Introduction
According to a study by the World Health
Organization's International Agency for Research on Cancer (IARC),
lung cancer has the highest incidence and mortality of all
malignant tumors (1). In the United
States, its incidence and mortality in the male population have
gradually decreased, the incidence in females has remained
unchanged, and the overall mortality rate has exhibited a downward
trend (1). However, in China,
because the number of smokers is still increasing, the incidence of
lung cancer is also increasing. According to an epidemiological
survey conducted in 2002, patients aged 65 years or older accounted
for 55% of the total number of lung cancers and, among people older
than 65 years, the incidence of lung cancer was significantly
higher in developed countries than in less developed countries
(1). The age of onset of lung
cancer has decreased in recent years, with the incidence rate curve
tending to move forward by ~5 to 10 years (2). In addition, as the population of China
ages, the predicted incidence of lung cancer will continue to
rise.
In recent decades, the diagnosis and treatment of
lung cancer have considerably improved due to better understanding
of lung cancer pathogenesis and clinical treatment research.
Treatments for patients with advanced non-small-cell lung cancer
(NSCLC) have been sequentially launched and include first-,
second-, and third-line mature chemotherapy regimens (3). Notably, targeted therapy with
molecular targets as a classification standard has become a
milestone in the treatment of NSCLC (4). In contrast to the continually updated
iterative treatments for NSCLC, there have been no major
breakthroughs in the treatment of small-cell lung cancer (SCLC).
Since chemotherapy with or without radiotherapy remains the
preferred option for SCLC patients, its failure may significantly
reduce survival (3). Therefore,
identification of new treatment options or adjuvant treatment
options for SCLC has urgent practical needs.
Resveratrol (Res), chemically known as
trans−3,4,5-trihydroxystilbene, is a small molecule
polyphenolic compound found in the dried rhizomes and roots of the
sylvestris plant, the skin and seeds of the fruit of the grape
family, legumes, and peanuts. Res is a natural antioxidant with
multiple effects that has been used in multi-system research
(5). Zeng et al (6) revealed that the anticancer activity of
Res affects the entire process of cancer development and can
inhibit the proliferation of cancer cells and induce their
apoptosis. Our previous research results (7) revealed that Res significantly
inhibited the viability of H446 SCLC cells and had a synergistic
effect on cisplatin, indicating that their combination could
enhance the anticancer effects of cisplatin on H446 cells.
Based on the previous results, the present study
further clarified that Res affects cell oxidative stress and
mitochondrial membrane potential by interfering with the PI3K/Akt
signaling pathway in H446 cells and ultimately inhibits the
viability and promotes the apoptosis of H446 cells.
Materials and methods
Cell culture
The human SCLC H446 cell line was provided by the
Department of Respiratory and Critical Care Medicine, Tangdu
Hospital, Air Force Military Medical University. The cells were
cultured in RPMI-1640 medium containing 10% fetal calf serum
(Gibco; Thermo Fisher Scientific, Inc.) in a 37°C incubator with 5%
CO2. Cells in the logarithmic growth phase were used for
the subsequent experimental studies. The cells were randomly
divided into a blank control group, Res group, Res+
N-acetyl-L-cysteine (NAC) group, and NAC group. The treatment
concentrations of Res and NAC [a reactive oxygen species (ROS)
inhibitor] were 40 µg/ml and 2 mmol/l, respectively, in line with
our previous research results (7,8). Cells
in the Res+NAC group were first treated with 2 mmol/l of NAC for 2
h and then with 40 µg/ml Res for 24 h.
Cell viability assay
Cell viability was assessed by the MTT method. H446
cells in the logarithmic growth phase were seeded in a 96-well
plate at a density of 5×104 cells/well. After treatment
of the cells in each group, MTT (5 mg/ml, 15 µl/well) was added to
each well and incubated at 37°C for 4 h. Then, the culture medium
in each well was changed to 200 µl DMSO and the optical density
(OD) value of each well was measured at 492 nm using a Bio-Rad 550
microplate reader (Bio-Rad Laboratories, Inc.). Cell survival rates
were calculated according to the OD value of each well.
Apoptosis detection
After the various treatments were completed, the
cells (1×106) in each group were assessed for apoptosis
using flow cytometry. Briefly, the detection process was as
follows: Each group of cells was co-incubated with propidium iodide
(PI) and Annexin V-FITC solution (BD Biosciences) and processed
according to the instructions of the kit. The apoptosis level of
each group of cells was examined by flow cytometry (BD FACSVerse™;
BD Biosciences) and the results were analyzed by FlowJo (Flow Jo
7.6; Tree Star Inc.).
Intracellular ROS content
detection
After the various treatments were completed, the
cells (1×106) from each group were collected and the
differences in the ROS content of each group were determined by
flow cytometry. Briefly, the 2′,7′-dichlorodihydrofluorescein
diacetate (DCF-DA) stain (Beyotime Institute of Biotechnology, 10
µmol/l, 20 min of incubation at 37°C) was used to detect the ROS
contents of the cells, and the cells were processed according to
the instructions of the kit. The fluorescence intensity of DCF was
detected by flow cytometry (BD-FACSVerse; BD Biosciences), and the
average DCF intensity level was considered as a reflection of the
ROS content of the cells in each group.
Mitochondrial membrane potential
detection
After the various treatments were completed, the
cells (1×106) from each group were collected, stained
with 10 µg/ml tetrachloro-tetraethyl benzimidazol carbocyanine
iodide (JC-1; Abcam), and incubated at 37°C for 45 min. Flow
cytometry (BD-FACSVerse; BD Biosciences) was used to determine and
FlowJo (Flow Jo 7.6; Tree Star Inc.) was used to analyze the
mitochondrial membrane potential (MMP) in the cells of each group
according to the instructions.
Western blotting
The aforementioned groups of cells were collected
and the expression levels of the target proteins in each group were
determined by western blotting. Total protein and nuclear protein
were extracted according to the procedure of the Protein Extraction
Kit (BestBio Institute of Biotechnology). The concentration of each
protein sample was analyzed by the BCA method and samples were
stored at −20°C after denaturation. A 12% SDS-PAGE separation gel
was prepared, and 20 µg of the protein sample was subjected to
SDS-PAGE and then transferred to a PVDF membrane (EMD Millipore).
The PVDF membrane was blocked in TBST containing 5% skim milk
powder (blocking solution) at room temperature for 2 h, and then
the corresponding primary antibody (Table I) was diluted in the blocking
solution to the desired concentration and incubated at 4°C
overnight. After three washes with the TBST solution, the PVDF
membrane was placed in a secondary antibody solution for 2 h at
room temperature. The protein expression signals were detected
using an ECL detection system (ChemiScope 5300 Pro; CLiNX) and
analyzed by ImageJ (version 1.8.0; National Institutes of
Health).
 | Table I.Antibodies used in the present
study. |
Table I.
Antibodies used in the present
study.
| Antibody | Source | Dilution ratio | Catalogue no. | Supplier |
|---|
| Anti-Bcl-2 | Mouse | 1:2,000 | ab182858 | Abcam |
| Anti-Bcl-xL | Rabbit | 1:2,000 | ab178844 | Abcam |
| Anti-Bax | Rabbit | 1:2,000 | ab32503 | Abcam |
|
Anti-cyto-c | Rabbit | 1:1,000 | ab13575 | Abcam |
| Anti-AIF | Mouse | 1:1,000 | PBO388 | Boster |
| Anti-p-Akt | Rabbit | 1:1,000 | ab38449 | Abcam |
| Anti-t-Akt | Rabbit | 1:2,000 | ab176463 | Abcam |
| Anti-PI3K | Rabbit | 1:2,000 | ab140347 | Abcam |
| Anti-GAPDH | Rabbit | 1:5,000 | ab181602 | Abcam |
| Anti-Lamin A/C | Rabbit | 1:5,000 | GB11407 | Servicebio |
| Goat anti-rabbit
IgG | Goat | 1:4,000 | BL003A | BioSharp |
| Rabbit anti-mouse
IgG | Rabbit | 1:4,000 | BL001A | BioSharp |
Statistical analysis
Statistical analysis was performed with GraphPad
Prism 6 (GraphPad Software, Inc.). Numerical variables are
expressed as the means ± standard deviations. Statistical
differences among experimental conditions were assessed by one-way
analysis of variance (ANOVA) followed by Dunnett's test. P<0.05
was considered to indicate a statistically significant
difference.
Results
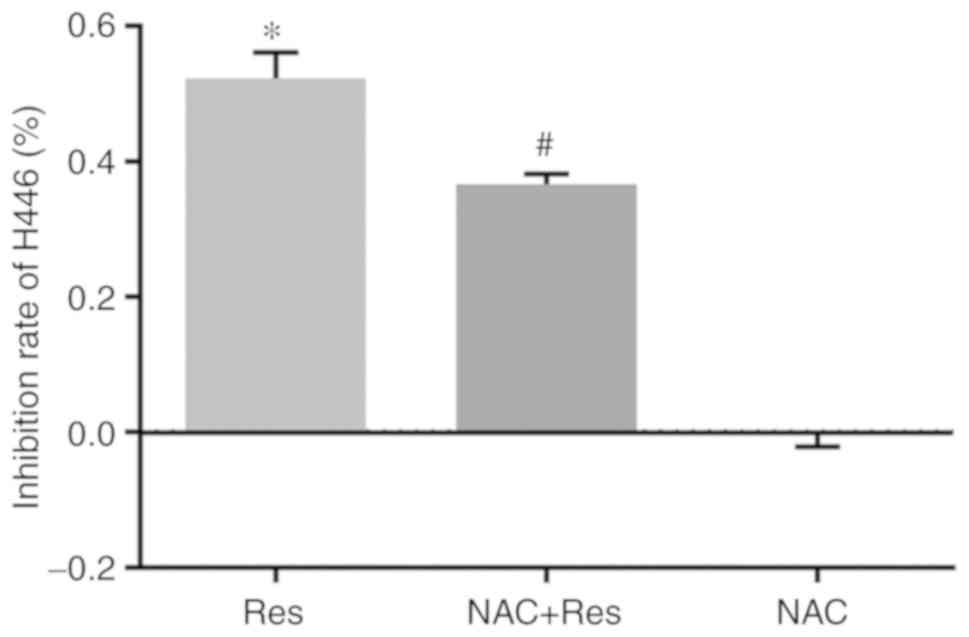
Effect of Res on the survival rate of
H446 cells
The cell survival rate of the blank control group
(Ctl) was regarded as ‘1’, and the OD values of the Res group, the
Res+NAC group, and the NAC group were compared with that of the Ctl
group, allowing calculation of the relative viability inhibition
rates of the cells in each group. As revealed in Fig. 1, the viability inhibition rate of 40
µg/ml Res in H446 cells was 51.33% (P<0.05 vs. the Ctl). After
pretreatment with NAC, the viability inhibition rate of 40 µg/ml
Res decreased to 36.61%. There was also a statistically significant
decrease in the viability inhibition rate of H446 cells in the
NAC+Res group compared with the Res group (P<0.05), but there
was no significant effect on cell survival with 2 mmol/l NAC
alone.
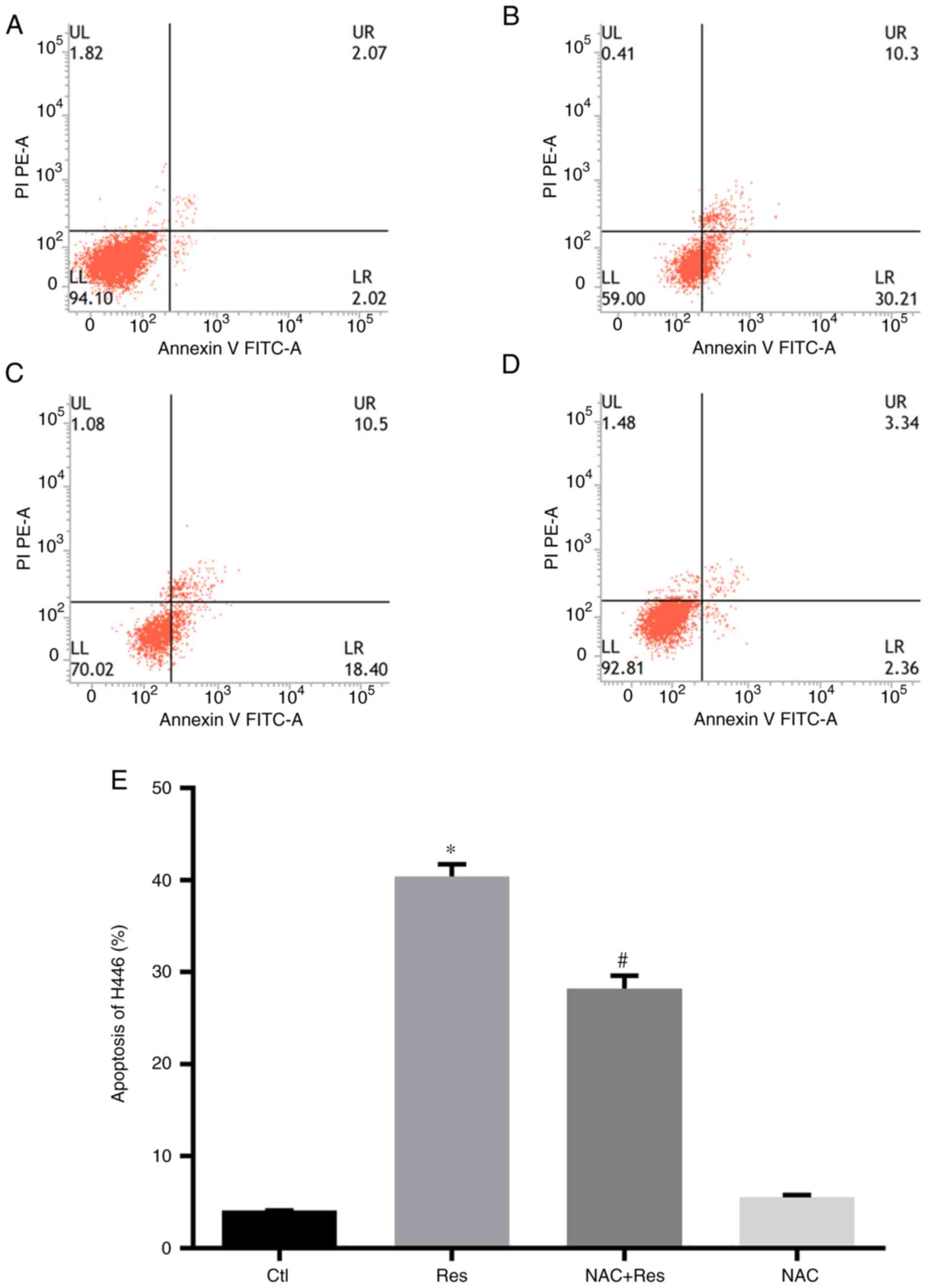
Effect of Res on H446 cell
apoptosis
Flow cytometry was used to determine the effect of
Res on the apoptosis of H446 cells with or without NAC
pretreatment. As revealed in Fig.
2, the apoptosis rate was 40.39% in H446 cells when they were
stimulated by 40 µg/ml Res for 24 h, which was significantly higher
than that in the Ctl group (P<0.05). However, after pretreatment
with NAC, 24 h of Res treatment resulted in 28.23% apoptosis in
H446 cells. In addition, compared with the Res group, the NAC+Res
group was significantly decreased (P<0.05). Treatment with 2
mmol/l NAC had no marked effect on apoptosis in H446 cells.
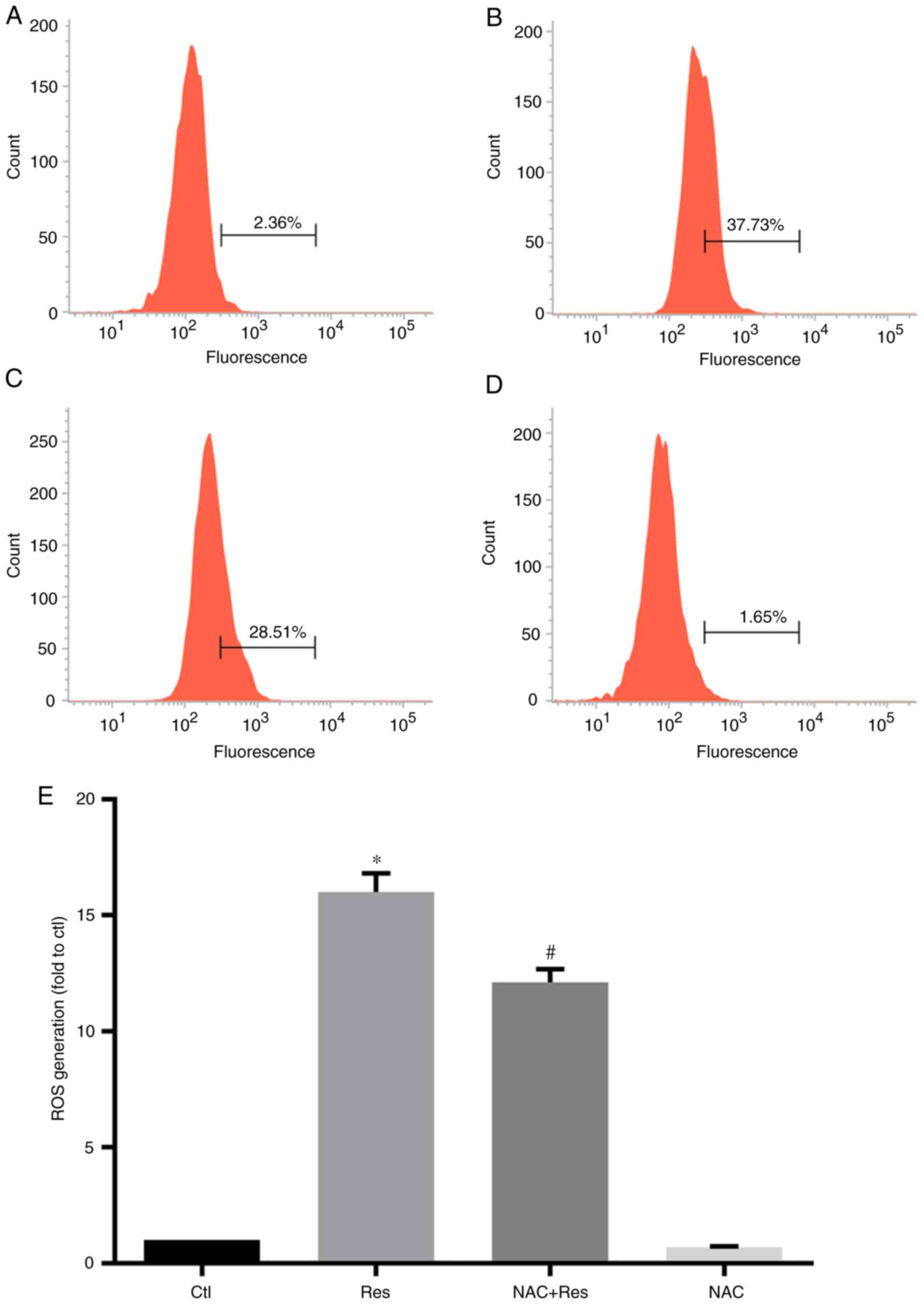
Effect of Res on ROS production in
H446 cells
Since Res inhibited the viability of H446 cells and
promoted their apoptosis, the effect of Res on ROS levels in H446
cells was further examined. The results are presented in Fig. 3. Following Res treatment, the level
of ROS in H446 cells was significantly increased, reaching ~16
times that of the Ctl group (P<0.05). In addition, Res treatment
also increased the ROS content in NAC-pretreated H446 cells, which
was 12.10 times that of the Ctl. However, the ROS level in cells
from the NAC+Res group was still significantly lower than that of
the Res group (P<0.05). Treatment of cells with NAC alone had no
significant effect on the level of ROS in H446 cells.
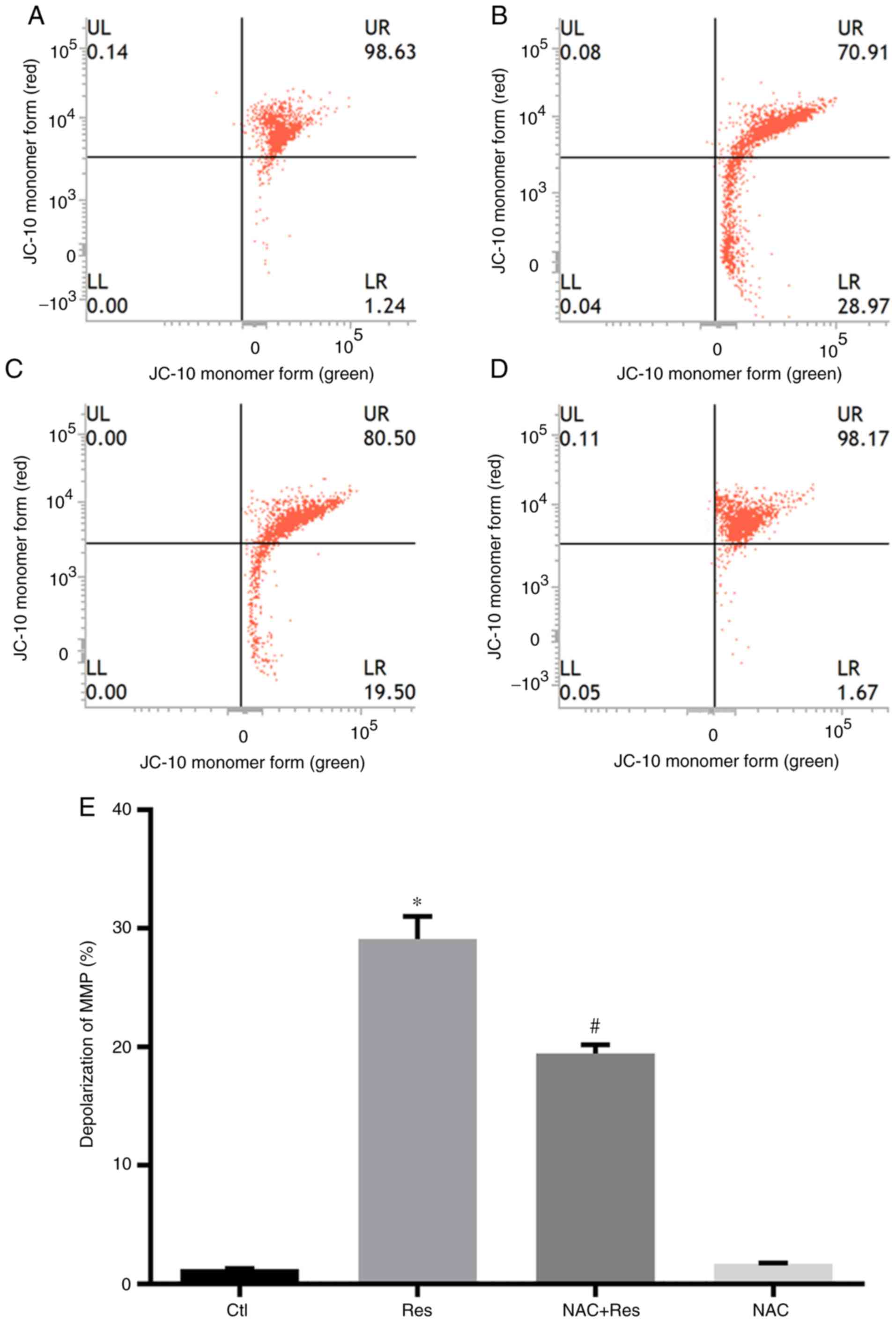
Effect of Res on the MMP of H446
cells
After confirming that Res inhibited the viability of
H446 cells and promoted apoptosis in a manner that was related to
ROS production, the changes in the MMP of H446 cells treated with
Res were further examined. Res at 40 µg/ml increased the amount of
H446 cells with depolarized MMP by 29.13% compared with the Ctl
group (P<0.05; Fig. 4). While
pretreatment of NAC at 2 mmol/l alleviated the effects of Res on
depolarization of MMP to 19.44% compared with that of Ctl group.
Statistical analysis also revealed that the NAC+Res group was
significantly lower compared with the Res group (P<0.05). NAC
treatment alone had no significant effect on the MMP of H446
cells.
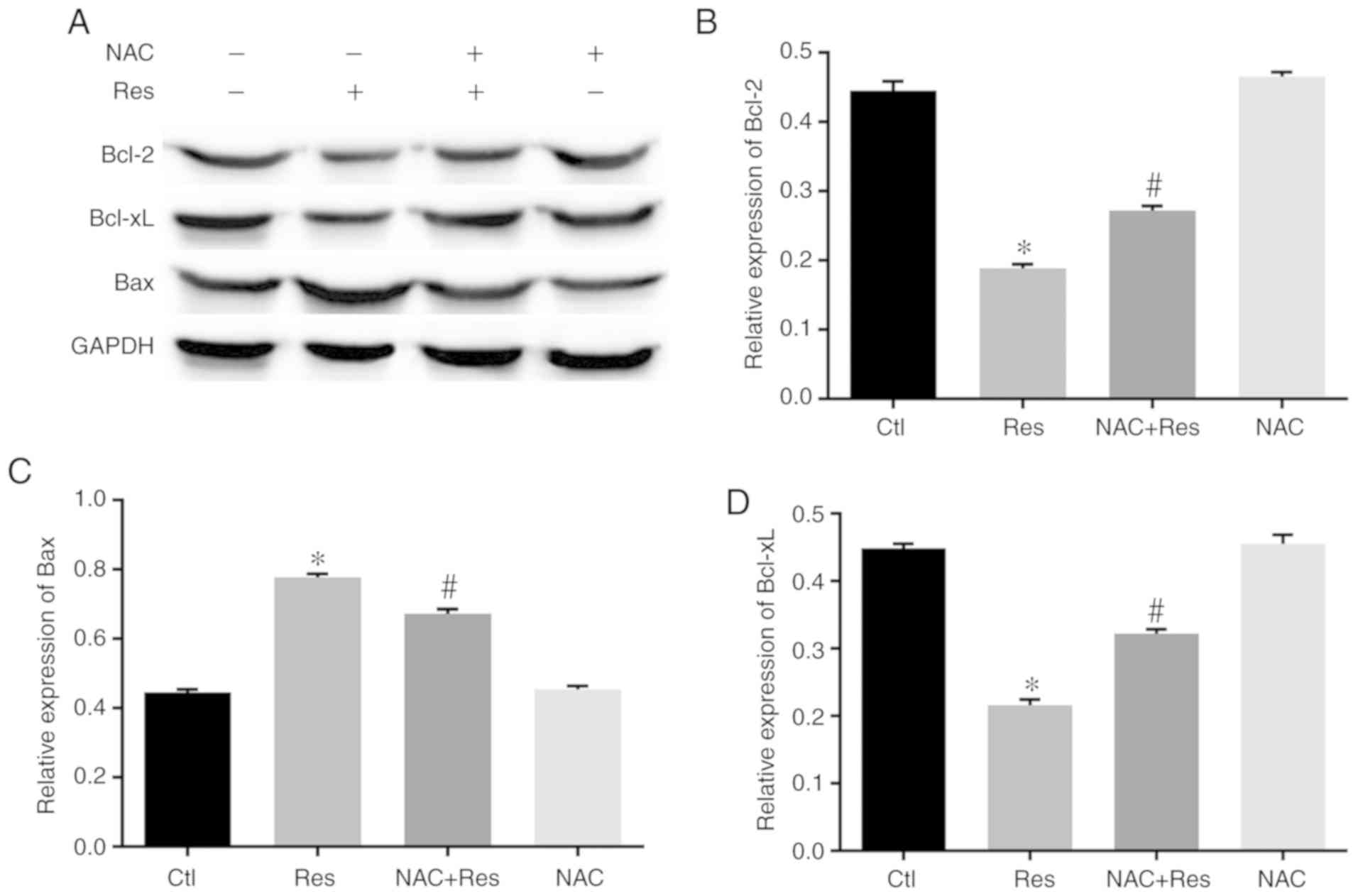
Effect of Res on the expression of
apoptotic proteins in H446 cells
After confirming that Res inhibited the viability of
H446 cells and promoted apoptosis through ROS production and MMP,
the effect of Res on the expression of apoptosis-related proteins
was further explored in H446 cells. As revealed in Fig. 5, after Res treatment of H446 cells,
the expression of Bcl-2 and Bcl-xL was significantly lower than
that of the Ctl group (P<0.05). However, after pretreatment with
NAC, the expression of Bcl-2 and Bcl-xL in the NAC+Res group was
significantly higher than that of the Res group (P<0.05). In
contrast to the expression of Bcl-2 and Bcl-xL, the expression of
Bax in H446 cells was significantly increased after Res treatment
compared with the Ctl group (P<0.05). Conversely, NAC
pretreatment significantly alleviated the increase in Bax
expression in Res-stimulated H446 cells (P<0.05).
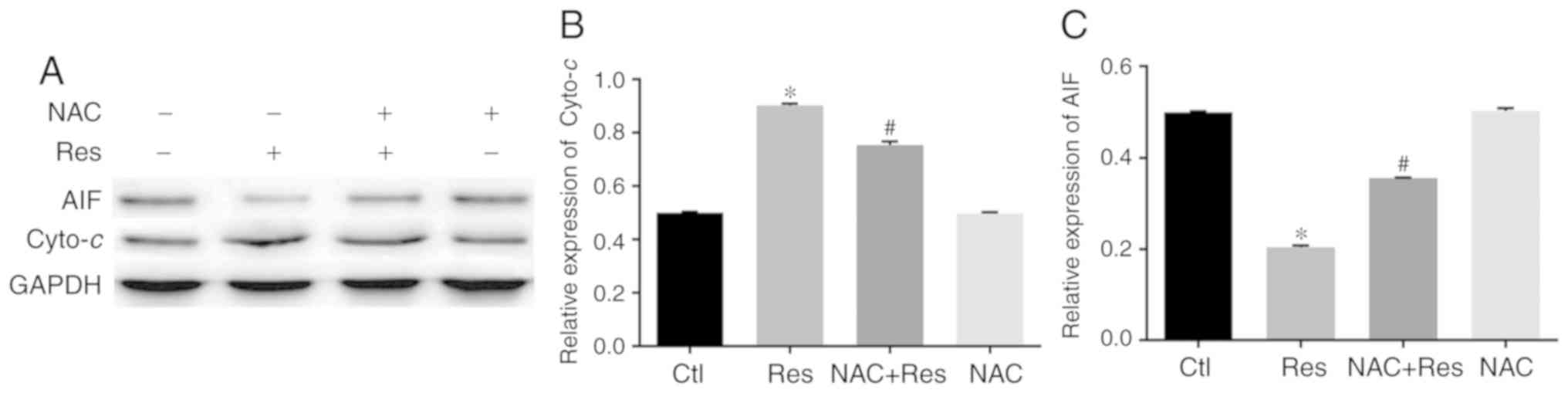
Effect of Res on the cytoplasmic
expression of cytochrome C in H446 cells
Based on the aforementioned results, the effect of
Res on the expression of cytochrome c (cyto-c) in the
H446 cytoplasm was further explored. Western blot analysis
(Fig. 6A and B) revealed that the
expression of cyto-c in the H446 cytoplasm was significantly
higher in Res-treated cells than in the control group (P<0.05).
However, in the NAC-pretreated N446 cells, the cytoplasmic
expression of cyto-c was significantly lower than that of
the Res group (P<0.05). NAC alone had no significant effect on
the expression of cyto-c in H446 cells.
Effect of Res on the expression of AIF
in H446 cells
The effect of Res stimulation on AIF expression in
H446 cells was further examined. The results revealed that the
cytoplasmic expression of AIF was significantly lower in
Res-stimulated cells than in the control group (P<0.05)
(Fig. 6A and C). However, the
expression of AIF in the NAC+Res group was significantly higher
than that of the Res group (P<0.05). In contrast, analysis of
AIF expression in the nuclear fraction of H446 cells (Fig. 7A) revealed that Res stimulation
increased the nuclear AIF expression of H446 cells compared with
the control group (P<0.05) and the expression of AIF in the
NAC+Res group was significantly lower than that of the Res group
(P<0.05). In addition, NAC treatment alone had no significant
effect on the expression of AIF in the H446 cytoplasm or
nucleus.
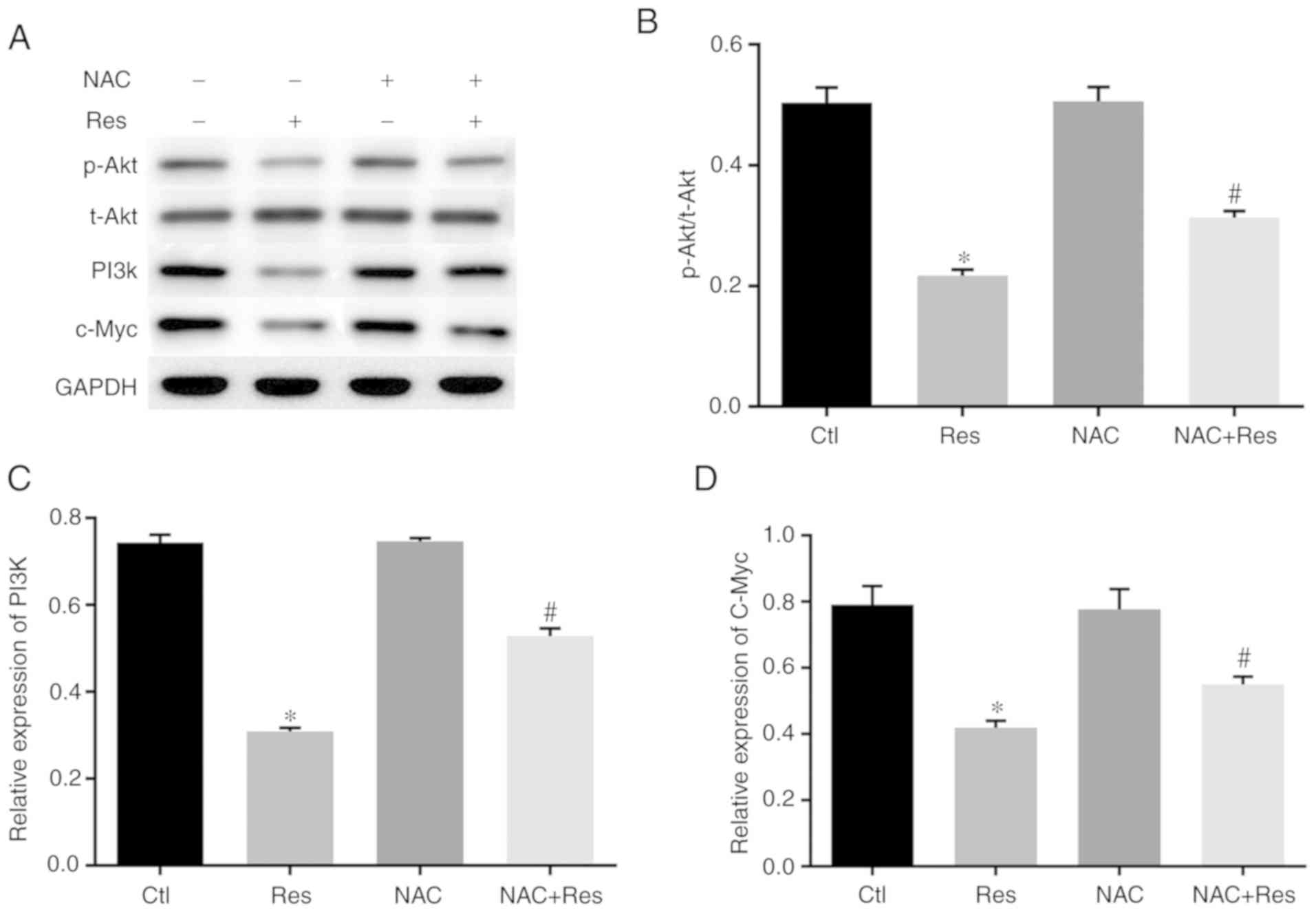
Effect of Res on the expression of
PI3K/Akt signaling pathway components
Based on the aforementioned experiments, the
mechanism by which Res affects SCLC H446 cells and its effect on
the PI3K/Akt signaling pathway were further investigated. As
revealed in Fig. 8A-C, the
expression levels of PI3K and p-Akt were significantly decreased in
Res-stimulated H446 cells (P<0.05 vs. the Ctl), the expression
of p-Akt and PI3K in the NAC+Res group was significantly higher
than that of the Res group (P<0.05). In addition, we assessed
the expression of c-Myc in H446 cells. The results (Fig. 7B and 8D) revealed that Res stimulation decreased
the expression of c-Myc in both the cytoplasm and nucleus but the
expression of c-Myc in the NAC+Res group was higher than that of
Res group (P<0.05).
Discussion
SCLC is a major type of primary malignant tumor in
the lungs, accounting for 15–20% of all lung cancers. This cancer
subtype has a high degree of malignancy, is prone to recurrence and
metastasis, and has a short survival period. A platinum-based
double-drug chemotherapy regimen has been recommended as the
standard treatment for lung cancer since its first application in
the 1970s, but it has not fundamentally improved the survival and
recovery of patients with SCLC (3).
Res is a natural plant-derived compound widely found in nature
(9). Since its recognition,
considerable research into its bioactivity has been conducted
(10). A number of studies have
found that Res has potential anticancer activity in tumors of
multiple systems in the human body (6,11),
although there are few studies on the anticancer effects of Res in
lung cancer. Previous research by our group revealed that Res
concentration- and time-dependently decreased the viability of H446
cells, promoted their apoptosis through the mitochondrial apoptosis
pathway, and boosted the antitumor effect of cisplatin on H446
cells (7).
In our previous research, the relationship between
the concentration of Res and toxicity in H446 cells was explored,
confirming that Res has potent toxic effects on the SCLC H446 cell
line (7). Based on these findings,
40 µg/ml Res was used in the present study to further explore the
anticancer effects of Res in H446 cells. It was confirmed that 40
µg/ml Res caused morphological damage, inhibited proliferation, and
induced the apoptosis of H446 cells. However, NAC pretreatment
attenuated the morphological changes and reduced the proliferative
capacity of Res-treated H446 cells. NAC is an antioxidant that can
significantly reduce the oxidative stress response of tissues and
cells (12). Therefore, the present
results further confirmed that the antitumor effects of Res were
related to oxidative stress.
ROS play a key role in regulating apoptosis, and the
aim of chemotherapy strategies is to alter the oxidative and
antioxidant balance by increasing ROS production in tumor cells,
which can further lead to tumor cell apoptosis (13). Excessive ROS production decreases
the MMP (14). Mitochondria are
dynamic intracellular organelles that provide 95% of the adenosine
triphosphate (ATP) required by the human body (15). A normal MMP is critical for
oxidative phosphorylation and ATP production, and a reduced MMP is
a hallmark of various early apoptotic pathways (16). In the present study, Res-treated
H446 cells generated more ROS than control cells and experienced
more apoptosis, as well as an MMP decrease. Conversely, after
pretreatment with NAC, Res-induced ROS production and MMP
depolarization were ameliorated to some extent, and assessment of
apoptosis also revealed that NAC reduced the number of apoptotic
H446 cells after Res treatment.
A lower MMP is often followed by the release of
cyto-c from the mitochondria to the cytoplasm (17), which mediates apoptosis with the
participation of Bcl-2 family proteins (18). Bcl-2 and Bcl-xL are generally
considered to play a role in preventing apoptosis and cell death
caused by various stressors (19).
In contrast, Bax homodimers with sequence homology to Bcl-2 are
important pro-apoptotic proteins (20). The results of the present study
indicated that Res stimulation promoted the release of
cyto-c from the mitochondria to the cytoplasm while
increasing the expression of the pro-apoptotic protein Bax and
decreasing the expression of the antiapoptotic proteins Bcl-2 and
Bcl-xL. AIF is a redox active enzyme on the mitochondrial membrane
that would be released into the cytoplasm and then enter the
nucleus after depolarization of the MMP. Once the AIF enters the
nucleus, the DNA is cleaved into 50-kb fragments and then caspase
family-independent apoptosis is triggered (21). After Res treatment of H446 cells,
the cytoplasmic expression of AIF decreased while its nuclear
expression increased, which is consistent with a study of AIF
involvement in apoptosis (22).
After NAC treatment, the inductive effect of Res on the release of
cyto-c and expression of Bax in H446 cells was alleviated.
Concurrently, Bcl-2 and Bcl-xL expression levels were maintained at
a relatively high level. In addition, Res slightly reversed the
change in AIF expression. These results are consistent with our
previous results (7) and those of
other researchers examining the antitumor mechanism of Res
(23).
The PI3K/Akt pathway is an important intracellular
signaling pathway that governs the cell cycle and is directly
involved in regulating numerous cell processes, including cell
growth, proliferation, differentiation, and migration (24). Phosphorylated Akt (p-Akt) activates
or inhibits downstream target proteins such as mTOR, caspase-9,
NF-κB, and p21 that are involved in cell proliferation (25). The expression of p-Akt is increased
in approximately 50% of SCLCs, and its overexpression is associated
with the prognosis of lung cancer. p-Akt inhibits the Bcl family
members Bad and Bax, inhibits p53-mediated apoptosis, activates
NF-κB, and increases the expression of Bcl-2 and Bcl-xL (26). In the present study it was revealed
that Res stimulation significantly decreased the expression levels
of PI3K and p-Akt in H446 cells, which was consistent with its
effects on cell viability and apoptosis and the expression of
related proteins. In addition, NAC pretreatment alleviated the
inhibitory effects of Res on PI3K and p-Akt expression. c-Myc
regulates the glycolytic activity of tumors (27) and is a key downstream molecule in
the PI3K/Akt pathway (28).
Overexpression of c-Myc has been observed in multiple tumors
(23), and inhibition of c-Myc
could inhibit the malignant biological behavior of tumor cells
(29). The present results
indicated that Res stimulation decreased the expression of c-Myc in
both the cytoplasm and nucleus of H446 cells, which was consistent
with our anticancer results for Res as well as those in another
study (30). However, the effect of
Res on c-Myc was markedly inhibited after NAC pretreatment,
indicating that Res exerts its antitumor effect through oxidative
stress.
In summary, the results from the present study
indicated that Res inhibited the viability and promoted the
apoptosis of human SCLC H446 cells, which may be related to the
oxidative stress level and abnormal mitochondrial membrane
potential of H446 cells during Res stimulation. Moreover, Res may
exert its antitumor effects through the PI3K/Akt/c-Myc pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LM designed the research and revised the manuscript.
FJ was involved in the conception of the study and helped modifying
the manuscript. WL and CL were the major contributors in conducting
the experiments, interpreting the data and drafting the manuscript
All authors read and approved the final manuscript and agree to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
Glossary
Abbreviations
Abbreviations:
|
SCLC
|
small-cell lung cancer
|
|
Res
|
resveratrol
|
|
NAC
|
N-acetyl-L-cysteine
|
|
OD
|
optical density
|
|
PI
|
propidium iodide
|
|
DCF-DA
|
2′,7′-dichlorodihydrofluorescein
diacetate
|
|
JC-1
|
tetrachloro-tetraethyl benzimidazol
carbocyanine iodide
|
|
MMP
|
mitochondrial membrane potential
|
|
cyto-c
|
cytochrome c
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burns DM, Anderson CM and Gray N: Has the
lung cancer risk from smoking increased over the last fifty years?
Cancer Causes Control. 22:389–397. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geddes D: The history of respiratory
disease management. Medicine (Abingdon). 48:239–243.
2020.PubMed/NCBI
|
|
4
|
Blumenthal GM, Zhang L, Zhang H,
Kazandjian D, Khozin S, Tang S, Goldberg K, Sridhara R, Keegan P
and Pazdur R: Milestone analyses of immune checkpoint inhibitors,
targeted therapy, and conventional therapy in metastatic non-small
cell lung cancer trials: A meta-analysis. JAMA Oncol.
3:e1710292017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cucciolla V, Borriello A, Oliva A,
Galletti P, Zappia V and Della Ragione F: Resveratrol: From basic
science to the clinic. Cell Cycle. 6:2495–2510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zeng Y, Li FD, Shi CW, Du JL, Xue YJ, Liu
XY, Cao X and Wei N: Mechanism and therapeutic prospect of
resveratrol combined with TRAIL in the treatment of renal cell
carcinoma. Cancer Gene Ther. Oct 30–2019.(Epub ahead of print).
|
|
7
|
Li W, Shi Y, Wang R, Pan L, Ma L and Jin
F: Resveratrol promotes the sensitivity of small-cell lung cancer
H446 cells to cisplatin by regulating intrinsic apoptosis. Int J
Oncol. 53:2123–2130. 2018.PubMed/NCBI
|
|
8
|
Wang R, Ma L, Weng D, Yao J, Liu X and Jin
F: Gallic acid induces apoptosis and enhances the anticancer
effects of cisplatin in human small cell lung cancer H446 cell line
via the ROS-dependent mitochondrial apoptotic pathway. Oncol Rep.
35:3075–3083. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ahmadi R and Ebrahimzadeh MA:
Resveratrol-A comprehensive review of recent advances in anticancer
drug design and development. Eur J Med Chem. 200:1123562020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muniraj N, Siddharth S and Sharma D:
Bioactive compounds: Multi-targeting silver bullets for preventing
and treating breast cancer. Cancers (Basel). 11:15632019.
View Article : Google Scholar
|
|
11
|
Ashrafizadeh M, Ahmadi Z, Farkhondeh T and
Samarghandian S: Resveratrol targeting the Wnt signaling pathway: A
focus on therapeutic activities. J Cell Physiol. 235:4135–4145.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu H and Tong C: Ratiometric fluorometric
determination of silver(I) by using blue-emitting silicon- and
nitrogen-doped carbon quantum dots and red-emitting
N-acetyl-L-cysteine-capped CdTe quantum dots. Mikrochim Acta.
186:7232019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zamzami N, Marchetti P, Castedo M,
Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B and
Kroemer G: Sequential reduction of mitochondrial transmembrane
potential and generation of reactive oxygen species in early
programmed cell death. J Exp Med. 182:367–377. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hamada M, Sumida M, Okuda H, Watanabe T,
Nojima M and Kuby SA: Adenosine
triphosphate-adenosine-5′-monophosphate phosphotransferase from
normal human liver mitochondria. Isolation, chemical properties,
and immunochemical comparison with Duchenne dystrophic serum
aberrant adenylate kinase. J Biol Chem. 257:13120–13128.
1982.PubMed/NCBI
|
|
16
|
Kalpage HA, Bazylianska V, Recanati MA,
Fite A, Liu J, Wan J, Mantena N, Malek MH, Podgorski I, Heath EI,
et al: Tissue-specific regulation of cytochrome c by
post-translational modifications: respiration, the mitochondrial
membrane potential, ROS, and apoptosis. FASEB J. 33:1540–1553.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Dong X, Wang W, You L, Yin X, Yang
C, Sai N, Leng X and Ni J: Molecular mechanisms of apoptosis in
HepaRG cell line induced by polyphyllin VI via the Fas death
pathway and mitochondrial-dependent pathway. Toxins (Basel).
10:2012018. View Article : Google Scholar
|
|
18
|
Samarghandian S, Nezhad MA and Mohammadi
G: Role of caspases, Bax and Bcl-2 in chrysin-induced apoptosis in
the A549 human lung adenocarcinoma epithelial cells. Anticancer
Agents Med Chem. 14:901–909. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang W, Guo Q, You Q, Zhang K, Yang Y, Yu
J, Liu W, Zhao L, Gu H, Hu Y, et al: Involvement of bax/bcl-2 in
wogonin-induced apoptosis of human hepatoma cell line SMMC-7721.
Anticancer Drugs. 17:797–805. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang B, Johnson TS, Thomas GL, Watson PF,
Wagner B, Furness PN and El Nahas AM: A shift in the Bax/Bcl-2
balance may activate caspase-3 and modulate apoptosis in
experimental glomerulonephritis. Kidney Int. 62:1301–1313. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ding L, Li J, Li W, Fang Z, Li N, Wu S, Li
J and Hong M: p53- and ROS-mediated AIF pathway involved in
TGEV-induced apoptosis. J Vet Med Sci. 80:1775–1781. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang T, Xu X, Ye D, Chen W, Gao B and
Huang Y: Caspase/AIF/apoptosis pathway: A new target of puerarin
for diabetes mellitus therapy. Mol Biol Rep. 46:4787–4797. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuan L, Zhou M, Huang D, Wasan HS, Zhang
K, Sun L, Huang H, Ma S, Shen M and Ruan S: Resveratrol inhibits
the invasion and metastasis of colon cancer through reversal of
epithelial- mesenchymal transition via the AKT/GSK-3β/Snail
signaling pathway. Mol Med Rep. 20:2783–2795. 2019.PubMed/NCBI
|
|
24
|
Zoncu R, Efeyan A and Sabatini DM: mTOR:
From growth signal integration to cancer, diabetes and ageing. Nat
Rev Mol Cell Biol. 12:21–35. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: An
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Manning BD and Toker A: AKT/PKB signaling:
Navigating the network. Cell. 169:381–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
An J, Li F, Qin Y, Zhang H and Ding S: Low
concentrations of FA exhibits the hormesis effect by affecting cell
division and the warburg effect. Ecotoxicol Environ Saf.
183:1095762019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang HF, Wu C, Alshareef A, Gupta N, Zhao
Q, Xu XE, Jiao JW, Li EM, Xu LY and Lai R: The PI3K/AKT/c-MYC axis
promotes the acquisition of cancer stem-like features in esophageal
squamous cell carcinoma. Stem Cells. 34:2040–2051. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang F, Li K, Yao X, Wang H, Li W, Wu J,
Li M, Zhou R, Xu L and Zhao L: A miR-567-PIK3AP1-PI3K/AKT-c-Myc
feedback loop regulates tumour growth and chemoresistance in
gastric cancer. Ebiomedicine. 44:311–321. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ko JH, Sethi G, Um JY, Shanmugam MK,
Arfuso F, Kumar AP, Bishayee A and Ahn KS: The role of resveratrol
in cancer therapy. Int J Mol Sci. 18:25892017. View Article : Google Scholar
|