Introduction
Laryngeal cancer is the second most common type of
head and neck cancer and is more prevalent in males compared with
females (1). Laryngeal squamous
cell carcinoma (LSCC) is the most common type of laryngeal
carcinoma. In 2018, there were 177,422 new cases and 94,771
cancer-associated deaths, worldwide, which accounted for 1% of all
types of cancer. The incidence rate of LSCC is increasing annually
worldwide (2). Approximately 60% of
patients with LSCC are diagnosed with advanced disease (stage III
or IV), which leads to poor treatment efficacy and worse prognosis
(3). There has been an improvement
in the treatment of LSCC; however, the survival rate of patients
with LSCC has remained low over the past few decades and has shown
a downward trend (4). Therefore, it
is important to investigate the pathogenesis and molecular
mechanism of LSCC, to identify novel prevention and treatment
strategies.
A study of the human genome reported that ~80% of
DNA are transcribed into RNA; however, only ~2% of RNA are
translated into proteins. RNAs that do not encode proteins are
termed non-coding RNAs (5). Long
non-coding (lnc)RNAs are a type of functional RNA and >200
nucleotides in length (6). lncRNAs
do not have protein-coding function; however, they can regulate
gene expression at both the transcriptional and
post-transcriptional levels (7). It
was demonstrated that lncRNAs may play key roles in the occurrence
and development of tumors and they could be potential biomarkers
for early diagnosis of multiple types of tumor and potential
therapeutic targets (8–10). However, the specific roles and
molecular mechanisms of lncRNAs in LSCC are limited. In our
previous study, the differential expression level of lncRNAs in
four LSCC and adjacent normal tissues was identified using a
microarray (11). It was verified
that the expression level of RASSF8-AS1 in tumor tissues was
significantly lower compared with that in paired normal tissues,
which was further validated using reverse
transcription-quantitative PCR (RT-qPCR). To the best of our
knowledge, there are few studies investigating the mechanism of
RASSF8-AS1 in other types of tumor. At present, the mechanism of
competitive endogenous (ce) RNA has become a hot topic in
lncRNA-mediated tumorigenesis. Recent studies have demonstrated
that lncRNAs have oncogenic or antitumor activities, by functioning
as microRNA (miRNA/miR) sponges, which suggest that they have
pivotal roles in the development of tumors (12–14).
For example, Song et al (12) reported that SPRY4-IT1 increased the
expression level of TCF7L2 by targeting miR-6882-3p, promoted the
proliferation and stemness of breast cancer cells, and promoted the
renewal ability and stemness maintenance of breast cancer stem
cells. Chen et al (13)
demonstrated that SNHG16 promoted the proliferation, migration and
invasion of hepatocellular carcinoma cells by negatively regulating
the expression level of miR-186, as a ceRNA. However, the mechanism
and function of lncRNAs as ceRNAs in LSCC have not been
investigated. Therefore, the present study aimed to verify the role
of lncRNA RASSF8-AS1 in LSCC through a ceRNA network. To the best
of our knowledge, this is the first time RASSF8-AS1 has been
investigated in LSCC. The results showed that the mRNA expression
level of RASSF8-AS1 was decreased in LSCC tissues and cell lines,
while the overexpression of RASSF8-AS1 inhibited the proliferation,
invasion and migration of LSCC cells by targeting the
miR-664b-3p/transducin-like enhancer of split 1 (TLE1) axis. These
findings provide a deeper understanding of the tumorigenic
mechanism and suggest a potential target for the treatment of
LSCC.
Materials and methods
Patients and tissue samples
A total of 72 pairs of LSCC tumor and adjacent
normal tissues were collected from the Second Hospital of Hebei
Medical University (Hebei, China) between October 2016 and March
2019. None of the patients with LSCC received radiotherapy and/or
chemotherapy prior to surgery. All procedures were conducted
following the ethical standards of the Institutional Research
Council of Hebei Medical University (Hebei, China) and the
Declaration of Helsinki from 2008. The present study was approved
by the Ethics Committee of the Second Hospital of Hebei Medical
University (Hebei, China). All the tissue specimens were stored at
−80°C at the Otorhinolaryngology Head and Neck Surgery Biobank of
Hebei Medical University for RNA extraction. Clinical features and
pathological diagnosis were collected from the hospital records
(Table I).
 | Table I.Information and clinicopathological
data of the 72 pairs of tumor and normal tissues obtained from the
patients with LSCC. |
Table I.
Information and clinicopathological
data of the 72 pairs of tumor and normal tissues obtained from the
patients with LSCC.
|
Characteristics | n (%) |
|---|
| Sex |
|
Male | 72 (100.0) |
|
Female | 0 (0.00) |
| Age (years) |
|
<60a | 27 (37.5) |
|
≥60 | 45 (62.5) |
| Smoking |
| No | 9 (12.5) |
|
Yes | 63 (87.5) |
| Alcohol
consumption |
| No | 31 (43.1) |
|
Yes | 41 (56.9) |
| TNM stage |
|
I+II | 30 (41.7) |
|
III+IV | 42 (58.3) |
| Cervical lymph node
metastasis |
| No | 37 (51.4) |
|
Yes | 35 (48.6) |
| Pathological
differentiation degree |
|
Well | 38 (52.8) |
|
Moderate/poor | 34 (47.2) |
Cell lines and cell culture
A total of 4 human LSCC cell lines (TU686, TU177,
TU212 and AMC-HN-8) and the 293T cell line were obtained from
Beijing Beina Chuanglian Institute of Biotechnology. The TU212 cell
line was authenticated using short tandem repeat (STR) profiling by
Shanghai Biowing Applied Biotechnology Co., Ltd. DNA was extracted
using an Axygen genomic extraction kit and amplified using a 20-STR
amplification protocol. STR loci and the gender gene, Amelogenin
were detected using an ABI 3730XL genetic analyzer. The AMC-HN-8
and 293T cells were cultured in DMEM, supplemented with 10% FBS,
while the TU686, TU177 and TU212 cells were cultured in RPMI-1640
medium, containing 10% FBS. The aforementioned media and reagents
were purchased from Gibco (Thermo Fisher Scientific, Inc.). The
cells were cultured at 37°C in a humidified incubator (Thermo
Fisher Scientific, Inc.) with 5% CO2.
RNA extraction and RT-qPCR assay
Total RNA was extracted from the LSCC tissues and
cells using an Eastep® Super Total RNA Extraction Kit
(Promega Corp.). The transcriptor First Strand cDNA synthesis kit
(Roche Diagnosis GmbH) was used for RT. RT-qPCR was performed using
a GoTaq® qPCR Master Mix (Promega Corp.). The following
thermocycling conditions were used: Initial denaturation at 95°C
for 2 min, followed by denaturation at 95°C for 15 sec. For
annealing to extension, selecting the suitable annealing
temperature according to different primers for 60 sec, for a total
of 40 cycles was carried out. lncRNA or mRNA expression was
normalized using GAPDH, while U6 was used as the internal control
for miRNA. Relative expression was normalized using the
2−∆∆Cq method (15). All
primers used are shown in Table
II.
 | Table II.Oligonucleotide sequences used in
this study. |
Table II.
Oligonucleotide sequences used in
this study.
| Gene | Sequence |
|---|
| RASSF8-AS1 | F:
5′-CAAAGGGTGACACACCAGGA-3′ |
|
| R:
5′-TGGTGATCAACACAAACTGGA-3′ |
| GAPDH | F:
5′-AGGTGAAGGTCGGAGTCAACG-3′ |
|
| R:
5′-AGGGGTCATTGATGGCAACA-3′ |
| miR-664b-3p
(stem-loop reverse transcription) |
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTGTAGGCT |
| miR-664b-3p | F:
5′-GCCGCGTTCATTTGCCTCCCAGCCT-3′ |
|
| R:
5′-GTGCAGGGTCCGAGGTATT-3′ |
| U6 | F:
5′-GCTTCGGCAGCACATATACTAAAAT-3′ |
|
| R:
5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
| TLE1 | F:
5′-GAGTCCCTGGACCGGATTAAA-3′ |
|
| R:
5′-AATACATCACATAGTGCCTCTGC-3′ |
| pmirGLO
RASSF8-AS1-wild | F:
5′-TCAGCTAGCAGTGGTCTCAGGACCATAAG-3′ |
|
| R:
5′-CGATCTAGAATACACAGAGTCGGGCAAG-3′ |
| pmirGLO
RASSF8-AS1-mut | F:
5′-CAGATTTTTAAACAAATAGGTATGTGGTGC-3′ |
|
| R:
5′-CATTCCATAGCCCCCCTAGACT-3′ |
| pmirGLO
TLE1-wild | F:
5′-CTACTCGAGGTTGTAACTTTAAAAGAG-3′ |
|
| R:
5′-GCAGTCGACCTCACACTTGGGCAAGG-3′ |
| pmirGLO
TLE1-mut | F:
5′-CTACATAGACCGACTAGAGCACCAAGG-3′ |
|
| R:
5′-ACAGGTGACTTTCTGCTG-3′ |
| miR-664b-3p
mimic |
5′-UUCAUUUGCCUCCCAGCCUACA-3′ |
| miR-664b-3p
inhibitor |
5′-UGUAGGCUGGGAGGCAAAUGAA-3′ |
| shRNA-TLE1-1 |
5′-GATCCGATCTGCACAACCAGACACTATTCAAG |
|
|
ACGTAGTGTCTGGTTGTGCAGATCTTTTTTGTCGACA-3′ |
| shRNA-TLE1-2 |
5′-AGCTTGTCGACAAAAAAGATCTGCACAACCAGACACTACGTCTTGAATAGTGTCTGGTTGTGCAGATCG-3 |
Subcellular fractionation
Subcellular fractionation was performed using a
PARIS™ Protein and RNA Isolation System (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. U6
and GAPDH were used as the cytoplasmic and nuclear controls,
respectively.
Cell transfection
For overexpression of RASSF8-AS1, the
pcDNA3.1-RASSF8-AS1 vector was purchased from Sangon Biotech Co.,
Ltd., while hsa-miR-664b-3p mimic/inhibitor/negative control (NC)
was synthesized by Guangzhou RiboBio Co., Ltd. For knockdown of
TLE1, the pGenesil-1 plasmid was used to construct the knockdown
plasmid to form pGenesil-1-TLE1 [short inhibiting (sh)-TLE1]. A
total of 2 primers (Table II) were
annealed to form the double-stranded DNA. After restriction
digestion (BamHI and HindIII) and purification of
pGenesil-1, double-stranded DNA was ligated into pGenesil-1 to
obtain a recombinant plasmid termed sh-TLE1, which was identified
by sequencing. The pcDNA3.1 was used as a negative control for
overexpression of RASSF8-AS1, and the pGenesil-1 was used as a
negative control for sh-TLE1. The TU177 and TU686 cell lines were
both seeded in separate 6-well plates and cultured to 70–80%
confluence. Then, TU177 and TU686 cells were transfected
respectively with pcDNA3.1-RASSF8-AS1 or sh-TLE1 using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instruction. AS
control, the pcDNA3.1 or pGenesil-1 empty vector were transfected
at the same time. hsa-miR-664b-3p mimic/inhibitor/negative control
(NC) were transfected into TU177 and TU686 cells according to the
manufacturer's instruction. Transfection efficiency was determined
using RT-qPCR.
Dual luciferase reporter assay
The TU177 and TU686 cell lines were seeded in
24-well plates separately, cultured for 24 h, and co-transfected
with pmirGLO-RASSF8-AS1-wild-type (WT) or pmirGLO-RASSF8-AS1-mutant
(MUT) reporter plasmids (Sangon Biotech, Co., Ltd.) and miR-664b-3p
mimic, inhibitor or NC. After 48 h of transfection, luciferase
activity was measured using a dual-luciferase reporter assay system
(Promega Corp.), while Renilla luciferase activity was used
for normalization. Using the same method, the TU177 or 293T cell
line was co-transfected, using Lipofectamine® 2000, with
pmirGLO-TLE1-WT or pmirGLO-TLE1-MUT reporter plasmids (Sangon
Biotech, Co., Ltd.) and miR-664b-3p mimic, inhibitor or NC. The
subsequent experiments were as aforementioned.
Colony formation assay
A total of 2×103 cells were seeded in
each well of a 6-well plate, and cultured at 37°C in a humidified
incubator with 5% CO2 for 10 days, 24 h following
transfection. Then, the LSCC cell lines were washed with PBS, fixed
with 4% paraformaldehyde for 20 min, and then stained with 0.5%
crystal violet for 20 min. Finally, we used a microscope (CKX53,
Olympus Corp.) at ×200 magnification to observe cells, colonies of
>50 cells per well were calculated.
Cell proliferation assay
The proliferation ability of the transfected LSCC
cell lines was detected using an MTS assay. After transfection for
24 h, 2×103 cells/per well were seeded into a 96-well
plate. Using a CellTiter 96® AQueous one solution cell
proliferation assay kit (Promega Corp.), 20 µl MTS reagent was
added into each well, after the cells were seeded for 0, 24, 48, 72
and 96 h and subsequently incubated for 2.5 h, according to the
manufacturer's instructions. The optical density (OD) was detected
at 490 nm.
Cell migration and invasion
assays
A Transwell chamber (Corning, Inc.) was placed into
a 24-well plate to detect the migration or invasion ability of the
transfected LSCC cells. For the migration assays, the transfected
LSCC cell lines were digested with pancreatin, and then suspended
in serum-free medium, following which a cell counter was used for
cell counting. A total of 1×105 cells were plated into
the upper chamber and 650 µl culture medium (including 10% FBS) was
added into the bottom chamber. After incubation at 37°C for 24 h,
the Transwell chamber was removed, washed with PBS, and fixed with
4% paraformaldehyde for 20 min, and then stained with 0.5% crystal
violet for 20 min. Then, the cells were observed and counted using
a microscope (CKX53; Olympus Corp.) at ×200 magnification. For the
invasion assay, 50 µl Matrigel was added to the upper chamber to
form a matrix barrier; the same protocol was then used for the
Transwell assay.
Western blot analysis
Total protein was extracted from the LSCC cell
linesusing RIPA buffer (Beijing Solarbio Science and Technology,
Co., Ltd.), supplemented with a protease inhibitor cocktail
(Promega Corp.). Protein samples (20 µg) were separated using 10%
SDS-PAGE and then transferred to a polyvinylidene fluoride membrane
(Bio-Rad Laboratories, Inc.). The membranes were incubated with
rabbit anti-human TLE1 (molecular weight, 83 kDa; dilution 1:1,500;
Abcam, cat no. ab183742) and rabbitanti-human GAPDH (molecular
weight, 37 kDa; dilution 1:5,000; ProteinTech Group, Inc., cat no.
10494-1-AP) overnight. Then, the protein band was visualized and
quantified using an enhanced chemiluminescence kit and a ChemiDoc™
XRS + system (Bio-Rad Laboratories, Inc.).
RNA immunoprecipitation (RIP)
assay
For the RIP assay, pSL-MS2-12X (Addgene, Inc.) was
double digested using BamHI and XhoI, and the MS2-12X
fragment was inserted to the pcDNA3.1-RASSF8-AS1 vector to form
pcDNA3.1-MS2-RASSF8-AS1. Then, pcDNA3.1-MS2-RASSF8-AS1 was mutated
using the Q5®Site-Directed Mutagenesis Kit (New England
Biolabs, Inc.) into pcDNA3.1-MS2-RASSF8-AS1-MUT. The LSCC cell
lines were co-transfected with pMS2-GFP (Addgene, Inc.) and
pcDNA3.1-MS2-RASSF8-AS1 or pcDNA3.1-MS2-RASSF8-AS1-MUT. After 48 h,
the LSCC cell lines were used for the RIP assay with a green
fluorescent protein antibody (Roche Diagnostics, Switzerland) and a
Magna RIP™ RNA-binding protein immunoprecipitation kit (EMD
Millipore) according to manufacturer's instructions (16).
Bioinformatic analysis
Targets of RASSF8-AS1 were obtained from
DIANA-LncBase Predicted v2 (http://carolina.imis.athena-innovation.gr/Diana_tools/web/index.php)
and the results showed has-miR-664b-3p might be a potential target
for RASSF8-AS1. Targets for has-miR-664b-3p were analyzed at
Starbase (http://starbase.sysu.edu.cn/index.php) and revealed
TLE1 was a potential target for has-miR-664b-3p.
Statistical analysis
All statistical analyses were performed using SPSS
software v21.0 (IBM Corp.) and GraphPad Prism v7 (GraphPad Software
Inc.). The data are presented as the mean ± standard deviation. The
figures were created using GraphPad Prism v7. The differences
between 2 groups were analyzed with the Student's t-test.
Differences between >2 groups were determined by one-way ANOVA
followed by Tukey's post hoc test. A Pearson's correlation test was
performed to determine the correlation between the mRNA expression
levels of RASSF8-AS1 and miR-664b-3p, or the correlation between
the mRNA expression levels of TLE1 and miR-664b-3p. P<0.05 was
considered to indicate a statistically significant difference.
Results
Silencing of RASSF8-AS1 in LSCC cell
lines and tissues
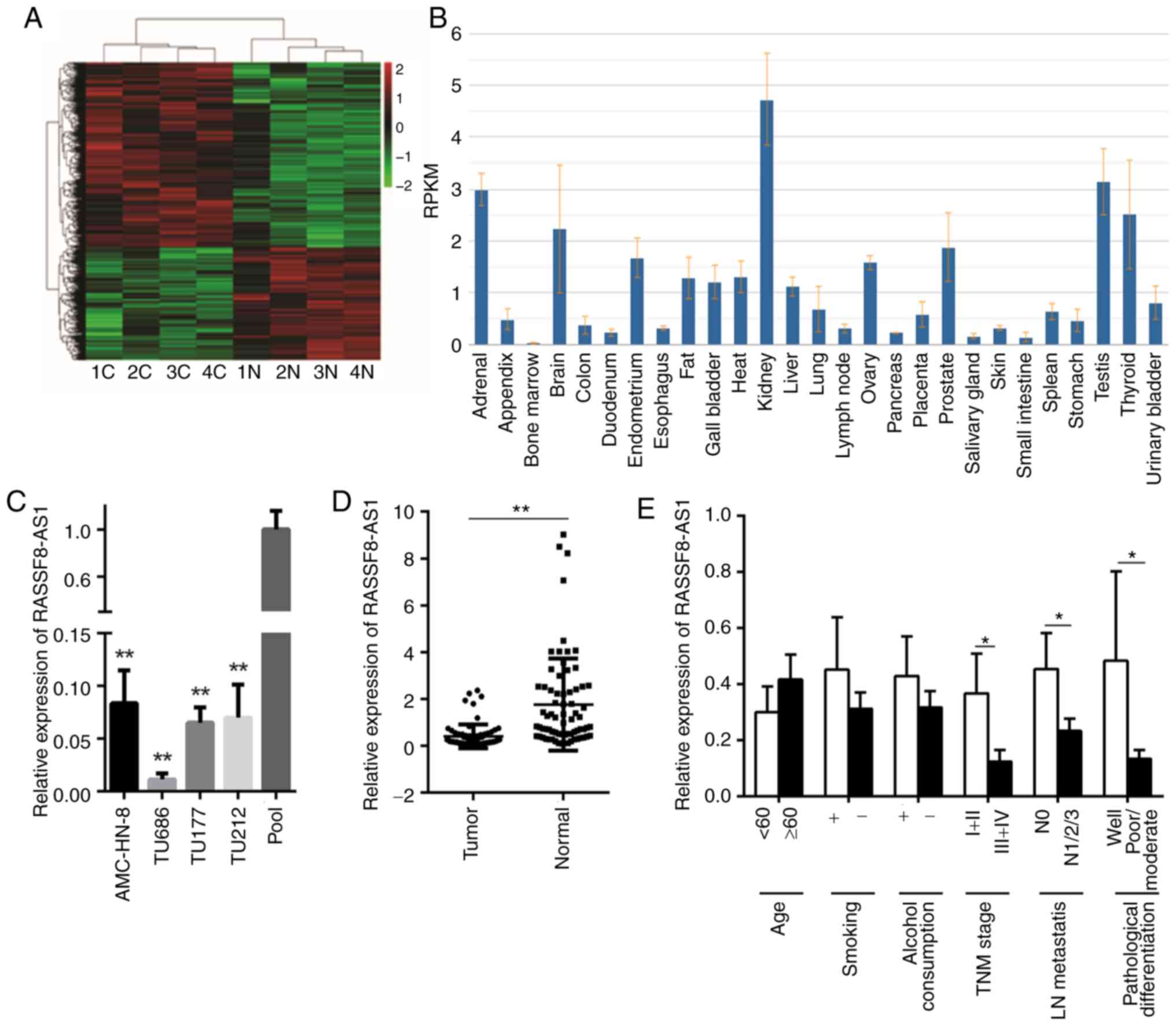
Microarray analysis was used to compare the lncRNA
expression levels between four pairs of LSCC tissues and adjacent
normal tissues to investigate the potential role of lncRNAs in LSCC
(11) (Fig. 1A). A lncRNA, RASSF8-AS1, which was
decreased in the microarray analysis was selected for further
experiments. Using National Centre for Biotechnology Information,
RASSF8-AS1 was found to be differently expressed in different types
of human tissue (Fig. 1B) and
subsequently, using RT-qPCR, the mRNA expression level of
RASSF8-AS1 was found to be significantly decreased in four LSCC
cell lines and 72 LSCC tissues (Fig. 1C
and D). The pool in Fig. 1C
represents the average value of lncRNA RASSF8-AS1 relative
expression from the normal tissues, and it was used as a control
for the laryngeal squamous cell lines, as described previously
(17). In the 72 tumor tissues, a
low mRNA expression level of RASSF8-AS1 was associated with
well-differentiated, lower lymph node metastasis and lower TNM
staging; however, no association was observed between RASSF8-AS1
mRNA expression level and age, smoking, or alcohol consumption
(Fig. 1E). These results suggest
that lncRNA RASSF8-AS1 could be a tumor-inhibiting factor in the
progression of LSCC.
Overexpression of RASSF8-AS1 reduces
proliferation and colony formation efficiency, and invasion and
migration abilities of the LSCC cell lines
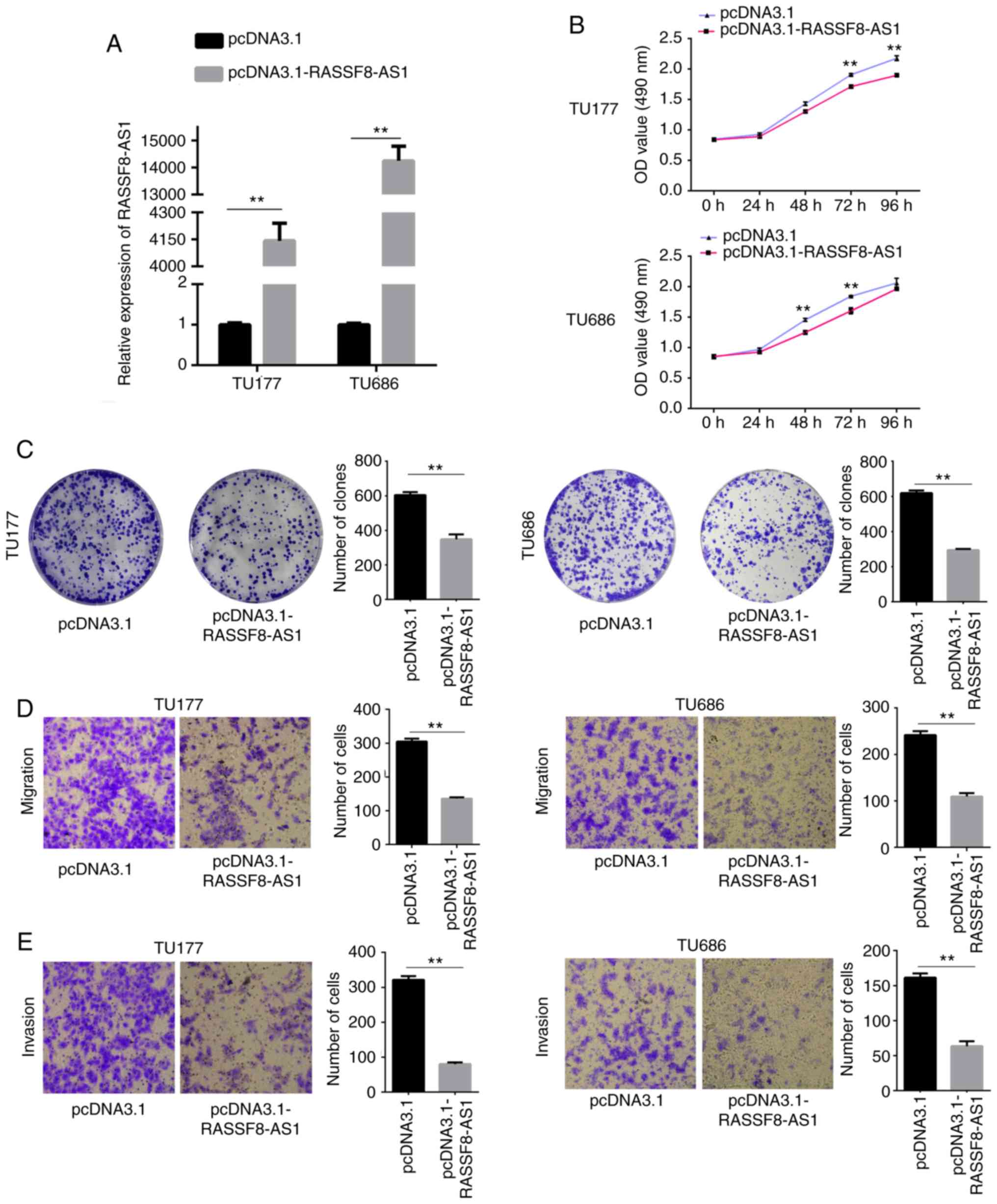
To further investigate the effect of RASSF8-AS1 in
the LSCC cell lines, pcDNA3.1-RASSF8-AS1 or pcDNA3.1 were
transfected into the TU177 and TU686 cell lines, as the expression
level of RASSF8-AS1 was lowest out of the LSCC cells investigated.
RT-qPCR analysis showed that the mRNA expression level of
RASSF8-AS1 was significantly increased by pcDNA3.1-RASSF8-AS1 in
both cell lines (Fig. 2A). Using an
MTS assay, the cells transfected with pcDNA3.1-RASSF8-AS1 exhibited
significantly reduced proliferative abilities compared with that in
the cells transfected with empty vectors (Fig. 2B). Overexpression of RASSF8-AS1
significantly reduced colony formation ability compared with that
in cells transfected with empty vectors using a colony formation
assay (Fig. 2C), while the cells
transfected with overexpression of RASSF8-AS1 had significantly
reduced migration and invasion abilities (Fig. 2D and E). Therefore, we hypothesized
that RASSF8-AS1 is a tumor inhibitor by suppressing the
proliferation, colony formation, migration and invasion abilities
of the LSCC cell lines.
lncRNA RASSF8-AS1 acts as a sponge for
miR-664b-3p
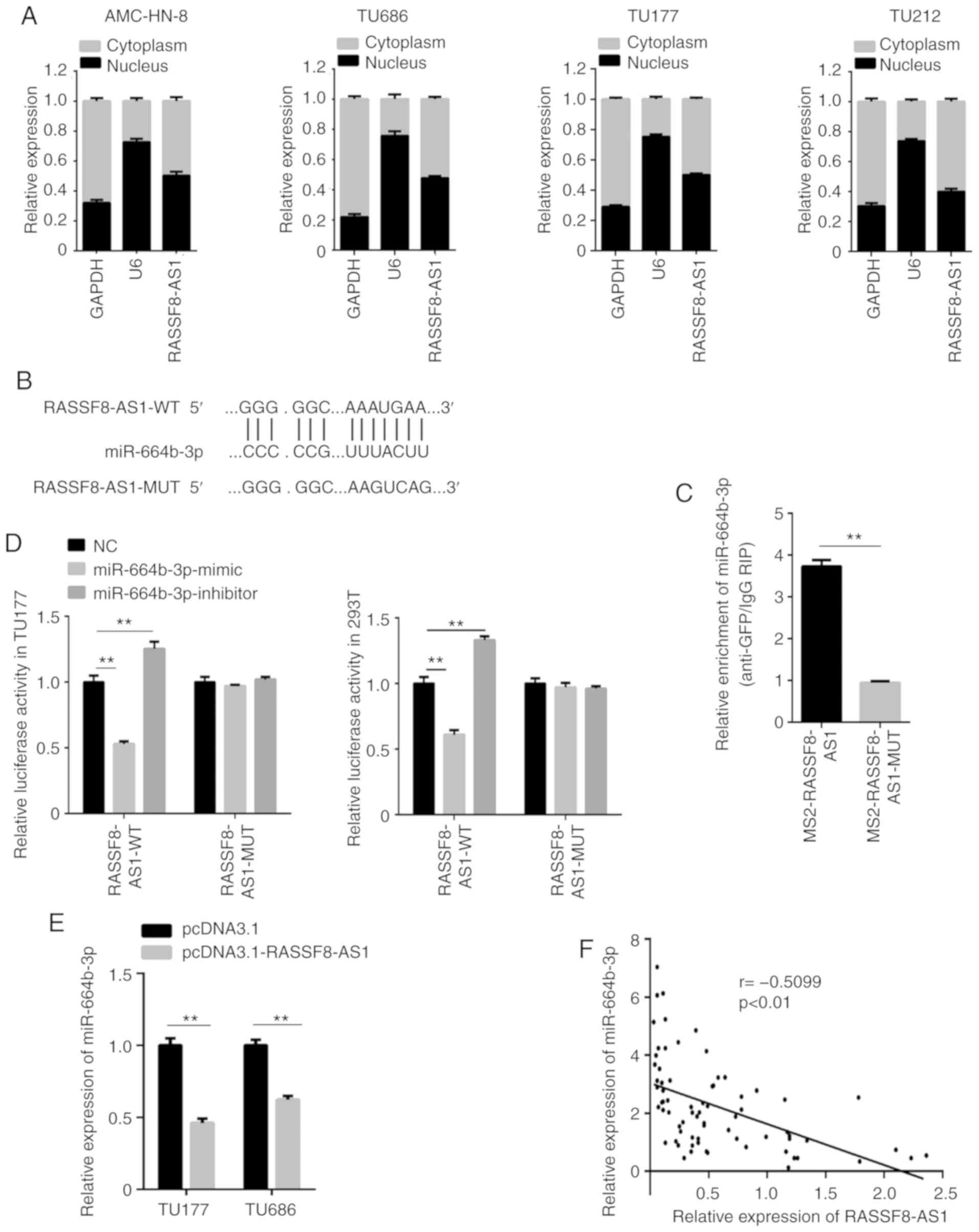
Recent studies have found that lncRNAs, which are
localized in the cytoplasm, are known as ceRNAs to regulate miRNAs
(18). The lncRNA, RASSF8-AS1 in
the four LSCC cell lines was found in both the cytoplasm and
nucleus; however, the expression level was slightly higher in the
cytoplasm (Fig. 3A). To discover
the ceRNA mechanism of RASSF8-AS1 in LSCC, the miRNA associated
with RASSF8-AS1 was investigated and the results showed that
RASSF8-AS1 was found to possess a conserved target site of
miR-664b-3p (Fig. 3B) with a high
score using bioinformatics miRNA target prediction tools (Starbase
v3.0 and DIANA). The RIP assay was then used to validate the
binding ability between miR-664b-3p and RASSF8-AS1. miR-664b-3p was
found to be markedly enriched in the TU177 cell line, which was
transfected with RASSF8-AS1-WT compared with that in cells
transfected with RASSF8-AS1-MUT (Fig.
3C). The interaction between miR-664b-3p and RASSF8-AS1 was
further confirmed using a dual luciferase reporter assay compared
with that in the control group, and the ratio of firefly luciferase
to Renilla activity was decreased following co-transfection
of the TU177 and 293T cell lines with miR-664b-3p mimic and
pmirGLO-RASSF8-AS1-WT. However, co-transfection of miR-664b-3p
mimic and pmirGLO-RASSF8-AS1-MUT did not decrease the firefly
luciferase to Renilla activity ratio. In addition, the
firefly luciferase to Renilla activity ratio was increased
in the cells co-transfected with miR-664b-3p inhibitor and
pmirGLO-RASSF8-AS1-WT, but not in cells transfected with
pmirGLO-RASSF8-AS1-MUT (Fig. 3D).
miR-664b-3p mRNA expression level was notably decreased in the
TU177 and TU686 cell lines transfected with pcDNA3.1-RASSF8-AS1
compared with that in cells transfected with the pcDNA3.1 vector
using RT-qPCR (Fig. 3E). Then,
further analysis using the LSCC tissues revealed that the
RASSF8-AS1 and miR-664b-3p mRNA expression levels were found to be
negatively correlated (Fig. 3F).
All the results revealed that RASSF8-AS1 was associated with
miR-664b-3p and acts as a ceRNA.
miR-664b-3p is upregulated in LSCC and
promotes proliferation, migration and invasion of LSCC cells
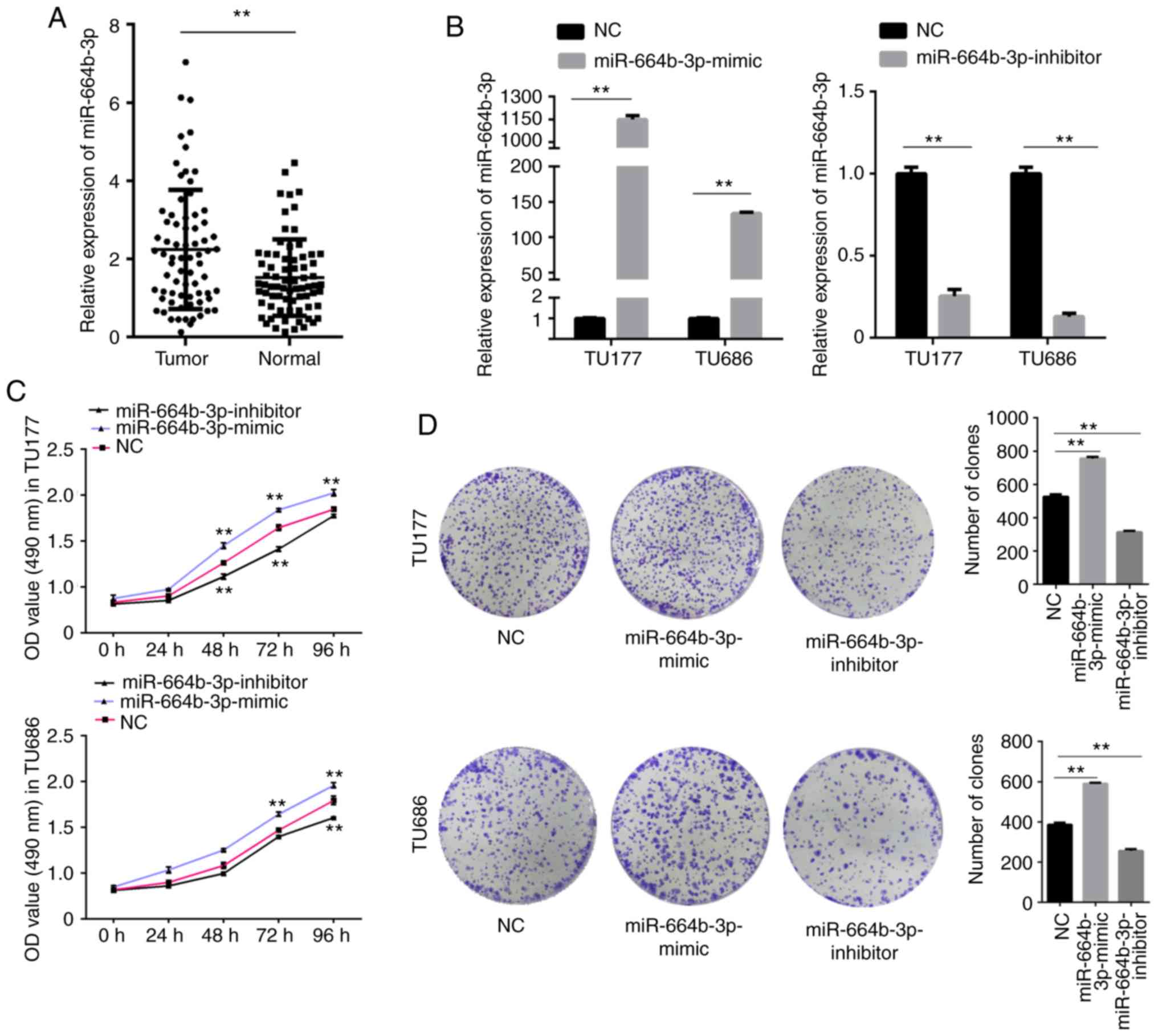
The aforementioned results showed that miR-664b-3p
is associated with RASSF8-AS1. The mRNA expression level of
miR-664b-3p was found to be increased in the LSCC tissues compared
with that in the paired normal tissues (Fig. 4A). To further investigate whether
miR-664b-3p affects the progression of LSCC, the function of
miR-664b-3p was determined. miR-664b-3p mimic, inhibitor and NC
were transfected into the TU177 and TU686 cell lines and the
transfection efficiency was determined using RT-qPCR (Fig. 4B). The MTS and colony formation
assays revealed that upregulation of miR-664b-3p notably increased
cell proliferation and colony forming abilities of the TU177 and
TU686 cell lines, whereas downregulation of miR-664b-3p had the
opposite effect (Fig. 4C and D).
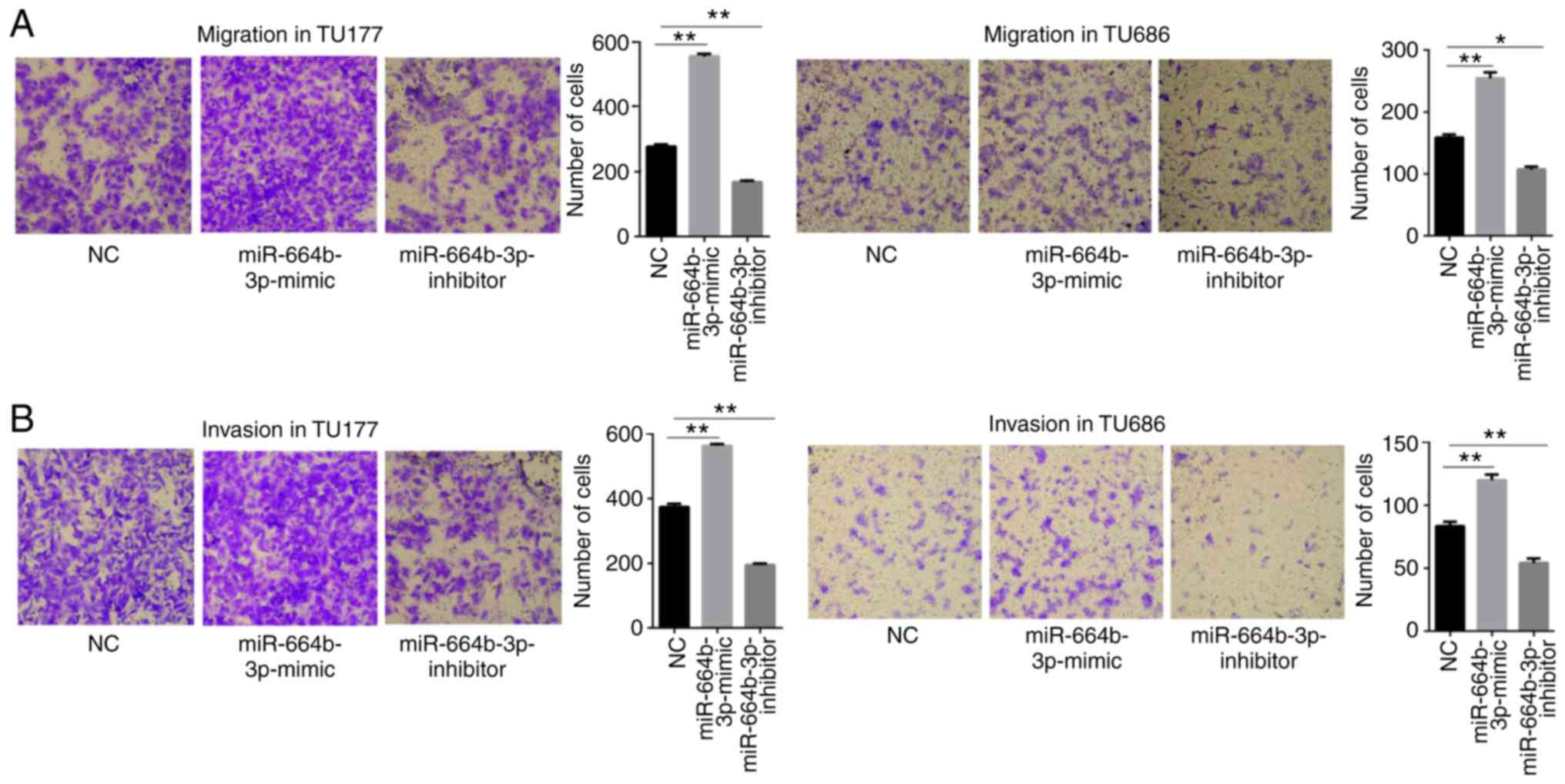
The migration and invasion assays revealed the same results
(Fig. 5A and B). Taken together,
all these results showed that miR-664b-3p promotes the
proliferation, migration and invasion abilities of the LSCC
cells.
Overexpression of RASSF8-AS1 partially
reverses the promoting effects of miR-664b-3p mimic in regards to
LSCC cell proliferation, migration, invasion and colony formation
efficiency
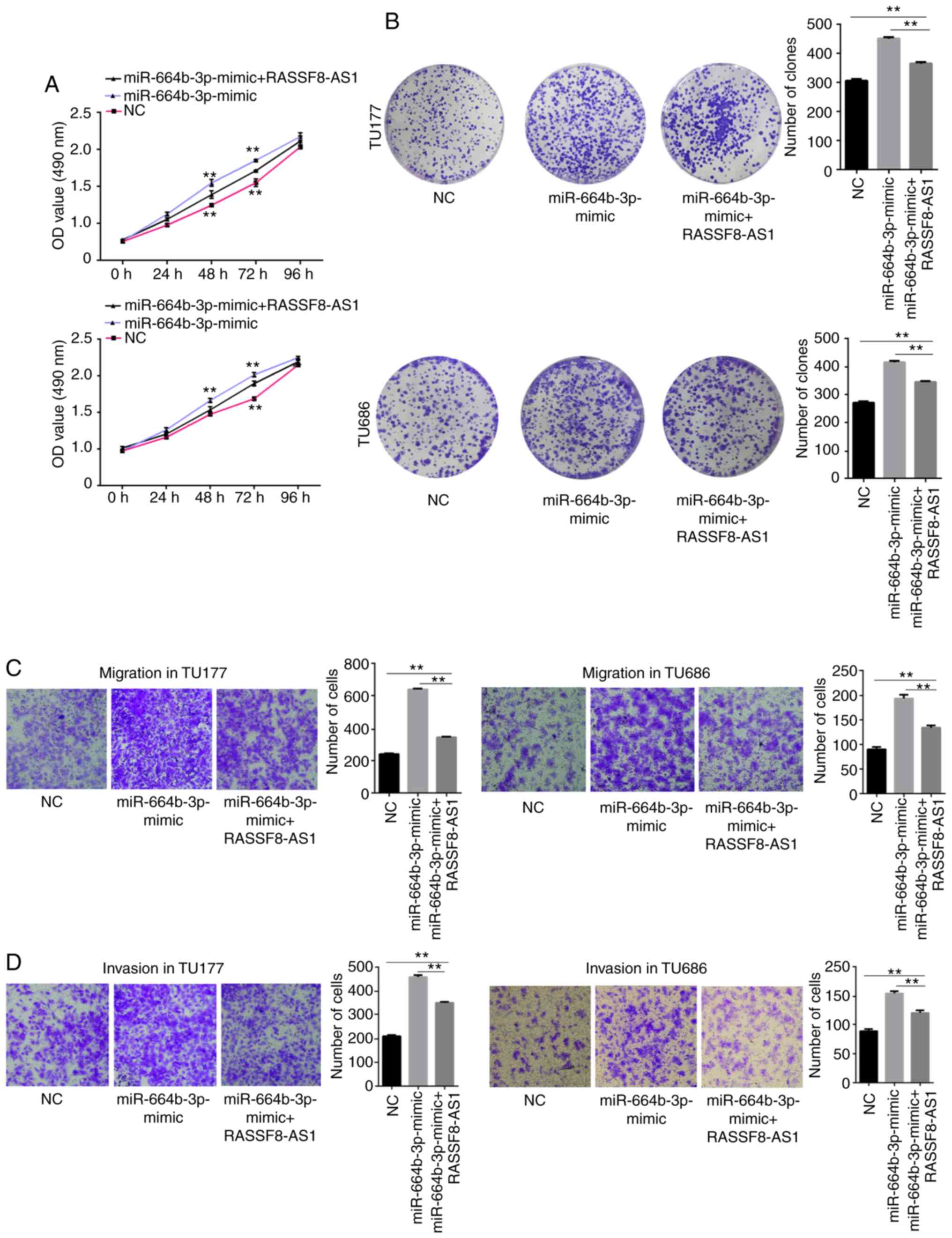
From the aforementioned results, it was found that
lncRNA RASSF8-AS1 sponges miR-664b-3p and has a negative
correlation with miR-664b-3p. Next, the regulatory effect of
RASSF8-AS1 on miR-664b-3p was verified using cell functional
experiments. NC and pcDNA3.1, miR-664b-3p mimics and pcDNA3.1 or
miR-664b-3p mimic and pcDNA3.1-RASSF8-AS1 were co-transfected into
the TU177 and TU686 cell lines. MTS, colony formation, migration
and invasion assays were performed and the results showed that the
cell proliferation, colony formation, migration and invasion
abilities were notably increased with the overexpression of
miR-664b-3p, and these were partially reversed by the
overexpression of RASSF8-AS1 (Fig.
6A-D). In summary, the results of the cell functional
experiments verified that RASSF8-AS1 could inhibit the tumor
promotion of LSCC cells by downregulating miR-664b-3p.
TLE1 is a potential target gene of
miR-664b-3p
To further investigate the mechanisms underlying the
effects of miR-664b-3p on LSCC, a potential target gene for
miR-664b-3p was subsequently determined. The Starbase v3.0 and
DIANA tools predicted that the 3′-untranslated region (UTR) of TLE1
aligned with miR-664b-3p (Fig. 7A).
To verify their relationship, the mRNA expression level of TLE1 was
detected following overexpression and knockdown of miR-664b-3p in
the TU177 and TU686 cell lines using RT-qPCR. The results suggested
that a significant reduction in the TLE1 mRNA expression level was
induced by overexpressing miR-664b-3p, while the result from
miR-664b-3p knockdown had the opposite effect (Fig. 7B). The overexpression of miR-664b-3p
in the TU177 and TU686 cell lines transfected with miR-664b-3p
mimic significantly decreased the protein expression level of TLE1;
however, knockdown of miR-664b-3p notably improved the protein
expression level of TLE1 in the LSCC cell lines (Fig. 7C). Detection of the TLE1 mRNA
expression level in the LSCC tissues showed a significantly low
expression compared with that in the adjacent normal tissues
(Fig. 7D) and exhibited a negative
correlation with the miR-664b-3p mRNA expression level in LSCC
tissues (Fig. 7E). To further
investigate whether miR-664b-3p regulates the expression of TLE1 by
binding to the 3′-UTR of TLE1, a luciferase assay was performed.
The firefly luciferase activity was reduced in the TU177 and 293T
cell lines, co-transfected with miR-664b-3p mimic and
pmirGLO-TLE1-WT compared with that in the control group, while
co-transfection of miR-664b-3p mimic and pmirGLO-TLE1-MUT did not
reduce the firefly luciferase to Renilla activity ratio
compared with that in the control group. The firefly luciferase to
Renilla activity ratio was increased in the cell lines,
which were co-transfected with the miR-664b-3p inhibitor and
pmirGLO-TLE1-WT, but not in cells co-transfected with the MUT
vector (Fig. 7F). Taken together,
the results suggest that TLE1 is a downstream target gene of
miR-664b-3p, and miR-664b-3p may be involved in downregulating the
TLE1 mRNA expression level.
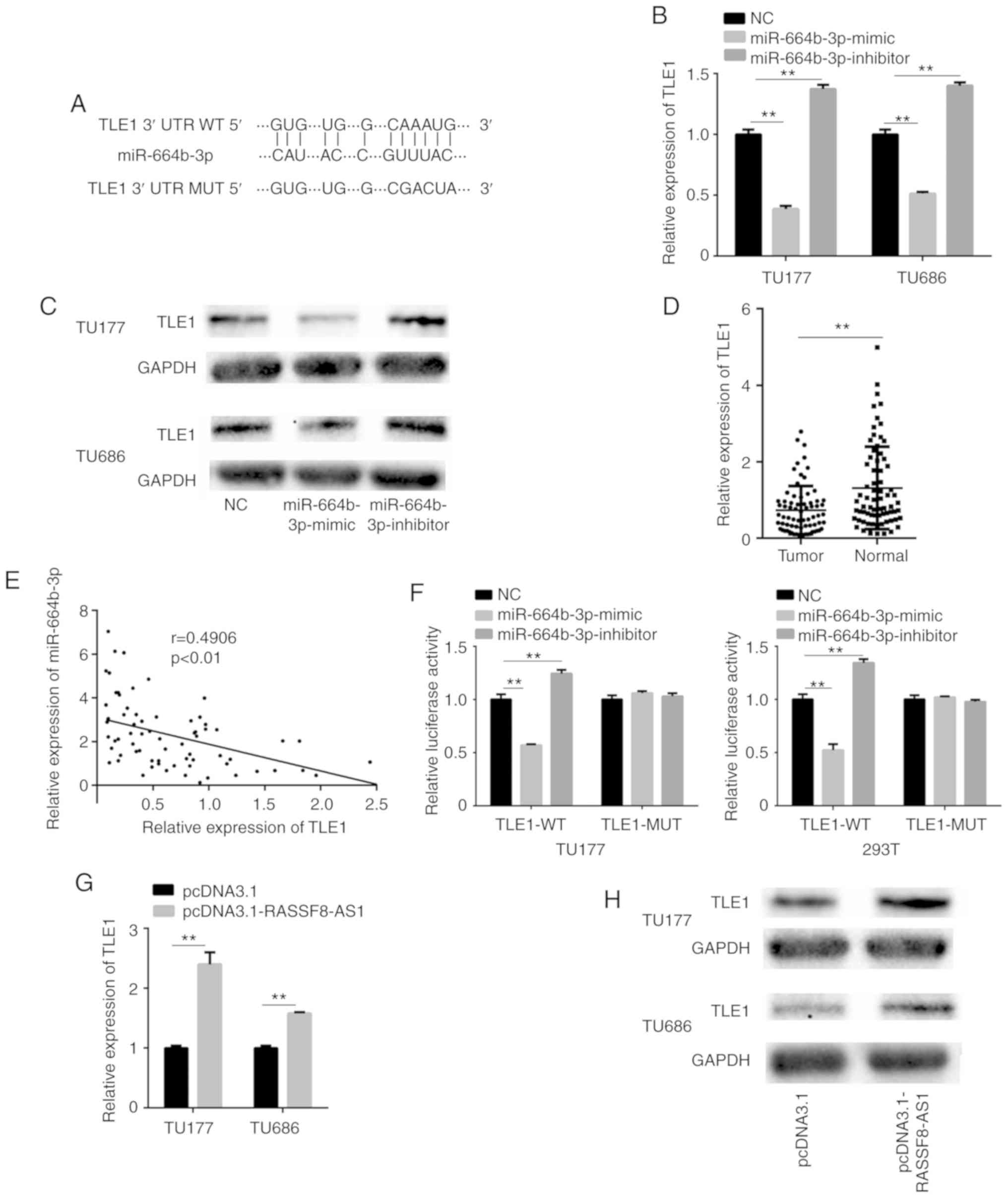 | Figure 7.TLE1 is a potential target gene of
miR-664b-3p and RASSF8-AS1 regulates TLE1 by binding to
miR-664b-3p. (A) The potential binding site of TLE1 and miR-664b-3p
predicted using bioinformatic analysis. The TLE1 (B) mRNA and (C)
protein expression level in the TU177 and TU686 cell lines
transfected with miR-664b-3p mimic and inhibitor performed using
RT-qPCR and western blot analysis, respectively. (D) The relative
mRNA expression level of TLE1 in LSCC and adjacent normal tissues.
(E) Correlation between TLE1 and miR-664b-3p in the LSCC tissues.
(F) The firefly luciferase to Renilla activity ratio of the
TU177 and 293T cell lines transfected with pmirGLO-TLE1-WT or
pmirGLO-TLE1-MUT and NC, miR-664b-3p mimic or miR-664b-3p inhibitor
in the dual-luciferase assays. The (G) mRNA and (H) protein
expression level of TLE1 in the TU177 and TU686 cell lines
transfected with pcDNA3.1-RASSF8-AS1 and pcDNA3.1 performed using
RT-qPCR and western blot analysis, respectively. Data are presented
as the mean ± SD from three independent experiments. **P<0.01
vs. NC group. LSCC, laryngeal squamous cell carcinoma; TLE1,
transducin-like enhancer of split 1; NC, negative control; WT,
wild-type; MUT, mutant; miR, microRNA; RT-qPCR, reverse
transcription-quantitative PCR. |
RASSF8-AS1 regulates TLE1 by binding
with miR-664b-3p
To verify the regulation of RASSF8-AS1 on TLE1, the
transcriptional and protein expression levels were investigated. At
the transcriptional level, the TLE1 mRNA expression level was
significantly increased by the overexpression of RASSF8-AS1 in the
TU177 and TU686 cell lines (Fig.
7G), while the protein expression level was also significantly
increased (Fig. 7H). Taken
together, these results indicate that RASSF8-AS1 upregulates TLE1
by binding with miR-664b-3p and acts as a ceRNA.
Knockdown of TLE1 partially reverses
the suppressive effects of pcDNA3.1-RASSF8-AS1 on LSCC cell
proliferation, migration, invasion and colony formation
efficiency
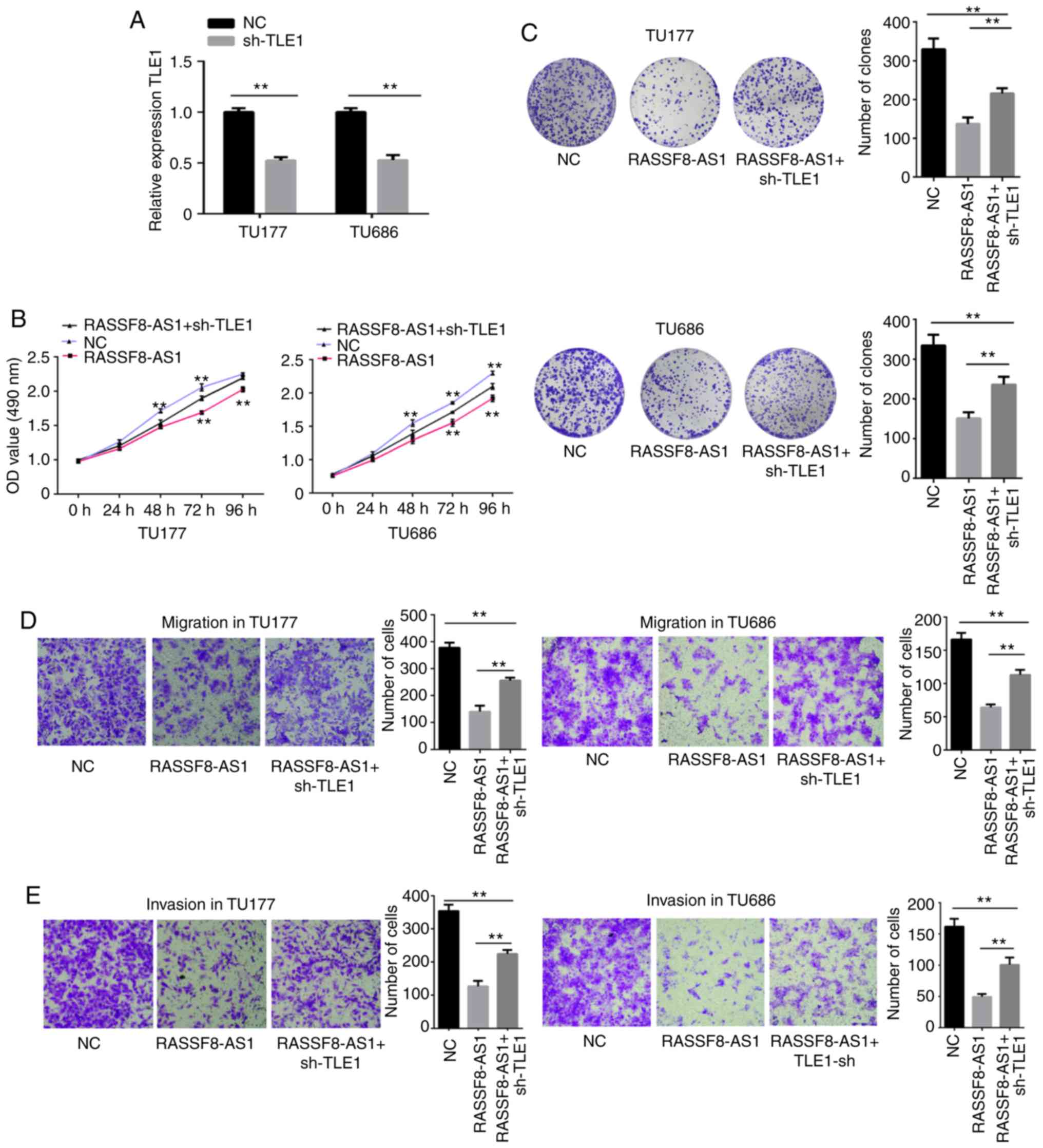
The aforementioned results proved that RASSF8-AS1
could regulate TLE1. In the following experiments, the association
between RASSF8-AS1 and TLE1 was verified using cell functional
experiments. pGenesil-1 and pcDNA3.1, pcDNA3.1-RASSF8-AS1 and
pGenesil-1 or sh-TLE1 and pcDNA3.1-RASSF8-AS1 were co-transfected
into the TU177 and TU686 cell lines. RT-qPCR analysis showed that
TLE1 mRNA expression level was reduced by sh-TLE1 (Fig. 8A). MTS, colony formation, migration
and invasion assays were then performed and the results showed that
the cell proliferation, colony formation, migration and invasion
abilities were notably suppressed following overexpression of
RASSF8-AS1, and these were partially reversed by TLE1 knockdown
(Fig. 8B-E). In summary, the
results of the cell functional experiments verified the association
between RASSF8-AS1 and TLE1.
Discussion
The importance of non-coding RNAs (lncRNAs) in
regulating tumor progression, as well as other diseases, has become
a hot topic, recently, and can be viewed as potential targets for
tumor diagnosis, prognosis and treatment targets (19). Recently, lncRNAs have been verified
to regulate laryngeal squamous cell carcinoma (LSCC) progression.
Gao et al (20) found that
the low expression of LOC285194 distinguished patients with LSCC
from a healthy control group, suggesting that LOC285194 may play an
anticancer role in LSCC. In addition, Meng et al (21) confirmed that aberrant methylation
and low expression of ZNF667-AS1 and ZNF667 may stimulate the
progression of LSCC. However, the roles and molecular mechanisms of
lncRNAs in LSCC require further elucidation.
In the present study, lncRNA RASSF8-AS1 was found to
be expressed at low levels in four LSCC tissues compared with that
in paired normal tissues using microarray assays. Then, low mRNA
expression level of RASSF8-AS1 was found in the 72 LSCC tissues and
4 LSCC cell lines. In addition, low mRNA expression level of
RASSF8-AS1 was associated with well-differentiated, lower lymph
node metastasis and lower TNM staging. Furthermore, RASSF8-AS1 was
found to play suppressive roles in the progression of LSCC by
reducing cell proliferation, colony formation, migration and
invasion in vitro. The aforementioned results suggest that
RASSF8-AS1 may be a biomarker for LSCC invasion and metastasis.
The competitive endogenous RNA (ceRNA) hypothesis is
considered to be a novel post-transcriptional approach, which
regulates genes by competing with miRNAs (22). The endogenous RNAs, containing
mRNAs, long non-coding, pseudogene and circular RNAs, are involved
in the development of different types of cancer by competitively
binding to miRNAs with miRNA response elements (23,24).
For example, Wu et al (25)
verified that the lncRNA SNHG20 could increasethe expression level
of SCGB2A1 to promote prostate cancer migration and invasion by
binding with miR-6516-5p. Han et al (26) reported that the lncRNA MYOSLID
regulated the MCL-1 expression level by sponging miR-29c-3p in
gastric cancer and promoted the progression of gastric cancer, as a
ceRNA. In the present study, the distribution of RASSF8-AS1 in the
4 LSCC cell lines was found to be in both the cytoplasm and the
nucleus, although there was slightly higher expression in the
cytoplasm, suggesting that the lncRNA RASSF8-AS1 may act as a
ceRNA. Then, bioinformatics analysis revealed that miR-664b-3p was
a potential target of RASSF8-AS1, which was confirmed using the
dual-luciferase reporter and RIP assays. In addition, correlation
analysis using LSCC tissues revealed that RASSF8-AS1 and
miR-664b-3p mRNA expression levels were negatively correlated.
These results showed that RASSF8-AS1 may act on LSCC by binding
with miR-664b-3p as a ceRNA and miR-664b-3p is a downstream target
of RASSF8-AS1.
In the present study, it was validated that the
expression level of miR-664b-3p was increased in the LSCC tissues.
The overexpression of miR-664b-3p promoted the proliferation,
migration and invasion abilities of the LSCC cell lines; however,
knockdown of miR-664b-3p had the opposite effect. As
aforementioned, miR-664b-3p may be a cancer-promoting gene and it
could play a carcinogenic role in LSCC. At the same time, cell
function rescue assays revealed that overexpression of RASSF8-AS1
partially reversed the increase in proliferation, migration and
invasion of the LSCC cell lines by miR-664b-3p mimic. It was also
confirmed that RASSF8-AS1 plays a key role in LSCC cells by
sponging miR-664b-3p during the progression of LSCC.
In general, lncRNAs play a role on downstream miRNA
targets by inhibiting miRNAs in the mechanism of ceRNAs. Therefore,
identifying the miRNA target is an important part of the ceRNA
network (22). To investigate the
downstream target gene of miR-664b-3p, bioinformatics tools were
used to reveal that transducin-like enhancer of split 1 (TLE1) is
one of the potential miR-664b-3p targets. TLE1 is a member of the
Groucho/transducin-like enhancer of split family, acts as a
corepressor for numerous transcription factors and is involved in
their development. It has been found that abnormal expression of
TLE1 may lead to the tumorigenesis and progression of multiple
types of tumors (27–29). For example, Brunquell et al
(30) discovered that TLE1
inhibited Bit1-mediated anoikis and induced Bit1-mediated anoikis
might be an effective strategy for breast cancer treatment. Lee
et al (31) found that in
patients with gastric cancer, the TLE1 expression level was also
associated with prognosis, indicating that the expression of TLE1
was a prognostic indicator of gastric cancer. In the present study,
it was verified that TLE1 mRNA expression level was decreased in
LSCC tissues compared with that in the paired normal tissues and
there was a negative correlation between TLE1 and miR-664b-3p mRNA
expression levels. Furthermore, to validate that miR-664b-3p
targets TLE1, a dual-luciferase reporter assay was used to confirm
that the 3′-UTR of TLE1 was the binding site for miR-664b-3p. In
addition, it was confirmed that miR-664b-3p regulates TLE1 at the
transcription and translation levels in the LSCC cell lines. These
results indicate that TLE1 is a downstream target gene of
miR-664b-3p. Then, it was confirmed that RASSF8-AS1 could
upregulate TLE1, both at the transcription and translation levels
in the TU686 and TU177 cell lines. Lastly, cell functional rescue
assays revealed that knockdown of TLE1 partially reversed the
suppression of proliferation, migration and invasion of the LSCC
cell lines by pcDNA3.1-RASSF8-AS1. The aforementioned results
confirmed that RASSF8-AS1 regulates TLE1 by reducing miR-664b-3p
expression level.
In conclusion, it was verified that lncRNA
RASSF8-AS1 is a suppressor gene that reduced the proliferation,
migration and invasion abilities of LSCC cell lines by regulating
TLE1 via binding with miR-664b-3p, to act as a ceRNA. The present
study provides a basis for a further understanding of the role of a
ceRNA network in the development of LSCC. RASSF8-AS1 may be a
potential important target for the prediction, diagnosis and
treatment of LSCC.
Acknowledgements
The authors would like to thank Professor W. Guo for
providing writing services.
Funding
This study was supported by grants from the Key
Program of Hebei Natural Science Foundation (grant no. H2017206391)
and National Natural Science Foundation of China (grant no.
81972553).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
BW conceived and designed the experiments. TL
performed the experiments, collected the data and wrote the paper.
HC and WM recruited the patients and collected the specimens. WCh,
LZ, WCu and HY performed experiments and produced the figures. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All procedures performed in this study were in
accordance with the ethical standards of the Institutional Research
Committee of Hebei Medical University (Hebei, China) and with the
Declaration of Helsinki (2008). The present study was approved by
the Ethics Committee of Hebei Medical University and the Second
Hospital of Hebei Medical University. Informed consent was provided
by all the patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
lncRNA
|
long non-coding RNA
|
|
LSCC
|
laryngeal squamous cell carcinoma
|
|
RT-qPCR
|
reverse transcription quantitative
polymerase chain reaction
|
|
TLE1
|
transducin-like enhancer of split
1
|
|
ceRNA
|
competitive endogenous RNA
|
|
PBS
|
phosphate-buffered saline
|
|
RIP
|
RNA immunoprecipitation
|
|
MRE
|
miRNA response element
|
|
NC
|
negative control
|
|
WT
|
wild-type
|
|
MUT
|
mutant type
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Groome PA, O'Sullivan B, Irish JC,
Rothwell DM, Schulze K, Warde PR, Schneider KM, Mackenzie RG,
Hodson DI, Hammond JA, et al: Management and outcome differences in
supraglottic cancer between Ontario, Canada, and the surveillance,
epidemiology, and end results areas of the United States. J Clin
Oncol. 21:496–505. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marur S and Forastiere AA: Forastiere,
Head and neck squamous cell carcinoma: Update on epidemiology,
diagnosis, and treatment. Mayo Clin Proc. 91:386–96. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Awan HM, Shah A, Rashid F and Shan G:
Primate-specific long non-coding RNAs and microRNAs. Genomics
Proteomics Bioinformatics. 15:187–195. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guttman M and Rinn JL: Modular regulatory
principles of large non-coding RNAs. Nature. 482:339–46. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parasramka MA, Maji S, Matsuda A, Yan IK
and Patel T: Long non-coding RNAs novel targets for therapy in
hepatocellular carcinoma. Pharmacol Ther. 161:67–78. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao D, Ding Q, Yu W, Gao M and Wang Y:
Long noncoding RNA SPRY4-IT1 promotes malignant development of
colorectal cancer by targeting epithelial-mesenchymal transition.
Onco Targets Ther. 9:5417–5425. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cui WN, Meng WX, Zhao L, Cao H, Chi W and
Wang BS: TGF-β-induced Long non-coding RNA MIR155HG promotes the
progression and EMT of laryngeal squamous cell carcinoma by
regulating themiR-155-5p SOX10 axis. Int J Oncol. 54:2005–2018.
2019.PubMed/NCBI
|
|
12
|
Song X, Zhang X, Wang X, Chen L, Jiang L,
Zheng A, Zhang M, Zhao L and Wei M: LncRNA SPRY4-IT1 regulates
breast cancer cell stemness through competitively binding
miR-6882-3p with TCF7L2. J Cell Mol Med. 24:772–784. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen H, Li ML and Huang P: LncRNA SNHG16
promotes hepatocellular carcinoma proliferation, migration and
invasion by regulating miR-186 expression. J Cancer. 10:3571–3581.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Huang YQ, Song W, Li Y, Wang H,
Wang WJ and Huang M: Comprehensive analysis of the
lncRNA-associated competing endogenous RNA network in breast
cancer. Oncol Rep. 42:2572–2582. 2019.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y,
Chen W, Liu F, Sun W, Li XF, et al: Exosome-transmitted lncARSR
promotes sunitinib resistance in renal cancer by acting as a
competing endogenous RNA. Cancer Cell. 29:653–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo W, Liang X, Liu L, Guo Y, Shen S,
Liang J and Dong Z: MiR-6872 host gene SEMA3B and its antisense
lncRNA SEMA3B-AS1 function synergistically to suppress gastric
cardia adenocarcinoma progression. Gastric Cancer. 22:705–722.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta stone of a hidden RNA
language. Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y, Li C, Pan Y, Han S, Feng B, Gao Y,
Chen J, Zhang K, Wang R and Chen L: The emerging role and promise
of long non coding RNAs in lung cancer treatment. Cell Physiol
Biochem. 38:2194–2206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao Y, Wang ZQ, Tong J and Zheng Y: LncRNA
loc285194 inhibits tumor growth of laryngeal squamous cell
carcinoma cells by downregulating hexokinase 2. Exp Ther Med.
18:2378–2384. 2019.PubMed/NCBI
|
|
21
|
Meng W, Cui W, Zhao L, Chi W, Cao H and
Wang B: Aberrant methylation and downregulation of ZNF667-AS1 and
ZNF667 promote the malignant progression of laryngeal squamous cell
carcinoma. J Biomed Sci. 26:132019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cesana M, Cacchiarelli D, Legnini I,
Santini T, Sthandier O, Chinappi M and Bozzoni I: A long non-coding
RNA controls muscle differentiation by functioning as a competing
endogenous RNA. Cell. 147:358–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sumazin P, Yang X, Chiu HS, Chung WJ, Iyer
A, Llobet-Navas D and Califano A: An extensive microRNA-mediated
network of RNA-RNA interactions regulates established oncogenic
pathways in glioblastoma. Cell. 147:370–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu XC, Xiao Y, Zhou Y, Zhou Z and Yan WG:
lncRNA SNHG20 promotes prostate cancer migration and invasion via
targeting the miR-6516-5p/SCGB2A1 axis. Am J Transl Res.
11:5162–5169. 2019.PubMed/NCBI
|
|
26
|
Han Y, Wu N, Jiang M, Chu Y, Wang Z, Liu
H, Cao J, Liu H, Xu B and Xie X: Long non-coding RNA MYOSLID
functions as a competing endogenous RNA to regulate MCL-1
expression by sponging miR-29c-3p in gastric cancer. Cell Prolif.
52:e126782019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaul A, Schuster E and Jennings BH: The
Groucho co-repressor is primarily recruited to local target sites
in active chromatin to attenuate transcription. PLoS Genet.
10:e10045952014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ramasamy S, Saez B, Mukhopadhyay S, Ding
D, Ahmed AM, Chen X, Pucci F, Yamin R, Wang J, Pittet MJ, et al:
Tle1 tumor suppressor negatively regulates inflammation in vivo and
modulates NF-κB inflammatory pathway. Proc Natl Acad Sci USA.
113:1871–1876. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Y, Dehni G, Purcell KJ, Sokolow J,
Carcangiu ML, Artavanis-Tsakonas S and Stifani S: Epithelial
expression and chromosomal location of human TLE genes:
Implications for notch signaling and neoplasia. Genomics. 31:58–64.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brunquell C, Biliran H, Jennings S,
Ireland SK, Chen R and Ruoslahti E: TLE1 is an anoikis regulator
and is downregulated by Bit1 in breast cancer cells. Mol Cancer
Res. 10:1482–1495. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee JH, Son MW, Kim KJ, Oh MH, Cho H, Lee
HJ, Jang SH and Lee MS: Prognostic and Clinicopathological
significance of transducer-like enhancer of split 1 expression in
gastric cancer. J Gastr Cancer. 16:21–27. 2016. View Article : Google Scholar
|