa in size (Fig. 4E). Therefore, fragmentation of NTH
α1(VI) into fragments of less than 10 kDa was responsible for the
enhanced proliferation of cancer cells.
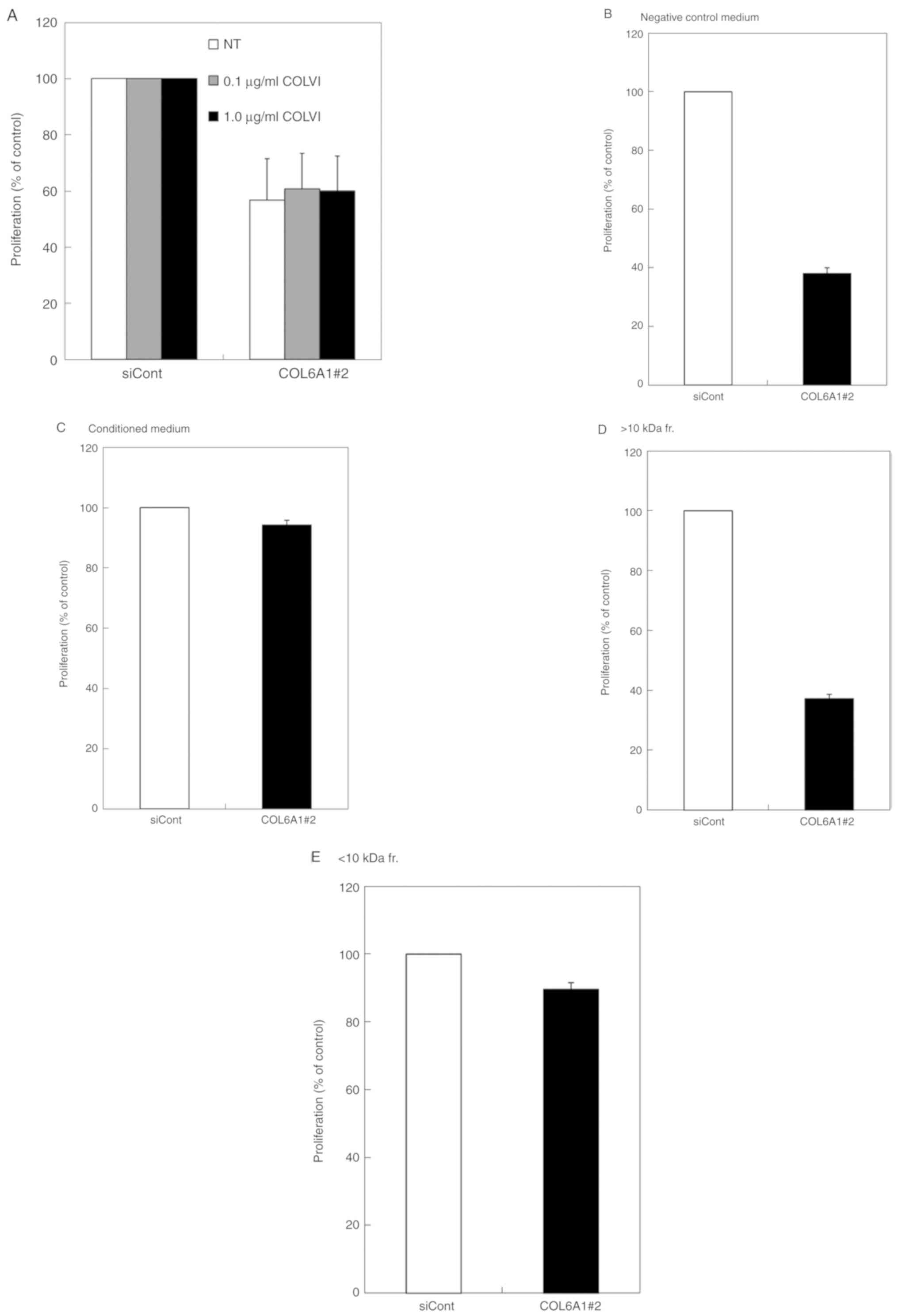 | Figure 3.Effect of differently sized fractions
from conditioned media on the proliferation of S2-VP10 cells. (A)
Effect of type VI collagen on the proliferation of S2-VP10 cells.
Transfection with COL6A1 siRNA#2 was the same as that shown in
Fig. 2. After siRNA transfection,
the indicated amount of type VI collagen (COLVI) was added to the
cells, and cell proliferation was determined by methylene blue
staining; data are expressed as the mean ± SD (n=3). (B-E) Rescue
activities of fractionated S2-VP10 conditioned media on the
proliferation of S2-VP10 cells; after siRNA transfection, COL6A1#2
transfected S2-VP10 cells were cultured for 72 h. Then, S2-VP10
cell proliferations using DMEM supplemented with 10% FBS (negative
control medium) (A), conditioned medium from S2-VP10 cells (B),
>10 kDa fraction (C), and <10 kDa fraction (D) were
determined by methylene blue staining. The fractions were prepared
using Amicon Ultra Centrifugal Filter Units. Data are expressed as
the mean ± SD (n=3). COL6A1, collagen type VI alpha 1 chain;
COL6A1#2, COL6A1#2 siRNA; fr., fraction; siCont, control siRNA; NT,
no treatment. |
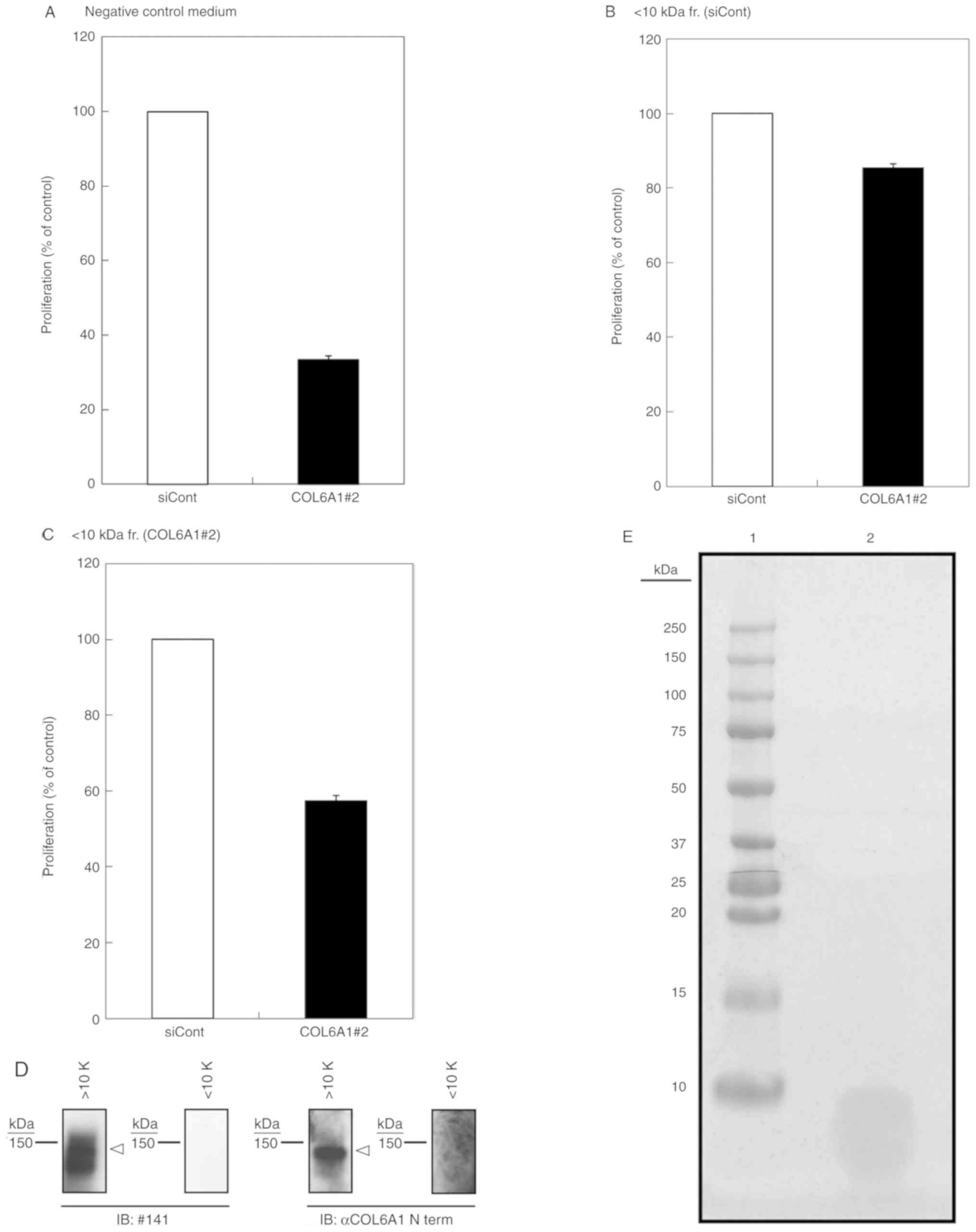 | Figure 4.Rescue activities of NTH
α1(VI)-derived peptides. (A-C) Rescue activities of
siRNA-transfected conditioned media. Transfection with COL6A1
siRNA#2 was the same as that shown in Fig. 2. After siRNA transfection, the cells
were cultured for 72 h, after which cell proliferation was
determined. The cell proliferations of S2-VP10 cells using DMEM
supplemented with 10% FBS as negative control (A), control
siRNA-treated <0 kDa fraction (B), and COL6A1 #2 siRNA-treated
<10 kDa fraction (C) were determined by methylene blue staining.
Data are expressed as the mean ± SD (n=3). (D) Representative
immunoblot images of the indicated fraction; the fraction
containing polypeptides >10 kDa (>10K) and polypeptides
<10 kDa (<10K) were subjected to western blotting under
reducing conditions, and probing was performed with antibody #141
(IB: #141) and anti-COL6A1 (N-term) antibody (IB: αCOL6A1 N term).
COL6A1 is indicated by white arrowheads. (E) Coomassie Brilliant
Blue (CBB) staining of the <10 kDa fraction. Acetone
precipitated <10 kDa fraction was subjected to SDS-PAGE and
stained with CBB staining. Lane 1, molecular weight marker; lane 2,
concentrated <10 kDa fraction. COL6A1, collagen type VI
alpha 1 chain; NTH α1(VI), non-triple helical type VI collagen α1
chain; COL6A1#2, COL6A1#2 siRNA; fr., fraction; IB, immunoblot;
siCont, control siRNA. |
Discussion
We showed that peptides of less than 10 kDa,
possibly derived from NTH α1(VI) fragmentation, are involved in the
proliferation of S2-VP10 cells. Higher expression of collagen type
VI alpha 1 chain (COL6A1) is observed in some tumors (4–6), and
its expression is associated with poor prognosis (7–9). Zhu
et al also indicated that COL6A1 overexpression promotes the
proliferation of prostate cancer LNCaP cells, and that COL6A1
expression is higher in patients with castration-resistant prostate
cancer (6). In addition, secretion
of the COL6A1 fragment is upregulated in some types of cancers,
including pancreatic cancer (16).
This suggests that the peptide fragments described herein are
involved in cell proliferation within tumors. Recently, Chen et
al reported that COL6A1 KD suppresses the proliferation and
migration of human aortic vascular smooth muscle cells (26), suggesting that the peptides are also
involved in vascular development.
Collagen-derived matricryptins (endostatin,
arresten, canstatin, and tumstatin) have anti-angiogenic and
antitumor activities (13,14). Hamano et al indicated that
tumstatin is generated by MMP-9 proteolysis in vivo
(27). It has also been reported
that S2-VP10 cells constitutively express many matrix
metalloproteases (MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, MMP-10, and
MMP-14) and show a highly metastatic phenotype (17). In addition, Willumsen et al
indicated that the serum level of MMP-generated COL6A1 fragments
was upregulated in some types of cancers (16). Similarly, the peptide fragments in
the present study may have been generated by one or more of these
proteases.
Our results suggest that the growth-promoting
mechanism of the peptides is not shared with type VI collagen. As
previously indicated, arresten, canstatin, and tumstatin show
anti-angiogenic and antitumor activities; these matricryptin
sequences are located in non-collagenous NC1 domains. The
biological activities of these matricryptins have also been
reported to be mediated through integrins (13,14).
In addition, endotrophin, the C5 Kunitz-like domain of COL6A3,
promotes malignant tumor progression via transforming growth factor
β signaling (15). This indicates
that non-collagenous regions in collagens have biological functions
and that these activities are mediated via specific receptors.
COL6A1 has three von Willebrand factor A (vWA) modules, one in its
N-terminus and two in its C-terminus (1,28,29).
Whittaker and Hynes suggested that the vWA modules in collagen
proteins, including COL6A1, are involved in protein-protein
interactions with other matrix proteins and possibly with cells
(30), suggesting that vWA modules
are important for peptide activity.
We showed that the mRNA and protein expression of
COL6A2 was low in S2-VP10 cells. This suggests that the chain
stoichiometry between COL6A1 and COL6A2 is different. Merl-Pham
et al also reported that the chain stoichiometry abnormality
between COL6A1 and COL6A2 was observed in the extracellular matrix
of human lung fibroblasts; they concluded that one of the causes of
the stoichiometry abnormality is the existence of NTH α1(VI)
(31). This strongly suggests that
the origin of the peptides is NTH α1(VI); however, the possibility
that the origin of the peptides is a different type VI collagen is
not completely excluded. Thus, it is essential to identify the
peptide sequences. We are currently investigating this fraction by
liquid chromatography-tandem mass spectrometry analysis; the
sequence may also provide information about peptide generation as
MMPs and other proteases contain consensus sequences for peptide
cleavage.
In conclusion, we demonstrated i) that peptides of
less than 10 kDa are involved in the proliferation of pancreatic
cancer S2-VP10 cells and ii) the peptides are derived from NTH
α1(VI) fragmentation.
Acknowledgements
The authors would like to thank Takashi Aijima, Aya
Kikitsu, and Naoko Takahara for their experimental support.
Funding
This work was supported in part by a grant from the
Strategic Research Foundation Grant-aided Project for Private
Universities from the Ministry of Education, Culture, Sport,
Science, and Technology, Japan, 2014–2018 (S1411005 to YI).
Availability of data and materials
The datasets and materials used during the present
study are available from the corresponding author upon reasonable
request.
Author's contributions
TS designed, conducted and performed the
experiments, and wrote the manuscript. KT, KS, HS, TH, YI and MM
designed, reviewed and edited the manuscript. MM designed,
conducted and supervised this project. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen P, Cescon M and Bonaldo P: Collagen
VI in cancer and its biological mechanisms. Trends Mol Med.
19:410–417. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iyengar P, Espina V, Williams TW, Lin Y,
Berry D, Jelicks LA, Lee H, Temple K, Graves R, Pollard J, et al:
Adipocyte-derived collagen VI affects early mammary tumor
progression in vivo, demonstrating a critical interaction in the
tumor/stroma microenvironment. J Clin Invest. 115:1163–1176. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
You WK, Bonaldo P and Stallcup WB:
Collagen VI ablation retards brain tumor progression due to
deficits in assembly of the vascular basal lamina. Am J Pathol.
180:1145–1158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujita A, Sato JR, Festa F, Gomes LR,
Oba-Shinjo SM, Marie SK, Ferreira CE and Sogayar MC: Identification
of COL6A1 as a differentially expressed gene in human astrocytomas.
Genet Mol Res. 7:371–378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Voiles L, Lewis DE, Han L, Lupov IP, Lin
TL, Robertson MJ, Petrache I and Chang HC: Overexpression of type
VI collagen in neoplastic lung tissues. Oncol Rep. 32:1897–1904.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu YP, Wan FN, Shen YJ, Wang HK, Zhang GM
and Ye DW: Reactive stroma component COL6A1 is upregulated in
castration-resistant prostate cancer and promotes tumor growth.
Oncotarget. 6:14488–14496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wan F, Wang H, Shen Y, Zhang H, Shi G, Zhu
Y, Dai B and Ye D: Upregulation of COL6A1 is predictive of poor
prognosis in clear cell renal cell carcinoma patients. Oncotarget.
6:27378–27387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Turtoi A, Blomme A, Bianchi E, Maris P,
Vannozzi R, Naccarato AG, Delvenne P, De Pauw E, Bevilacqua G and
Castronovo V: Accessibilome of human glioblastoma:
Collagen-VI-alpha-1 is a new target and a marker of poor outcome. J
Proteome Res. 13:5660–5669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hou T, Tong C, Kazobinka G, Zhang W, Huang
X, Huang Y and Zhang Y: Expression of COL6A1 predicts prognosis in
cervical cancer patients. Am J Transl Res. 8:2838–2844.
2016.PubMed/NCBI
|
|
10
|
Owusu-Ansah KG, Song G, Chen R, Edoo MIA,
Li J, Chen B, Wu J, Zhou L, Xie H, Jiang D and Zheng S: COL6A1
promotes metastasis and predicts poor prognosis in patients with
pancreatic cancer. Int J Oncol. 55:391–404. 2019.PubMed/NCBI
|
|
11
|
Sato T, Takano R, Tokunaka K, Saiga K,
Tomura A, Sugihara H, Hayashi T, Imamura Y and Morita M: Type VI
collagen α1 chain polypeptide in non-triple helical form is an
alternative gene product of COL6A1. J Biochem. 164:173–181. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davis GE, Bayless KJ, Davis MJ and
Meininger GA: Regulation of tissue injury responses by the exposure
of matricryptic sites within extracellular matrix molecules. Am J
Pathol. 156:1489–1498. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Monboisse JC, Oudart JB, Ramont L,
Brassart-Pasco S and Maquart FX: Matrikines from basement membrane
collagens: A new anti-cancer strategy. Biochim Biophys Acta.
1840:2589–2598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ricard-Blum S and Vallet SD: Matricryptins
network with matricellular receptors at the surface of endothelial
and tumor cells. Front Pharmacol. 7:112016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park J and Scherer PE: Adipocyte-derived
endotrophin promotes malignant tumor progression. J Clin Invest.
122:4243–4256. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Willumsen N, Bager C and Karsdal MA:
Matrix metalloprotease generated fragments of type VI collagen have
serum biomarker potential in cancer-a proof of concept study.
Transl Oncol. 12:693–698. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kitamura N, Iwamura T, Taniguchi S,
Yamanari H, Kawano MA, Hollingsworth K and Setoguchi T: High
collagenolytic activity in spontaneously highly metastatic variants
derived from a human pancreatic cancer cell line (SUIT-2) in nude
mice. Clin Exp Metastasis. 18:561–571. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Morita M, Sugihara H, Tokunaka K, Tomura
A, Saiga K, Sato T, Imamura Y and Hayashi T: Preparation and
partial characterization of monoclonal antibodies specific for the
nascent non-triple helical form of the type IV collagen alpha 1
chain. Biochem Biophys Rep. 9:128–132. 2016.PubMed/NCBI
|
|
19
|
Sato T, Takano R, Takahara N, Tokunaka K,
Saiga K, Tomura A, Sugihara H, Hayashi T, Imamura Y and Morita M:
Identification of a common epitope in the sequences of COL4A1 and
COL6A1 recognized by monoclonal antibody #141. J Biochem.
165:85–95. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cailleau R, Olivé M and Cruciger QV:
Long-term human breast carcinoma cell lines of metastatic origin:
Preliminary characterization. In Vitro. 14:911–915. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Elliott WM and Auersperg N: Comparison of
the neutral red and methylene blue assays to study cell growth in
culture. Biotech Histochem. 68:29–35. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dror R, Lederman M, Umezawa K, Barak V,
Pe'er J and Chowers I: Characterizing the involvement of the
nuclear factor-kappa B (NF kappa B) transcription factor in uveal
melanoma. Invest Ophthalmol Vis Sci. 51:1811–1816. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kaufmann SH, Ewing CM and Shaper JH: The
erasable western blot. Anal Biochem. 161:89–95. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Allamand V, Briñas L, Richard P, Stojkovic
T, Quijano-Roy S and Bonne G: ColVI myopathies: Where do we stand,
where do we go? Skelet Muscle. 1:302011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Z, Wu Q, Yan C and Du J: COL6A1
knockdown suppresses cell proliferation and migration in human
aortic vascular smooth muscle cells. Exp Ther Med. 18:1977–1984.
2019.PubMed/NCBI
|
|
27
|
Hamano Y, Zeisberg M, Sugimoto H, Lively
JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A and Kalluri R:
Physiological levels of tumstatin, a fragment of collagen IV alpha3
chain, are generated by MMP-9 proteolysis and suppress angiogenesis
via alphaV beta3 integrin. Cancer Cell. 3:589–601. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chu ML, Mann K, Deutzmann R,
Pribula-Conway D, Hsu-Chen CC, Bernard MP and Timpl R:
Characterization of three constituent chains of collagen type VI by
peptide sequences and cDNA clones. Eur J Biochem. 168:309–317.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cescon M, Gattazzo F, Chen P and Bonaldo
P: Collagen VI at a glance. J Cell Sci. 128:3525–3531. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Whittaker CA and Hynes RO: Distribution
and evolution of von Willebrand/integrin A domains: Widely
dispersed domains with roles in cell adhesion and elsewhere. Mol
Biol Cell. 13:3369–3387. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Merl-Pham J, Basak T, Knüppel L, Ramanujam
D, Athanason M, Behr J, Engelhardt S, Eickelberg O, Hauck SM,
Vanacore R and Staab-Weijnitz CA: Quantitative proteomic profiling
of extracellular matrix and site-specific collagen
post-translational modifications in an in vitro model of lung
fibrosis. Matrix Biol Plus. 1:1000052019. View Article : Google Scholar
|