Introduction
Colorectal cancer (CRC) is the third most prevalent
cancer worldwide, with 935,000 annual deaths in 2020 and an
incidence estimated to increase to 2.5 million new cases by 2035
(1,2).
CRC has incidence and mortality rates of 9.8 and 9.2%,
respectively, globally with ~25% fewer cases in females compared
with in males in 2020 (1). This
difference suggests that sex hormones might have an influence on
the development of CRC, and this role is most likely protective in
females (3). Thus, an improved
understanding of the effect of sex hormones on CRC progression is
urgently needed to facilitate the exploration of new therapeutic
targets and the development of effective treatment strategies for
CRC.
Progesterone, an important natural sex hormone, is
involved in the menstrual cycle, pregnancy and embryogenesis of
humans (4). Its cellular effects are
mediated by binding to the progesterone receptor (PGR) and
regulating hormone response target genes in several cancer types,
such as liver (5), ovarian (6), gastric (7)
and breast (8) cancer. Furthermore,
progesterone regulates various cancer cell phenotypes, including
proliferation, apoptosis, angiogenesis and autophagy (9). Studies have suggested that progesterone
promotes apoptosis and an inhibitory effect is observed on cell
proliferation in endometrial cancer (10,11).
Progesterone-induced apoptosis occurs by arresting the progression
from the G1 phase to S phase (12). Thus, in clinical settings,
progesterone has been used in patients who exhibit
well-differentiated endometrial cancer, or patients with recurrence
(13). Although there is accumulating
data suggesting that progesterone and other hormone replacement
therapy contributes to the decreased progression of CRC (11,14,15), the
individual effect of progesterone on CRC progression or cell lines
has been minimally studied. Progesterone-related gene variants
increase the risk of developing CRC in women, predicting a
therapeutic target (11). During the
treatment of CRC with folic acid, PGR activation is required for
its anti-proliferative effect, which results from cell cycle arrest
(16). Although progesterone has been
preliminarily confirmed to have an inhibiting effect on CRC cell
proliferation and tumor progression, respectively, the specific
mechanism by which progesterone inhibits CRC progression remains to
be elucidated (17).
Through triggering a variety of molecular responses
leading to cell proliferation in CRC, the mitogen activated protein
kinase (MAPK) pathway has recently been proposed to be involved in
apoptosis and cell cycle regulation by various compounds (18). As a member of MAPK family of proteins,
the c-Jun N-terminal kinase (JNK) has a specific role in mediating
apoptosis in several types of cancer cell (19). G2/M phase arrest and
apoptosis are induced via ROS-mediated p38 and JNK signalling
pathways in human colon cancer cells (20). Human CRC cells are inhibited by JNK1
pathway-induced apoptosis (21). The
levels of phosphorylated ERK and JNK reportedly increase when
treated with a combination of 17β-estradiol and progesterone
(22). The up-regulation of
progesterone-induced decidual protein is also accompanied by the
activation of the ERK pathway, leading to senescence in colon
cancer cells (23). However, whether
anticancer activity against CRC is induced by progesterone through
the JNK pathway has not been determined.
The present study aimed to reveal the potential
mechanism by which progesterone inhibits CRC progression. First,
the expression of progesterone and PGR was evaluated. Second, the
role of progesterone in several cancer-related processes in
vitro and in vivo were investigated. Finally, the
specific mechanism by which progesterone inhibited CRC progression
was analysed.
Materials and methods
Samples from patients with CRC
In total, 77 pre-existing blood and
paraffin-embedded tissue samples were used from patients with CRC
who underwent colectomy at The General Surgery Center, the General
Hospital of Western Theater Command (Chengdu, China) between
January 2015 and January 2020. All CRC tissue samples had paired
adjacent non-cancerous tissues. Patient follow-up time ranged from
0.54 to 60 months, with a median follow-up time of 25.24 months.
The clinical features of patients with CRC were collected,
including age, gender, tumor size, tumor number, differentiation,
vascular invasion, and tumor node metastasis (TNM) (24). Written informed consent was obtained
from all patients during the admission. The study was performed in
accordance with clinical study protocols and the principles of the
Declaration of Helsinki (modified 2018) and was approved by The
Research Care and Ethics Committee of the General Hospital of
Western Theater Command (approval no. SPPHCT2015-0117) (25).
Cell culture
LoVo, SW620, HT29, HCT116 and SW480 CRC cell lines
were purchased from The Cell Bank of Shanghai Institute of Cell
Biology. The cell lines were tested and were free from mycoplasma
contamination, and had also passed STR identification without
errors. The cell lines were maintained in complete Dulbecco's
Modified Eagle's Medium (high glucose) supplemented with 10% foetal
bovine serum (both Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
of penicillin and 100 µg/ml of streptomycin at 37°C in 5%
CO2. Trypsin-EDTA (0.05%) (Thermo Fisher Scientific,
Inc.) was used for cell passages.
Flow cytometry
Flow cytometry was performed to observe cell cycle
distribution and apoptosis of LoVo and SW620 cells (26). Cells were treated with 250 µM
progesterone for 48 h before the start of the experiment to ensure
cell synchronisation. The cells were collected through
trypsinization and fixed in 90% 4°C ethanol for 30 min. The fixed
cells were washed in phosphate-buffered saline (PBS) and stained
with 50 µg/ml propidium iodide (PI; Sigma-Aldrich; Merck KGaA) for
30 min at 37°C. A cell cycle detection kit (cat. no. KGA511;
Nanjing KeyGen Biotech Co., Ltd.) was used to detect changes in
cell cycle progression in each group. Fluorescence-activated cell
sorting (FACS) was performed using a FACSCalibur flow cytometer.
The proportion of apoptotic cells in each group was measured using
an Annexin V-kFluor647/PI double-stained Apoptosis Detection kit
(cat. no. KGAV113; Nanjing KeyGen Biotech Co., Ltd.). The cells
were detached using 0.25% trypsin without EDTA, washed twice in
cold PBS, and resuspended in 100 µl binding buffer. The cells were
then incubated with 5 µl Annexin V-kFluor647 and 5 µl PI for 10 min
at room temperature in the dark and detected with a FACSCalibur
flow cytometer. The data of cell cycle analysis and apoptosis was
analysed using CELLQuest (version no. 0.9.13α; BD Biosciences).
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay (cat. no. BS350B, Anhui Biosharp
Co., Ltd.) was performed to monitor cell proliferation. Cells were
plated at a density of 5,000 cells/well in 96-well plates. After 24
h in culture, the cells were treated with increasing concentrations
of progesterone (0, 125, 250 and 500 µM) for 72 h. The control
group was treated with PBS. Samples were collected at 24, 48 and 72
h. After adding 10 µl of CCK-8 solution per well and incubating for
1 h, absorbance was measured at 450 nm using an enzyme calibrator
(BioTek Instruments, Inc.). Experiments were performed in
triplicate.
Small interfering (si)RNA
transfection
siRNA targeting GADD45α
(5′-GGAGGAAGUGCUCAGCAAA-dTdT-3′) and scrambled siRNA
(5′-UUCUCCGAACGUGUCACGU-3′) were synthesised by GenScript Co.,
Ltd.. The cells were transfected with siGADD45α or scrambled siRNA
using Entranster™-R4000 (Engreen Biosystem Co., Ltd.) according to
the manufacturer's instructions. Cells were confluent to be 80% for
transfection. One µl Entranster™-R4000 Reagent was diluted in 25 µl
Opti-MEM™ medium (cat. no. 11058021, Gibco Co., Ltd.) and mixed
well. The siRNA (0.6 µg or 50 pmol) was diluted in 25 µl Opti-MEM™
medium, then diluted siRNA was added to tube of diluted
Entranster™-R4000 Reagent (1:1 ratio) and incubated for 10 min at
room temperature. Cells were incubated subsequently for 2 days at
37°C. Then, the transfected cells were used for subsequent
experimentation.
Histology and immunohistochemistry
(IHC) analysis
Tissue samples from patients with CRC were cut into
4-µm thick sections, deparaffinized with xylene and rehydrated
through graded ethanol (cat. no. 0012036210; Fuyu Chemistry Co.,
Ltd.) washes (from 100, 100, 95, 85 and 75%). Then the sections
were immersed in sodium citrate antigen retrieval solution (cat.
no. C1032; Solarbio) at 100°C for 5 min, and then were cooled to
room temperature. Tumour tissues from mice were directly fixed in
OCT Compound (cat. no. 4583; Sakura Finetek, Inc.) at room
temperature for 4 h, frozen at −80°C for 2 h and sectioned into
4-µm thick sections using a freezing microtome (Leica Microsystems
GmbH). Tissues were blocked with 5% BSA (cat. no. A8020; Beijing
Solarbio Science & Technology Co., Ltd.) at room temperature
for 20 min. To detect the expression of PGR and Ki67 in CRC tissue,
the sections were incubated with an anti-PGR antibody (1:500; cat.
no. ab32085; Abcam) or an anti-Ki67 antibody (1:500; cat. no.
ab21700; Abcam) overnight at 4°C. Slides were rinsed in PBS and the
immunoreactive signals were visualised using a DAKO EnVision
Detection system (Fuzhou MXB® Biotechnology Development
Co., Ltd.). IHC staining scores demonstrated variable expression
levels of PGR in tissue samples from patients with CRC and were
used to define high and low expression of PGR in CRC tissues.
Scores (1–3 and 4) were used to represent at least 10, 30, 50 and
70% of malignant cells exhibiting positive PGR staining,
respectively. A score of 0 was used to define <10% of cells
showing positive PCR staining. A high expression level was defined
as a staining score of ≥3 with at least 50% of the malignant cells
exhibiting positive PGR staining, while a low expression level was
defined as a staining score ≤2 with <50% of the malignant cells
showing nuclear staining. Four views were randomly collected from
each sample and images were captured using a light microscope
(n=3). PGR expression data extracted from The Cancer Genome Atlas
(TCGA) database (UALCAN, http://ualcan.path.uab.edu/) was analysed to display
its relationship with normal tissue, primary tumours and the stage
of cancer.
Experimental animals and xenograft
model
The study was approved by The Animal Research Ethics
Committee of the General Hospital of Western Theater Command
(Chengdu, China) and complied with the Guidelines for Animal
Experiments on Laboratory Animals (27). In total, 12 nude mice, aged 7 weeks
old and weighing 18–22 g were purchased from Chengdu Dashuo
Biotechnology Co., Ltd. (China; license no. SCXK 2008-24). All mice
were maintained on a 12/12-h light dark cycle with free access to
standard laboratory feed and water. In order to reduce the
suffering in nude mice, xenograft model was established under
sodium pentobarbital anaesthesia (50 mg/kg) by intraperitoneal
injection. SW620 cells (1×105) were implanted into the
dorsa subcutaneous tissue of mice to study tumour growth. Tumour
volume was monitored by measuring the diameter each week
(volume=length × width2/2) for 7 weeks after which the
mice were sacrificed. Lack of responsiveness to manual stimulation
and an inability to eat or drink in mice, a tumor burden >10%
body weight, and ulcerated, nectrotic or infected tumors were
considered as humane endpoints. Inhalation of CO2 (20%
of the displacement volume per min) was used for euthanasia, and
death was confirmed by parameters including no movement, no
breathing or dilated pupils. Tumour weights were measured at the
end of the 7-week observation. Harvested tumours were fixed in 4%
paraformaldehyde for 24 h at room temperature, and stored in 75%
ethanol at 4°C. After this, the tissues were embedded in paraffin
and subsequently sliced into 4-µm thick sections for further
analysis.
Enzyme-linked immunosorbent assay
(ELISA)
The concentration of progesterone was measured using
an ELISA kit (cat. no. JL45435; Jianglai Biology; http://www.jonln.com/191804/). All serum samples from
patients with CRC were placed at room temperature (22–25°C) for 2
h, and then centrifuged at 1,000 × g for 20 min at 4°C, and the
supernatant was collected and stored at −80°C. ELISA was performed
according to the manufacturer's instructions and each sample was
evaluated in triplicate. The conjugate reagent was then incubated
for 2 h at 4°C, followed by incubation with the substrate solution
for 30 min at room temperature in the dark. The reaction was
terminated using a stop solution, and the optical density was
measured at a wavelength of 450 nm using a Varioskan™ Flash
Multimode Reader (Thermo Fisher Scientific, Inc.). The best
standard curve was constructed. Progesterone levels were determined
based on the standard curve.
Western blot analysis
Total protein was extracted from tumour samples
collected from patients with CRC, mice or cells. The total protein
was extracted with the protein extraction kit (cat. no. KGP250;
Nanjing KeyGen Biotech Co., Ltd.), and the protein content was
detected using the BCA method. The protein (10 µg per lane) was
separated using 10% SDS-PAGE gels. The samples were separated and
transferred to nitrocellulose membranes. The membranes were blocked
for 2 h at room temperature and then incubated overnight at 4°C
with one of the following primary antibodies: Mouse monoclonal
anti-PGR (cat. no. ab32085; Abcam); rabbit monoclonal anti-B cell
lymphoma-2 (BCL-2; cat. no. ab182858 Abcam), rabbit monoclonal
anti-BCL2-Associated X (BAX; cat. no. ab182733; Abcam), rabbit
monoclonal anti-cleaved caspase-3 (cat. no. ab2302; Abcam), rabbit
polyclonal anti-GADD45α (cat. no. ab180768; Abcam), mouse
monoclonal anti-c Jun N terminal kinase 1/2 (JNK1/JNK2 cat. no.
AHO1362; Invitrogen; Thermo Fisher Scientific, Inc.), rabbit
polyclonal anti-phosphorylated-JNK1/JNK2 (cat. no. 44682G;
Invitrogen; Thermo Fisher Scientific, Inc.), rabbit monoclonal
anti-c-Jun(cat. no. ab280089; Abcam) and rabbit monoclonal
anti-Ki67 (cat. no. ab15580; Abcam). All antibodies were diluted by
TBST (cat. no. T1086; Solarbio) to 1:1,000. After washing, the
membranes were incubated for 2 h with goat anti-mouse IgG antibody
(cat. no. A9917; Sigma-Aldrich; Merck KGaA) and goat anti-rabbit
IgG antibody (cat. no. SAB3700870; Sigma-Aldrich; Merck KGaA).
Protein intensity was determined using Clarity Western ECL
Substrate (Bio-Rad Laboratories, Inc.) and measured using Image
Lab™ software (version 2.0.1; cat. no. 1709690; Bio-Rad
Laboratories, Inc.).
Reverse transcription-quantitative
(RT-q)PCR analysis
The SW620 cell line was treated with progesterone
(250 µM) for 48 h. Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), and
reverse-transcribed using the PrimeScript™ II 1st Strand cDNA
Synthesis kit (Takara Bio, Inc.) according to the manufacturer's
instructions. The sequences of the primers used are shown in
Table I. The optimal primer
concentrations were determined based on the optimisation protocols
provided in the Applied Biosystems SYBR-Green PCR Master mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) manual. Each
PCR amplification and detection was carried out using a CFX96 Real
Time PCR Detection system (Bio-Rad Laboratories, Inc.). The cycling
conditions used were as follows: 95°C For 130 sec, 60°C for 30 sec,
72°C for 30 sec and 72°C for 40 cycles. GAPDH was used as an
internal control to normalise the mRNA expression of each gene. All
reactions were performed in triplicate. The results were calculated
as previously described using the 2−ΔΔCq method
(28).
 | Table I.Primers used for reverse
transcription-quantitative PCR. |
Table I.
Primers used for reverse
transcription-quantitative PCR.
| Sequence,
5′-3′ | Primer name | Product length,
bp |
|---|
| Cyclin A1 |
| 82 |
|
Forward |
GAGGTCCCGATGCTTGTCAG |
|
|
Reverse |
GTTAGCAGCCCTAGCACTGTC |
|
| Cyclin B1 |
| 111 |
|
Forward |
AATAAGGCGAAGATCAACATGGC |
|
|
Reverse |
TTTGTTACCAATGTCCCCAAGAG |
|
| Cyclin D1 |
| 135 |
|
Forward |
GCTGCGAAGTGGAAACCATC |
|
|
Reverse |
CCTCCTTCTGCACACATTTGAA |
|
| Cyclin E1 |
| 80 |
|
Forward |
AAGGAGCGGGACACCATGA |
|
|
Reverse |
ACGGTCACGTTTGCCTTCC |
|
| C-Myc |
| 175 |
|
Forward |
ATGGCCCATTACAAAGCCG |
|
|
Reverse |
TTTCTGGAGTAGCAGCTCCTAA |
|
| c-Jun |
| 78 |
|
Forward |
TCCAAGTGCCGAAAAAGGAAG |
|
|
Reverse |
CGAGTTCTGAGCTTTCAAGGT |
|
| JNK1 |
| 179 |
|
Forward |
GGGTATGCCCAAGAGGACAGA |
|
|
Reverse |
GTGTTGGAAAAGTGCGCTGG |
|
| JNK2 |
| 207 |
|
Forward |
GAAACTAAGCCGTCCTTTTCAGA |
|
|
Reverse |
TCCAGCTCCATGTGAATAACCT |
|
| GADD45α |
| 68 |
|
Forward |
GGATGCCCTGGAGGAAGTG |
|
|
Reverse |
CTTCGTACACCCCGACAGTGA |
|
| P15 |
| 97 |
|
Forward |
GGGACTAGTGGAGAAGGTGC |
|
|
Reverse |
CATCATCATGACCTGGATCGC |
|
| BAX |
| 155 |
|
Forward |
CCCGAGAGGTCTTTTTCCGAG |
|
|
Reverse |
CCAGCCCATGATGGTTCTGAT |
|
| BCL2L1 |
| 136 |
|
Forward |
CATGCTGGGAGCGTCACAT |
|
|
Reverse |
CTCCACTGAACTCGTACAAACTT |
|
| BCL2 |
| 147 |
|
Forward |
GCTACCGTCGTGACTTCGC |
|
|
Reverse |
CCCCACCGAACTCAAAGAAGG |
|
| Ki67 |
| 104 |
|
Forward |
ATCATTGACCGCTCCTTTAGGT |
|
|
Reverse |
GCTCGCCTTGATGGTTCCT |
|
| GAPDH |
| 197 |
|
Forward |
GGAGCGAGATCCCTCCAAAAT |
|
|
Reverse |
GGCTGTTGTCATACTTCTCATGG |
|
PCR microarray
PCR microarray was performed to screen 80 genes
involved in the regulation of progesterone-inhibited CRC
progression by Wcgene Biotechnology. SW620 cells were cultured in a
10-cm2 dish and incubated overnight. After intervention
with progesterone (250 µM) for 48 h, the cells were washed and RNA
was extracted and purified using TRIzol. Then, 1 µg RNA was used to
synthesise cDNA using a reverse transcription kit (Thermo Fisher
Scientific, Inc.), and stored at −20°C. First strand cDNA was added
to the PCR microarray precoated with specific primers. PCR array
was performed under the following conditions: 95°C For 10 min, then
40 cycles at 95°C for 15 sec and finally at 60°C for 1 min. GAPDH
was used for homogenisation. PCR microarray analysis was performed
using the GeneAmp7300 RT-PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The results were calculated as previously
described and the level of change (fold-change >1 or <-1) was
identified as the different significance (28).
Statistical analysis
Statistical analysis was performed using SPSS
version 19.0 (IBM, Corp.). All data are expressed as mean ±
standard deviation (unless otherwise shown). The correlations and
associations between clinical characteristics in patients with CRC
were analysed using Pearson's correlation, Spearman's correlation,
unpaired Student's t-test or χ2 test. Survival data were
used to draw Kaplan-Meier curves, and differences between the
groups were analysed using the log-rank test. Student's t-tests
were used to determine the significance of differences between two
groups, and one-way ANOVA and Tukey's post hoc test were used to
determine differences among multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
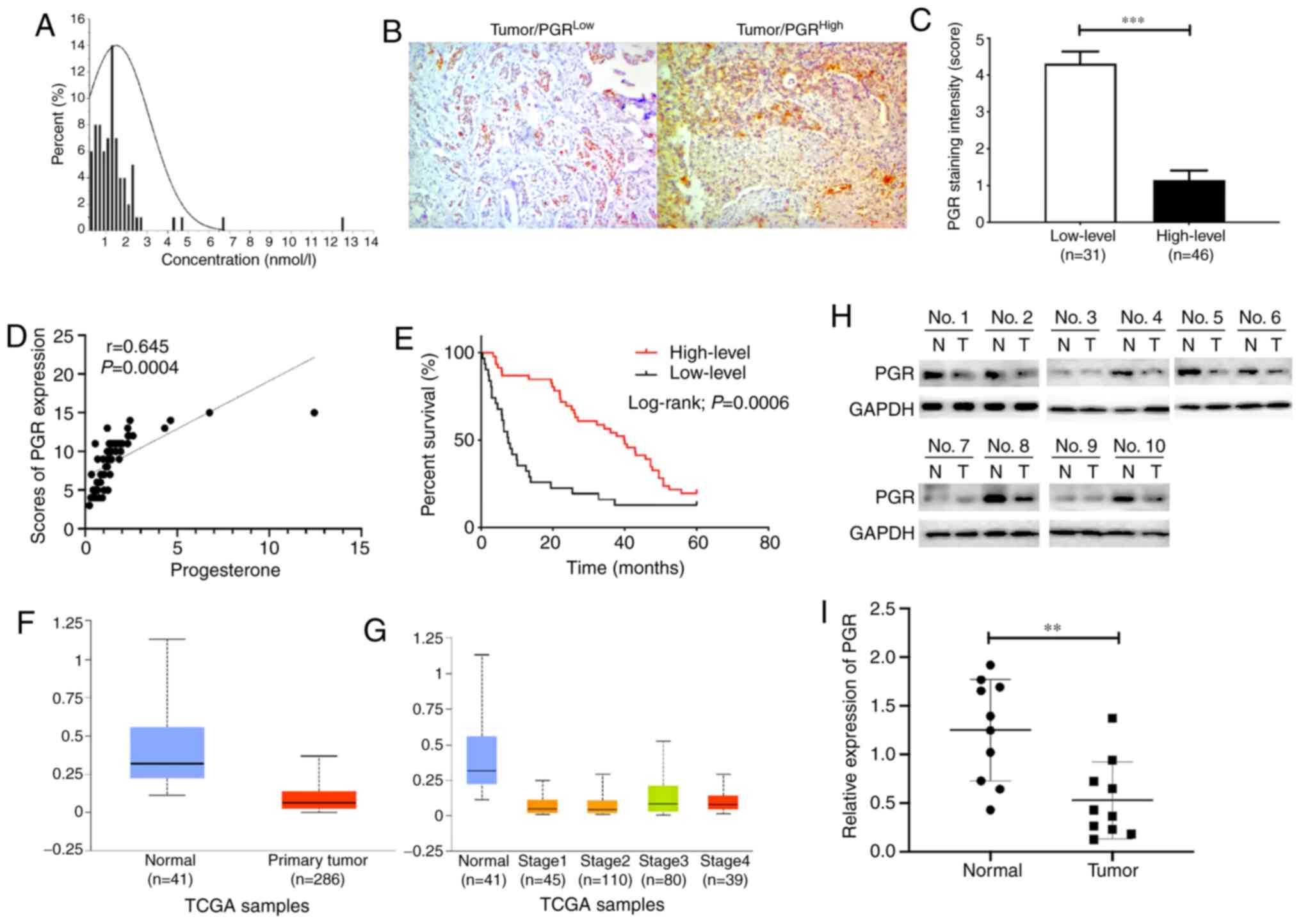
Progesterone and PGR levels were
negatively correlated with CRC prognosis
To examine the correlation between progesterone
levels and CRC prognosis, the concentration of progesterone was
measured in 77 patients (median, 1.23; interquartile range,
0.737–1.715 nmol/l) (Fig. 1A).
Progesterone plays an essential role in physiological and
pathological processes through activation of the PGR (5). Thus, IHC staining was performed in
colorectal tumour tissues (Fig. 1B),
and the scores demonstrated the variable expression level of PGR
and were used to define high and low PGR levels (Fig. 1C). Furthermore, correlation between
the expression of progesterone and its receptor was analysed. The
results indicated that progesterone level was positively associated
with the IHC staining scores of PGR in patient samples (r=0.645,
P=0.0004; Fig. 1D). Correlations
between progesterone and clinical characteristics were also
analysed (Table II). Statistical
analysis showed significant correlations in the number of tumours
(r=−0.243, P=0.033), tumour differentiation (r=−0.254, P=0.026) and
survival time (r=0.383, P=0.001). When association between PGR and
clinical characteristics of CRC patients were analysed, it was
found that high levels of PGR expression were associated with
tumour size (P=0.001), differentiation (P=0.011), vascular invasion
(P=0.005) and tumour stage (P=0.001) (Table III). In addition, the results
indicated that patients with higher expression levels of PGR had
longer short-term survival times (P=0.0006; Fig. 1E). The 1-year, 3-year and 5-year
survival rates were 86.96, 78.26, and 19.57%, respectively, in the
high expression group, and 35.48, 16.13 and 12.90%, respectively,
in the low expression group. The results from UALCAN showed that
PGR expression was higher in normal tissues compared with that in
primary tumours, regardless of tumour stage (Fig. 1F and G). Further analyses revealed
that PGR protein levels were lower in 10 randomly selected paired
specimens, which were analysed via western blotting (P<0.01;
Fig. 1H and I). In summary, these
data confirmed that low progesterone levels in serum and low PGR
expression in CRC tissue led to poor prognosis in patients with
CRC.
 | Table II.Correlation analysis of progesterone
expression and clinical characteristics of 77 patients with
colorectal cancer. |
Table II.
Correlation analysis of progesterone
expression and clinical characteristics of 77 patients with
colorectal cancer.
| Variable | r or t-value | P-value |
|---|
| Age | −0.158 | 0.169 |
| Sex,
male/female | 0.682a | 0.499 |
| Tumor size | −0.170 | 0.139 |
| Number of
tumors | −0.243 | 0.033b |
| Differentiation,
high/moderate/low | −0.254 | 0.026b |
| Vascular invasion,
+/- | 0.313a | 0.755 |
| TNM,
I/II/III/IV | −0.198 | 0.084 |
| Survival time | 0.383 | 0.001b |
 | Table III.Relationship between progesterone
receptor and clinical characteristics of 77 patients with
colorectal cancer. |
Table III.
Relationship between progesterone
receptor and clinical characteristics of 77 patients with
colorectal cancer.
|
|
| Expression of the
PGR |
|
|---|
|
|
|
|
|
|---|
| Variable | Value, n | Low level,
n=31 | High level,
n=46 | P-value |
|---|
| Age, years |
|
|
|
|
|
<60 | 28 | 14 | 14 | 0.188 |
|
≥60 | 49 | 17 | 32 |
|
| Sex |
|
|
|
|
|
Male | 43 | 13 | 30 | 0.044a |
|
Female | 34 | 18 | 16 |
|
| Tumor size, cm |
|
|
|
|
|
<5 | 38 | 8 | 30 | 0.001b |
| ≥5 | 39 | 23 | 16 |
|
| Number of
tumors |
|
|
| 0.279 |
|
Single | 34 | 16 | 18 |
|
|
Multiple | 43 | 15 | 28 |
|
|
Differentiation |
|
|
| 0.011a |
|
High | 29 | 13 | 16 |
|
|
Moderate | 26 | 14 | 12 |
|
|
Low | 22 | 4 | 18 |
|
| Vascular
invasion |
|
|
| 0.005b |
| No | 40 | 10 | 30 |
|
|
Yes | 37 | 21 | 16 |
|
| TNM stage |
|
|
| 0.001b |
|
I/II | 42 | 10 | 32 |
|
|
III/IV | 35 | 21 | 14 |
|
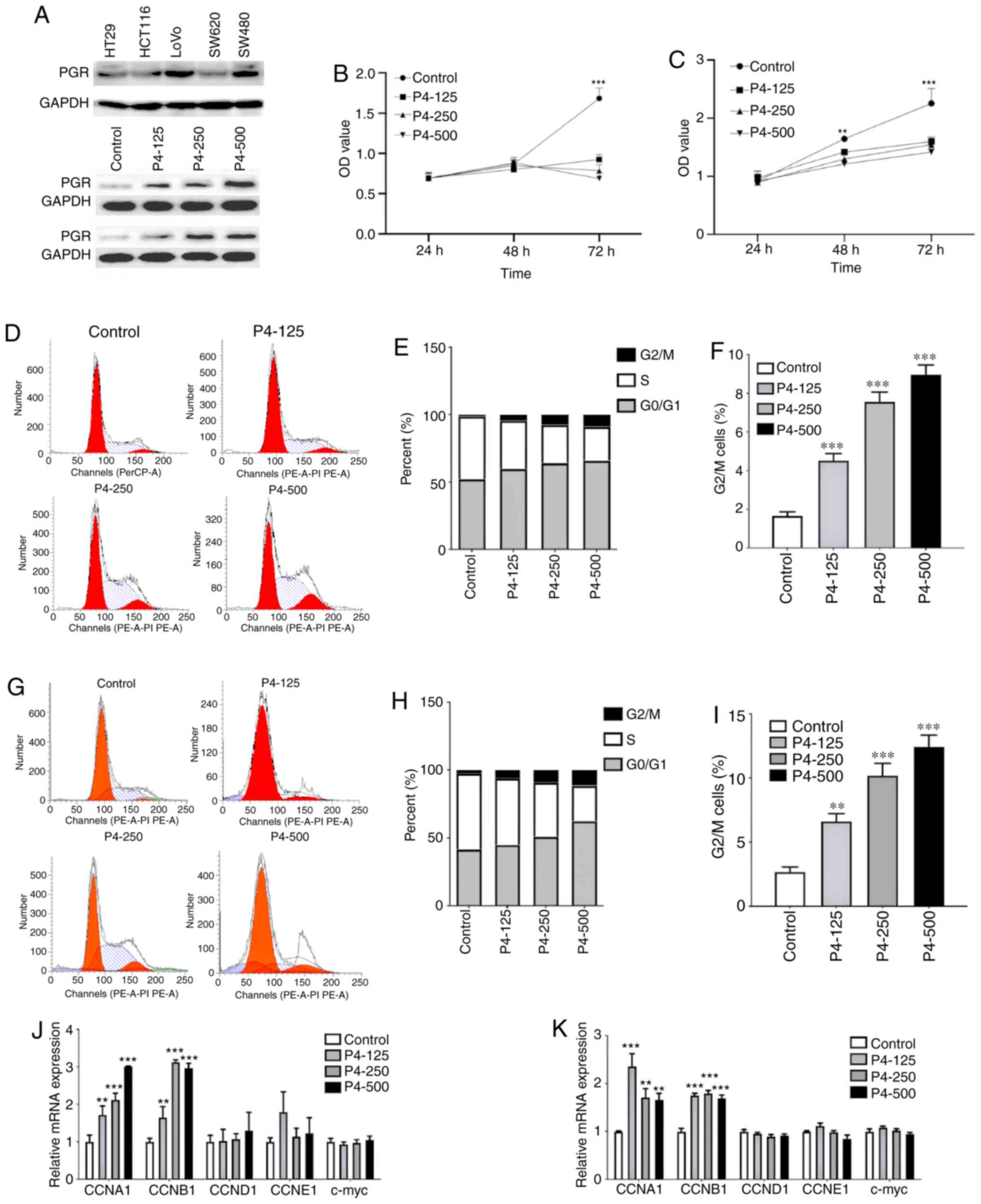
Progesterone inhibited CRC cell
proliferation by arresting the cell cycle and inducing
apoptosis
The study sought to further identify the biological
function of progesterone in CRC cell proliferation by studying the
association between progesterone and CRC prognosis. First, PGR
expression was analysed in various cell lines. LoVo cells exhibited
relatively higher PGR expression levels, while expression in SW620
cells was lower (Fig. 2A). When
treated with gradually increasing concentrations of progesterone,
PGR expression was enhanced in LoVo and SW620 cell lines (Fig. 2A). When treated with different
concentrations of progesterone (125, 250 and 500 µM), proliferation
at 72 h was significantly inhibited in both LoVo and SW620 cells
(both P<0.001; Fig. 2B and C,
respectively), and proliferation at 48 h was significantly
inhibited in SW620 cells compared with the control (P<0.01;
Fig. 2C). Cell cycle analyses were
performed using a flow cytometer following propidium iodide
staining (Fig. 2D), and the cell
phase distribution at 48 h was analysed (Fig. 2E). Increasing doses of progesterone
significantly increased the percentage of LoVo cells in the
G2/M phase (Fig. 2F) and
SW620 CELLS (Fig. 2I). To verify cell
cycle arrest, cell cycle-related cytokines, including CCNA1, CCNB1,
CCND1, CCNE1 and c-myc, were detected at the transcriptional level.
The results showed that CCNA1 and CCNB1 were significantly
up-regulated in progesterone-treated LoVo (P<0.01; Fig. 2J) and SW620 (P<0.01; Fig. 2K) cells.
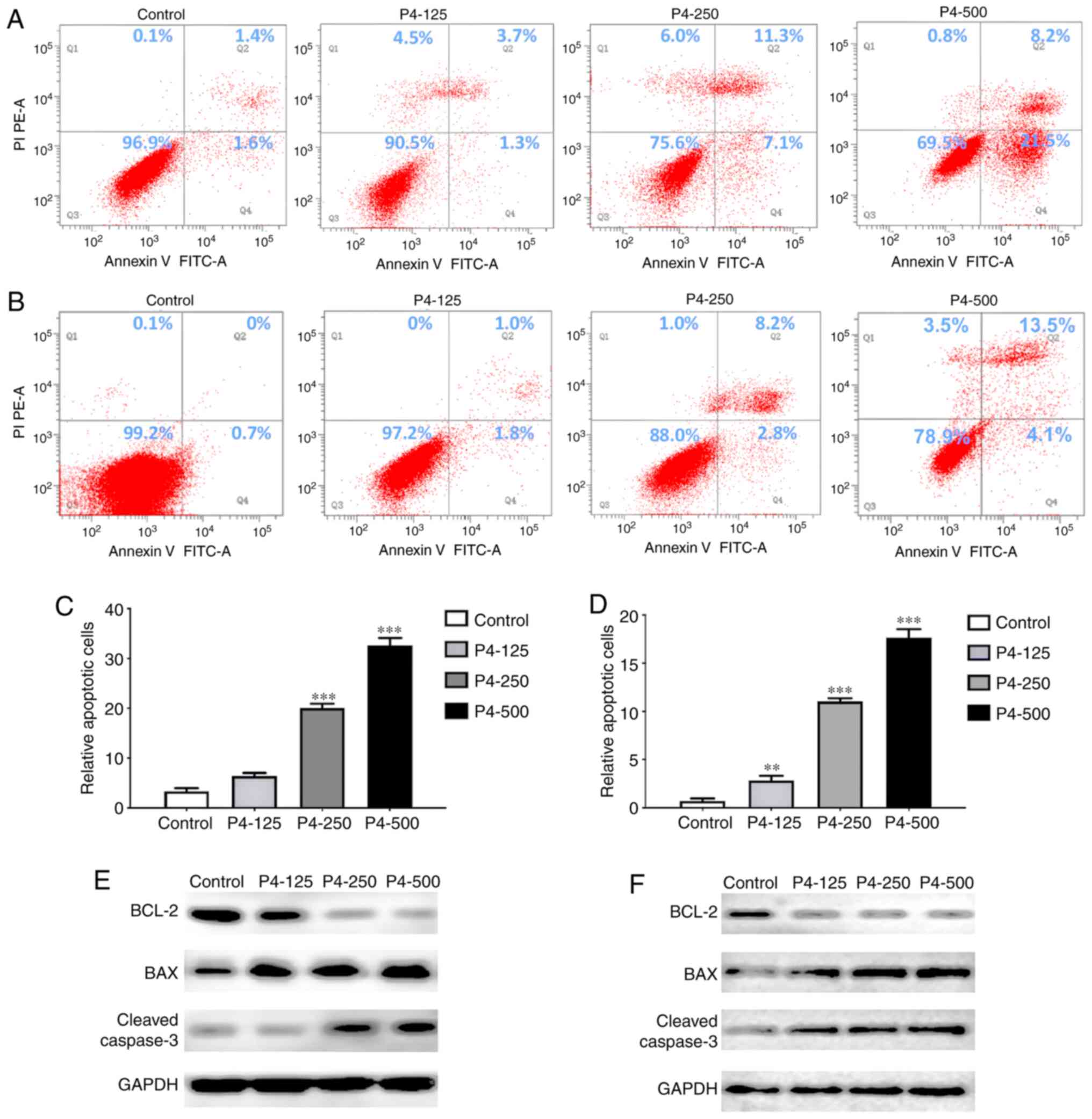
Furthermore, flow cytometry was used to analyse the
apoptotic process in LoVo and SW620 cell lines 48 h after
progesterone intervention (Fig. 3A and
B). A significant increase in the apoptosis rate was observed
in LoVo cells, with 2-, 6- and 10-fold higher apoptosis rates in
the 125, 250 and 500 µM progesterone groups, respectively, compared
with the control group (P<0.001; Fig.
3C). Significant increases in apoptosis rates were also
observed in SW620 cells, with 3-, 5- and 10-fold higher rates in
the 125, 250 and 500 µM progesterone groups, respectively, compared
with the control group (P<0.01; Fig.
3D). To verify this variation in apoptosis, the apoptosis
inhibitory protein BCL-2, apoptosis-promoting protein BAX and
cleaved caspase-3 levels were measured. The results showed
down-regulated BCL-2 and up-regulated cleaved caspase-3 in LoVo
cells, and down-regulated BCL-2 and BAX and up-regulated cleaved
caspase-3 in SW620 cells (Fig. 3E and
F). The aforementioned results indicated that progesterone
arrested the cell cycle mainly in the G2/M phase and
promoted apoptosis, inducing the inhibition of CRC cell
proliferation.
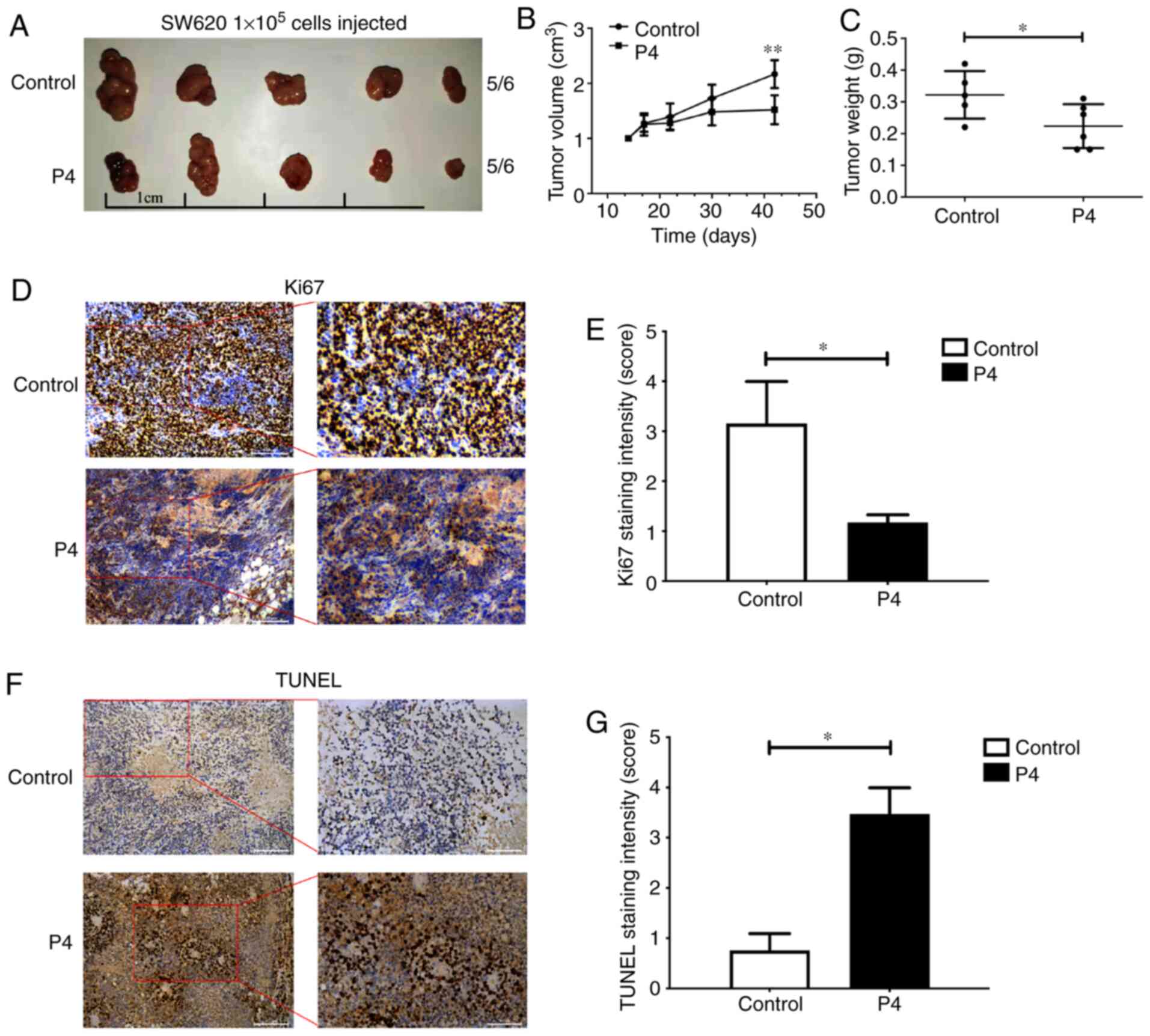
Progesterone inhibits tumour growth in
vivo
A xenograft tumour model was established to
determine whether progesterone affected tumour growth in
vivo (Fig. 4A). The volume of
implanted tumours reduced significantly following 40 days of
progesterone intervention (P<0.01; Fig. 4B). Progesterone treatment also
resulted in tumours with lower weights (P<0.05; Fig. 4C) and light staining of Ki-67 in
tumours (P<0.05; Fig. 4D and E).
It was also observed that the number of TUNEL-positive cells in the
tumour tissue was prominently enhanced in the progesterone-treated
mice compared with control mice (P<0.05; Fig. 4F and G). Collectively, these data
showed that progesterone was essential for inhibiting CRC
growth.
Progesterone up-regulates the JNK
pathway via GADD45α to inhibit CRC progression
The JNK signalling pathway plays an essential role
in regulating cell proliferation, migration and invasion. It is
associated with reduced cell proliferation, arrested cell cycle and
increased apoptosis following overactivation with progesterone
(16). A PCR microarray was performed
to investigate progesterone-induced changes in the expression of
genes related to cell proliferation. As shown in Fig. 5A, a noticeable number of genes were
altered by progesterone intervention (250 µM). In total, 34 genes
whose levels changed the most (fold-change >1 or <-1) are
depicted in Fig. 5B. In SW620 cells,
changes in the expression of P15, c-Jun, GADD45A, CCL18, neutrophil
cytosol factor 1 and matrix metalloproteinase 3 were most
significant. Progesterone considerably increased the expression of
P15 (5.0-fold change), c-Jun (4.0-fold change) and GADD45A
(3.9-fold change). RT-qPCR was performed to verify the differences
in the genes. The gene with the most considerable change was
GADD45α, with a 2.5-fold increase in expression. Increased
expression of c-Jun (2.3-fold change) and JNK1 (2.2-fold change),
and decreased expression of BCL2L1 (2.0-fold change), were also
noted (Fig. 5C). The similar results
were also observed in LoVo cells (Fig.
5D). Furthermore, the transcription levels of GADD45α, JNK1,
JNK2, c-Jun, BCL-2 and Ki67 were detected with the intervention of
progesterone (250 µM) in SW620 (Fig.
5E) and LoVo (Fig. 5G) cells.
GADD45α, JNK1 and c-Jun were significantly up-regulated, while
BCL-2 and Ki67 were significantly down-regulated. To verify that
GADD45α was the main factor involved in the regulation of the JNK
pathway, siGADD45α and progesterone were simultaneously used to
detect the significant changes. Inhibition of GADD45α strongly
suppressed GADD45A and c-Jun and promoted BCL-2 and Ki67 compared
with progesterone only treatment. The aforementioned cytokines,
which showed significant changes, were analysed at the protein
level in SW620 (Fig. 5F) and LoVo
(Fig. 5H) cells. GADD45α,
phosphorylation of JNK and c-Jun were enhanced with progesterone
intervention but decreased when siGADD45α was added. In contrast to
this, decreased levels of BCL-2 and Ki67 were observed and were
increased with the addition of siGADD45α. Collectively, these data
suggested that progesterone inhibited CRC cell proliferation by
arresting the cell cycle and inducing apoptosis through the
activated GADD45α/JNK/c-Jun pathway.
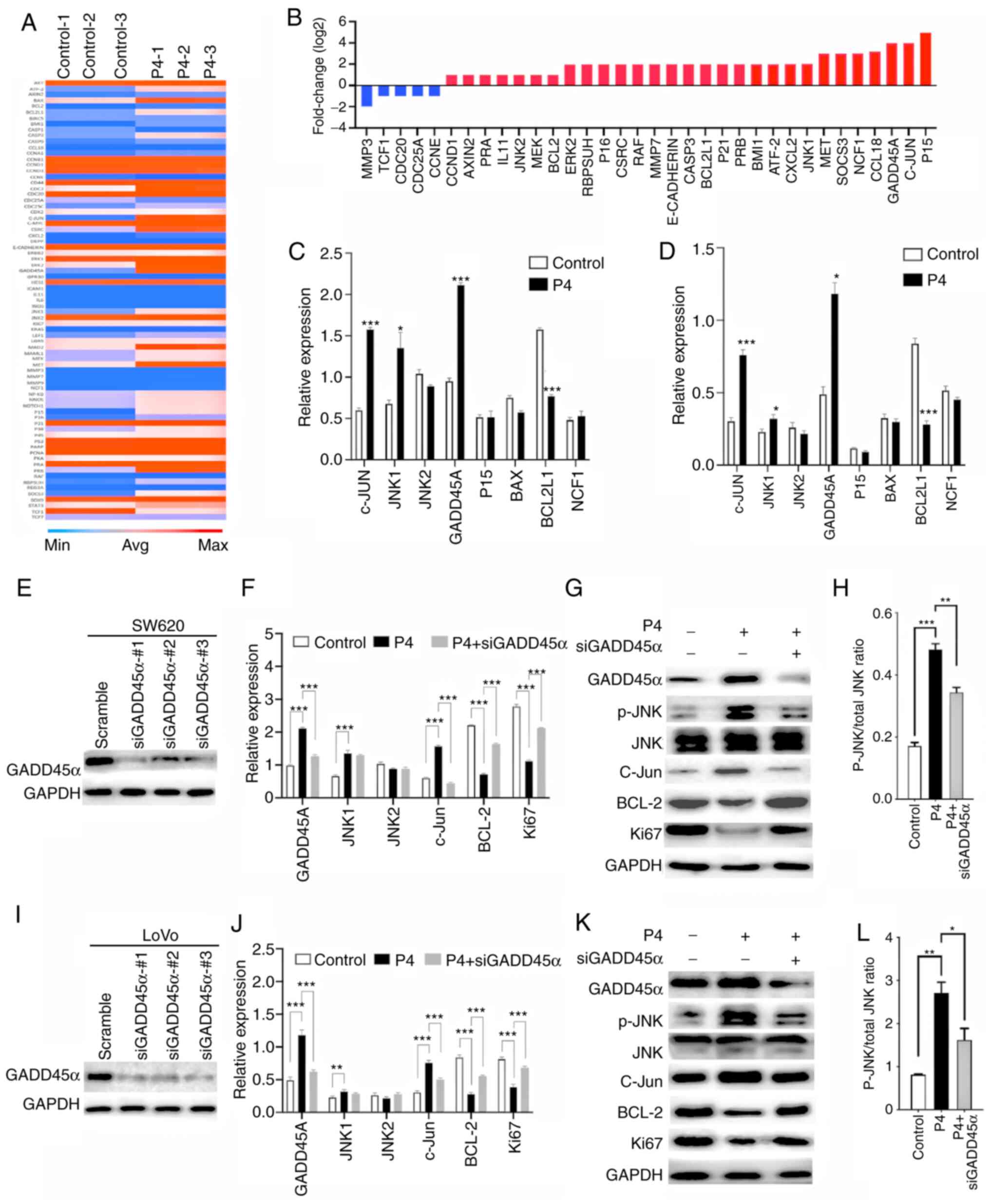 | Figure 5.Progesterone up-regulates the JNK
pathway via GADD45α activation to inhibit progression of colonic
carcinoma. (A) Heat map depicting progesterone-induced changes in
the expression profile of genes that were assessed on the PCR
microarray. Blue and red represent low and high gene expression
levels in pinnae, respectively. (B) Selection of genes that were
altered the most in SW620 cells. Genes are presented
alphabetically. RT-PCR was used to investigate the effect of
progesterone on proliferation-related genes such as c-Jun, JNK1,
JNK2, GADD45α, P15, BAX, BCL2L1 and NCF1 in (C) SW620 and (D) LoVo
cells Efficiency of knockdown GADD45α in (E) SW620 and (I) LoVo
cells, respectively. Expression of GADD45α, JNK1, JNK2, c-Jun,
BCL-2, and Ki67 in (F) SW620 and (J) LoVo cells was analysed using
RT-PCR, and GADD45α, phosphorylation of JNK, JNK, c-Jun, BCL-2 and
Ki67 in (G) SW620 and (K) LoVo cells were analysed using western
blot. Relative expression of phosphorylation of JNK to total
protein of JNK in (H) SW620 and (L) LoVo cells. *P<0.05,
**P<0.01 and ***P<0.001 vs. control. GADD45α, growth arrest
and DNA damage-inducible protein α; JNK, c-Jun N-terminal kinase;
BCL-2, B cell lymphoma-2; NCF1, neutrophil cytosol factor 1. |
Discussion
Although some evidence indicates that progesterone
is associated with decreased incidence of CRC (3), other reports have correlated
progesterone with later cancer stage and increased mortality from
CRC (15). Data explaining this
contradiction is currently lacking. This may be because the effects
of synthetic progestins are different from those of natural
progesterone (29). Progesterone has
been shown to participate in various cancer-associated processes in
different cancer types. Progesterone activates the angiogenesis
pathway involved in vascular endothelial growth factor stimulation
to promote breast cancer progression (30), and interfers with the progression of
ovarian cancer by down=-regulating the phosphorylation of Src and
focal adhesion kinase (31). However,
information on its cellular effects on CRC cells is limited
(32). The present study reported
that low levels of progesterone in patients with CRC were
consistent with the PGR level in CRC tissues and correlated with
poor prognosis of CRC. Treatment with an increased concentration of
progesterone resulted in the inhibition of CRC cell proliferation
in vitro and in vivo. Furthermore, progesterone
up-regulated GADD45α and c-Jun and promoted the phosphorylation of
JNK. These results suggested that progesterone inhibited the
proliferation of CRC cells to reduce the malignant progression of
CRC by up-regulating the JNK pathway via GADD45α.
Progesterone is dysregulated in several types of
cancer and is associated with cancer progression (29). Studies in humans and monkeys have
suggested that oral micronized progesterone has a more favourable
effect on risk biomarkers for postmenopausal breast cancer
(33–35). Progesterone prevents high-grade serous
ovarian cancer by inducing necroptosis, and inhibits migration and
invasion, revealing its mechanism of action (36). Progesterone also reduces the invasive
potential of endometrial cancer cells (37). However, the specific mechanism by
which progesterone inhibits the malignant progression of CRC is
unclear. In line with previous reports, the current results
confirmed that low progesterone expression was associated with poor
prognosis of CRC (11,38). Moreover, the relationship with PGR was
revealed, and the growth inhibitory effects in CRC were analysed.
PGR has been identified in normal and malignant tissues and is a
highly structured transcription factor that regulates diverse
physiological processes (39,40). However, the reported relationship
between PGR expression and cancer progression is still
contradictory. In ovarian cancer, higher levels of PGR predict
favourable survival (41). However,
PRG expression is decreases by 30–40% in prostate cancer-associated
stroma compared with benign stroma (42). Higher tumour grades are associated
with increased PGR expression in astrocytomas (43). This variation may be due to
differences in the way specimens were prepared, the method or
antibodies used, the location of the lesions or the level of
staining required to consider a sample positive (44). In CRC tumours, low PGR levels are
inversely associated with extensive primary tumours, poor prognosis
and high recurrence rate of CRC (38,39,45). In
addition, mifepristone, the PGR agonist, protects against CRC by
modulating the effects of oestrogen on carcinogenesis (46). In contrast, PGR is detected in normal
and cancer tissues, but without any significant relationship with
clinicopathological tumour characteristics and patient outcome
(47,48). Recently, lower PGR expression levels
are associated with more extensive CRC primary tumours and poorer
prognosis (39). As the range of
actions of PGR have limited its clinical use thus far, the present
results revealed a positive correlation with progesterone level and
a negative correlation with clinical characteristics. PGR
expression in specimens (normal and tumour tissue) was also
confirmed, which was consistent with results from TCGA
database.
A number of investigations have shown that
progesterone signals negatively regulates cell cycle progression by
suppressing S and G2/M phases and downregulating cell
cycle phase-specific cyclins/CDKs (49). Progesterone also inhibits CRC by
regulating proliferation and apoptosis (16,50). In
parallel with a previous study, progesterone also inhibited CRC
cell proliferation in this study (17). Cell growth factors were also analysed.
CCNA1, CCNB1 and cleaved caspase-3 were up-regulated, while BCL-2
and BAX were down-regulated. These data indicated that progesterone
inhibited CRC proliferation by arresting the G2/M phase
and inducing apoptosis. In contrast, previous studies indicated
that progesterone does not inhibit the proliferation of CRC cell
lines (51,52). These contradictory results may be due
to the use of different types of cell lines, different
concentrations of progesterone or different PGR expression
patterns.
Although the role of progesterone in the progression
of CRC has been evaluated, the specific mechanism by which
progesterone regulates CRC remains to be explored. Progesterone has
the ability to bind to nuclear or membrane receptors to regulate
cancer development via the classical and non-classical pathways
(34). The classical signalling
induced by progesterone leads to the decomposition of heat shock
protein, PGR dimerization, and progesterone response element (PRE),
and initiates the transcription of downstream effector targets
cyclin D1 and P4 mediator receptor activator of nuclear factor κB
ligand. In non-classical pathway, PRE binds to proto-oncogene
tyrosine-protein Src, mitogen activated protein kinase and protein
kinase B, and then activates downstream effector targets
wingless-type MMTV integration site family member 1, cyclin D1,
epidermal growth factor receptor and transcription of p21.
Progesterone exerts a tumour suppressive effect by inducing
apoptosis via activation of the MAPK pathway (10). The apoptosis of CRC cells is due to
the up-regulation of GADD45α and activation of MAPKs (JNK, p38 and
ERK) (53). In addition, GADD45α was
also reported to act as a regulator of DNA damage and S-phase
arrest in hepatoma cells (54). The
present additional investigation using a PCR microarray revealed
that GADD45α JNK, and c-Jun expressions were up-regulated following
progesterone intervention. Since the mechanism of JNK-induced
apoptosis and GADD45α-mediated cell cycle have been widely verified
(21,55,56), it
was hypothesised that intervention with progesterone regulated the
link between cell cycle arrest and GADD45α and apoptosis in
response to the activation of JNK/c-Jun pathway. Some studies have
shown that GADD45α activates the JNK pathway (56,57),
whereas others have suggested that GADD45α is downstream of JNK/p38
pathways, and is associated with cell viability, DNA damage and the
cell cycle (58,59). In addition, an in vitro
experiments have indicated the existence of a feedback loop between
GADD45α and the JNK pathway (53).
The current study observed enhanced expression of GADD45α,
phosphorylation of JNK and c-Jun and inhibition of BCL-2 in
progesterone-treated SW620 cells. The down-regulated
phosphorylation of JNK and c-Jun and up-regulated expression of
BCL-2 was verified when these cells were transfected with GADD45α
siRNA. These results implied that the JNK pathway was activated by
progesterone-induced GADD45α to inhibit the malignant progression
of CRC.
Overall, the present investigations demonstrated
that progesterone and PGR acted as inhibiting factors for poor
prognosis of CRC. Progesterone intervention in CRC cells suppressed
proliferation by arresting the cell cycle and inducing apoptosis.
Moreover, progesterone-induced inhibition of CRC progression was
regulated by GADD45α/JNK/c-Jun signalling. These findings suggested
that progesterone plays an efficient role in CRC inhibition, which
might be used in the treatment of CRC patients with progesterone
deficiency. However, several missing links remain to be filled in
future studies, including the molecular mechanism responsible for
progesterone-mediated upregulation of GADD45α.
Acknowledgements
The authors would like to thank the critical
revisions by Professor Nalu Navarro-Alvarez from College of
Medicine, Harvard Medical School, and give thanks to Professor
Yun-ming Li from General Hospital of Western Theater Command in
giving statistical guidance for this manuscript.
Funding
This work was supported by The Sichuan Major
Scientific and Technological Projects (grant no. 2017SZDZX0022),
The Sichuan Science and Technology Plan (grant nos. 2018GZ0240 and
2019YFS0542) and The Chengdu Technology Innovation and Development
Project (grant no. 2019-YF05-01161-SN).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. The datasets generated and/or analyzed during the current
study are available in the UALCAN repository (http://ualcan.path.uab.edu/).
Authors' contributions
YLZ, LFZ and YHH designed the study. XDW, XG and PTZ
contributed to the acquisition, analysis and interpretation of data
from patients. YLZ, XDW, XG, WL, TTW, PTZ and SQH performed the
experiments. LFZ was in charge of statistical analysis. YLZ, XDW
and XG drafted the manuscript. SQH, TTW and PTZ took charge of
critical revision of the manuscript and language polishing. YHH and
LFZ provided administrative and technical supervision. XDW and PTZ
confirm the authenticity of all raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was performed in accordance with clinical
study protocols and the principles of the Declaration of Helsinki
(modified 2000) and was approved by The Research Care and Ethics
Committee of the General Hospital of Western Theater Command
(approval no. SPPHCT2015-0117). Patients or their families gave
signed informed consent to participate in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BCL-2
|
B cell lymphoma-2
|
|
CCK-8
|
Cell Counting Kit-8
|
|
CRC
|
colorectal cancer
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
IHC
|
immunohistochemistry
|
|
JNK
|
c-Jun N-terminal kinases
|
|
MAPK
|
mitogen-activated protein kinase
|
|
PBS
|
phosphate buffer saline
|
|
PGR
|
progesterone receptor
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
PRE
|
progesterone response element
|
|
TNM
|
Tumor-Node-Metastasis
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. Feb 4–2021.(Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dekker E, Tanis P, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Folkerd EJ and Dowsett M: Influence of sex
hormones on cancer progression. J Clin Oncol. 28:4038–4044. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fonseca EB, Celik E, Parra M, Singh M and
Nicolaides KH; Fetal Medicine Foundation Second Trimester Screening
Group, : Progesterone and the risk of preterm birth among women
with a short cervix. N Engl J Med. 357:462–469. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsai HW, Ho CL, Cheng SW, Lin YJ, Chen CC,
Cheng PN, Yen CJ, Chang TT, Chiang PM, Chan SH, et al: Progesterone
receptor membrane component 1 as a potential prognostic biomarker
for hepatocellular carcinoma. World J Gastroenterol. 24:1152–1166.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Syed V, Mukherjee K, Lyons-Weiler J, Lau
KM, Mashima T, Tsuruo T and Ho SM: Identification of ATF-3,
caveolin-1, DLC-1, and NM23-H2 as putative antitumorigenic,
progesterone-regulated genes for ovarian cancer cells by gene
profiling. Oncogene. 24:1774–1787. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim HW, Kim JH, Lim BJ, Kim H, Kim H, Park
JJ, Youn YH, Park H, Noh SH, Kim JW and Choi SH: Sex disparity in
gastric cancer: Female sex is a poor prognostic factor for advanced
gastric cancer. Ann Surg Oncol. 23:4344–4351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aktas B, Kasimir-Bauer S, Müller V, Janni
W, Fehm T, Wallwiener D, Pantel K and Tewes M; DETECT Study Group,
: Comparison of the HER2, estrogen and progesterone receptor
expression profile of primary tumor, metastases and circulating
tumor cells in metastatic breast cancer patients. BMC Cancer.
16:5222016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Núñez C, Capelo JL, Igrejas G, Alfonso A,
Botana LM and Lodeiro C: An overview of the effective combination
therapies for the treatment of breast cancer. Biomaterials.
97:34–50. 2016. View Article : Google Scholar
|
|
10
|
Kong X, Li M, Shao K, Yang Y, Wang Q and
Cai M: Progesterone induces cell apoptosis via the
CACNA2D3/Ca2+/p38 MAPK pathway in endometrial cancer. Oncol Rep.
43:121–132. 2020.PubMed/NCBI
|
|
11
|
Lin JH, Manson JE, Kraft P, Cochrane BB,
Gunter MJ, Chlebowski RT and Zhang SM: Estrogen and
progesterone-related gene variants and colorectal cancer risk in
women. BMC Med Genet. 12:782011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu Y, Tian LB, Yang HY and Zhang HP:
Effects of estradiol and progesterone on the growth of HeLa
cervical cancer cells. Eur Rev Med Pharmacol Sci. 21:3959–3965.
2017.PubMed/NCBI
|
|
13
|
Park JY, Kim DY, Kim TJ, Kim JW, Kim JH,
Kim YM, Kim YT, Bae DS and Nam JH: Hormonal therapy for women with
stage IA endometrial cancer of all grades. Obstet Gynecol.
122:7–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Simon MS, Chlebowski RT, Wactawskiwende J,
Johnson KC, Muskovitz A, Kato I, Young A, Hubbell FA and Prentice
RL: Estrogen plus progestin and colorectal cancer incidence and
mortality. J Clin Oncol. 30:3983–3990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schürmann R, Cronin M and Meyer JU:
Estrogen plus progestin and colorectal cancer in postmenopausal
women. N Engl J Med. 350:2417–2419. 2004. View Article : Google Scholar
|
|
16
|
Kuo CT and Lee WS: Progesterone receptor
activation is required for folic acid-induced anti-proliferation in
colorectal cancer cell lines. Cancer Lett. 378:104–110. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Motylewska E and Mełeń-Mucha G: Estrone
and progesterone inhibit the growth of murine MC38 colon cancer
line. J Steroid Biochem Mol Biol. 113:75–79. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mauri G, Bonazzina E, Amatu A, Tosi F,
Bencardino K, Gori V, Massihnia D, Cipani T, Spina F, Ghezzi S, et
al: The evolutionary landscape of treatment for BRAF (V600E) mutant
metastatic colorectal cancer. Cancers (Basel). 13:1372021.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu X, Jiang X, Duan C, Li A, Sun Y, Qi Q,
Liu Y, Li S and Zhao Z: S-Allylmercaptocysteine induces G2/M phase
arrest and apoptosis via ROS-mediated p38 and JNK signaling pathway
in human colon cancer cells in vitro and in vivo. RSC Adv.
7:49151–49158. 2017. View Article : Google Scholar
|
|
21
|
Chen K, Chu BZ, Liu F, Li B, Gao CM, Li
LL, Sun QS, Shen ZF and Jiang YY: New benzimidazole acridine
derivative induces human colon cancer cell apoptosis in vitro via
the ROS-JNK signaling pathway. Acta Pharmacol Sin. 36:1074–1084.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou WJ, Hou XX, Wang XQ and Li DJ:
Fibroblast growth factor 7 regulates proliferation and
decidualization of human endometrial stromal cells via ERK and JNK
pathway in an autocrine manner. Reprod Sci. 24:1607–1619. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Ma L, Su M, Zhou Y, Mao K, Li C,
Peng G, Zhou C, Shen B and Dou J: Baicalin induces cellular
senescence in human colon cancer cells via upregulation of DEPP and
the activation of Ras/Raf/MEK/ERK signaling. Cell Death Dis.
9:2172018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin M and Frankel W: Lymph node metastasis
in colorectal cancer. Surg Oncol Clin N Am. 27:401–412. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
World Medical Association Inc., .
Declaration of Helsinki. Ethical principles for medical research
involving human subjects. J Indian Med Assoc. 107:403–405.
2009.PubMed/NCBI
|
|
26
|
Lee JA, Spidlen J, Boyce K, Cai J, Crosbie
N, Dalphin M, Furlong J, Gasparetto M, Goldberg M, Goralczyk EM, et
al: MIFlowCyt: The minimum information about a flow cytometry
experiment. Cytometry A. 73A:926–930. 2010. View Article : Google Scholar
|
|
27
|
Percie du Sert N, Hurst V, Ahluwalia A,
Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl
U, et al: The ARRIVE guidelines 2.0: Updated guidelines for
reporting animal research. J Cereb Blood Flow Metab. 40:1769–1777.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using Real-Time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lieberman A and Curtis L: In defense of
progesterone: A review of the literature. Altern Ther Health Med.
23:24–32. 2017.PubMed/NCBI
|
|
30
|
Zhou L, Zhou W, Zhang H, Hu Y, Yu L, Zhang
Y, Zhang Y, Wang S, Wang P and Xia W: Progesterone suppresses
triple-negative breast cancer growth and metastasis to the brain
via membrane progesterone receptor α. Int J Mol Med. 40:755–761.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lima MA, Silva SV, Jaeger RG and Freitas
VM: Progesterone decreases ovarian cancer cells migration and
invasion. Steroids. 161:1086802020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tanaka Y, Kato K, Mibu R, Uchida S,
Asanoma K, Hashimoto K, Nozaki M and Wake N: Medroxyprogesterone
acetate inhibits proliferation of colon cancer cell lines by
modulating cell cycle-related protein expression. Menopause.
15:442–453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bluming AZ: Progesterone and breast cancer
pathogenesis. J Mol Endocrinol. 66:C1–C2. 2021. View Article : Google Scholar
|
|
34
|
Pedroza DA, Subramani R and Lakshmanaswamy
R: Classical and non-classical progesterone signaling in breast
cancers. Cancers (Basel). 12:24402020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wood CE, Register TC, Lees CJ, Chen H,
Kimrey S and Cline JM: Effects of estradiol with micronized
progesterone or medroxyprogesterone acetate on risk markers for
breast cancer in postmenopausal monkeys. Breast Cancer Res Treat.
101:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu NY, Huang HS, Chao TH, Chou HM, Fang C,
Qin CZ, Lin CY, Chu TY and Zhou HH: Progesterone prevents
high-grade serous ovarian cancer by inducing necroptosis of
p53-defective fallopian tube epithelial cells. Cell Rep.
18:2557–2565. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Waheed S, Dorjbal B, Hamilton CA, Maxwell
GL, Rodriguez GC and Syed V: Progesterone and calcitriol reduce
invasive potential of endometrial cancer cells by targeting ARF6,
NEDD9 and MT1-MMP. Oncotarget. 8:113583–113597. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu D: Gene signatures of estrogen and
progesterone receptor pathways predict the prognosis of colorectal
cancer. FEBS J. 283:3115–3133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Abd ElLateef AAE, Mohamed AES, Elhakeem AA
and Ahmed SF: Estrogen and progesterone expression in colorectal
carcinoma: A clinicopathological study. Asian Pac J Cancer Prev.
21:1155–1162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim JJ, Kurita T and Bulun SE:
Progesterone action in endometrial cancer, endometriosis, uterine
fibroids, and breast cancer. Endocr Rev. 34:130–162. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao D, Zhang F, Zhang W, He J, Zhao Y and
Sun J: Prognostic role of hormone receptors in ovarian cancer: A
systematic review and meta-analysis. Int J Gynecol Cancer.
23:25–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu Y, Yang O, Fazli L, Rennie PS, Gleave
ME and Dong X: Progesterone receptor expression during prostate
cancer progression suggests a role of this receptor in stromal cell
differentiation. Prostate. 75:1043–1050. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tavares CB, Gomes-Braga FD, Costa-Silva
DR, Escórcio-Dourado CS, Borges US, Conde-Junior AM,
Barros-Oliveira Mda C, Sousa EB, Barros Lda R, Martins LM, et al:
Expression of estrogen and progesterone receptors in astrocytomas:
A literature review. Clinics (Sao Paulo). 71:481–486. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jiang H, Teng R, Wang Q, Zhang X, Wang H,
Wang Z, Cao J and Teng L: Transcriptional analysis of estrogen
receptor alpha variant mRNAs in colorectal cancers and their
matched normal colorectal tissues. J Steroid Biochem Mol Biol.
112:20–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Qasim BJ, Ali HH and Hussein AG:
Immunohistochemical expression of estrogen and progesterone
receptors in human colorectal adenoma and carcinoma using specified
automated cellular image analysis system: A clinicopathological
study. Oman Med J. 26:307–314. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Koo JH and Leong RW: Sex differences in
epidemiological, clinical and pathological characteristics of
colorectal cancer. J Gastroenterol Hepatol. 25:33–42. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Berta L, Fronticelli Baldelli C, Fazzari
A, Radice E, Bargoni A, Frairia R and Gaetini A: Sex steroid
receptors, secondary bile acids and colorectal cancer. A possible
mechanism of interaction. Panminerva Med. 45:261–266.
2003.PubMed/NCBI
|
|
48
|
Ye SB, Cheng YK, Zhang L, Wang XP, Wang L
and Lan P: Prognostic value of estrogen receptor-α and progesterone
receptor in curatively resected colorectal cancer: A retrospective
analysis with independent validations. BMC Cancer. 19:9332019.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yazdani S, Kasajima A, Onodera Y, McNamara
KM, Ise K, Nakamura Y, Tachibana T, Motoi F, Unno M and Sasano H:
Progesterone arrested cell cycle progression through progesterone
receptor isoform A in pancreatic neuroendocrine neoplasm. J Steroid
Biochem Mol Biol. 178:243–253. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sasso CV, Santiano FE, Campo Verde Arboccó
F, Zyla LE, Semino SN, Guerrero-Gimenez ME, Pistone Creydt V, López
Fontana CM and Carón RW: Estradiol and progesterone regulate
proliferation and apoptosis in colon cancer. Endocr Connect.
8:217–229. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nakayama Y, Sakamoto H, Satoh K and
Yamamoto T: Tamoxifen and gonadal steroids inhibit colon cancer
growth in association with inhibition of thymidylate synthase,
survivin and telomerase expression through estrogen receptor beta
mediated system. Cancer Lett. 161:63–71. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lointier P, Wildrick DM and Boman BM: The
effects of steroid hormones on a human colon cancer cell line in
vitro. Anticancer Res. 12:1327–1330. 1992.PubMed/NCBI
|
|
53
|
Su MQ, Zhou YR, Rao X, Yang H, Zhuang XH,
Ke XJ, Peng GY, Zhou CL, Shen BY and Dou J: Baicalein induces the
apoptosis of HCT116 human colon cancer cells via the upregulation
of DEPP/Gadd45a and activation of MAPKs. Int J Oncol. 53:750–760.
2018.PubMed/NCBI
|
|
54
|
Li D, Dai C, Yang X, Li B, Xiao X and Tang
S: GADD45a regulates olaquindox-induced DNA damage and S-phase
arrest in human hepatoma G2 cells via JNK/p38 pathways. Molecules.
22:1242017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Song L, Li J, Zhang D, Liu ZG, Ye J, Zhan
Q, Shen HM, Whiteman M and Huang C: IKKbeta programs to turn on the
GADD45alpha-MKK4-JNK apoptotic cascade specifically via p50
NF-kappaB in arsenite response. J Cell Biol. 175:607–617. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ueda T, Kohama Y, Kuge A, Kido E and
Sakurai H: GADD45 family proteins suppress JNK signaling by
targeting MKK7. Arch Biochem Biophys. 635:1–7. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Camilleri-Robles C, Serras F and Corominas
M: Role of D-GADD45 in JNK-dependent apoptosis and regeneration in
drosophila. Genes. 10:3782019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Choi HJ, Kang KS, Fukui M and Zhu BT:
Critical role of the JNK-p53-GADD45α apoptotic cascade in mediating
oxidative cytotoxicity in hippocampal neurons. Br J Pharmacol.
162:175–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yin F, Bruemmer D, Blaschke F, Hsueh WA,
Law RE and Herle AJ: Signaling pathways involved in induction of
GADD45 gene expression and apoptosis by troglitazone in human MCF-7
breast carcinoma cells. Oncogene. 23:4614–4623. 2004. View Article : Google Scholar : PubMed/NCBI
|