Introduction
Endometrial cancer is one of the three commonly
diagnosed gynecological malignancies of the genital tract.
According to US cancer statistics, endometrial cancer ranks fourth
among women's invasive tumors worldwide, and there were an
estimated 61,880 new patients and 12,160 deaths in 2019 (1). In China, the incidence of endometrial
cancer is increasing and the age of onset is becoming younger
(2,3). Contrary to the overall downward trend
in the most common cancers, mortality from endometrial cancer
increased by about 2% per year between 2011 and 2015 (4). Patients with early endometrial cancer
have a relatively good prognosis, but those with advanced
endometrial cancer have a poor response to treatment and their
prognoses are worse. The prognosis of endometrial cancer is
affected by many factors including tumor grade, stage, histologic
subtype, lymph node metastasis and myometrial invasion. Lack of
effective treatment for patients with advanced endometrial cancer
reflects the need to study the molecular mechanisms of endometrial
cancer occurrence and development (5).
Cyclin-dependent kinases (CDKs) are a family of
serine/threonine kinases whose activity is derived from
heterodimeric complexes composed of catalytic kinase subunits and
regulatory cyclin subunits (6,7). They
play an important role in controlling the cell cycle,
transcription, and neuronal function (6). Multiple genetic and epigenetic events
lead to a general overactivity of the cell cycle CDK in human
cancers, and its inhibition may lead to both cell cycle arrest and
apoptosis (8). With regard to the
role of CDKs in the cell cycle or transcriptional regulation, CDK1,
CDK2, CDK3, CDK4 and CDK6 drive cell cycle, CDK7 regulates cell
cycle, CDK7, CDK8, CDK9, CDK10 and CDK11 regulate transcription,
and CDK5 regulates neuronal differentiation (6). Of the multiple CDKs involved in
transcriptional regulation, CDK9 is considered to be the most
important one (9). CDK9 is a
Cdc2-like Ser/Thr kinase that mainly binds to cyclin T1 and
constitutes the basal transcription factor, positive transcription
elongation factor b (p-TEFb), which phosphorylates the
carboxy-terminal domain (CTD) of the large subunit of RNA
polymerase II (RNAPII) and reaches the RNA transcription
elongation, thereby regulating cell proliferation, differentiation,
apoptosis, and DNA repair (10,11).
CDK9 is involved in the transcription of most eukaryotic cells,
including human tissues (12). CDK9
expression is significantly increased in different cellular
processes and different tissues, and has an important contribution
to the progression of various types of cancer (10). Recently, studies have found that
CDK9 is of critical importance in the development of many cancers,
including leukemia, cervical cancer, prostate cancer, glioblastoma,
breast cancer, melanoma and lung cancer (13–19).
In the present study, in order to investigate the
role of CDK9 in the progression of endometrial cancer, we firstly
evaluated its expression in endometrial cancer tissue, and then
used siRNA and inhibitors to suppress CDK9.
Materials and methods
Human endometrial cancer tissues
The specimens used in this study were collected from
32 patients with endometrial cancer treated at the Second Xiangya
Hospital of Central South University (Changsha, Hunan, China) from
January 2002 to December 2017. The age of the patients ranged from
39 to 69 years (average, 60.8 years). All patients accepted primary
surgery; the primary and metastatic tumor tissues were obtained by
the primary surgery. Upon tumor recurrence, 15 patients had pelvic
recurrence, 7 patients had vaginal metastasis, 1 patient had vulvar
metastasis, 3 patients had inguinal lymph node metastases, 3
patients had bone metastases, 2 patients had lung metastases, and 1
patient had liver metastases. The recurrent tumor tissues were
obtained by second surgery or biopsy. The tumor tissues of these 32
patients were collected at three different stages, including:
Primary tumor tissue, postoperative metastatic tissue, and
recurrent tumor tissue. The tumor tissues were fixed with
formaldehyde and embedded in paraffin. We collected data concerning
patient age; surgical method; histologic subtype;
surgical-pathological stage; pathological grade; recurrence site;
recurrence tissue acquisition method; patient survival status at
the end of follow-up: Progression-free survival (PFS), defined as
the interval between the date of primary surgery and the objective
tumor progression or death; overall survival (OS), defined as the
interval between the date of primary surgery to last follow-up or
death (Table I). The study was
approved by the Ethics Committee of The Second Xiangya Hospital of
Central South University (IRB protocol number: Study 181). All
patients signed a consent form for their tissues and clinical
information to be used for this research.
 | Table I.Clinical data for the endometrial
cancer patients (N=32). |
Table I.
Clinical data for the endometrial
cancer patients (N=32).
| Patient | Age (years) | Primary
surgery | Histologic
subtype | Stage | Grade | Recurrence
site | Method of recurrent
tissue obtained | PFS (months) | OS (months) | Patient status |
|---|
| 1 | 54 | TH and BSO and
lymphadenectomy | Carcinosarcoma | IVB | 3 | Vaginal | Biopsy | 4 | 9 | Deceased |
| 2 | 63 | RH and BSO and
lymphadenectomy | Clear cell
carcinoma | IVB | 3 | Vaginal | Biopsy | 6 | 11 | Deceased |
| 3 | 64 | RH and BSO and
lymphadenectomy | Mixed cell
tumors | IVA | 3 | Vaginal | Biopsy | 7 | 14 | Deceased |
| 4 | 58 | RH and BSO and
lymphadenectomy | Serous
carcinoma | IVB | 3 | Vaginal | Biopsy | 8 | 17 | Deceased |
| 5 | 55 | TH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIC2 | 2 | Pelvic | Biopsy | 9 | 20 | Deceased |
| 6 | 66 | TH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIC1 | 3 | Vaginal | Biopsy | 11 | 25 | Deceased |
| 7 | 68 | RH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIB | 2 | Pelvic | Biopsy | 12 | 26 | Deceased |
| 8 | 53 | RH and BSO and
lymphadenectomy | Serous
carcinoma | IVB | 3 | Bone | Biopsy | 13 | 27 | Deceased |
| 9 | 67 | TH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIC2 | 3 | Pelvic | Biopsy | 13 | 28 | Deceased |
| 10 | 61 | RH and BSO and
lymphadenectomy | Mucinous
carcinoma | IIIB | 2 | Inguinal lymph
node | Biopsy | 16 | 30 | Deceased |
| 11 | 39 | TH and BSO and
lymphadenectomy | Carcinosarcoma | IVB | 3 | Lung | Biopsy | 17 | 31 | Deceased |
| 12 | 59 | RH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIC1 | 2 | Vaginal | Biopsy | 16 | 36 | Deceased |
| 13 | 67 | RH and BSO and
lymphadenectomy | Serous
carcinoma | IVB | 3 | Liver | Biopsy | 20 | 37 | Deceased |
| 14 | 62 | TH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIA | 2 | Pelvic | Biopsy | 12 | 40 | Deceased |
| 15 | 69 | RH and BSO and
lymphadenectomy | Serous
carcinoma | IVB | 3 | Inguinal lymph
node | Biopsy | 13 | 54 | Deceased |
| 16 | 65 | RH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIA | 2 | Pelvic | Biopsy | 15 | 55 | Deceased |
| 17 | 63 | TH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIC2 | 2 | Pelvic | Biopsy | 16 | 57 | Deceased |
| 18 | 67 | RH and BSO and
lymphadenectomy | Mixed cell
tumors | IVA | 3 | Lung | Biopsy | 16 | 63 | Deceased |
| 19 | 63 | TH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIC1 | 2 | Pelvic | Biopsy | 18 | 70 | Deceased |
| 20 | 62 | RH and BSO and
lymphadenectomy | Clear cell
carcinoma | IIIB | 2 | Bone | Biopsy | 20 | 72 | Deceased |
| 21 | 60 | TH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIB | 3 | Pelvic | Biopsy | 25 | 80 | Deceased |
| 22 | 61 | TH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIC1 | 2 | Pelvic | Biopsy | 26 | 85 | Deceased |
| 23 | 67 | RH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIA | 3 | Pelvic | Biopsy | 30 | 96 | Deceased |
| 24 | 66 | TH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIC1 | 3 | Bone | Biopsy | 33 | 97 | Deceased |
| 25 | 53 | RH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIC1 | 2 | Pelvic | Biopsy | 40 | 109 | Deceased |
| 26 | 64 | RH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIA | 3 | Inguinal lymph
node | Biopsy | 43 | 120 | Deceased |
| 27 | 69 | TH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIB | 2 | Pelvic | Biopsy | 43 | 127 | Deceased |
| 28 | 60 | RH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIA | 3 | Vaginal | Biopsy | 51 | 139 | Deceased |
| 29 | 59 | TH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIC1 | 3 | Pelvic | Biopsy | 53 | 140 | Deceased |
| 30 | 58 | TH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIC1 | 3 | Vulvar | Biopsy | 53 | 148 | Deceased |
| 31 | 52 | TH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIB | 1 | Pelvic | Biopsy | 53 | 155 | Alive |
| 32 | 51 | RH and BSO and
lymphadenectomy | Endometrioid
carcinoma | IIIA | 2 | Pelvic | Biopsy | 57 | 163 | Alive |
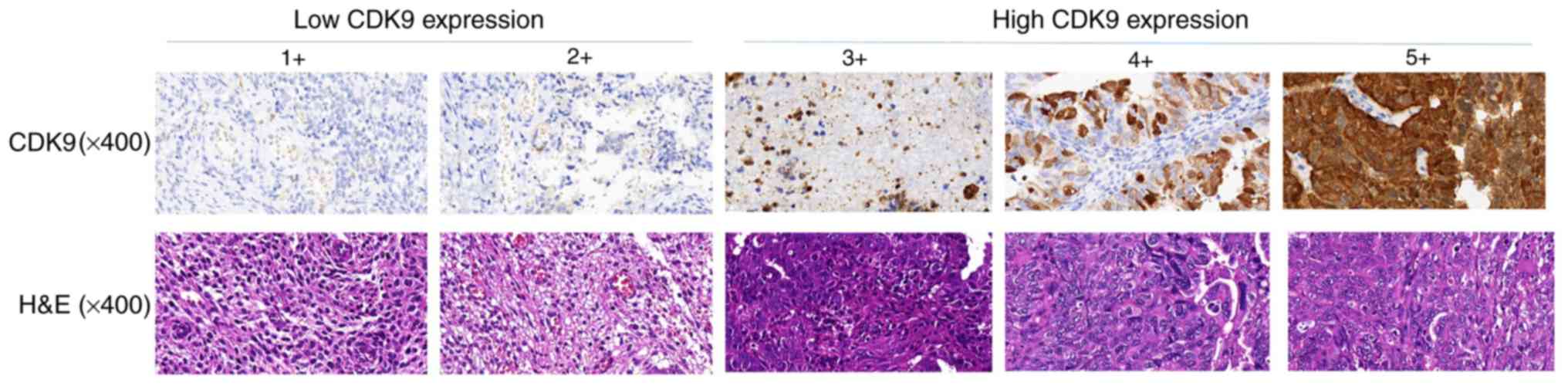
Immunohistochemistry
CDK9 expression was determined by standard
immunohistochemical protocol. In short, paraffin-embedded slides (4
µM) were baked at 60°C for 1 h and then dewaxed in xylene and
rehydrated by fractionated ethanol (100 and 95%). Antigen was
extracted by Dako Target Retrieval Solution (Dako; Agilent
Technologies, Inc.), and incubated with 3%
H2O2 for 10 min to eliminate endogenous
peroxidase activity. Thereafter, the slides were sealed with goat
serum for 1 h, and then polyclonal rabbit antibodies against human
CDK9 (cat. no. 2316; 1:50 dilution in 1% bovine serum albumin; Cell
Signaling Technology, Inc.) were added and incubated overnight. The
slides were fully covered with the anti-rabbit
SignalStain® Boost Detection Reagent (Cell Signaling
Technology) and placed in a humid chamber for 30 min, and then
SignalStain® DAB (Cell Signaling Technology) was added
to the slides to reveal the staining intensity. Subsequently, the
slides were counterstained with Hematoxylin QS (Vector
Laboratories, Burlingame, CA, USA) and fixed with VectaMount AQ
(Vector Laboratories, Inc.) for long-term storage. Two independent
pathologists evaluated the percentage of cells with positive
nuclear staining on the immunostained slides. The expression of
CDK9 was divided into five levels: i) 1+, <10% positive cells;
ii) 2+, 10–25% positive cells; iii) 3+, 26–50% positive cells; iv)
4+, 51–75% positive cells; v) 5+, >75% positive cells. Based on
the CDK9 expression scores of the primary endometrial cancer
samples, patients were divided into the following two groups: Low
CDK9 group (CDK9 staining score ≤2) and high CDK9 group (CDK9
staining score ≥3). CDK9 stained images were captured with an
Olympus BX51microscope (original magnification, ×400) (Olympus
Corporation of the Americas) and a Spot RT digital camera
(Diagnostic Instruments).
Endometrial cancer cell lines and
reagents
The human endometrial cancer cell lines HEC-1A,
ARK-2H, EC-1B, RL95-2 and SPAC1S were obtained from the American
Type Culture Collection (ATCC; Rockville, MD, USA). AN3CA was
obtained from the German Collection of Microorganisms and Cell
Cultures (DSMZ; Braunschweig, Germany). Ishikawa cells were
obtained from the Central Cell Services Facility at the Cancer
Research UK (CRUK). All endometrial cancer cell lines were
maintained in RPMI-1640 (Invitrogen; Life Technologies) containing
10% fetal bovine serum (FBS) and 1% penicillin/streptomycin.
Monoclonal rabbit anti-human CDK9 antibody (cat. no. 2316; Cell
Signaling Technology, Inc.) was purchased from Cell Signaling
Technology, Inc.. The highly selective CDK9 inhibitor LDC000067
(abbreviated as LDC067) was purchased from Selleck Chemicals. Human
non-specific small interfering RNA (siRNA) and CDK9-targeting siRNA
(59-GCUGCUAAUGUGCUUAUCA-39) were purchased from Merck KGaA.
Lipofectamine RNAiMax was purchased from Thermo Fisher Scientific,
Inc. Apoptosis-related antibodies were obtained from Cell Signaling
Technology, Inc..
Western blotting
Total protein lysate was extracted from cells using
RIPA lysis buffer (Sigma-Aldrich; Merck KGaA). Thirty micrograms of
the protein sample was separated on NuPage 4–12% Bis-Tris gel
(Thermo Fisher Scientific, Inc.) and transferred to nitrocellulose
membranes (Bio-Rad Laboratories, Inc.), and then incubated with the
following primary antibodies in 5% milk with tris-buffered saline
Tween 20: CDK9 (cat. no. 2316; 1:1,000 dilution; Cell Signaling
Technology, Inc.), Mcl-1 (cat. no. 39224; 1:1,000 dilution; Cell
Signaling Technology, Inc.), PARP (cat. no. 9532; 1:1,000 dilution;
Cell Signaling Technology, Inc.), Bax (cat. no. 2772; 1:1,000
dilution; Cell Signaling Technology, Inc.) and tubulin (cat. no.
3873; 1:1,000 dilution; Cell Signaling Technology, Inc.). Next, the
membranes were further incubated with a goat anti-rabbit IRDye
800CW (926–32,211; 1:5,000 dilution; Li-Cor Biosciences) or goat
anti-mouse IRDye 680LT secondary antibody (926–68,020; 1:15,000
dilution; Li-Cor Biosciences) at room temperature for 2 h. The
membranes were then scanned by an Odyssey CLx device (Li-Cor
Biosciences) to detect bands. Finally, protein bands were
quantified by densitometry with Odyssey v. 3.0 software (Li-Cor
Biosciences).
Knockout of CDK9 through siRNA
transfection and MTT assay
CDK9 in endometrial cancer cells was knocked out by
transfection of synthetic CDK9 siRNA. AN3CA and SPAC1S cells were
seeded into 12-well plates at a density of 4×104
cells/well or into 96-well plates at a density of 2×103
cells/well. CDK9 siRNA (5′-GCUGCUAAUGUGCUUAUCAUCA-3′) was
synthesized with Lipofectamine RNAiMax reagent (Thermo Fisher
Scientific, Inc.) at concentrations of 10, 20 and 40 nM,
respectively, according to the protocol provided by the
manufacturer. Negative control group used non-specific siRNA (40
nM). Five days after CDK9 siRNA transfection, proteins from AN3CA
and SPAC1S cells were extracted for analysis of cell proliferation
by MTT assay. Then, at the end of cell transfection, 20 µl MTT
reagent (5 mg/ml; Merck KGaA) was added to each plate, and then
cells were incubated at 37°C for another 4 h. After removing the
supernatant, the obtained intracellular formazan crystals were
dissolved in 100 µl per well of acid isopropanol. SpectraMax 340PC
microplate reader (Molecular Devices, LLC) was used to evaluate the
absorbance of the samples.
Suppression of CDK9 expression by
LDC067 inhibitor and MTT assay
LDC067 is able to decrease the expression of CDK9 in
various cancer cell lines at a concentration of 10 µM (20). AN3CA and SPAC1S cells were plated
into a 96-well plate at a density of 4×103 cells/well,
or a 6-well plate at a density of 6×105 cells/well.
Prior to subsequent experiments, the cells were incubated for 2, 3,
or 5 days with increasing LDC067 concentrations (0, 1.25, 2.5, 5.0
and 10 µM). After 5 days of LDC067 treatment, MTT assay was used to
study cell proliferation of AN3CA and SPAC1S cells.
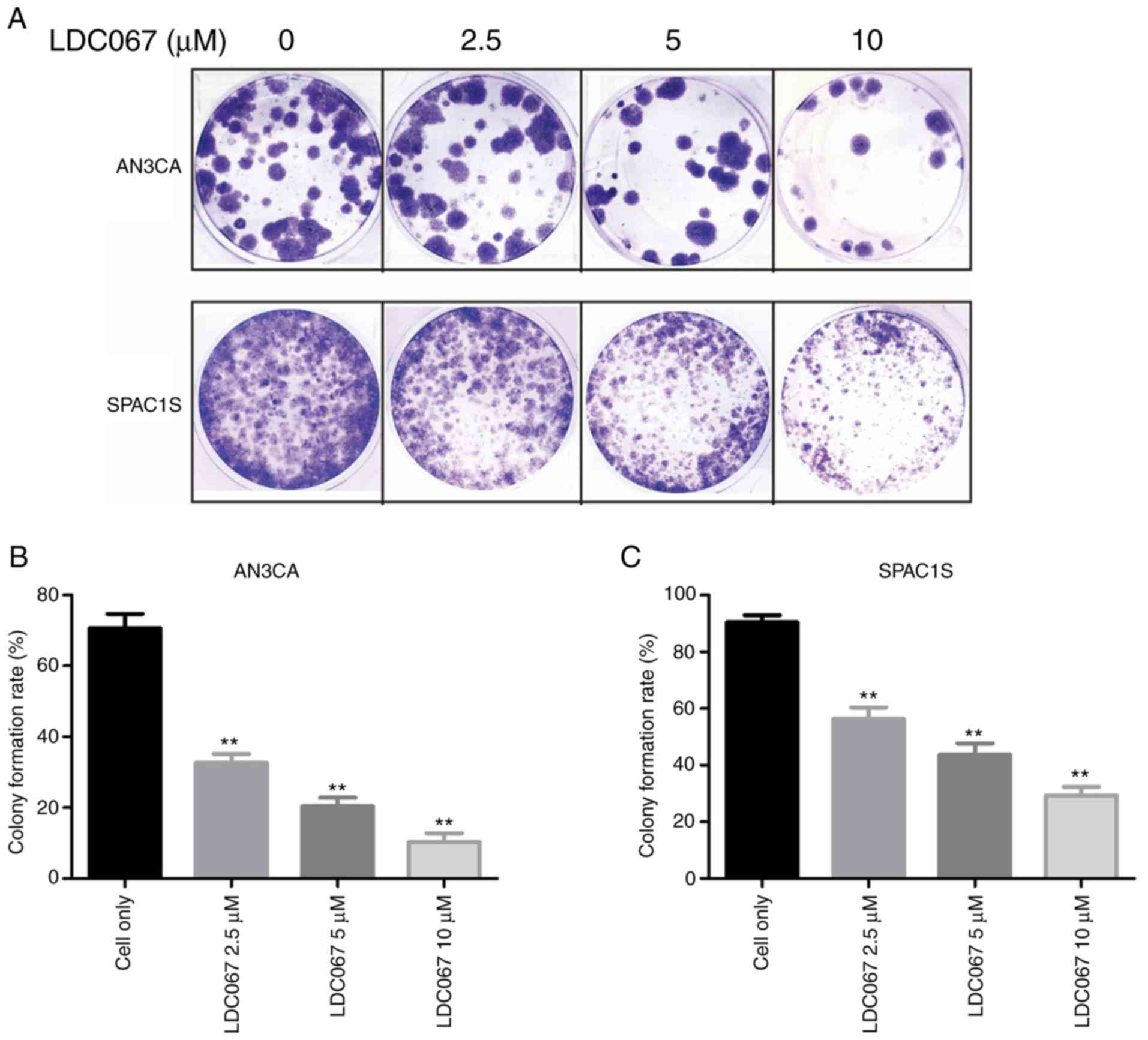
Colony formation assay
Colony formation assay is a commonly used method to
assess cell viability and proliferation capacity. AN3CA and SPAC1S
cells were seeded into 12-well plates at a density of 100
cells/well and treated with different concentrations of CDK9
inhibitor LDC067 (0, 2.5, 5 and 10 µM). Incubation lasted for 14
days, and then methanol was used to fix these colonies for 10 min.
After being washed three times with PBS, the cells were stained
with 10% Giemsa solution (Merck KGaA) for 20 min. Colonies were
rinsed under running water and then dried naturally. Finally, an
Olympus digital camera was used to capture images of the (original
magnification, ×1) colonies.
Wound-healing assay
Wound healing assay was used to analyze the effect
of CDK9 inhibitor LDC067 on the migration activity of endometrial
cancer cells. AN3CA and SPAC1S cells were plated into 6-well at a
density of 4×105 cells/well plates and incubated
overnight. A 30-µl sterile pipette was used to scrape two parallel
lines at the adhered cell layer. Next, 5 µM of LDC067 was added to
the medium, and incubated in a low serum medium containing 2% fetal
calf serum for 72 h (21,22). Although the serum-free medium can
prevent cell proliferation, it also increases the number of
apoptotic cells, which can affect the conclusion of the wound
healing assay. After 0, 24 and 48 h of treatment with LDC067,
images of the wounds were obtained respectively, with a Nikon
microscope (original magnification, ×200) (diagnostic instrument)
equipped with Zen Imaging software (Carl Zeiss). Wound width was
assessed by the distance between the two edges of the scratch at 5
sites in each image. The relative cell migration distance was
calculated by the following formula: Wound width at the 0 h time
point-Wound width at the observed time point/Wound width at the 0 h
time point.
Statistical analysis
Statistical analysis was carried out with the
assistance of GraphPad Prism 7 (GraphPad Software, Inc.) or SPSS
26.0 (IBM, Corp.). CDK9 scores in primary tumor tissue, recurrent
tumor tissue, and metastatic tumor tissue were compared through
Friedman's test. PFS and OS were analyzed using Kaplan-Meier
survival curves with log-rank tests for significance. The
χ2 test was used to evaluate the relationship between
CDK9 expression and endometrial cancer clinical-pathological
parameters. One-way ANOVA was used for the group comparison, and
Tukey's test was performed for pairwise comparison. We considered a
difference statistically significant at P-value <0.05.
Results
Expression of CDK9 in primary
endometrial cancer tissue and its matched metastasis and recurrent
endometrial cancer tissue
We used immunohistochemistry to compare the
expression of CDK9 in different endometrial cancer tissues. Based
on CDK9 expression scores in primary endometrial cancer samples,
patients were divided into the following two groups: Low CDK9 group
(CDK9 staining score ≤2; 59.4%) and high CDK9 group (CDK9 staining
score ≥3; 40.6%) (Fig. 1). There
was no statistical difference in the surgical-pathological stage
(P=0.5993, based on the χ2 test), pathological grade
(P=0.8206, based on the χ2 test), and histologic subtype
(P=0.7224, based on the χ2 test) between the patients
with low CDK9 expression and those with high CDK9 expression
(Table II). We examined the
expression of CDK9 in primary, metastatic, and recurrent tumor
specimens from 32 patients with endometrial cancer. CDK9 is mainly
located in the nucleus. Although the expression level of CDK9 in
metastatic and recurrent endometrial cancer tissues was not
statistically different (P>0.999, based on the Friedman's test),
the CDK9 expression level in primary endometrial cancer tissues was
significantly lower than that in metastatic and recurrent
endometrial cancer tissues. These differences were statistically
significant (metastatic vs. primary, P<0.001; recurrent vs.
primary, P<0.001, based on Friedman's test) (Fig. 2A).
 | Table II.Association between CDK9 expression
and clinicopathological features of the endometrial cancer
cases. |
Table II.
Association between CDK9 expression
and clinicopathological features of the endometrial cancer
cases.
|
|
| CDK9
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | No. of cases | Low | High | P-value |
|---|
| All patients | 32 | 19 | 13 |
|
| Stage |
|
|
|
|
| IV | 9 | 6 | 3 | 0.5993 |
|
III | 23 | 13 | 10 |
|
| Grade |
|
|
|
|
| 3 | 18 | 11 | 7 | 0.8206 |
| ≤2 | 14 | 8 | 6 |
|
| Histologic
subtype |
|
|
|
|
|
Endometrioid carcinoma | 21 | 12 | 9 | 0.7224 |
|
Mucinous carcinoma | 1 | 1 |
|
|
| Serous
carcinoma | 4 | 3 | 1 |
|
| Clear
cell carcinoma | 2 | 2 | 0 |
|
| Mixed
cell tumors | 2 | 1 | 1 |
|
|
Carcinosarcoma | 2 | 0 | 2 |
|
Relationship between CDK9 expression
and patient prognosis
Kaplan-Meier survival curve showed that compared
with patients with endometrial cancer in the CDK9 low expression
group (CDK9 staining score ≤2), those in the CDK9 high expression
group (CDK9 staining score ≥3) had significantly shorter PFS and
OS, and the differences were statistically significant
(P<0.0001, based on the log-rank test) (Fig. 2B and C). Specifically, the median
PFS and OS of the CDK9 low-expression group were 39 and 96 months,
while the median PFS and OS of the CDK9 high-expression group were
14 and 26 months. These results suggest that elevated CDK9
expression is closely associated with poor prognosis in patients
with endometrial cancer.
Expression of CDK9 protein in various
endometrial cancer cell lines
We detected the expression of CDK9 protein in
different endometrial cancer cell lines (AN3CA, ARK-2, HEC-1A,
HEC-1B, lshikawa, RL95-2 and SPAC1S) by western blot analysis. The
results showed that CDK9 was expressed in all endometrial cancer
cell lines, as shown in Fig. 3.
Effect of CDK9 siRNA on the
proliferation of endometrial cancer cells
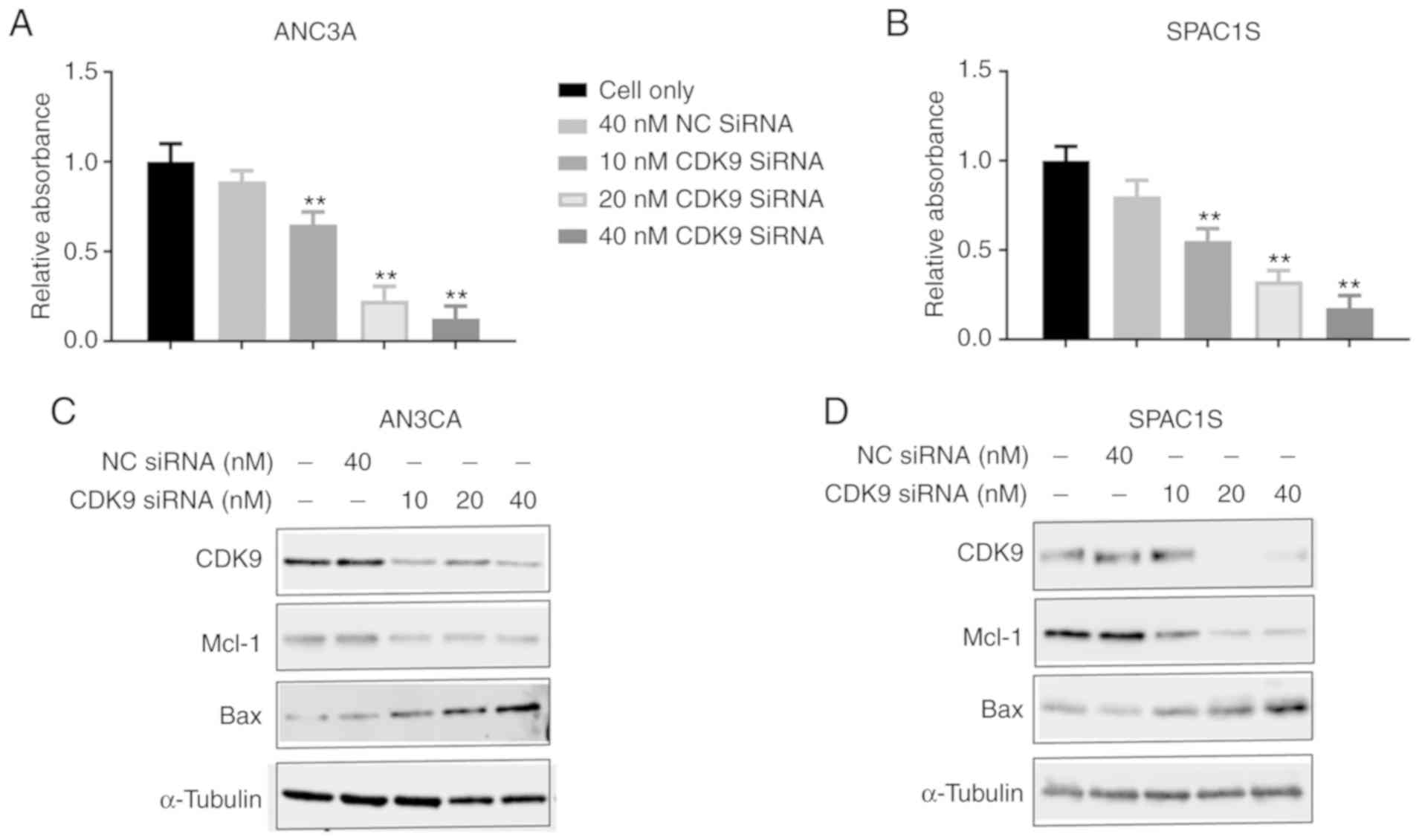
We transfected AN3CA and SPAC1S cell lines with CDK9
siRNA to knock down CDK9 expression to confirm the function of CDK9
in the proliferation and progression of endometrial cancer cells.
The cell lines were transfected with increasing concentrations of
CDK9 siRNA (10–40 nM) for 5 days, and we discovered that cell
viability of both cell lines was significantly and dose-dependently
inhibited. This was absent in the non-specific siRNA transfected
cells (40 nM NC siRNA) (Fig. 4A and
B). Western blot analysis confirmed that non-specific siRNA (NC
siRNA) had no effect on CDK9 expression, while CDK9 siRNA
significantly reduced CDK9 expression during a 2-day observation
period. Knockdown of CDK9 reduced the level of anti-apolipoprotein
myeloid cell leukemia-1 (Mcl-1) and increased the level of the
proapoptotic protein BCL2 associated X, apoptosis regulator (Bax)
(Fig. 4C and D). These data
illustrate the key role of CDK9 in the growth and proliferation of
endometrial cancer cells.
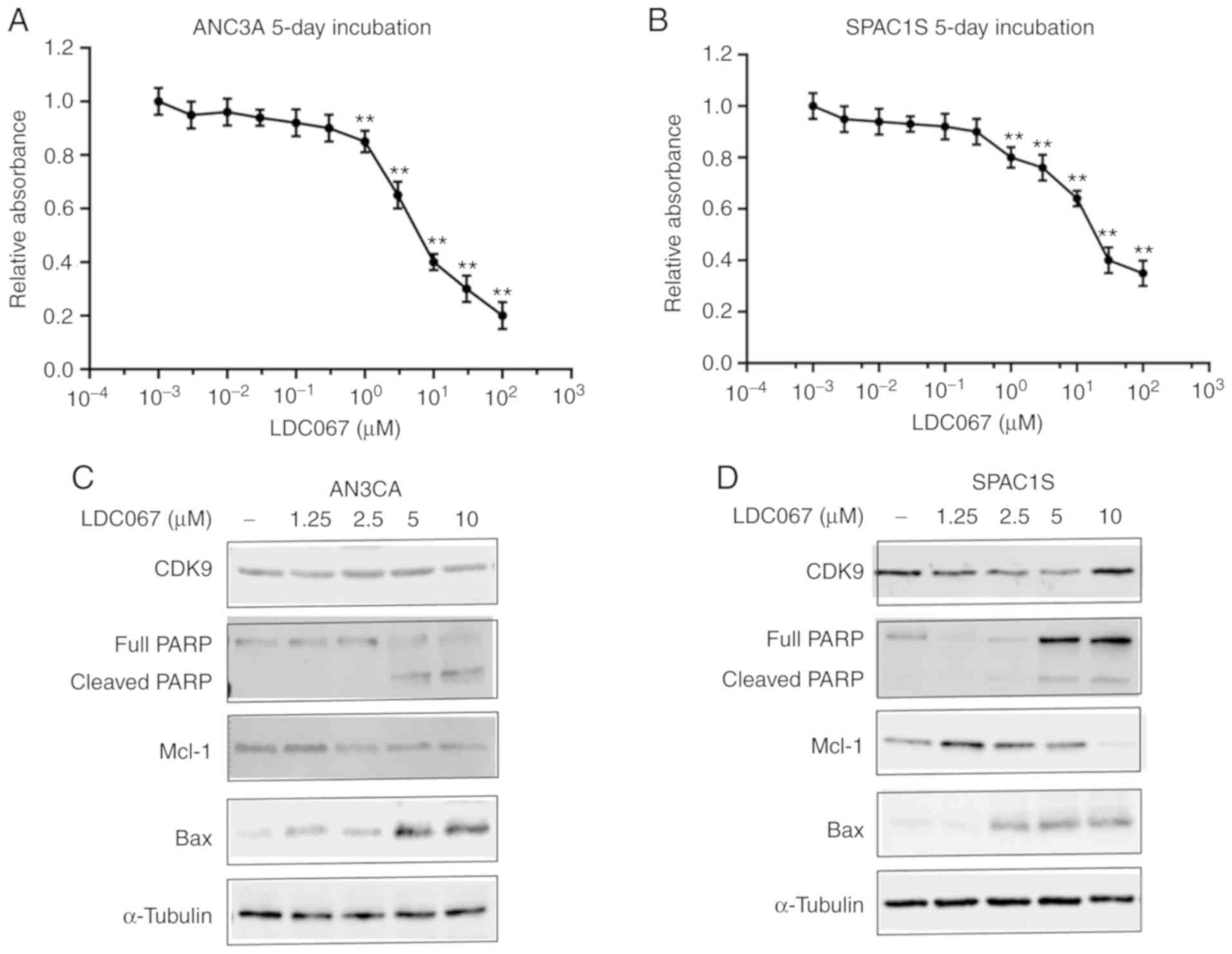
Effect of CDK9 inhibitor on
endometrial cancer cell proliferation
Subsequently, we examined the effects of a novel
CDK9-specific inhibitor LDC067 on the proliferation of endometrial
cancer cells. Similar to CDK9 siRNA transfection, CDK9 selective
inhibitor LDC067 decreased AN3CA and SPAC1S cell viability in a
dose-dependent manner during a specified 5-day observation period
(Fig. 5A and B). To further verify
the function of LDC067 on the transcriptional regulation of
endometrial cancer cells, we investigated the expression of
apoptosis-related proteins. AN3CA and SPAC1S cell lines were
incubated with 1.25, 2.5, 5.0 and 10 µM LDC067 for 48 h. The
results showed that LDC067 could increase the expression of
pro-apoptotic proteins cleaved poly(ADP-ribose) polymerase (PARP)
and Bax, while inhibiting the expression of anti-apoptotic protein
Mcl-1 in a concentration-dependent manner. Importantly, LDC067 only
inhibited the activity of CDK9, and there was no significant
difference in protein expression (Fig.
5C and D).
Effect of CDK9 inhibitor on
endometrial cancer cell colony formation
Colony formation assay is a commonly used method to
assess cell viability and proliferation capacity. Endometrial
cancer AN3CA and SPAC1S cells were exposed to 2.5, 5.0 and 10.0 µM
of LDC067 for 14 days. Colony formation assay showed that compared
with the untreated cells, both the numbers and sizes of colonies
formed in the LDC067 treatment group were significantly decreased
in a dose-dependent manner (P<0.01). These results suggest that
LDC067 can inhibit the ability of endometrial cancer to form
colonies in a concentration-dependent manner (Fig. 6).
Effect of CDK9 inhibitor on the
migration ability of endometrial cancer cells
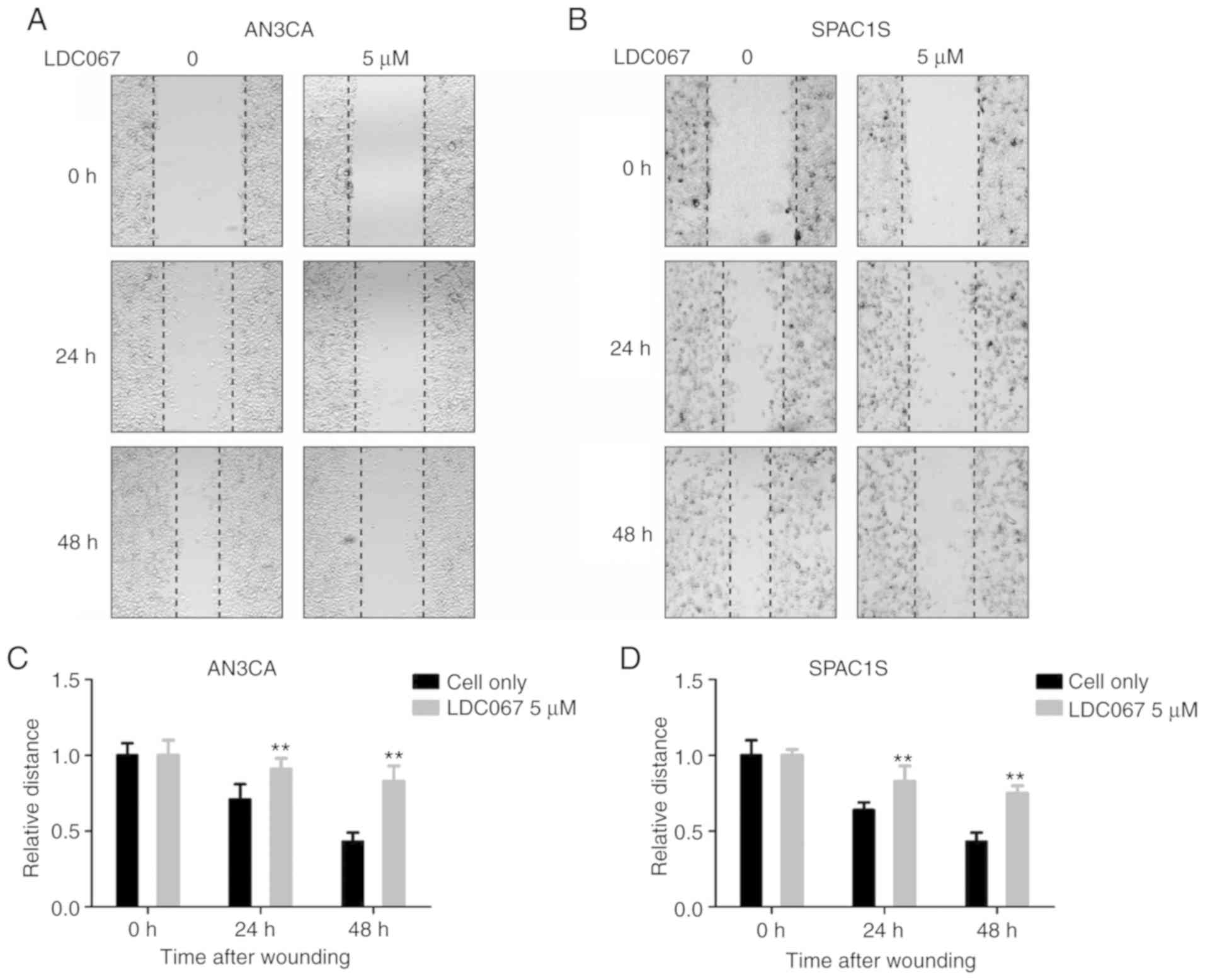
Tumor cell migration plays a significant role in
cancer metastasis and recurrence. We analyzed the effect of LDC067
on inhibiting the migration activity of endometrial cancer cells
through wound-healing assay. As shown in Fig. 7, after 24 and 48 h, the addition of
5 µM LDC067 brought about a time-dependent decrease in the
migration capacity of the two cell lines compared to the untreated
cells (P<0.05). In summary, the results indicate that LDC067
inhibits the migration capacity of endometrial cancer cells in a
time-dependent manner.
Discussion
Surgery is the main treatment for endometrial
cancer. Most patients in the early stage can be cured by surgery,
but treatment is limited for patients with metastasis and
recurrence (23). It is widely
recognized that laparoscopy is superior to laparotomy in most
patients with endometrial cancer. Laparoscopy can reduce
invasiveness, but still maintains safety and surgical
effectiveness, optimizes surgical outcomes, and improves the
cosmetic effect. The endoscopic single-site (LESS) surgery, 3 mm
laparoscopy (MiniLPS) and percutaneous system (PSS) include the
principal innovations in ultra-minimally invasive surgery
introduced during the last few years. Even when reducing the port
number or instrument size to reduce invasiveness, these
improvements still maintain the same surgical technique, efficacy
and safety of standard laparoscopy (LPS) (24–27).
Although there exists continuous research concerning the
tumorigenesis and development of endometrial cancer, it still
remains unclear which signaling pathways or related molecules
promote the occurrence and development of endometrial cancer. Many
studies confirm that overexpression of cyclin-dependent kinase 9
(CDK9) is directly related to tumor development (13–19). A
recent study found that CDK9 inhibition dephosphorylates the
SWI/SNF protein BRG1 (ATP-dependent helicase) and reactivates
epigenetic silenced genes in cancer, thereby restoring
tumor-suppressor gene expression (28). In the present study, we found that
CDK9 was overexpressed in metastatic and recurrent tissue samples
from patients with endometrial cancer, and survival analysis showed
that CDK9 can be used as a prognostic indicator for endometrial
cancer patients. This finding is consistent with that of Wang et
al who revealed that CDK9 expression is elevated in human
ovarian cancer cell lines and is also increased in metastatic and
recurrent ovarian tumor tissue compared with patient-matched
primary ovarian tumor tissue. In addition, increased CDK9 was
significantly associated with poor patient prognosis (29). This also is in accordance with
earlier observations, which showed that during the progression of
cervical cancer from pre-invasive lesions to squamous cell
carcinoma, the intracellular concentration of CDK9 increases
(30). Our results provide evidence
that CDK9 has the opportunity to become a potential prognostic
biomarker for endometrial cancer.
Research has shown that the CDK9 signaling system
may have important significance in the development of malignant
tumors and/or maintaining the cell phenotype (31). Inhibition of CDK9 kinase activity
significantly affects the gene transcription regulation, which
could be used as a strategy for cancer treatment interventions
(29). The antitumor effect of CDK9
inhibition has been attributed to MYC proto-oncogene, BHLH
transcription factor (MYC) and/or Mcl-1) inhibition (28). CDK9 is involved in many types of
cancer by recruiting p-TEFb to transcribe the downstream
proto-oncogene MYC involved in cell growth and cell cycle
progression (32). In addition,
over-stimulation of CDK9 increases the inhibitory effect of Mcl-1
on apoptosis, which will lead to cell survival and subsequent
cancer progression (33).
It has also been reported that in ovarian cancer,
leukemia, breast cancer, prostate cancer and liver cancer, reducing
the expression of CDK9 by siRNA can inhibit cell proliferation and
induce apoptosis (28,34–37).
We used liposome-mediated transfection of synthetic CDK9 siRNA to
silence or reduce CDK9 gene expression. The results demonstrated
that CDK9 siRNA transfection significantly reduced the expression
of CDK9 in a concentration-dependent manner. CDK9 siRNA can inhibit
the phosphorylation of RNAPII, resulting in the overall decrease in
mRNA levels, thereby reducing the level of anti-apoptotic protein
Mcl-1 and increasing the level of pro-apoptotic protein Bax. With
the increase in CDK9 siRNA transfection concentration, the
proliferation of endometrial cancer cells was significantly
inhibited.
CDK4/6 is part of the core cell cycle mechanism that
drives cell proliferation. In many types of human tumors, CDK4 and
CDK6 are abnormally activated through different mechanisms. The
chemical inhibitors of CDK4/6, palbociclib, ribociclib and
abemaciclib, have been used in multiple clinical trials worldwide
to treat more than 30 different types of tumors, including
endometrial cancer (38). A recent
study demonstrated that increased expression of phosphorylated
NPM/B23 (at Thr199) in cancer cells promotes its interaction with
CDK6 and plays a key role in endometrial tumorigenesis (39). By inhibiting CDK4/6 kinase,
palbociclib can inhibit the phosphorylation of NPM/B23 (Thr199) and
promote the expression of estrogen receptor (ER)α, ultimately
making hormone-refractory endometrial cancer sensitive to endocrine
therapy (39). Yet, clinical trials
of treatment with CDK9 inhibitors remain unsuccessful and involve
many adverse effects (40). LDC067
is a novel CDK9-specific inhibitor that has been used in different
types of cancer (20). In the
present study, LDC067 reduced the phosphorylation of RNAPII,
induced apoptosis in endometrial cancer cells, and inhibited cell
proliferation activity in a dose-dependent manner. These results
are consistent with those of Albert et al who found LDC067
targeted the ser2 of the RNAPII CTD, prevented phosphorylation and
induced apoptosis (20). Unlike
CDK9 siRNA, LDC067 only inhibits the activity of CDK9, while the
protein expression of CDK9 is not significantly reduced. It was
reported that gene expression profiling of tumor cells treated with
CDK9 inhibitor LDC067 showed a selective reduction of short-lived
mRNAs, including the MYC and Mcl-1 genes that regulate
proliferation and apoptosis (20).
In the clone formation and wound healing assays of
two endometrial cancer cell lines, the average number and size of
colonies formed after LDC067 treatment were significantly
inhibited, and the cell migration ability was also restrained in a
time-dependent manner. In a recent study, CDK9 knockout in
osteosarcoma cell lines and ovarian cancer cell lines triggered an
effective antitumor response (29,41).
These results are consistent with what we have observed.
Collectively, these results support CDK9 as a potential factor for
the progression of endometrial cancer.
In conclusion, our research demonstrated that high
expression of CDK9 is closely connected with the poor prognosis of
human endometrial cancer. In addition, inhibition of CDK9
significantly decreased endometrial cancer cell proliferation by
suppressing the phosphorylation of RNAPII. Our research indicates
that CDK9 may be a potential marker for the prognosis of
endometrial cancer and may become a new direction for
tumor-targeted therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (nos. 81801425, 81671437,
81771558 and 81702582) and the Natural Science Foundation of Hunan
Province in China (2018JJ3739).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SH wrote the manuscript and substantially
contributed to the design of the study and the interpretation of
the data. XF performed the literature search for this article. XX
and TH revised and corrected the manuscript critically for
important intellectual content. XX and TH assisted in analyzing the
data for the study and also contributed to the conception of the
study. TZ conceived and designed the study. All authors read and
approved the final manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Second Xiangya Hospital, Central South University (Changsha,
China) (IRB protocol number: Study 181), and written informed
consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wong YF, Cheung TH, Lo KW, Yim SF, Siu NS,
Chan SC, Ho TW, Wong KW, Yu MY, Wang VW, et al: Identification of
molecular markers and signaling pathway in endometrial cancer in
Hong Kong Chinese women by genome-wide gene expression profiling.
Oncogene. 26:1971–1982. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Liu Z, Yu X, Zhang X, Lü S, Chen
X and Lü B: The association between metabolic abnormality and
endometrial cancer: A large case-control study in China. Gynecol
Oncol. 117:41–46. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boren T, Xiong Y, Hakam A, Wenham R, Apte
S, Wei Z, Kamath S, Chen DT, Dressman H and Lancaster JM: MicroRNAs
and their target messenger RNAs associated with endometrial
carcinogenesis. Gynecol Oncol. 110:206–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Malumbres M and Barbacid M: Mammalian
cyclin-dependent kinases. Trends Biochem Sci. 30:630–641. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malumbres M: Cyclin-dependent kinases.
Genome Biol. 15:1222014. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shapiro GI: Cyclin-dependent kinase
pathways as targets for cancer treatment. J Clin Oncol.
24:1770–1783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang S and Fischer PM: Cyclin-dependent
kinase 9: A key transcriptional regulator and potential drug target
in oncology, virology and cardiology. Trends Pharmacol Sci.
29:302–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Franco LC, Morales F, Boffo S and Giordano
A: CDK9: A key player in cancer and other diseases. J Cell Biochem.
119:1273–1284. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kohoutek J: P-TEFb- the final frontier.
Cell Div. 4:192009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bagella L, MacLachlan TK, Buono RJ, Pisano
MM, Giordano A and De Luca A: Cloning of murine CDK9/PITALRE and
its tissue-specific expression in development. J Cell Physiol.
177:206–213. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brägelmann J, Dammert MA, Dietlein F,
Heuckmann JM, Choidas A, Böhm S, Richters A, Basu D, Tischler V,
Lorenz C, et al: Systematic kinase inhibitor profiling identifies
CDK9 as a synthetic lethal target in NUT midline carcinoma. Cell
Rep. 20:2833–2845. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rahaman MH, Kumarasiri M, Mekonnen LB, Yu
M, Diab S, Albrecht H, Milne RW and Wang S: Targeting CDK9: A
promising therapeutic opportunity in prostate cancer. Endocr Relat
Cancer. 23:T211–T226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mitra P, Yang RM, Sutton J, Ramsay RG and
Gonda TJ: CDK9 inhibitors selectively target estrogen
receptor-positive breast cancer cells through combined inhibition
of MYB and MCL-1 expression. Oncotarget. 7:9069–9083. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Whittaker SR, Barlow C, Martin MP, Mancusi
C, Wagner S, Self A, Barrie E, Te Poele R, Sharp S, Brown N, et al:
Molecular profiling and combinatorial activity of CCT068127: A
potent CDK2 and CDK9 inhibitor. Mol Oncol. 12:287–304. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baker A, Gregory GP, Verbrugge I, Kats L,
Hilton JJ, Vidacs E, Lee EM, Lock RB, Zuber J, Shortt J, et al: The
CDK9 inhibitor dinaciclib exerts potent apoptotic and antitumor
effects in preclinical models of MLL-rearranged acute myeloid
leukemia. Cancer Res. 76:1158–1169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Su YT, Chen R, Wang H, Song H, Zhang Q,
Chen LY, Lappin H, Vasconcelos G, Lita A, Maric D, et al: Novel
targeting of transcription and metabolism in glioblastoma. Clin
Cancer Res. 24:1124–1137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ajiro M, Sakai H, Onogi H, Yamamoto M,
Sumi E, Sawada T, Nomura T, Kabashima K, Hosoya T and Hagiwara M:
CDK9 inhibitor FIT-039 suppresses viral oncogenes E6 and E7 and has
a therapeutic effect on HPV-induced neoplasia. Clin Cancer Res.
24:4518–4528. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Albert TK, Rigault C, Eickhoff J, Baumgart
K, Antrecht C, Klebl B, Mittler G and Meisterernst M:
Characterization of molecular and cellular functions of the
cyclin-dependent kinase CDK9 using a novel specific inhibitor. Br J
Pharmacol. 171:55–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baranyi U, Winter B, Gugerell A, Hegedus
B, Brostjan C, Laufer G and Messner B: Primary human fibroblasts in
culture switch to a myofibroblast-like phenotype independently of
TGF beta. Cells. 8:7212019. View Article : Google Scholar
|
|
22
|
Lay V, Yap J, Sonderegger S and
Dimitriadis E: Interleukin 11 regulates endometrial cancer cell
adhesion and migration via STAT3. Int J Oncol. 41:759–764. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De U, Son JY, Sachan R, Park YJ, Kang D,
Yoon K, Lee BM, Kim IS, Moon HR and Kim HS: A new synthetic histone
deacetylase inhibitor, MHY2256, induces apoptosis and autophagy
cell death in endometrial cancer cells via p53 acetylation. Int J
Mol Sci. 19:27432018. View Article : Google Scholar
|
|
24
|
Espedal H, Fonnes T, Fasmer KE, Krakstad C
and Haldorsen IS: Imaging of preclinical endometrial cancer models
for monitoring tumor progression and response to targeted therapy.
Cancers (Basel). 11:18852019. View Article : Google Scholar
|
|
25
|
Gueli Alletti S, Rossitto C, Cianci S,
Restaino S, Costantini B, Fanfani F, Fagotti A, Cosentino F and
Scambia G: Telelap ALF-X vs standard laparoscopy for the treatment
of early-stage endometrial cancer: A single-institution
retrospective cohort study. J Minim Invasive Gynecol. 23:378–383.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scaletta G, Dinoi G, Capozzi V, Cianci S,
Pelligra S, Ergasti R, Fagotti A, Scambia G and Fanfani F:
Comparison of minimally invasive surgery with laparotomic approach
in the treatment of high risk endometrial cancer: A systematic
review. Eur J Surg Oncol. 46:782–788. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rossitto C, Cianci S, Gueli Alletti S,
Perrone E, Pizzacalla S and Scambia G: Laparoscopic,
minilaparoscopic, single-port and percutaneous hysterectomy:
Comparison of perioperative outcomes of minimally invasive
approaches in gynecologic surgery. Eur J Obstet Gynecol Reprod
Biol. 216:125–129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang H, Pandey S, Travers M, Sun H,
Morton G, Madzo J, Chung W, Khowsathit J, Perez-Leal O, Barrero CA,
et al: Targeting CDK9 reactivates epigenetically silenced genes in
cancer. Cell. 175:1244–1258.e26. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang J, Dean DC, Hornicek FJ, Shi H and
Duan Z: Cyclin- dependent kinase 9 (CDK9) is a novel prognostic
marker and therapeutic target in ovarian cancer. FASEB J.
33:5990–6000. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ramdass B, Maliekal TT, Lakshmi S, Rehman
M, Rema P, Nair P, Mukherjee G, Reddy BK, Krishna S and
Radhakrishna Pillai M: Coexpression of Notch1 and NF-kappaB
signaling pathway components in human cervical cancer progression.
Gynecol Oncol. 104:352–361. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Romano G: Deregulations in the
cyclin-dependent kinase-9- related pathway in cancer: Implications
for drug discovery and development. ISRN Oncol.
2013:3053712013.PubMed/NCBI
|
|
32
|
Lu H, Xue Y, Yu GK, Arias C, Lin J, Fong
S, Faure M, Weisburd B, Ji X, Mercier A, et al: Compensatory
induction of MYC expression by sustained CDK9 inhibition via a
BRD4-dependent mechanism. Elife. 4:e065352015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yin T, Lallena MJ, Kreklau EL, Fales KR,
Carballares S, Torrres R, Wishart GN, Ajamie RT, Cronier DM,
Iversen PW, et al: A novel CDK9 inhibitor shows potent antitumor
efficacy in preclinical hematologic tumor models. Mol Cancer Ther.
13:1442–1456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Caracciolo V, Laurenti G, Romano G,
Carnevale V, Cimini AM, Crozier-Fitzgerald C, Gentile Warschauer E,
Russo G and Giordano A: Flavopiridol induces phosphorylation of AKT
in a human glioblastoma cell line, in contrast to siRNA-mediated
silencing of Cdk9: Implications for drug design and development.
Cell Cycle. 11:1202–1216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Walsby E, Pratt G, Shao H, Abbas AY,
Fischer PM, Bradshaw TD, Brennan P, Fegan C, Wang S and Pepper C: A
novel Cdk9 inhibitor preferentially targets tumor cells and
synergizes with fludarabine. Oncotarget. 5:375–385. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rajput S, Khera N, Guo Z, Hoog J, Li S and
Ma CX: Inhibition of cyclin dependent kinase 9 by dinaciclib
suppresses cyclin B1 expression and tumor growth in triple negative
breast cancer. Oncotarget. 7:56864–56875. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang CH, Lujambio A, Zuber J,
Tschaharganeh DF, Doran MG, Evans MJ, Kitzing T, Zhu N, de
Stanchina E, Sawyers CL, et al: CDK9-mediated transcription
elongation is required for MYC addiction in hepatocellular
carcinoma. Genes Dev. 28:1800–1814. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fassl A and Sicinski P: Chemotherapy and
CDK4/6 inhibition in cancer treatment: Timing is everything. Cancer
Cell. 37:265–267. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lin CY, Lee LY, Wang TH, Hsu CL, Tsai CL,
Chao A and Lai CH: Palbociclib promotes dephosphorylation of
NPM/B23 at threonine 199 and inhibits endometrial cancer cell
growth. Cancers (Basel). 11:10252019. View Article : Google Scholar
|
|
40
|
Morales F and Giordano A: Overview of CDK9
as a target in cancer research. Cell Cycle. 15:519–527. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ma H, Seebacher NA, Hornicek FJ and Duan
Z: Cyclin-dependent kinase 9 (CDK9) is a novel prognostic marker
and therapeutic target in osteosarcoma. EBioMedicine. 39:182–193.
2019. View Article : Google Scholar : PubMed/NCBI
|